Published online Sep 25, 2022. doi: 10.5501/wjv.v11.i5.221
Peer-review started: March 13, 2022
First decision: April 8, 2022
Revised: May 14, 2022
Accepted: August 10, 2022
Article in press: August 10, 2022
Published online: September 25, 2022
Processing time: 195 Days and 10.2 Hours
Based on mucosal immunization to promote both mucosal and systemic immune responses, next-generation coronavirus disease 2019 (COVID-19) vaccines would be administered intranasally or orally. The goal of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines is to provide adequate immune protection and avoid severe disease and death. Mucosal vaccine candidates for COVID-19 including vector vaccines, recombinant subunit vaccines and live attenuated vaccines are under development. Furthermore, subunit protein vac-cines and virus-vectored vaccines have made substantial progress in preclinical and clinical settings, resulting in SARS-CoV-2 intranasal vaccines based on the previously successfully used nasal vaccines. Additional to their ability to trigger stable, protective immune responses at the sites of pathogenic infection, the development of ‘specific’ mucosal vaccines targeting coronavirus antigens could be an excellent option for preventing future pandemics. However, their efficacy and safety should be confirmed.
Core Tip: Oral or nasal vaccination against coronavirus disease 2019 (COVID-19) would stimulate both the humoral and cellular immune responses and may exert many socioeconomic benefits. Mucosal vaccines are promising for preventing infections and reducing the transmission, morbidity and mortality of COVID-19. Mucosal vaccination may be used prophylactically in human populations at high risk for severe acute respiratory syndrome coronavirus 2. Currently, only a limited number of oral vaccines are approved for human use, and some others are included in preclinical and clinical trials to validate their efficacy and safety.
- Citation: Miteva D, Peshevska-Sekulovska M, Snegarova V, Batselova H, Alexandrova R, Velikova T. Mucosal COVID-19 vaccines: Risks, benefits and control of the pandemic. World J Virol 2022; 11(5): 221-236
- URL: https://www.wjgnet.com/2220-3249/full/v11/i5/221.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i5.221
The current coronavirus disease 2019 (COVID-19) pandemic, characterized by the ongoing rapid spread and high mutation rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emphasizes the need for more efficient vaccinations to avoid preventable illness and mortality. In addition, SARS-CoV-2 is a mucosal pathogen that spreads via person-to-person respiratory droplets[1] and infects human respiratory epithelial cells and gastrointestinal tract by attaching to angiotensin-converting enzyme 2 via the spike (S) receptor-binding domain[2]. Thus, mucosal immunity will be primary for adequate and long-term viral protection[3].
To date, there are over 300 potential anti-SARS-CoV-2 vaccines at various stages of preclinical and clinical trials and 24 vaccines approved for emergency use in humans[4-6], (https://covid19.trackvaccines.org/trials-vaccines-by-country/). Approved vaccines and most of the preparations under development are intended to be administered intramuscularly to provide high levels of antibodies against systemic viral infection[7]. This method of administration is the most common immunization method. While it is not the most efficient option to protect against pathogens entering through the mucous membranes, it is still an effective method.
Thus, current vaccines against COVID-19 fail to fully prevent viral infection, which is partly due to the lack of mucosal immune activation. On the other hand, mucosal immunization has the ability to promote both mucosal and systemic immune responses[8].
Over 10 different vaccines against SARS-CoV-2 are in various stages of development, including virus-based vaccines, recombinant subunit vaccines and live attenuated vaccines[9-13]. Their development and application are encouraging because of the expected efficacy of the mucosal and systemic immune response they will elicit.
Despite the emergence of SARS-CoV-2 variants, people will prefer the next generation COVID-19 vaccine (i.e. intranasal immunization). This vaccine is expected to be very effective in producing both mucosal and systemic immune responses[13].
Various intranasal vaccines against SARS-CoV-2 are now being studied, even though they are not yet approved, with 12 candidates advancing to multiple stages of clinical trials, including virus-vectored vaccines, recombinant subunit vaccines and live attenuated vaccines[14].
The rationale for the need for effective COVID-19 vaccines that elicit mucosal immunity is to use early mucosal immune responses against the virus to prevent the virus from entering mucosal layers and causing infection. This is also called “sterilizing immunity”[15]. So far, the data show that people naturally infected with SARS-CoV-2 produce mucosal immunoglobulin (Ig)A antibodies (e.g., saliva, nasal swab/wash or bronchoalveolar lavage fluid) and systemic IgG antibodies[16,17].
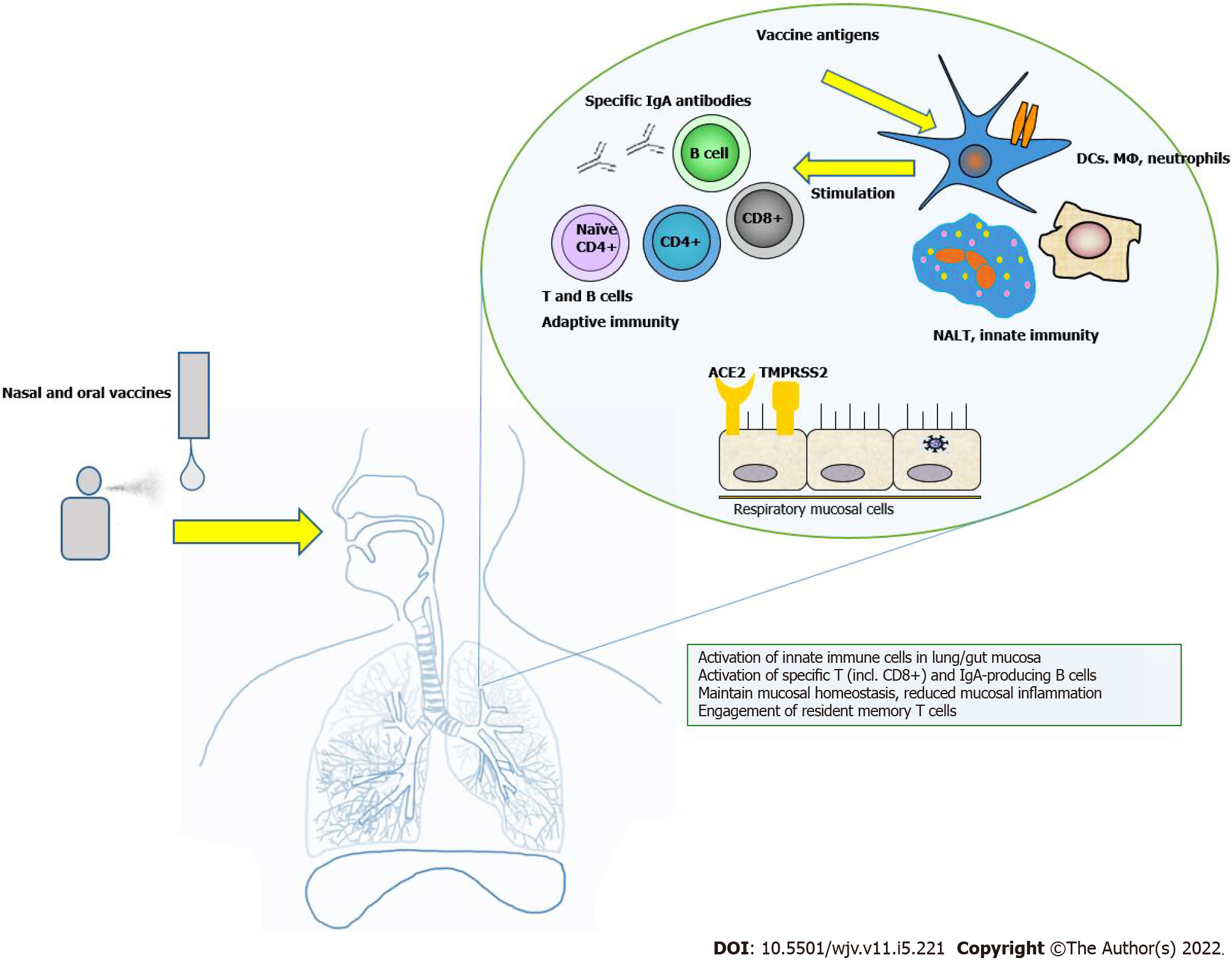
However, firstly, when SARS-CoV-2 infiltrates the nasal and/or oral cavities, nasopharynx-associated lymphoid tissue, bronchial-associated lymphoid tissue and mucosa-associated lymphoid tissue act as the first line of defense against viral infection[18]. In addition, all components of the innate immunity of the upper respiratory tract and/or the gastrointestinal tract (phagocytic neutrophils, macrophages, dendritic cells, resident microfolded M cells, innate lymphoid cells, natural killer cells and mast cells)[19] and immune molecules (i.e. galectins, collectins, cytokines and others) are involved in the immune response against the virus in various ways[20]. Additionally, T helper (Th)1- and Th2 cells, IgA-switched B cells are also rapidly activated after the initial interaction of SARS-CoV-2 with the innate immunity of the host[21].
These immune cells can work together to produce an integrated system that includes pattern-recognition receptors such as toll-like receptor 7 or toll-like receptor 8[22]. They identify molecular patterns (i.e. single-stranded RNA) associated with viral pathogens, resulting in increased production of proinflammatory cytokines such as type I interferon. Interferons have an essential role in the early stages of viral infection[23]. However, SARS-CoV-2 possesses the ability to suppress the production of interferons. The complement system is a vital part of innate immunity against SARS-CoV-2, which contributes to acute respiratory distress syndrome and cytokine storm[24]. Therefore, it is important to consider antibody-based treatments and vaccines when developing strategies to fight SARS-CoV-2.
After the innate immune system activation through dendritic cells, T and subsequent B cells specific to SARS-CoV-2 are recruited, mainly in the systemic bloodstream[25]. However, the simultaneous expansion of CD4+ T-helper cells, CD8+ cytotoxic T cells and plasma cells is crucial for viral elimination. Specific SIgA protects against SARS-CoV-2 by neutralizing it, suppressing its adhesion ability and agglutinating. This allows for a stronger anti-inflammatory response[26].
Traditional injectable vaccines are not very effective at inducing mucosal immunity. Furthermore, the benefits of such vaccination that leads to mucosal (SIgA) and circulating (IgG and IgA) antibody formation as well as SARS-specific effector and memory T cell responses have not been demonstrated in conventional vaccines[27,28].
However, a study showed induced S1-specific neutralizing IgA and IgG responses in the nasal mucosa following BNT162b2 but not after inactivated virus vaccine[29]. Additionally, it was shown that nasal immunization after an intramuscular vaccine could induce robust mucosal immunity to prevent mucosal pathogen entrance and development. A recent animal study showed promising results for using mucosal booster immunizations after mRNA priming to elicit mucosal immunity in addition to systemic responses[30].
There is evidence that SARS-CoV-2 nasal vaccination provides protection against both ancestral and mutant strains (i.e. variants of concern, B.1.1.7 and B.1.351)[31]. Furthermore, the authors suggest that adenovirus (Ad)-vectored multivalent vaccination delivered via the respiratory mucosa is a viable next-generation COVID-19 vaccine approach for inducing overall mucosal immunity against existing and future variants of concern[31].
Similarly, a combination of mucosal prime and systemic booster vaccines has been shown to increase the lifespan of lung CD8+ resident memory T cells[32]. In addition, CD4+ resident memory T cells are essential to developing protective CD8+ memory cells and B lymphocytes[33,34]. The ability of nasal vaccinations to induce resident memory T cells in the respiratory and gastrointestinal tract considerably increases their effectiveness. The development of nasal vaccines also relies on the data that mucosal immunization can elicit a wide range of adaptive immune responses, including SIgA antibodies and resident memory T cells[35]. Nasopharynx-associated lymphoid tissue is an important location for the induction of mucosal immune responses. Th1- and Th2-polarized lymphocytes, as well as IgA-secreting B cells, proliferate there. SIgA antibodies neutralize toxins and pathogens via immunological exclusion, antigen excretion and intracellular neutralization[36-38].
Additionally, we must keep in mind that mucosal SIgA levels raise up rapidly in babies, and these levels reach adult levels early in the childhood[39]. This should be considered when developing vaccines for children[40]. Also, although the titers of protection are not known now, virus-neutralizing antibodies are needed to protect and control the infection[41].
Nasal vaccination successfully stimulates resident memory T cell production, and persistent antigens in the lungs and gut can support long-term memory cell maintenance[42]. Resident memory T cells (especially CD8+) in the mucosa may help protect the body against virus infection by producing cytokines that mediate tissue antiviral resistance and chemokines that attract additional immune cells[43]. It is known that resident memory T cells are more effective at protecting the lungs than circulating T cells[44]. Furthermore, these memory cells can move from the lungs to mediastinal lymph nodes via a mechanism known as “retrograde migration” to maintain the memory phenotype and provide long-term protection[45].
The principle of intranasal vaccines, the vaccine-induced immune responses, mucosal involvement and benefits are shown in Figure 1.
Intranasal and oral COVID-19 vaccines promise to generate both local and systemic immune responses. Fortunately, the local activation of innate antigen-presenting cells by viral antigens leads to the stimulation of adaptive immune cells and an efficient immune response against the virus. Once this occurs, this local immune response has the potential to spread to other mucosal surfaces in the organism. It is assumed that local immunity will prevent virus entry and shedding and keep low levels of inflammation in the mucous membranes. Some adaptive cells remain in the mucosa and act as effector T cells or specific IgA-plasmacytes. Some of them exert systemic antiviral effects by going to the periphery. Additionally, activated innate and adaptive immune cells can clear the virus at the infection site, leading to undetectable viral RNA in airways and gut mucosa, leading to long-term immunity.
Humans have been licensed to eight oral and one intranasal vaccines against various mucosal infections. All of these vaccines are complete viral vaccines[1,32]. These types of vaccines are exceptionally preferred since they do not involve needles. In addition, subunit protein vaccines and virus-vector vaccines have significant advantages. Therefore, using all the scientific data and knowledge gained over the years about these types of vaccines, the scientific community is trying to create nasal vaccines SARS-CoV-2 based on already used vaccines in human history[32].
Oral vaccination can be used prophylactically in human populations at high risk for SARS-CoV-2. Thus, it could be the most cost-effective and efficient way to reduce the transmission of infection and morbidity. As we already stated, a needle-free vaccine eliminates the risk of transmitting blood-borne infections. Another benefit is that healthcare staff can perform oral vaccination without medical training. Pain and discomfort from a needle stick are avoided, as is the need to monitor side effects[32].
Type 1 and 2 monovalent oral poliovirus (OPV) vaccines (serotypes 1 or 3) and bivalent containing serotypes 1 and 3 were approved and licensed in 1961 and type 3 monovalent OPV vaccine in 1962. A trivalent OPV vaccine was approved in 1963. The World Health Organization (WHO) has announced that types 2 and 3 have been eradicated in 2015 and 2019, respectively. It turns out that OPV is the most effective and successful polio vaccine by inducing poliovirus-specific mucosal immunity[46].
The OPV contains a live poliovirus strains (Sabin). The strains are derived from wild polioviruses and have been reduced in virulence. Poliovirus is a member of the enterovirus subgroup of the Picornaviridae family. Picornaviruses are small viruses with an RNA genome, characterized by three poliovirus serotypes (type1, type 2 and type 3). Scientists have proven that immunity to one serotype does not confer significant immunity to other serotypes[47,48]. The virus enters the mouth and spreads throughout the oropharynx and gastrointestinal tract. The poliovirus is usually present in the nasopharynx for 1 wk to 2 wk and can be excreted in the feces for several weeks after infection. Even people with mild symptoms or without illness can be sources of infection[49].
In 2020, a global campaign was launched to end OPV use and switch to inactivated polio vaccination. But the last reports show that the neutralizing antibodies found in the nasopharynx of patients treated with OPV were more than those treated with inactivated polio vaccination[50].
After being ingested, the OPV vaccine replicates in the intestinal mucosa and lymphoid cells in the oropharynx and intestine. It behaves similarly to wild poliovirus. Vaccine strains are excreted in the feces of the vaccinated individual up to 6 wk after a dose, with maximum excretion occurring in the first 1-2 wk.
The OPV vaccine is very effective in protecting people from poliovirus. Interference among serotypes was observed during replication in the gut. A single dose of trivalent OPV elicits immune responses to all three vaccine viruses in half of recipients[51].
It is crucial that the OPV vaccine produces localized immunity in the intestines. This decreases the amount of virus that is shed when someone is re-infected with the same poliovirus serotype and reduces the chance of potential transmission. Subsequent vaccine doses reduce interference during gut replication. In contrast, three doses of vaccine provide immunity to all three poliovirus serotypes in more than 95% of recipients in industrialized countries. The immunity from the OPV is probably lifelong[52,53].
The OPV vaccine has been proven to have many benefits over the years, including providing non-specific protection against other infections. In addition, various studies have been conducted to research the effects of OPV and live enterovirus vaccines on the induction of non-specific immune responses, which show the non-reactogenicity and safety of vaccines[54-57].
All these studies demonstrated that cytopathic agents in the gastrointestinal tract decrease and reduce isolated infections of influenza, Ad, parainfluenza, herpesviruses, etc. According to these findings, OPV may offer protection against other viral respiratory infections.
In 2015, another research group conducted a retrospective cohort study in Denmark. They studied how the incidence of infection among the children with various infections changes depending on the last vaccine children received: OPV, DTap- inactivated polio vaccination-Hib (diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemo type b) or measles, mumps, rubella (MMR)[58]. A similar study was conducted in the United States. The results show the most significant reduction in non-specific infections with live vaccines[59].
When COVID-19 cases began to rise worldwide, the researchers began studying the effects of OPV vaccines in symptomatic and asymptomatic patients because the SARS-CoV-2 virus suppresses the innate immune system, which affects adaptive immunity[60]. Suppose the damage to the innate immune system is crucial for the transmission and infection of SARS-CoV-2. In that case, it may be suggested that preparing the immune system before infection can alleviate the course of the COVID-19 disease. Furthermore, evidence suggests that the prophylactic use of OPV or other live vaccines prior to COVID-19 may activate innate immunity and strengthen the immune system for the subsequent SARS-CoV-2 infection[61-63]. Therefore, it is necessary to consider the potential benefits of the OPV vaccine and its application before or together with the available COVID-19 vaccines.
Since the start of the COVID-19 pandemic, scientists have been scrutinizing rotavirus vaccines. Rotavirus is a double-stranded RNA virus (Reoviridae family). The outer capsid contains two important proteins, VP7 (G-protein) and VP4 (P-protein), which stimulate neutralizing antibodies. It is believed that they play an important role in immune protection[64]. The scientists proved that up to 60%-70% of children with severe rotavirus gastroenteritis demonstrate rotavirus antigen and RNA in serum (antigenemia). However, the immune correlates of protection for rotavirus are still not fully understood[64].
The antibodies against VP7 and VP4 that are found in the serum and mucosa probably play a crucial role in protecting against disease. Cell-mediated immunity probably helps to protect from infection and recover from it. Unfortunately, immunity usually does not last long after a vaccine is given. Re-infection can happen at any age[64].
Two live oral rotavirus vaccines, RV5 (RotaTeq) and RV1 (Rotarix), are currently approved for use[65]. In Finland and the United States, Phase III clinical efficacy trials of the RV5 vaccine were conducted. The data proved 74% efficacy after a 3-dose series against G1-G4 rotavirus gastroenteritis and 98% against severe G1-G4 rotavirus gastroenteritis, during the first entire rotavirus season after vaccination. Furthermore, scientists observed children during the first 2 years of life in a large health care utilization study. Among them, the RV5 vaccine decreased the incidence of G1-G4 rotavirus gastroenteritis: medical visits by 86%, emergency department visits by 94% and hospitalizations by 96%[66].
In Latin America and Europe, phase III clinical efficacy trials of RV1 vaccine were conducted. This study found that the 2-dose series against severe rotavirus gastroenteritis is 85% effective to age 1 year. The European study estimated the vaccines efficacy against severe rotavirus gastroenteritis is 96% through the first rotavirus season and 87% against any rotavirus gastroenteritis. The trial data also showed that vaccinating against rotavirus resulted in a 96% reduction in the number of hospitalizations for rotavirus gastroenteritis in the second season after vaccination[67].
In the United States, several RV5 and RV1 case-control vaccine effectiveness evaluations have been conducted among children between 2 years or 3 years or younger. The scientists found that the vaccine effectiveness against the combined outcome of emergency department visits or hospital admission for rotavirus was estimated at 84% for the RV5 and 83% for the RV1 vaccine. Evaluations of vaccine effectiveness tends to increase as the severity of rotavirus disease. Both vaccines have been shown to be effective against a wide range of rotavirus genotypes[68].
The exact duration of immunity with rotavirus vaccine is still unknown. However, effectiveness has been demonstrated in the first 2 years to 3 years of life in the United States. Vaccine efficacy was generally lower in the 2nd year of life than in the 1st year in low-income countries[66,69,70].
In the last 2 years, two more vaccines have received much attention concerning COVID-19. These are the tuberculosis vaccine Bacille Calmette-Guerin (BCG) and MMR vaccine.
BCG vaccination is an effective intervention against tuberculosis, and the researchers could make an effort to create a novel BCG-based vaccine for COVID-19. However, many studies reported non-specific cross-protective effects of the vaccine against other infectious diseases. For example, in 1932 the BCG vaccine was introduced for tuberculosis prevention in Northern Sweden[71]. Later, two groups studied the protective effect of BCG and, for the first time, reported a 45% reduction in child mortality from respiratory infections in West Africa[72,73]. Other examples of BCG-mediated non-specific effects were also reported by Stensballe et al[74] and Wardhana et al[75].
Furthermore, the BCG vaccine has recently been found to protect against different virus infections such as influenza virus, herpes simplex virus, human papillomavirus, respiratory syncytial virus and virus for yellow fever[76].
With the worldwide occurrence of the SARS-CoV-2, different agencies, including the WHO, have called to explore every possible solution, even already approved therapies and vaccines, to slow transmission and reduce the effects of the COVID-19 pandemic. However, the obtained data suggest that BCG does not reduce COVID-19 mortality. Still, BCG vaccination may reduce the incidence of frequency during the COVID-19 crisis[77,78].
Only randomized controlled trials will show whether BCG reduces the frequency and severity of COVID-19. A recent study, a phase III ACTIVATE trial (NCT03296423), confirmed that adults over 65 years who have recently been vaccinated against BCG are less likely to get new virus infections. The study found that the incidence of new respiratory infections after receiving a placebo vaccine (42.3%) was different from the incidence of new respiratory infections after receiving BCG vaccine (25.0%)[78].
Another clinical trial in Brazil, BATTLE (NCT04369794), is designed to test BCG-like therapeutic vaccination. The aim is to show if it affects the elimination of SARS-CoV-2 and the degree of seroconversion and titration (IgA, IgG and IgM)[79]. In murine models, the new BCG:CoVac form, which combines BCG with the stable form of S protein, simultaneously stimulates SARS-CoV-2 and T-cell responses even at levels equivalent to or higher than expected by current vaccines[80].
In March 2020, with the rise of COVID-19 cases in the United Arab Emirates, the Emirates International Hospital Safety Committee decided to offer a BCG booster vaccination to hospital staff, which is 280 people. Seventy-one received the BCG vaccine. None of the 71 people who received the BCG booster vaccine tested positive for the SARS-CoV-2 virus. For the other 209 individuals that had not received booster BCG, there were 18 positive PCR cases of COVID-19. There were no available reports of complications with the BCG booster group[81]. In conclusion, BCG vaccination may protect medical staff who work or who are vulnerable to SARS-CoV-2 infection. Further studies are needed to determine if BCG vaccine is effective against COVID-19.
MMR (measles-mumps-rubella) vaccine is another childhood vaccine relevant to the COVID-19 pandemic. Homologies of the amino acid sequence between SARS-CoV-2 and measles, rubella and mumps viruses have been found[82,83]. A study found that there is a strong correlation between mumps IgG titers and the severity of COVID-19 in people vaccinated with the MMR vaccine in childhood[84]. There are also data that recently vaccinated MMR people had less severe COVID-19 and lower mortality rate[85].
Until the end of 2021, a placebo-controlled randomized clinical trial was conducted with 30000 individuals to investigate the protective effect of MMR vaccination after a positive test and symptomatic COVID-19[86]. A case-control study indicated that there may be a protective effect of the MMR vaccine against SARS-CoV-2 in males but not in females[87]. Several other studies have shown that recently receiving the MMR vaccine may protect against SARS-CoV-2 and/or the development of severe COVID-19[88-90]. They showed that the MMR vaccine can stimulate innate immunity inducing non-specific protection against other infections. Compared with those in the placebo group, participants who received at least one dose of MMR had a significantly decreased risk for symptomatic COVID-19 and need for treatment.
These data were used to make assumptions about the potential efficacy of COVID-19 vaccine administered live or nasal/oral. We summarize the information in Table 1.
| Name of vaccine | Form | Immunity | Dosage | Route |
| OPV (oral poliovirus vaccine) | Live attenuated poliovirus (Sabin strain types 1, 2 or 3) | Poliovirus-specific mucosal immunity | 2 doses | Oral |
| BCG (Bacille Calmette-Guerin) | Live attenuated bacteria Mycobacterium bovis | Mycobacterium-specific mucosal and systemic immunity | 0.05 mL until 1 yr of age; 0.1 mL thereafter | Intradermal injection subcutaneous |
| MMR (measles, mumps and rubella vaccines) | Weakened forms of the measles, mumps and rubella viruses | Measles, mumps and rubella-specific systemic and mucosal immunity | 2 doses | Subcutaneous injection |
| RV1 (Rotarix®) | Live-attenuated rotavirus | Rotavirus-specific mucosal immunity | 2 doses | Oral |
| RV5 (RotaTeq®) | Live-attenuated rotavirus | Rotavirus-specific mucosal immunity | 3 doses | Oral |
Since the discovery of the first vaccine, it has always been a question about the benefits and risks of vaccines. However, over the years, vaccination programs that have been introduced and updated have managed to achieve their goals: Smallpox has been eradicated, polio and measles have been almost eradicated, and other diseases have been controlled[91].
The vaccines being administered now are given by injection. The mucosal vaccines can be superior to this process because they will elicit protective immune responses from the mucosa, blocking infection at the site of infection. The nature of the infection should be well known when developing mucosal vaccines: invasive (in intestinal pathogens), locally invasive (in shigellosis) or strictly mucosal (in cholera)[36,91,92]. This will affect the proper access of the circulating antibodies as well as the longevity of the immune response.
A large part of the population is willing to accept vaccines, but the claims about their risks have a greater impact than before. Therefore, the risks associated with a potential decision must be discussed in light of the best available scientific information.
When countries are faced with the decision to include a new vaccine in their national immunization programs, the relevant scientific, clinical, epidemiological and economic factors of the immunization program need to be considered.
Today, many vaccine production platforms vary in complexity and cost[93]. For example, the live attenuated OPV has a significantly lower cost of production. In contrast, the highly complex pneumococcal conjugate vaccine is much more expensive[94]. Financial cost-effectiveness is one of the most important factors when choosing a financial product or service. When a vaccine is cost-effective, it can help to manage both the health and financial consequences in a country. Oral vaccines offer great potential for preventing pandemics because they are very efficient, low cost, require no medical personnel and can elicit both systemic and mucosal immune responses. This type of vaccine is one of the most successful and cost-effective public health investments a country can make to improve people’s health.
Oral vaccines require protection in the harsh environment of the gastrointestinal tract, where the pH is low and the proteases are present. Under normal circumstances, antigens that enter orally are treated as nutrients. If a vaccine does not trigger the appropriate danger signals, it is recognized as non-pathogenic by the intestinal tissue[95]. High doses are usually required for successful immunization, but this may increase the risk of tolerance[96]. These barriers are the main reasons there are so few effective oral vaccines.
Mucosal vaccines against SARS-CoV-2 are incredibly challenging to develop and confirm their safety. However, they will offer the ability to trigger stable, protective immune responses at the sites of pathogenic infection. Unfortunately, mortality and morbidity associated with various infectious diseases caused by mucosal pathogens have remained very high over the last 10 years.
Data so far demonstrated several attempts to develop intranasal vaccines against SARS-CoV and Middle East respiratory syndrome, based on viral vector, subunit, DNA, virus-like particle, inactivated and live-attenuated, described extensively elsewhere[97-101]. Based on our experience with mucosal vaccine platforms for SARS and Middle East respiratory syndrome, effective mucosal vaccines against SARS-CoV-2 could be developed. There are different types of correlates of protection, both humoral and cellular, that are associated with different goals of vaccination: prevention of infection at the mucosal or systemic level. However, until their efficacy and safety have been proven in clinical trials, their use is not recommended.
According to WHO data from 2020, lower respiratory tract infections are the fourth leading cause of death worldwide[102]. Developing an effective vaccine to protect against SARS-CoV-2 infection is a worthwhile endeavor. An extensive risk-benefit analysis of COVID-19 vaccines was published in 2021[103]. The study was focused on thrombocytopenia and thromboembolism. It demonstrated that the risks of thrombocytopenia, venous or arterial thromboembolism, cerebral venous sinus thrombosis and ischemic stroke were much higher after SARS-CoV-2 infection than after vaccination.
The COVID-19 pandemic will continue and will hit low-income countries. Although there are already effective vaccines against SARS-CoV-2, mass production is still difficult, with no global coverage. The development of ‘specific’ mucosal vaccines targeting coronavirus antigens could be an excellent option for preventing future pandemics.
As mentioned earlier, intramuscular injections are not effective at reducing viral replication or nasal secretions in the upper respiratory tract. This leads to asymptomatic or mild symptomatic disease, which helps to spread the virus. On the other hand, intranasal vaccinations may generate sterilizing immunity against mucosal infections[104]. In addition, the principle of antigens exposed at the initial site of the viral infection will help to elicit a stable immune response in the mucosa[105]. The systemic immune response induced by intranasal vaccination is equivalent to or even stronger than the response caused by intramuscular immunization. This suggests that a lower dose will be needed to increase the efficacy and safety of vaccination.
Intranasal vaccination with chimpanzee Ad vector SARS-CoV-2 vaccine (ChAd-SARS-CoV-2-S) was shown to generate more significant levels of S-specific neutralizing antibodies in hamsters[106]. Such vaccination can produce pan-reactive antibodies[107], which is particularly attractive given the emergence of new SARS-CoV-2 mutants. The development of this type of vaccine would have a significant socioeconomic impact. A huge population could be vaccinated in a very short time in a global pandemic, such as COVID-19. The vaccines are supplied with nasal devices, which are preferred and convenient for patients. It is unnecessary from very low storage temperatures and a sterile environment that make them suitable for use. Furthermore, mucosal vaccination may hasten herd immunity, owing to its ease of delivery to impoverished individuals in low- and middle-income countries[98].
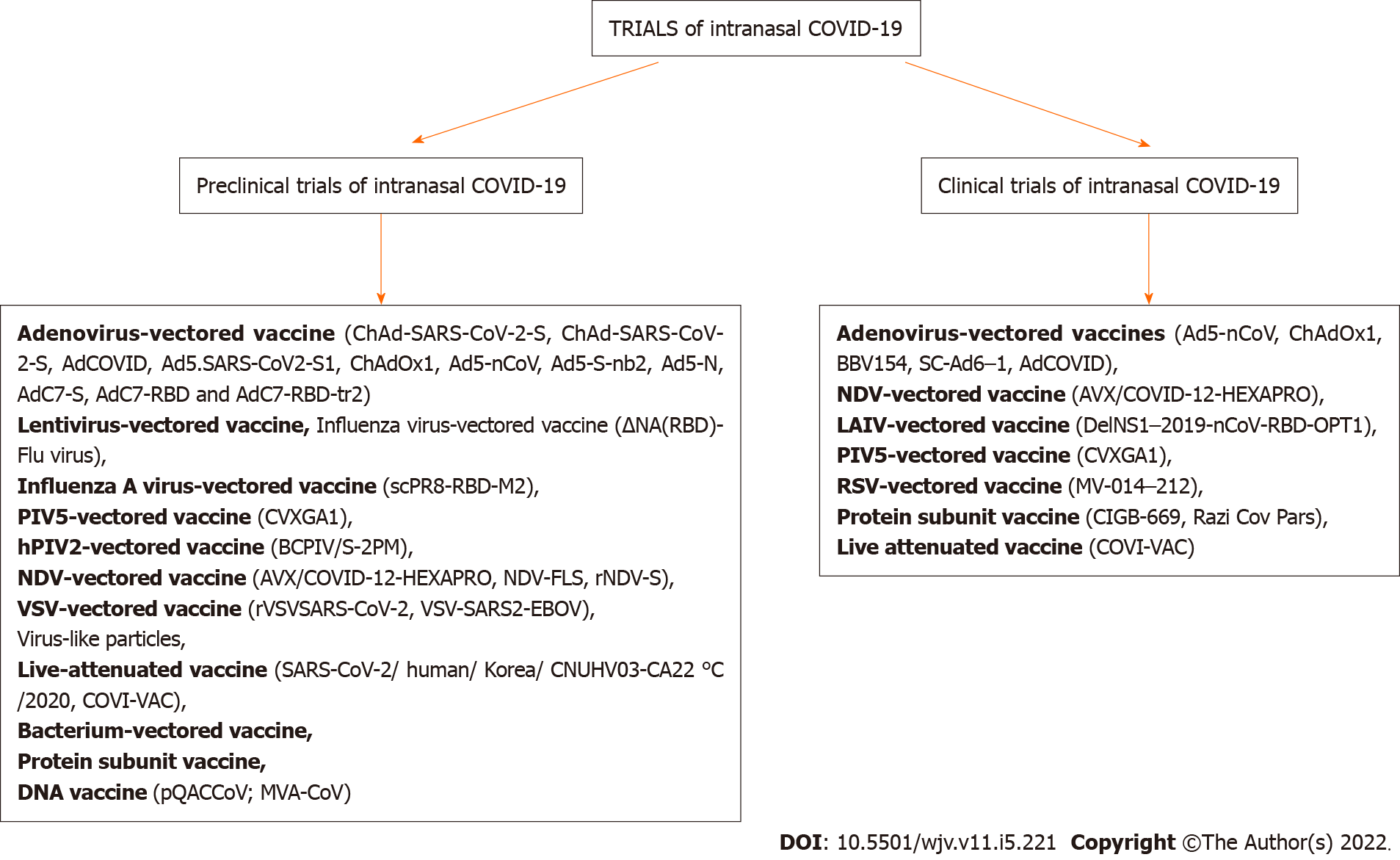
Because most clinical trial results have not yet been available, preclinical research is required to investigate the immunogenicity and safety of intranasal COVID-19 vaccines.
Preclinical studies of intranasal COVID-19 vaccines include a variety of mechanisms, which have been extensively described by Alu et al[8]. Studies on intranasal/mucosal COVID-19 vaccines, both preclinical and clinical trials[8], are shown schematically in Figure 2.
Vaxart’s vaccine is an tablet vaccine that contains an adenoviral vector. The vector encodes the SARS-CoV-2 S and nucleocapsid proteins. The vaccine has progressed to a phase I trial (NCT04563702). Therefore, the film-coated tablets provide mucosal immunity by dissolving in the digestive tract. In addition, the active ingredient is protected from the aggressive action of the stomach’s acidic environment by its enteric coating[7].
Studies show a significant increase in the titer of neutralizing antibodies against SARS-CoV-2 2 wk after the first vaccination in all animals that received the vaccine compared to the unvaccinated group[108]. Comparing the mucosal application of full-length wild-type S-protein antigens and those of the S1-domain or stabilized S-antigen, mucosal administration induced higher neutralizing antibody titers in the lungs and periphery.
Vaxart’s tablet vaccine studies have shown that both low and high doses induce antigen-specific CD4+ and CD8+ cells. It is in the process of undergoing clinical phase evaluation[108].
Another interesting project underway is the IosBio Pharma’s vaccine (United Kingdom). They also participated in the rat race for the golden choice SARS-CoV-2 vaccine by developing an oral dual-antigen COVID-19 vaccine in capsule form called OraPro-COVID-19[109]. This candidate is based on a human adenoviral vector (hAd5). It expresses modified SARS-CoV-2 S protein and nucleocapsid protein genes with enhanced T-cell stimulation domain, which is predicted to enhance major histocompatibility class II responses[110]. Gabitzsch et al[111] first investigated this adenoviral vector platform against various viral antigens such as influenza, HIV-1 and Lassa fever. Their previous and current results show that this immunization model both promotes humoral and cell-mediated immunity[111-114].
In investigating the role of T-cell-mediated immunity in SARS-CoV-2 infection, Sekine et al[115] highlighted its importance by detecting virus-specific T-cells in the serum of patients with SARS-CoV-2 negative antibodies, including asymptomatic individuals or exposed family members.
Gabitzsch et al[116] also developed a dual vaccine model to ensure T-cell activation and more durative protective immunity. First, they studied a murine model using hAd5 S-Fusion + nucleocapsid protein genes with enhanced T-cell stimulation domain. They proved that this type of immunization elicits not only a protective humoral but also a Th1-dominant T-cell response. Furthermore, they found that if the vaccine was stored at room temperature, subcutaneous application followed by oral boost elicited both antibody-mediated and T-cell responses. In addition, they also demonstrated that applications of hAd5 S-Fusion + nucleocapsid protein genes with enhanced T-cell stimulation domain inhibited viral replication in both nasal and pulmonary mucosa within 24 h, with complete clearance 7 d after administration.
Human clinical trials are still underway to determine dose strengths and the number of vaccine applications. However, more research is needed to determine if this oral dual vaccine model is effective in managing COVID-19.
DNA vaccines have some limitations, such as the need for high doses, suitable adjuvants and unique technologies for delivering them to specific sites in the body[117,118]. So poliovirus has been used as a vaccine vector to overcome these barriers due to its safety, low cost and ability to be used orally.
One of the potential platforms for developing an effective vaccine against COVID-19 for oral mucosa is based on the Sabin-1 poliovirus cDNA-based recombinant poliovirus Sabin 1 (RPS) vector system. Sabin-1 is one of three attenuated poliovirus serotypes (OPV). The Sabin strains are safe and easy to store and manipulate experimentally. Therefore, they are ideal vaccine vectors for foreign antigen expression. There are two variants of this system: RPS-Vax and RPS-cytoplasmic transduction peptide (CTP). The RPS-Vax has the multiple cloning site and 3C-protease cutting site, which allow the cloning of a vaccine gene and the release of the vaccine protein from the viral particle during replication[119]. The RPS-CTP vector system is a modified version of the RPS-Vax vector system, which contains CTP right above the multiple cloning site.
Based on the RPS-CTP platform, the vaccine is designed to be used orally instead of parenterally or intramuscularly. In this regard, it has advantages that can make it convenient for patients with COVID-19: easy to apply and no loss during application[120]. In addition, it has also been well established that OPV can induce long T-cell and B-cell memory[121]. Therefore, OPV is an effective and preferred vaccine in most of the world because it has the potential to quickly halt viral transmission. Furthermore, it successfully mimics infection that is naturally acquired due to its oral application.
OPV also has the hypothetical ability to “vaccinate” indirectly through close contact of vaccine recipients, who spread OPV through nasopharyngeal secretions and feces[122]. These results suggest that the vector system RPS-CTP can be used to develop preventive or therapeutic mucosal vaccines against COVID-19 and other diseases.
Although challenging, oral vaccination has many socioeconomic benefits and stimulates both the humoral and cellular immune responses. They are easy to use, even in areas without medical staff, with relatively few side effects and lower cost. Although there are many oral vaccines currently undergoing clinical trials, only a limited number of these vaccines have been approved for human use. According to the WHO, most COVID-19 vaccines are designed to be administered by the intramuscular route[123] in order to produce high titers of neutralizing antibodies. It is well established that mucosal vaccines offer robust protective potential in pathogen infection sites. Additionally, the adaptive immunity induction at mucosal sites comprises secretory antibody production and T cell responses, preventing infection and developing disease symptoms[7]. These data support developing an oral or nasal mucosal vaccine against SARS-CoV-2, as this type of vaccine is known to activate the mucosal immune system and have been successful in protecting people from other infections in the past[124].
Identifying safe and effective mucosal adjuvants allied to innovative antigen delivery plays a crucial role in advancing mucosal COVID-19 vaccines. The complex mechanisms of innate and adaptive mucosal immunity regulation are not yet fully understood. But the significant progress that has been made in recent years will help create more effective oral vaccines. In addition, oral tablet versions of a COVID-19 vaccine will also reach regions without healthcare staff and healthcare infrastructure.
In addition, because the gut is already colonized with microorganisms, oral vaccines do not require extensive and expensive antigen purification. This simplifies the entire production process and reduces the cost. These benefits of oral vaccination may be preferred over conventional vaccination methods during pandemic situations like COVID-19.
The production of effective oral vaccines for COVID-19 must comply with high safety standards, stability and immunogenicity. When oral vaccines succeed in generating protective and therapeutic immune responses, we will be able to overcome the global COVID-19 pandemic that has changed people’s lives worldwide[125].
A mucosal SARS-CoV-2 vaccine that targets the mucosal surfaces such as the nose or mouth would be ideal if it were shown to be safe. However, there are still major regulatory issues concerning stability and effectiveness. It is fascinating to see if the intranasal application of SARS-CoV-2 mRNA vaccines may induce resident memory T cells and B cells and protect the lungs and gut. Recent and ongoing studies highlight the importance of understanding local immune responses and suggest that mucosal, innate and vaccine-mediated immunity to SARS-CoV-2 has enormous therapeutic implication value.
| 1. | Tiboni M, Casettari L, Illum L. Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines? Int J Pharm. 2021;603:120686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 2. | Hsieh CL, Goldsmith JA, Schaub JM, DiVenere AM, Kuo HC, Javanmardi K, Le KC, Wrapp D, Lee AG, Liu Y, Chou CW, Byrne PO, Hjorth CK, Johnson NV, Ludes-Meyers J, Nguyen AW, Park J, Wang N, Amengor D, Maynard JA, Finkelstein IJ, McLellan JS. Structure-based Design of Prefusion-stabilized SARS-CoV-2 Spikes. bioRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Wang S, Liu H, Zhang X, Qian F. Intranasal and oral vaccination with protein-based antigens: advantages, challenges and formulation strategies. Protein Cell. 2015;6:480-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | WHO’s landscape of COVID-19 vaccine candidates. 24-November 2021. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines. |
| 5. | Registry data of COVID-19 vaccine candidates. Last access 15-May-2022. Available from: https://covid19.trackvaccines.org/. |
| 6. | Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 374] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 7. | Ashraf MU, Kim Y, Kumar S, Seo D, Ashraf M, Bae YS. COVID-19 Vaccines (Revisited) and Oral-Mucosal Vector System as a Potential Vaccine Platform. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Alu A, Chen L, Lei H, Wei Y, Tian X, Wei X. Intranasal COVID-19 vaccines: From bench to bed. EBioMedicine. 2022;76:103841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 176] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 9. | Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001;14:430-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, Aileni VK, Kanungo S, Rai S, Reddy P, Verma S, Singh C, Redkar S, Mohapatra S, Pandey A, Ranganadin P, Gumashta R, Multani M, Mohammad S, Bhatt P, Kumari L, Sapkal G, Gupta N, Abraham P, Panda S, Prasad S, Bhargava B, Ella K, Vadrevu KM; COVAXIN Study Group. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398:2173-2184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 11. | Karczmarzyk K, Kęsik-Brodacka M. Attacking the Intruder at the Gate: Prospects of Mucosal Anti SARS-CoV-2 Vaccines. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 12. | WHO—COVID19 Vaccine Tracker. (accessed on 12 May 2022). Available from: https://covid19.trackvaccines.org/agency/who. |
| 13. | Matuchansky C. Mucosal immunity to SARS-CoV-2: a clinically relevant key to deciphering natural and vaccine-induced defences. Clin Microbiol Infect. 2021;27:1724-1726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Clinical trials register. (accessed on 12 May 2022). Available from: https://clinicaltrials.gov/ct2/results?term=COVID-19+vaccine&recrs=a&cond=Covid19. |
| 15. | Kyei-Barffour I, Addo SA, Aninagyei E, Ghartey-Kwansah G, Acheampong DO. Sterilizing Immunity against COVID-19: Developing Helper T cells I and II activating vaccines is imperative. Biomed Pharmacother. 2021;144:112282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 721] [Cited by in RCA: 699] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 17. | Velikova T. Infection-acquired vs vaccine-induced immunity against COVID-19. Cent Asian J Med Hypotheses Ethics. 2021;2:29-35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Villena J, Kitazawa H. The Modulation of Mucosal Antiviral Immunity by Immunobiotics: Could They Offer Any Benefit in the SARS-CoV-2 Pandemic? Front Physiol. 2020;11:699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1078] [Cited by in RCA: 1178] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 20. | Yuan Q, Walker WA. Innate immunity of the gut: mucosal defense in health and disease. J Pediatr Gastroenterol Nutr. 2004;38:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Velikova TV, Kotsev SV, Georgiev DS, Batselova HM. Immunological aspects of COVID-19: What do we know? World J Biol Chem. 2020;11:14-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 22. | Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8061] [Cited by in RCA: 8983] [Article Influence: 449.2] [Reference Citation Analysis (1)] |
| 23. | Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8:911-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 592] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 24. | Kurtovic L, Beeson JG. Complement Factors in COVID-19 Therapeutics and Vaccines. Trends Immunol. 2021;42:94-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Velikova T, Snegarova V, Kukov A, Batselova H, Mihova A, Nakov R. Gastrointestinal mucosal immunity and COVID-19. World J Gastroenterol. 2021;27:5047-5059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Su F, Patel GB, Hu S, Chen W. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum Vaccin Immunother. 2016;12:1070-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int. 2021;41:509-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 28. | Azzi L, Dalla Gasperina D, Veronesi G, Shallak M, Ietto G, Iovino D, Baj A, Gianfagna F, Maurino V, Focosi D, Maggi F, Ferrario MM, Dentali F, Carcano G, Tagliabue A, Maffioli LS, Accolla RS, Forlani G. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine. 2022;75:103788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 29. | Chan RWY, Liu S, Cheung JY, Tsun JGS, Chan KC, Chan KYY, Fung GPG, Li AM, Lam HS. The Mucosal and Serological Immune Responses to the Novel Coronavirus (SARS-CoV-2) Vaccines. Front Immunol. 2021;12:744887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 30. | Lapuente D, Fuchs J, Willar J, Vieira Antão A, Eberlein V, Uhlig N, Issmail L, Schmidt A, Oltmanns F, Peter AS, Mueller-Schmucker S, Irrgang P, Fraedrich K, Cara A, Hoffmann M, Pöhlmann S, Ensser A, Pertl C, Willert T, Thirion C, Grunwald T, Überla K, Tenbusch M. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat Commun. 2021;12:6871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 31. | Afkhami S, D'Agostino MR, Zhang A, Stacey HD, Marzok A, Kang A, Singh R, Bavananthasivam J, Ye G, Luo X, Wang F, Ang JC, Zganiacz A, Sankar U, Kazhdan N, Koenig JFE, Phelps A, Gameiro SF, Tang S, Jordana M, Wan Y, Mossman KL, Jeyanathan M, Gillgrass A, Medina MFC, Smaill F, Lichty BD, Miller MS, Xing Z. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. 2022;185:896-915.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 227] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 32. | Lavelle EC, Ward RW. Mucosal vaccines - fortifying the frontiers. Nat Rev Immunol. 2022;22:236-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 482] [Article Influence: 96.4] [Reference Citation Analysis (0)] |
| 33. | Swarnalekha N, Schreiner D, Litzler LC, Iftikhar S, Kirchmeier D, Künzli M, Son YM, Sun J, Moreira EA, King CG. T resident helper cells promote humoral responses in the lung. Sci Immunol. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 34. | Son YM, Cheon IS, Wu Y, Li C, Wang Z, Gao X, Chen Y, Takahashi Y, Fu YX, Dent AL, Kaplan MH, Taylor JJ, Cui W, Sun J. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci Immunol. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 35. | Li Y, Jin L, Chen T. The Effects of Secretory IgA in the Mucosal Immune System. Biomed Res Int. 2020;2020:2032057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 36. | Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8:656-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 37. | Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 454] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 38. | Rogier EW, Frantz AL, Bruno ME, Kaetzel CS. Secretory IgA is Concentrated in the Outer Layer of Colonic Mucus along with Gut Bacteria. Pathogens. 2014;3:390-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Nurkic J, Numanovic F, Arnautalic L, Tihic N, Halilovic D, Jahic M. Diagnostic Significance of Reduced IgA in Children. Med Arch. 2015;69:236-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Fischer A. Resistance of children to Covid-19. How? Mucosal Immunol. 2020;13:563-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Slabakova Y, Gerenska D, Ivanov N, Velikova T. Immune titers of protection against severe acute respiratory syndrome coronavirus 2: are we there yet? Explor Immunol. 2022;2:9-24. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Uddbäck I, Cartwright EK, Schøller AS, Wein AN, Hayward SL, Lobby J, Takamura S, Thomsen AR, Kohlmeier JE, Christensen JP. Long-term maintenance of lung resident memory T cells is mediated by persistent antigen. Mucosal Immunol. 2021;14:92-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 43. | Rakhra K, Abraham W, Wang C, Moynihan KD, Li N, Donahue N, Baldeon AD, Irvine DJ. Exploiting albumin as a mucosal vaccine chaperone for robust generation of lung-resident memory T cells. Sci Immunol. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 44. | Slütter B, Pewe LL, Kaech SM, Harty JT. Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus. Immunity. 2013;39:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 45. | Stolley JM, Johnston TS, Soerens AG, Beura LK, Rosato PC, Joag V, Wijeyesinghe SP, Langlois RA, Osum KC, Mitchell JS, Masopust D. Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 46. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1286] [Cited by in RCA: 1544] [Article Influence: 257.3] [Reference Citation Analysis (1)] |
| 47. | CDC. Poliomyelitis prevention in the United States: updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2000;49:1-22. |
| 48. | CDC. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding routine poliovirus vaccination. MMWR. 2009;58:829-830. |
| 49. | Chard AN, Datta SD, Tallis G, Burns CC, Wassilak SGF, Vertefeuille JF, Zaffran M. Progress Toward Polio Eradication - Worldwide, January 2018-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:784-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 50. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14570] [Article Influence: 2428.3] [Reference Citation Analysis (3)] |
| 51. | Vidor E. Poliovirus vaccine—live. In Plotkin S, Orenstein W, Offit P, eds. Plotkin’s Vaccines. 7th ed. Philadelphia, PA: Elsevier; 2018: 841-865.e10. [DOI] [Full Text] |
| 52. | Wallace GS, Curns AT, Weldon WC, Oberste MS. Seroprevalence of Poliovirus Antibodies in the United States Population, 2009-2010. BMC Public Health. 2016;16:721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Wattigney WA, Mootrey GT, Braun MM, Chen RT. Surveillance for poliovirus vaccine adverse events, 1991 to 1998: impact of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine. Pediatrics. 2001;107:E83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Voroshilova MK. Potential use of nonpathogenic enteroviruses for control of human disease. Prog Med Virol. 1989;36:191-202. [PubMed] |
| 55. | Terekhov SN, Stepanchuk VA. Acute respiratory diseases. Materials of the Institute of Microbiology and Epidemiology 1971; 5: 62–6. |
| 56. | Prijmyagi LS, Grinshpoon LE. Use of live enterovirus vaccines for urgent prophylaxis of influenza. In: Chumakov ed. Medical Virology 1973; 21:29–37. |
| 57. | Chumakov MP, Voroshilova MK, Antsupova AS, Boĭko VM, Blinova MI, Priĭmiagi LS, Rodin VI, Seĭbil' VB, Siniak KM, Smorodintsev AA. Live enteroviral vaccines for the emergency non-specific prevention of mass respiratory diseases during fall-winter epidemics of influenza and acute respiratory diseases [in Russian]. Zh Mikrobiol Epidemiol Immunobiol. 1992;11-12: 37. [PubMed] |
| 58. | Sørup S, Stensballe LG, Krause TG, Aaby P, Benn CS, Ravn H. Oral Polio Vaccination and Hospital Admissions With Non-Polio Infections in Denmark: Nationwide Retrospective Cohort Study. Open Forum Infect Dis. 2016;3:ofv204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Bardenheier BH, McNeil MM, Wodi AP, McNicholl JM, DeStefano F. Risk of Nontargeted Infectious Disease Hospitalizations Among US Children Following Inactivated and Live Vaccines, 2005-2014. Clin Infect Dis. 2017;65:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Sallenave JM, Guillot L. Innate Immune Signaling and Proteolytic Pathways in the Resolution or Exacerbation of SARS-CoV-2 in Covid-19: Key Therapeutic Targets? Front Immunol. 2020;11:1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 61. | Benn CS, Fisker AB, Rieckmann A, Sørup S, Aaby P. Vaccinology: time to change the paradigm? Lancet Infect Dis. 2020;20:e274-e283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 62. | Chang AY, Aaby P, Avidan MS, Benn CS, Bertozzi SM, Blatt L, Chumakov K, Khader SA, Kottilil S, Nekkar M, Netea MG, Sparrow A, Jamison DT. One vaccine to counter many diseases? Modelling the economics of oral polio vaccine against child mortality and COVID-19, medRxiv 2022.22269560. [DOI] [Full Text] |
| 63. | Comunale BA, Engineer L, Jiang Y, Andrews JC, Liu Q, Ji L, Yurkovich JT, Comunale RA, Xie Q. Poliovirus Vaccination Induces a Humoral Immune Response That Cross Reacts With SARS-CoV-2. Front Med (Lausanne). 2021;8:710010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Kimberlin D, Brady M, Jackson M, eds. American Academy of Pediatrics. Rotavirus infections. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL: American Academy of Pediatrics; 2018: 700–704. [DOI] [Full Text] |
| 65. | Patel MM, Glass R, Desai R, Tate JE, Parashar UD. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure? Lancet Infect Dis. 2012;12:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 66. | Pindyck T, Tate JE, Parashar UD. A decade of experience with rotavirus vaccination in the United States - vaccine uptake, effectiveness, and impact. Expert Rev Vaccines. 2018;17:593-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 67. | Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, López P, Macías-Parra M, Ortega-Barría E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavía-Ruz N, Salmerón J, Rüttimann R, Tinoco JC, Rubio P, Nuñez E, Guerrero ML, Yarzábal JP, Damaso S, Tornieporth N, Sáez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O'Ryan M; Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1316] [Cited by in RCA: 1308] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 68. | CDC. Prevention of rotavirus gastroenteritis among infants and children recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2009;58 (No.RR-2):1-25. |
| 69. | Baker JM, Tate JE, Steiner CA, Haber MJ, Parashar UD, Lopman BA. Longer-term Direct and Indirect Effects of Infant Rotavirus Vaccination Across All Ages in the United States in 2000-2013: Analysis of a Large Hospital Discharge Data Set. Clin Infect Dis. 2019;68:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Bowen MD, Mijatovic-Rustempasic S, Esona MD, Teel EN, Gautam R, Sturgeon M, Azimi PH, Baker CJ, Bernstein DI, Boom JA, Chappell J, Donauer S, Edwards KM, Englund JA, Halasa NB, Harrison CJ, Johnston SH, Klein EJ, McNeal MM, Moffatt ME, Rench MA, Sahni LC, Selvarangan R, Staat MA, Szilagyi PG, Weinberg GA, Wikswo ME, Parashar UD, Payne DC. Rotavirus Strain Trends During the Postlicensure Vaccine Era: United States, 2008-2013. J Infect Dis. 2016;214:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Vaccination préventive de la tuberculose de l'homme et des animaux par le B C G. Rapports et documents provenant des divers pays (la France exceptée) transmis à l'Institut Pasteur en 1932. JAMA. 1932;99:940-941. [DOI] [Full Text] |
| 72. | Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ. 2000;321:1435-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 316] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 73. | Garly ML, Martins CL, Balé C, Baldé MA, Hedegaard KL, Gustafson P, Lisse IM, Whittle HC, Aaby P. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine. 2003;21:2782-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 74. | Stensballe LG, Nante E, Jensen IP, Kofoed PE, Poulsen A, Jensen H, Newport M, Marchant A, Aaby P. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005;23:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 75. | Wardhana, Datau EA, Sultana A, Mandang VV, Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43:185-190. [PubMed] |
| 76. | Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25:1473-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 326] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 77. | Moorlag SJCFM, van Deuren RC, van Werkhoven CH, Jaeger M, Debisarun P, Taks E, Mourits VP, Koeken VACM, de Bree LCJ, Ten Doesschate T, Cleophas MC, Smeekens S, Oosting M, van de Veerdonk FL, Joosten LAB, Ten Oever J, van der Meer JWM, Curtis N, Aaby P, Stabell-Benn C, Giamarellos-Bourboulis EJ, Bonten M, van Crevel R, Netea MG. Safety and COVID-19 Symptoms in Individuals Recently Vaccinated with BCG: a Retrospective Cohort Study. Cell Rep Med. 2020;1:100073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 78. | Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Domínguez-Andrés J, Kyriazopoulou E, Gkavogianni T, Adami ME, Damoraki G, Koufargyris P, Karageorgos A, Bolanou A, Koenen H, van Crevel R, Droggiti DI, Renieris G, Papadopoulos A, Netea MG. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell. 2020;183:315-323.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 315] [Article Influence: 52.5] [Reference Citation Analysis (4)] |
| 79. | Gonzalez-Perez M, Sanchez-Tarjuelo R, Shor B, Nistal-Villan E, Ochando J. The BCG Vaccine for COVID-19: First Verdict and Future Directions. Front Immunol. 2021;12:632478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 80. | Counoupas C, Johansen MD, Stella AO, Nguyen DH, Ferguson AL, Aggarwal A, Bhattacharyya ND, Grey A, Hutchings O, Patel K, Siddiquee R, Stewart EL, Feng CG, Hansbro NG, Palendira U, Steain MC, Saunders BM, Low JKK, Mackay JP, Kelleher AD, Britton WJ, Turville SG, Hansbro PM, Triccas JA. A single dose, BCG-adjuvanted COVID-19 vaccine provides sterilising immunity against SARS-CoV-2 infection. NPJ Vaccines. 2021;6:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 81. | Amirlak L, Haddad R, Hardy JD, Khaled NS, Chung MH, Amirlak B. Effectiveness of booster BCG vaccination in preventing Covid-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 82. | Sidiq KR, Sabir DK, Ali SM, Kodzius R. Does Early Childhood Vaccination Protect Against COVID-19? Front Mol Biosci. 2020;7:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Young A, Neumann B, Mendez RF, Reyahi A, Joannides A, Modis Y, Franklin RJM. Homologous protein domains in SARS-CoV-2 and measles, mumps and rubella viruses: preliminary evidence that MMR vaccine might provide protection against COVID-19. medRxiv. 2020;2005: 3207. [DOI] [Full Text] |
| 84. | Gold JE, Baumgartl WH, Okyay RA, Licht WE, Fidel PL Jr, Noverr MC, Tilley LP, Hurley DJ, Rada B, Ashford JW. Analysis of Measles-Mumps-Rubella (MMR) Titers of Recovered COVID-19 Patients. mBio. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 85. | Ashford JW, Gold JE, Huenergardt MA, Katz RBA, Strand SE, Bolanos J, Wheeler CJ, Perry G, Smith CJ, Steinman L, Chen MY, Wang JC, Ashford CB, Roth WT, Cheng JJ, Chao S, Jennings J, Sipple D, Yamamoto V, Kateb B, Earnest DL. MMR Vaccination: A Potential Strategy to Reduce Severity and Mortality of COVID-19 Illness. Am J Med. 2021;134:153-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | Avidan M. An International, Multi-site, Bayesian Platform Adaptive, Randomized, Placebo-controlled Trial Assessing the Effectiveness of Candidate Agents in Mitigating COVID-19 Disease in Adults, 2021 Mar. Report No.: NCT04333732. Available from: https://clinicaltrials.gov/ct2/show/NCT04333732. |
| 87. | Lundberg L, Bygdell M, Stukat von Feilitzen G, Woxenius S, Ohlsson C, Kindblom JM, Leach S. Recent MMR vaccination in health care workers and Covid-19: A test negative case-control study. Vaccine. 2021;39:4414-4418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 88. | De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 89. | Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O'Doherty M, Brown K, Cameron C, Stockton D, McMenamin J, Ramsay M. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 738] [Cited by in RCA: 737] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 90. | Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, Heath R, Tuner A, Friedrich Z, Morrison L, Ruffino G, Antico R, Adegbite D, Szasz-Benczur Z, Garcia Gonzalez M, Oliver J, Danon L, Finn A. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021;21:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 91. | Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 615] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 92. | Perez-Lopez A, Behnsen J, Nuccio SP, Raffatellu M. Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol. 2016;16:135-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 282] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 93. | Robinson JM. Vaccine production: main steps and considerations. In: Bloom B, Lambert PH, editors. The vaccine book. 2nd ed. Academic Press; San Diego: 2016. pp. 77–96; Gomez PL, Robinson JM, Rogalewicz JA. Vaccine Manufacturing. Vaccines, 6th ed. In: Plotkin S, Orenstien W, Offit P. Orlando, editors. WB Saunders Company; 2013: 44–57. |
| 94. | Smith J, Lipsitch M, Almond JW. Vaccine production, distribution, access, and uptake. Lancet. 2011;378:428-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 95. | Tordesillas L, Berin MC. Mechanisms of Oral Tolerance. Clin Rev Allergy Immunol. 2018;55:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 96. | Mestecky J, Russell MW, Elson CO. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J Immunol. 2007;179:5633-5638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 97. | Mudgal R, Nehul S, Tomar S. Prospects for mucosal vaccine: shutting the door on SARS-CoV-2. Hum Vaccin Immunother. 2020;16:2921-2931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 98. | Liu HL, Yeh IJ, Phan NN, Wu YH, Yen MC, Hung JH, Chiao CC, Chen CF, Sun Z, Jiang JZ, Hsu HP, Wang CY, Lai MD. Gene signatures of SARS-CoV/SARS-CoV-2-infected ferret lungs in short- and long-term models. Infect Genet Evol. 2020;85:104438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 99. | Wu YH, Yeh IJ, Phan NN, Yen MC, Hung JH, Chiao CC, Chen CF, Sun Z, Hsu HP, Wang CY, Lai MD. Gene signatures and potential therapeutic targets of Middle East respiratory syndrome coronavirus (MERS-CoV)-infected human lung adenocarcinoma epithelial cells. J Microbiol Immunol Infect. 2021;54:845-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Ko M, Chang SY, Byun SY, Ianevski A, Choi I, Pham Hung d'Alexandry d'Orengiani AL, Ravlo E, Wang W, Bjørås M, Kainov DE, Shum D, Min JY, Windisch MP. Screening of FDA-Approved Drugs Using a MERS-CoV Clinical Isolate from South Korea Identifies Potential Therapeutic Options for COVID-19. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 101. | Pei H, Liu J, Cheng Y, Sun C, Wang C, Lu Y, Ding J, Zhou J, Xiang H. Expression of SARS-coronavirus nucleocapsid protein in Escherichia coli and Lactococcus lactis for serodiagnosis and mucosal vaccination. Appl Microbiol Biotechnol. 2005;68:220-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | WHO. The top 10 causes of death. (2020). World Health Organization. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. |
| 103. | Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, Zaccardi F, Watkinson P, Shankar-Hari M, Doidge J, Harrison DA, Griffin SJ, Sheikh A, Coupland CAC. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 104. | Hassan AO, Kafai NM, Dmitriev IP, Fox JM, Smith BK, Harvey IB, Chen RE, Winkler ES, Wessel AW, Case JB, Kashentseva E, McCune BT, Bailey AL, Zhao H, VanBlargan LA, Dai YN, Ma M, Adams LJ, Shrihari S, Danis JE, Gralinski LE, Hou YJ, Schäfer A, Kim AS, Keeler SP, Weiskopf D, Baric RS, Holtzman MJ, Fremont DH, Curiel DT, Diamond MS. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell. 2020;183:169-184.e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 472] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 105. | Du Y, Xu Y, Feng J, Hu L, Zhang Y, Zhang B, Guo W, Mai R, Chen L, Fang J, Zhang H, Peng T. Intranasal administration of a recombinant RBD vaccine induced protective immunity against SARS-CoV-2 in mouse. Vaccine. 2021;39:2280-2287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 106. | Bricker TL, Darling TL, Hassan AO, Harastani HH, Soung A, Jiang X, Dai YN, Zhao H, Adams LJ, Holtzman MJ, Bailey AL, Case JB, Fremont DH, Klein R, Diamond MS, Boon ACM. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 2021;36:109400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 107. | Lijek RS, Luque SL, Liu Q, Parker D, Bae T, Weiser JN. Protection from the acquisition of Staphylococcus aureus nasal carriage by cross-reactive antibody to a pneumococcal dehydrogenase. Proc Natl Acad Sci U S A. 2012;109:13823-13828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 108. | Vaxart’s Oral COVID-19 Tablet Vaccine to Enter Clinical Trials. [(accessed on 19 November 2020)]; Available from: https://www.biopharma-reporter.com/Article/2020/09/15/Vaxart-First-tablet-COVID-19-vaccine-to-enter-clinical-trials. |
| 109. | Preclinical Studies of a Recombinant Adenoviral Mucosal Vaccine to Prevent SARS-CoV-2 Infection. [(accessed on 23 December 2020)]; Available from: https://www.biorxiv.org/content/10.1101/2020.09.04.283853v1. |
| 110. | Kumar A, Kumar A. Mucosal and transdermal vaccine delivery strategies against COVID-19. Drug Deliv Transl Res. 2022;12:968-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Gabitzsch ES, Xu Y, Yoshida LH, Balint J, Amalfitano A, Jones FR. Novel Adenovirus type 5 vaccine platform induces cellular immunity against HIV-1 Gag, Pol, Nef despite the presence of Ad5 immunity. Vaccine. 2009;27:6394-6398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Niazi KR, Ochoa MT, Sieling PA, Rooke NE, Peter AK, Mollahan P, Dickey M, Rabizadeh S, Rea TH, Modlin RL. Activation of human CD4+ T cells by targeting MHC class II epitopes to endosomal compartments using human CD1 tail sequences. Immunology. 2007;122:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 113. | Gabitzsch ES, Xu Y, Yoshida LH, Balint J, Gayle RB, Amalfitano A, Jones FR. A preliminary and comparative evaluation of a novel Ad5 [E1-, E2b-] recombinant-based vaccine used to induce cell mediated immune responses. Immunol Lett. 2009;122:44-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Gabitzsch ES, Xu Y, Balint JP Jr, Balcaitis S, Sanders-Beer B, Jones FR. Induction and comparison of SIV immunity in Ad5 naïve and Ad5 immune non-human primates using an Ad5 [E1-, E2b-] based vaccine. Vaccine. 2011;29:8101-8107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A, Llewellyn-Lacey S, Kamal H, Bogdanovic G, Muschiol S, Wullimann DJ, Kammann T, Emgård J, Parrot T, Folkesson E; Karolinska COVID-19 Study Group, Rooyackers O, Eriksson LI, Henter JI, Sönnerborg A, Allander T, Albert J, Nielsen M, Klingström J, Gredmark-Russ S, Björkström NK, Sandberg JK, Price DA, Ljunggren HG, Aleman S, Buggert M. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183:158-168.e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1425] [Cited by in RCA: 1425] [Article Influence: 237.5] [Reference Citation Analysis (0)] |
| 116. | Gabitzsch E, Safrit JT, Verma M, Rice A, Seiling P, Zakin L, Shin A, Morimoto B, Adisetiyo H, Wong R, Bezawada A, Dinkins K, Balint J, Peykov V, Garban H, Liu P, Bacon A, Drew J, Spilman P, Sender L, Rabizadeh S, Niazi K. Complete protection of nasal and lung airways against SARS-CoV-2 challenge by antibody plus Th1 dominant N- and S-specific T-cell responses to subcutaneous prime and thermally-stable oral boost bivalent hAd5 vaccination in an NHP study. bioRxiv. 2021;. [DOI] [Full Text] |
| 117. | Ulaeto D, Hruby DE. Uses of vaccinia virus in vaccine delivery. Curr Opin Biotechnol. 1994;5:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 118. | Wang Q, Jiang W, Chen Y, Liu P, Sheng C, Chen S, Zhang H, Pan C, Gao S, Huang W. In vivo electroporation of minicircle DNA as a novel method of vaccine delivery to enhance HIV-1-specific immune responses. J Virol. 2014;88:1924-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 119. | Han SS, Lee J, Jung Y, Kang MH, Hong JH, Cha MS, Park YJ, Lee E, Yoon CH, Bae YS. Development of oral CTL vaccine using a CTP-integrated Sabin 1 poliovirus-based vector system. Vaccine. 2015;33:4827-4836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 120. | Xiao Y, Daniell H. Long-term evaluation of mucosal and systemic immunity and protection conferred by different polio booster vaccines. Vaccine. 2017;35:5418-5425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Okayasu H, Sutter RW, Czerkinsky C, Ogra PL. Mucosal immunity and poliovirus vaccines: impact on wild poliovirus infection and transmission. Vaccine. 2011;29:8205-8214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 122. | Heymann DL, Murphy K, Brigaud M, Aymard M, Tembon A, Maben GK. Oral poliovirus vaccine in tropical Africa: greater impact on incidence of paralytic disease than expected from coverage surveys and seroconversion rates. Bull World Health Organ. 1987;65:495-501. [PubMed] |
| 123. | WHO, Landscape of COVID-19 Candidate Vaccines; 2020. Last accessed on 15th of July. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. |
| 124. | Vela Ramirez JE, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv Drug Deliv Rev. 2017;114:116-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 125. | Focosi D, Maggi F, Casadevall A. Mucosal Vaccines, Sterilizing Immunity, and the Future of SARS-CoV-2 Virulence. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tan JK, Malaysia; Wang CY, Taiwan S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH