Published online Nov 25, 2022. doi: 10.5501/wjv.v11.i6.467
Peer-review started: September 13, 2022
First decision: September 29, 2022
Revised: October 8, 2022
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: November 25, 2022
Processing time: 70 Days and 18.6 Hours
Most of the antiseizure medications (ASMs) are metabolized in liver and many of them particularly first-generation ASMs have the potential to increase liver enzymes or induce liver injury. Hence, treatment of new onset seizures or epilepsy by ASMs during the course of coronavirus disease 2019 (COVID-19), which could potentially be complicated by hepatic dysfunction, is a challenging clinical issue. Intravenous form of levetiracetam which has no significant hepatic metabolism or drug-drug interaction is often a favorable option to control seizures in acute phase of COVID-19. Administration of enzyme inducer ASMs and valproate with the well-known hepatotoxicity and common drug interactions is not generally recommended. In patients with epilepsy who are under control with potentially hepatotoxic ASMs, close observation and cautious dose reduction or drug switch should be considered if any evidence of hepatic impairment exists. However, risks of possible breakthrough seizures should be weighed against benefits of lowering the hazard of liver injury. In patients with epilepsy who receive polytherapy with ASMs, transient dose modification with the tendency to increase the dose of ASMs with more favorable safety profile and less drug interaction and decrease the dose of drugs with main hepatic metabolism, high protein binding, potential to cause liver injury and known drug-drug reaction should be considered. Finally, decision making should be individualized based on patients’ conditions and course of illness.
Core Tip: Most of antiseizure medications (ASMs) are metabolized in liver and many of them particularly first-generation ASMs have the potential to increase liver enzymes or induce liver injury. Hence, treatment of new onset seizures or epilepsy by ASMs during the course of coronavirus disease 2019 (COVID-19), which could potentially be complicated by hepatic dysfunction, is a challenging clinical issue. In this review, we aimed to discuss the potential risks of liver injury in patients with COVID-19 who are under treatment for epilepsy or need to receive ASMs to subside acute symptomatic seizures.
- Citation: Tabrizi N, Sharifi-Razavi A. Potential risk of liver injury in epileptic patients during COVID-19 pandemic. World J Virol 2022; 11(6): 467-476
- URL: https://www.wjgnet.com/2220-3249/full/v11/i6/467.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i6.467
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread across the globe, creating the coronavirus disease 2019 (COVID-19) pandemic[1]. Despite the advent of COVID-19 vaccines, the global pandemic continues[2,3]. Although, the lungs are the main target organs infected during COVID-19[4,5] and the initial reported symptoms of disease focused on the respiratory system[6]; this coronavirus can also invade multiple systems (immune and nervous systems) and target several organs and tissues (brain, liver, heart, lung, intestine, muscle, kidney, and gastrointestinal tract[3,7,8]). Liver is one of the most frequently impaired organs and elevation of serum aminotransferases has been recorded in some patients with COVID-19[9-11]. Most COVID-19 patients with liver dysfunction present elevations in one or more aminotransferases, with less than a three-fold increase from the normal values[12,13]. In most patients, liver injury seems to be self-limiting, neither requiring any specific intervention, nor is associated with acute liver failure[14,15]. Chen et al[4], in a retrospective study on 830 cases, reported 27.3% of the COVID-19 patients presented with mild abnormalities in the liver function and approximately 3.9% eventually developed liver insufficiency[4]. Yip et al[16], reported 23% elevation of liver enzymes and 2% acute liver injury in a cohort study of 1040 patients[16]. In another meta-analysis, approximately 25% of COVID-19 patients experienced elevation of liver enzymes which was directly correlated to the severity of COVID-19 disease[17]. Liver dysfunction could also increase the mortality rate in these patients[18].
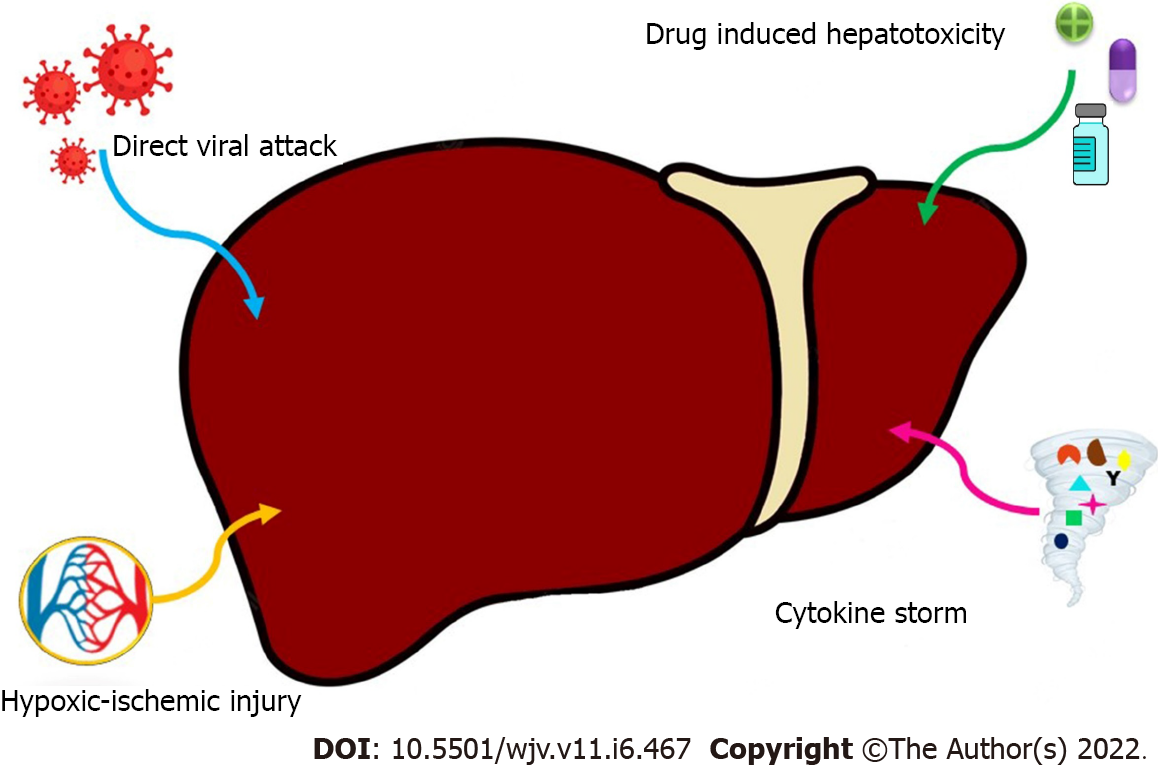
Possible mechanisms of liver injury are complex and include direct viral attack, hypoxic/ischemic injury, COVID-19 hyperinflammatory response and potential hepatotoxicity from therapeutic drugs[19,20] (Figure 1).
With the advent of the COVID-19, another health burden involved around 50 million people with epilepsy worldwide[21]. Epilepsy does not make patients more vulnerable to COVID-19 or its severe manifestations[22]. But, management of COVID-19 in patients with epilepsy needs special considerations. Many antiseizure medications (ASMs) have interactions with drugs commonly used for treatment of COVID-19[23]. Many patients with autoimmune epilepsy are under treatment with corticosteroids and other immunosuppressive drugs which might affect the defense ability of immune system[24]. On the other hand, seizure and status epilepticus as neurological manifestations of COVID-19 have been reported in patients with and without epilepsy[25-29]. In certain types of epilepsy particularly Dravet syndrome, fever might trigger seizures. Meanwhile, usual antipyretic and antihistaminic medications might lower seizure threshold in patients with epilepsy[24].
Most of the ASMs have hepatic metabolism and many of them especially older ASMs have the potential to increase hepatic enzymes or cause severe liver injury[30]. Treatment with these ASMs in patients with COVID-19 who have a potential predisposition to hepatic dysfunction, should proceed cautiously considering certain characteristics of medications and disease course. In this review, we aimed to discuss the potential risks of liver injury in patients with COVID-19 who are under treatment for epilepsy or need to receive ASMs to subside acute symptomatic seizures.
Several mechanisms might be involved in occurrence of acute symptomatic seizures during COVID-19 infection. SARS-CoV-2 could directly invade central nervous system by targeting angiotensin-converting-enzyme-2 (ACE-2) receptor and consequent meningoencephalitis could be a potential etiology of seizure[31-34]. Also, three indirect mechanisms including down-regulation of ACE-2 expression, cytokine storm and hypoxia could precipitate seizures[31]. Metabolic derangement and organ failure are among the other possible causes of seizure in patients with COVID-19. Detection and management of etiology often need serum metabolic and electrolyte investigation, cerebrospinal fluid analysis and brain imaging[28]. Short-term use of ASMs is often recommended to manage seizures in acute phase of COVID-19[35]. However, judicious selection of the ASMs is necessary to prevent exacerbation of organ failure, particularly liver dysfunction and also to decrease the possible drug interactions.
Moreover, new-onset refractory status epilepticus with a mortality rate of 10% to 20% has been reported secondary to COVID-19. Considering the undesirable response to ASMs, plasma exchange, intravenous immunoglobulin, steroids and immunosuppressives have been used for management of these patients with different success rates. There is no definite approach to manage these patients and the suggested treatment algorithm should be modified individually based on patient’s conditions[29].
The COVID-19 pandemic has had several negative impacts on patients with epilepsy, which are beyond the scope of this paper. In patients with epilepsy who experience COVID-19 infection, breakthrough seizures might occur for at least three reasons. Firstly, predisposing factors such as sepsis, sleep deprivation, metabolic derangement and electrolyte imbalance along with previously mentioned direct and indirect mechanisms of acute phase seizures, could precipitate breakthrough seizures in patients with epilepsy[31]. Secondly, fever can trigger seizures in certain types of epilepsy particularly Dravet syndrome[24]. Finally, common medications used for treatment of COVID-19 could induce seizure via lowering seizure threshold or decreasing the efficacy of ASMs through drug-drug interaction[36]. Thus, recognition and addressing all possible causes are necessary to control the seizures and prevent the consequent morbidity and mortality. This point is of the greatest importance in patients with drug-resistant epilepsy.
Furthermore, previously used ASMs in patients with epilepsy, might need modifications when COVID-19 complications such as cardiac, hepatic or renal dysfunction occur. Dose adjustment of ASMs should be considered in patients with hepatic or renal impairment. On the other hand, drug switch or dose reduction might be necessary if ASMs have the potential to aggravate organ failure. However, the possible risk of uncontrolled seizures induced by changes in type and dose of ASMs, should be weighed against the benefits of modifications and it might be injudicious for some ASMs with a high risk of withdrawal seizure and status epilepticus such as barbiturates.
Valproate is a broad-spectrum ASM with a high bioavailability (90%) and high protein binding (74%-93%). It has several mechanisms of action including increase in gamma amino butyric acid (GABA) activity and blockage of voltage-gated Na+, Ca2+ and K+ channels. Valproate extensively metabolized in the liver via glucuronidation, β-oxidation and oxidation by cytochrome P450[37]. Valproate inhibits CYP2C9, uridine glucoronate-glucuronsyl transferase (UGT), and epoxide hydrolase[22]. Protease inhibitors, such as lopinavir/ritonavir could increase metabolism of valproate by induction of valproate glucuronidation[37]. In contrast, valproate decreases the plasma concentrations of darunavir/cobicistat and increases the concentrations of lopinavir/ritonavir[36]. Valproate has no significant interaction with the other anti-COVID-19 drugs. However, there is a red flag for using this ASM in patients with abnormal liver function. Hepatotoxicity is a well-known adverse event of valproate[38]. It might occur through different mechanisms such as formation of valproate reactive metabolites, inhibition of fatty acid β-oxidation and excessive oxidative stress[39,40]. Valproate-induced liver injury has different degrees. The most common type is asymptomatic increase in liver enzymes. More than 3 times increase in liver function tests makes drug discontinuation necessary. The known risk factors for valproate hepatotoxicity are young age, polytherapy, developmental delay, metabolic disorders, febrile illness and polymerase gamma 1 related disorders[38]. Furthermore, valproate can cause hyperammonemic encephalopathy which presents as progressive confusional state leading to coma[41,42]. This condition could easily be neglected in a critically ill patient with COVID-19.
In conclusion, despite the high efficacy in treatment of various type of seizures, factors including possible drug interaction, potential to cause liver injury, exacerbation of underlying liver dysfunction and induction of hyperammonemia, have limited the use of valproate as the first line treatment in patients with COVID-19 and new onset seizures. However, in patients with epilepsy and COVID-19 who were under control by valproate, decision making is more challenging. The clinician might choose not to switch the medication at the first step; but the possibility of interaction with mentioned anti-COVID drugs should be closely observed by therapeutic drug monitoring. In addition, in case of any evidence of liver dysfunction, there should be a low threshold to lower the dose or switch the drug.
Phenytoin, carbamazepine, phenobarbital and primidone are among the first generation of ASMs. Their strong potential to induce various cytochrome p450 enzymes often causes several drug-drug interactions[43-46].
Phenytoin is one of the oldest ASMs which plays its antiseizure role by enhancing rapid inactivation of voltage-gated sodium channels[47]. Phenytoin has a high protein binding (> 90%) and 70%-100% bioavailability. It is metabolized by CYP2C9 and CYP2C19 hepatic isoenzymes.
It induces CYP1A2, CYP2B, CYP2C, CYP3A4, and UGT[22]. Phenytoin significantly decreases the serum concentration of atazanavir, darunavir/cobicistat, remdesivir, chloroquine and hydroxychloroquine and has a potential to decrease serum level of lopinavir/ritonavir. Nitazoxanide partially increases and tocilizumab weakly decreases the serum concentration of phenytoin. Phosphenytoin, the water-soluble prodrug of phenytoin has the same drug-drug interactions[36].
Hepatotoxicity is a well-known adverse effect of phenytoin which probably occurs through increase in reactive oxygen species formation and cellular oxidized glutathione, decrease in intracellular reduced glutathione, enhancement of lipid peroxidation and mitochondrial damage[48,49]. Phenytoin-induced liver injury could have a broad spectrum from mild asymptomatic elevation in liver function tests to severe hepatotoxicity which is often associated with hypersensitivity reactions[37,48,50,51]. Although the cosmetic and systemic adverse events have limited its use in chronic epilepsy, phenytoin is commonly used to abort focal and generalized seizures and also status epilepticus in emergency department[52,53]. However, it is not a good option to control seizures in patients with COVID-19. Phenytoin might cause cardiorespiratory depression which is potentially harmful in critically ill patients, elderly and underlying cardiac disease[54]. The potential for hepatotoxicity and increase in free drug level in hepatic and renal impairment[55] also limited its use in COVID-19. Moreover, significant dug-drug reaction with anti-COVID-19 agents could be challenging.
Carbamazepine is an effective ASM with a high bioavailability (75%-85%) and high protein binding (70%-80%). Its mechanism of action is similar to phenytoin. Carbamazepine is metabolized in liver by CYP3A4 and CYP2C8 enzymes[37]. It induces CYP1A2, CYP2C, CYP3A4 and UGT[22] and so, has multiple drug-drug interactions with anti-COVID medications. It significantly decreases the serum concentration of atazanavir, darunavir/cobicistat, remdesivir, chloroquine and hydroxychloroquine. Co-administration of carbamazepine with lopinavir/ritonavir also might lead to decrease in serum level of anti-COVID agent. Atazanavir, darunavir/cobicistat and lopinavir/ritonavir could increase serum concentration of carbamazepine and cause toxicity. In addition, tocilizumab has the potential to decrease carbamazepine concentration[36].
In a report of ASM-induced liver injury by FDA, carbamazepine had the highest odds ratio (2.92) among the other ASMs of first generation[30] and hepatotoxicity is a well-known adverse effect of this potent ASM[56]. Metabolic activation and following immune responses are reported as possible mechanisms of carbamazepine-induced liver injury[57].
Carbamazepine has no parenteral formulation and needs about 3 to 5 wk to reach the steady state. So, it is not commonly used for treatment of seizures in acute phase. However, many of patients with epilepsy are under treatment with this ASM. When comorbidity with COVID-19 occurs in these patients, higher doses of antiviral agents might be needed to compensate the decrement of serum concentration caused by carbamazepine. On the other hand, patients should be closely observed for sign and symptoms of carbamazepine toxicity in co-administration of atazanavir, darunavir/cobicistat and lopinavir/ritonavir. In critical patients with increased liver enzymes, reduction of carbamazepine dosage is generally recommended to prevent harmful increase in carbamazepine concentration and also further liver damage.
Phenobarbital, one of the first ASMs used to manage epilepsy, is of limited use currently. But it is still recommended as an alternative therapy in first and second line management of status epilepticus. Phenobarbital is also prescribed in some patients with epilepsy especially in countries with limited resources[58]. It plays its antiseizure role by affecting GABA-A receptors which leads to increase in chloride ions and consequently reduction of neuronal excitability. Phenobarbital has a high bioavailability (> 90%) and moderate protein binding (55%)[59].
Primidone is another old ASM which affects synaptic and extrasynaptic GABA receptors[42]. It is metabolized to phenobarbital and phenylethylmalonamide by CYP2C9, CYP2C19, and CYP2E1 enzymes[22]. Primidone is still prescribed for patients with epilepsy; but it has some other certain indications such as essential tremor as well[60]. It has a high bioavailability (> 90%) with a low plasma protein binding (10%)[59]. Phenobarbital and primidone induce CYP1A2, CYP2A6, CYP2B, CYP2C, CYP3A4, and UGT. Similar to other enzyme inducer ASMs, these two drugs considerably decrease the serum concentration of atazanavir, darunavir/cobicistat, remdesivir, chloroquine and hydroxychloroquine and could possibly decrease serum level of lopinavir/ritonavir. Darunavir/cobicistat significantly decreases the serum concentration of phenobarbital, but has no effect on primidone. Lopinavir/ritonavir might decrease primidone level[36].
Phenobarbital can cause large spectrum of hepatic adverse effects which could be various from asymptomatic increase in liver enzymes, to devastating hepatitis and acute liver failure. A possible mechanism of liver injury by phenobarbital is oxidative stress in hepatic mitochondria[38,61,62]. Due to availability of newer effective ASMs with more favorable safety profile in recent decade, phenobarbital has been less frequently administered in acute phase seizures. IV phenobarbital has the potential to cause cardiorespiratory depression[63] and elevation of liver enzymes[38] in critically ill patients with COVID-19 who are potentially in a compromised respiratory and hepatic state. Hence, phenobarbital is an inappropriate choice for treatment of seizures in COVID-19. In patients with epilepsy who are under treatment with phenobarbital and primidone, serious drug-drug interaction with anti-COVID-19 agents should be considered. Since, rapid taper and switch of these 2 drugs are impossible due to high risk of withdrawal seizure and status epilepticus, they should be continued cautiously with slight dose reduction in hepatic impairment and therapeutic drug monitoring. According dose modification of anti-COVID agents is also indispensable.
Several factors should be considered in treatment of seizures in acute phase of COVID-19. The selected ASM/ASMs should have the parenteral formulation to achieve a rapid appropriate serum level. The safety profile and low risk for systemic adverse effects are also very important; particularly if the disease course is already complicated with organ failure. Most of ASMs have hepatic metabolism and many of them could potentially cause hepatotoxicity which makes judicious selection and dose modification necessary. Moreover, several drug-drug interactions are expected between ASMs and anti-COVID drugs which could form a more complicated clinical scenario. The possible drug-drug interactions of common ASMs and anti-COVID-19 agents have been summarized in Table 1.
| ATV | DRV/c | LPV/r | RDV | FAV | HCLQ/CLQ | TCZ | IFN-β-1α | |
| Brivaracetam | Mi | - | Mi | - | - | Mo | - | - |
| Carbamazepine | S | S | Mo | S | - | S | Mi | Mo |
| Clobazam | Mo | Mo | Mo | - | - | - | - | - |
| Diazepam | Mo | Mo | Mo | - | - | - | - | - |
| Eslicarbazepine | Mo | Mo | Mo | Mo | - | Mo | - | - |
| Ethosuximide | Mo | Mo | Mo | - | - | - | - | - |
| Gabapentin | - | - | - | - | - | - | - | - |
| Lacosamide | Mi | Mo | Mi | - | - | - | - | - |
| Lamotrigine | - | Mo | Mo | - | - | - | - | - |
| Levetiracetam | - | - | - | - | - | - | - | - |
| Lorazepam | - | - | - | - | - | - | - | - |
| Oxcarbazepine | Mo | Mo | Mo | Mo | - | Mo | - | Mo |
| Perampanel | Mo | Mo | Mo | - | - | - | - | - |
| Phenytoin | S | S | Mo | S | - | S | Mi | Mo |
| Phenobarbital | S | S | Mo | S | - | S | Mi | Mo |
| Pregabalin | - | - | - | - | - | - | - | - |
| Primidone | S | S | Mo | S | - | S | Mi | - |
| Rufinamide | Mo | Mo | Mo | Mo | - | Mo | - | - |
| Topiramate | - | Mo | - | - | - | - | - | - |
| Valproic acid | - | Mo | Mo | - | - | - | - | Mo |
| Vigabatrin | - | - | - | - | - | - | - | - |
| Zonisamide | - | Mo | - | - | - | - | - | - |
Levetiracetam, lorazepam, gabapentin, vigabatrin and pregabalin are ASMs which have no interaction with anti-COVID drugs[36]. Among these ASMs, only levetiracetam and lorazepam have the parenteral form. IV lorazepam is the first line treatment to abort generalized convulsive seizure[64]. Benzodiazepines predominantly have hepatic metabolism. Metabolism of lorazepam is not significantly affected by liver dysfunction and the possibility of liver injury is very low with its administration[65]. However, it might cause transient respiratory depression and exacerbation of hepatic encephalopathy[22]. So, cautious use of lorazepam is acceptable for first-line treatment of seizure; but it could not be used as maintenance therapy to prevent further seizures.
Levetiracetam is an efficient broad spectrum ASM which is commonly used in treatment of epilepsy, acute phase seizures and status epilepticus[66-69]. It has a high bioavailability (> 95%) and a very low protein binding (< 10%). Less than 2% of levetiracetam is metabolized in liver which makes it a safe drug with no significant pharmacokinetic interaction[22]. It is postulated that levetiracetam mainly presents its antiseizure effect by targeting the synaptic vesicle glycoprotein SV2A[70]. Levetiracetam is a safe ASM for patients with liver dysfunction. There are very rare reports of levetiracetam-induced liver injury and elevation of liver enzymes[30]. No significant difference in pharmacokinetic of levetiracetam is expected in patients with mild to moderate hepatic impairment. But 50% reduction in total dose is recommended due to decreased drug clearance in patients with severe hepatic failure (Child-Pugh Class C)[65]. Overall, IV formulation of levetiracetam is a safe and efficient choice for treatment of acute onset seizures in COVID-19.
Eslicarbazepine acetate, oxcarbazepine, lacosamide, lamotrigine, clobazam, perampanel, rufinamide, tiagabine, topiramate, and zonisamide have mild to moderate interaction with anti-COVID drugs[22]. Among these ASMs, lacosamide is available in IV form and commonly has been used in treatment of seizure and status epilepticus[71,72]. Lacosamide has an almost complete bioavailability and a very low (< 15%) protein binding. It is metabolized to inactive O-desmethyl derivatives by CYP2C19 in liver[37]. Lacosamide enhances the slow inactivation of voltage-gated sodium channels[73]. In patients with mild to moderate hepatic impairment, reduction to 75% of maximum dose is recommended. But, lacosamide should not be administered in patients with severe hepatic dysfunction. Lacosamide-induced liver injury has not been reported in the literature[30]. So, IV lacosamide is an appropriate choice for aborting seizure in patients with epilepsy and COVID-19; but dose adjustment in hepatic dysfunction, interaction with darunavir/cobicistat and potential PR prolongation in coadministration with atazanavir and lopinavir/ritonavir should be cautiously considered[22].
Among previously mentioned ASMs with the higher probability of liver injury, IV formulations of valproate, phenytoin and phenobarbital are available. However, use of these ASMs should be limited to special conditions such as unavailability of new generation ASMs and refractoriness of seizures.
Many patients with epilepsy need long-term treatment with ASMs and drug withdrawal or switch might lead to breakthrough seizures or status epilepticus for them. Since, anti-COVID drugs-which have the most interaction with ASMs-are generally administered for a short course, mild to moderate drug-drug interactions could be cautiously managed by close observation, therapeutic drug monitoring and dose modifications. However, concurrent administration of drugs with severe interactions is not recommended. In these cases, the medical team should evaluate the risk and benefits of choosing a safer anti-COVID drug over switching the ASM.
In patients with controlled epilepsy who suffer from liver dysfunction during COVID-19 infection, appropriate dose adjustment of ASMs is the first step[22]. This approach could prevent serum concentration of drugs to reach the toxic level and also could protect liver from further injury. In this stage, there should be a low threshold to reduce the dose or switch ASMs with a high potential of hepatotoxicity. In patients with drug-resistant epilepsy or those who are on polytherapy with ASMs, transient dose reduction of hepatotoxic drugs and increase in dose of ASMs with more favorable profile might help the patients to pass the critical course without experiencing breakthrough seizures.
However, if severe liver injury occurs, some ASMs should be inevitably discontinued. Appropriate replacement of these drugs by safer ASMs such as levetiracetam could prevent seizure recurrence and subsequent complications.
COVID-19 pandemic has affected many people all over the world. Liver injury is a well-known complication of this infection and have an impact on management of patients with comorbidities. Particularly, management of seizure and epilepsy in patients with COVID-19 and liver injury could be challenging. Certain considerations should be taken in account in selection of ASMs for patients with new-onset seizures. Avoidance of ASMs with potential of hepatotoxicity, reasonable dose adjustment and monitoring of drug interactions with anti-COVID-19 drugs are necessary. Furthermore, in patients with epilepsy, cautious changes in dose and type of previously used ASMs are sometimes necessary. The possibility of drug-drug interactions along with the other comorbidities of patients should also be considered. Decision making by a medical team consists of different related specialties is often necessary to choose the best treatment method for the patients.
| 1. | Sasanejad P, Afshar Hezarkhani L, Arsang-Jang S, Tsivgoulis G, Ghoreishi A, Barlinn K, Rahmig J, Farhoudi M, Sadeghi Hokmabadi E, Borhani-Haghighi A, Sariaslani P, Sharifi-Razavi A, Ghandehari K, Khosravi A, Smith C, Nilanont Y, Akbari Y, Nguyen TN, Bersano A, Yassi N, Yoshimoto T, Lattanzi S, Gupta A, Zand R, Rafie S, Pourandokht Mousavian S, Reza Shahsavaripour M, Amini S, Kamenova SU, Kondybayeva A, Zhanuzakov M, Macri EM, Nobleza COS, Ruland S, Cervantes-Arslanian AM, Desai MJ, Ranta A, Moghadam Ahmadi A, Rostamihosseinkhani M, Foroughi R, Hooshmandi E, Akhoundi FH, Shuaib A, Liebeskind DS, Siegler J, Romano JG, Mayer SA, Bavarsad Shahripour R, Zamani B, Woolsey A, Fazli Y, Mojtaba K, Isaac CF, Biller J, Di Napoli M, Azarpazhooh MR. Safety and Outcomes of Intravenous Thrombolytic Therapy in Ischemic Stroke Patients with COVID-19: CASCADE Initiative. J Stroke Cerebrovasc Dis. 2021;30:106121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Jiang SX, Schwab K, Enns R, Ko HH. Survey of the Impact of COVID-19 on Chronic Liver Disease Patient Care Experiences and Outcomes. J Can Assoc Gastroenterol. 2022;18:gwac022. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Bouare N, Minta DK, Dabo A, Gerard C. COVID-19: A pluralistic and integrated approach for efficient management of the pandemic. World J Virol. 2022;11:20-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (8)] |
| 4. | Chen F, Chen W, Chen J, Xu D, Xie W, Wang X, Xie Y. Clinical features and risk factors of COVID-19-associated liver injury and function: A retrospective analysis of 830 cases. Ann Hepatol. 2021;21:100267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Sharifi-Razavi A, Karimi N, Zarvani A, Cheraghmakani H, Baghbanian SM. Ischemic stroke associated with novel coronavirus 2019: a report of three cases. Int J Neurosci. 2021;131:1243-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Amiri HA, Razavi AS, Tabrizi N, Cheraghmakani H, Baghbanian SM, Sedaghat-Chaijan M, Zarvani A, Ghazaeian M, Hosseinnataj A. The Effects of COVID-19 on Patients with Acute Ischemic and Hemorrhagic Stroke. J Stroke Cerebrovasc Dis. 2022;31:106512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Sharifi-Razavi A, Sedaghat Z, Baziboroun M, Karimi N. COVID-19 accompanied with intracerebral hemorrhage: A case series. Arch Clin Infect Dis. 2020;15:e104877. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | John KJ, Mishra AK, Ramasamy C, George AA, Selvaraj V, Lal A. Heart failure in COVID-19 patients: Critical care experience. World J Virol. 2022;11:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1214] [Article Influence: 202.3] [Reference Citation Analysis (0)] |
| 10. | Mohammed SA, Eid KM, Anyiam FE, Wadaaallah H, Muhamed MAM, Morsi MH, Dahman NBH. Liver injury with COVID-19: laboratory and histopathological outcome-systematic review and meta-analysis. Egypt Liver J. 2022;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Kumar R, Kumar V, Arya R, Anand U, Priyadarshi RN. Association of COVID-19 with hepatic metabolic dysfunction. World J Virol. 2022;11:237-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30462] [Article Influence: 5077.0] [Reference Citation Analysis (12)] |
| 13. | Yu D, Du Q, Yan S, Guo XG, He Y, Zhu G, Zhao K, Ouyang S. Liver injury in COVID-19: clinical features and treatment management. Virol J. 2021;18:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 14. | Ghoda A, Ghoda M. Liver Injury in COVID-19 Infection: A Systematic Review. Cureus. 2020;12:e9487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 364] [Article Influence: 60.7] [Reference Citation Analysis (4)] |
| 16. | Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 17. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Macías-Rodríguez RU, Solís-Ortega AA, Ornelas-Arroyo VJ, Ruiz-Margáin A, González-Huezo MS, Urdiales-Morán NA, Román-Calleja BM, Mayorquín-Aguilar JM, González-Regueiro JA, Campos-Murguía A, Toledo-Coronado IV, Chapa-Ibargüengoitia M, Valencia-Peña B, Martínez-Cabrera CF, Flores-García NC. Prognostic performance of an index based on lactic dehydrogenase and transaminases for patients with liver steatosis and COVID-19. World J Gastroenterol. 2022;28:5444-5456. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (4)] |
| 19. | Zghal M, Bouhamed M, Mellouli M, Triki M, Kallel R, Ayedi L, Boudawara TS, Makni S. Liver injury in COVID-19: pathological findings. Pan Afr Med J. 2022;41:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | McConnell MJ, Kondo R, Kawaguchi N, Iwakiri Y. Covid-19 and Liver Injury: Role of Inflammatory Endotheliopathy, Platelet Dysfunction, and Thrombosis. Hepatol Commun. 2022;6:255-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 21. | GBD 2016 Epilepsy Collaborators. Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:357-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 656] [Cited by in RCA: 669] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 22. | Asadi-Pooya AA, Attar A, Moghadami M, Karimzadeh I. Management of COVID-19 in people with epilepsy: drug considerations. Neurol Sci. 2020;41:2005-2011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Kuroda N. Epilepsy and COVID-19: Updated evidence and narrative review. Epilepsy Behav. 2021;116:107785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | French JA, Brodie MJ, Caraballo R, Devinsky O, Ding D, Jehi L, Jette N, Kanner A, Modi AC, Newton CR, Patel AA, Pennell PB, Perucca E, Sander JW, Scheffer IE, Singh G, Williams E, Wilmshurst J, Cross JH. Keeping people with epilepsy safe during the COVID-19 pandemic. Neurology. 2020;94:1032-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 25. | Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1282] [Cited by in RCA: 1454] [Article Influence: 242.3] [Reference Citation Analysis (0)] |
| 26. | Lu L, Xiong W, Liu D, Liu J, Yang D, Li N, Mu J, Guo J, Li W, Wang G, Gao H, Zhang Y, Lin M, Chen L, Shen S, Zhang H, Sander JW, Luo J, Chen S, Zhou D. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia. 2020;61:e49-e53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 27. | Dono F, Nucera B, Lanzone J, Evangelista G, Rinaldi F, Speranza R, Troisi S, Tinti L, Russo M, Di Pietro M, Onofrj M, Bonanni L, Assenza G, Vollono C, Anzellotti F, Brigo F. Status epilepticus and COVID-19: A systematic review. Epilepsy Behav. 2021;118:107887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Asadi-Pooya AA, Simani L, Shahisavandi M, Barzegar Z. COVID-19, de novo seizures, and epilepsy: a systematic review. Neurol Sci. 2021;42:415-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Arif A, Chavarria Y, Qamar MA, Tebha SS, Butt M, Qamar K, Yosufi A. New-Onset Refractory Status Epilepticus Secondary to COVID-19 Infection in Adults: A Systematic Review. Neuropsychiatr Dis Treat. 2022;18:1951-1961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Kamitaki BK, Minacapelli CD, Zhang P, Wachuku C, Gupta K, Catalano C, Rustgi V. Drug-induced liver injury associated with antiseizure medications from the FDA Adverse Event Reporting System (FAERS). Epilepsy Behav. 2021;117:107832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Narula N, Joseph R, Katyal N, Daouk A, Acharya S, Avula A, Maroun R. Seizure and COVID-19: association and review of potential mechanism. Neurol Psychiatry Brain Res. 2020;38:49-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, Talbot PJ. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 590] [Cited by in RCA: 717] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 33. | Fotuhi M, Mian A, Meysami S, Raji CA. Neurobiology of COVID-19. J Alzheimers Dis. 2020;76:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 34. | Iroegbu JD, Ifenatuoha CW, Ijomone OM. Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2. Neurol Sci. 2020;41:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Asadi-Pooya AA. Seizures associated with coronavirus infections. Seizure. 2020;79:49-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 36. | Russo E, Iannone L. 2020. Clinically relevant Drug-Drug interaction between AEDs and medications used in the treatment of COVID-19 patients. [cited 20 August 2022]. Available from: ilae.org/files/dmfile/Antiepileptic-drugs-interactions_in_COVID-19.pdf. |
| 37. | Karaźniewicz-Łada M, Główka AK, Mikulska AA, Główka FK. Pharmacokinetic Drug-Drug Interactions among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Vidaurre J, Gedela S, Yarosz S. Antiepileptic Drugs and Liver Disease. Pediatr Neurol. 2017;77:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Guo HL, Jing X, Sun JY, Hu YH, Xu ZJ, Ni MM, Chen F, Lu XP, Qiu JC, Wang T. Valproic Acid and the Liver Injury in Patients with Epilepsy: An Update. Curr Pharm Des. 2019;25:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 40. | Fu D, Cardona P, Ho H, Watkins PB, Brouwer KLR. Novel Mechanisms of Valproate Hepatotoxicity: Impaired Mrp2 Trafficking and Hepatocyte Depolarization. Toxicol Sci. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Habhab SF, Ulvin LB, Taubøll E, Svalheim S, Olsen KB, Horn MA, Heuser K. Influence of valproate-induced hyperammonemia on treatment decision in an adult status epilepticus cohort. Epilepsy Behav. 2020;111:107193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Smith KM, Britton JW, Hocker SE, Toledano M. Hyperammonemia in Patients With Status Epilepticus Treated With or Without Valproic Acid. Neurologist. 2021;26:80-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Kanner AM, Bicchi MM. Antiseizure Medications for Adults With Epilepsy: A Review. JAMA. 2022;327:1269-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 269] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 44. | Zaccara G, Perucca E. Interactions between antiepileptic drugs, and between antiepileptic drugs and other drugs. Epileptic Disord. 2014;16:409-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 45. | Brodie MJ. Sodium Channel Blockers in the Treatment of Epilepsy. CNS Drugs. 2017;31:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 46. | Lee-Lane E, Torabi F, Lacey A, Fonferko-Shadrach B, Harris D, Akbari A, Lyons RA, Rees MI, Sawhney I, Halcox J, Powell R, Pickrell WO. Epilepsy, antiepileptic drugs, and the risk of major cardiovascular events. Epilepsia. 2021;62:1604-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Patocka J, Wu Q, Nepovimova E, Kuca K. Phenytoin - An anti-seizure drug: Overview of its chemistry, pharmacology and toxicology. Food Chem Toxicol. 2020;142:111393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Eghbal MA, Taziki S, Sattari MR. Mechanisms of phenytoin-induced toxicity in freshly isolated rat hepatocytes and the protective effects of taurine and/or melatonin. J Biochem Mol Toxicol. 2014;28:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Wall M, Baird-Lambert J, Buchanan N, Farrell G. Liver function tests in persons receiving anticonvulsant medications. Seizure. 1992;1:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Sasaki E, Matsuo K, Iida A, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. A novel mouse model for phenytoin-induced liver injury: involvement of immune-related factors and P450-mediated metabolism. Toxicol Sci. 2013;136:250-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Crawshaw AA, Cock HR. Medical management of status epilepticus: Emergency room to intensive care unit. Seizure. 2020;75:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 53. | Nevitt SJ, Marson AG, Tudur Smith C. Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review. Cochrane Database Syst Rev. 2019;7:CD001911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Mathews SR, Badyal DK, Mathew R. Phenytoin-induced bradycardia and hypotension. Indian J Pharmacol. 2019;51:120-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Patsalos PN, Spencer EP, Berry DJ. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther Drug Monit. 2018;40:526-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 325] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 56. | Pal R, Singh K, Khan SA, Chawla P, Kumar B, Akhtar MJ. Reactive metabolites of the anticonvulsant drugs and approaches to minimize the adverse drug reaction. Eur J Med Chem. 2021;226:113890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Higuchi S, Yano A, Takai S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Metabolic activation and inflammation reactions involved in carbamazepine-induced liver injury. Toxicol Sci. 2012;130:4-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Farhat S, Nasreddine W, Alsaadi T, Beydoun AA, Arabi M, Beydoun A. Treatment of generalized convulsive status epilepticus: An international survey in the East Mediterranean Countries. Seizure. 2020;78:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Hakami T. Neuropharmacology of Antiseizure Drugs. Neuropsychopharmacol Rep. 2021;41:336-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 60. | Delgado N, Berry DS, Hernandez DI, Louis ED. Prospective, longitudinal analysis of medication use in a cohort of elderly essential tremor cases. J Neurol Sci. 2022;442:120387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Di Mizio G, Gambardella A, Labate A, Perna A, Ricci P, Quattrone A. Hepatonecrosis and cholangitis related to long-term phenobarbital therapy: an autopsy report of two patients. Seizure. 2007;16:653-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Santos NA, Medina WS, Martins NM, Rodrigues MA, Curti C, Santos AC. Involvement of oxidative stress in the hepatotoxicity induced by aromatic antiepileptic drugs. Toxicol In Vitro. 2008;22:1820-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Byun JI, Chu K, Sunwoo JS, Moon J, Kim TJ, Lim JA, Jun JS, Lee HS, Lee WJ, Lee DY, Jeon D, Lee ST, Jung KH, Jung KY, Lee SK. Mega-dose phenobarbital therapy for super-refractory status epilepticus. Epileptic Disord. 2015;17:444-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Kamdar HA, Hamed M, Smetana KS, Shanmugam K, Peters E, Yasin R, Thakur G, Gopal M, Sawalha K, Greene-Chandos D, Hussein O. Lorazepam timing for acute convulsive seizure control (LoTASC). Seizure. 2020;83:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Ahmed SN, Siddiqi ZA. Antiepileptic drugs and liver disease. Seizure. 2006;15:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Smith PEM. Initial Management of Seizure in Adults. N Engl J Med. 2021;385:251-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Haller JT, Bonnin S, Radosevich J. Rapid administration of undiluted intravenous levetiracetam. Epilepsia. 2021;62:1865-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Chamberlain JM, Kapur J, Shinnar S, Elm J, Holsti M, Babcock L, Rogers A, Barsan W, Cloyd J, Lowenstein D, Bleck TP, Conwit R, Meinzer C, Cock H, Fountain NB, Underwood E, Connor JT, Silbergleit R; Neurological Emergencies Treatment Trials; Pediatric Emergency Care Applied Research Network investigators. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020;395:1217-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 69. | Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D, Shinnar S, Conwit R, Meinzer C, Cock H, Fountain N, Connor JT, Silbergleit R; NETT and PECARN Investigators. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N Engl J Med. 2019;381:2103-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 365] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 70. | Löscher W, Gillard M, Sands ZA, Kaminski RM, Klitgaard H. Synaptic Vesicle Glycoprotein 2A Ligands in the Treatment of Epilepsy and Beyond. CNS Drugs. 2016;30:1055-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 71. | Strzelczyk A, Zöllner JP, Willems LM, Jost J, Paule E, Schubert-Bast S, Rosenow F, Bauer S. Lacosamide in status epilepticus: Systematic review of current evidence. Epilepsia. 2017;58:933-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 72. | Eilam A, Khmeliov N, Penker D, Gilad R. Intravenous Lacosamide in Seizure Clusters: Dose and Efficacy. Clin Neuropharmacol. 2021;44:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Rogawski MA, Tofighy A, White HS, Matagne A, Wolff C. Current understanding of the mechanism of action of the antiepileptic drug lacosamide. Epilepsy Res. 2015;110:189-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kar SK, India; Parvizpour F, Iran S-Editor: Fan JR L-Editor: A P-Editor: Fan JR