Published online Nov 25, 2021. doi: 10.5501/wjv.v10.i6.301
Peer-review started: March 24, 2021
First decision: May 5, 2021
Revised: May 18, 2021
Accepted: August 13, 2021
Article in press: August 13, 2021
Published online: November 25, 2021
Processing time: 242 Days and 11.3 Hours
Coronavirus disease 2019 (COVID-19) has caused a global pandemic unprece
Core Tip: The outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has posed a critical threat to global public health. Beyond the respiratory symptoms, some patients with COVID-19 show liver damage. In this scenario, it has been suggested that there might be a specific relationship between SARS-CoV-2 infection and liver injury.
- Citation: Gato S, Lucena-Valera A, Muñoz-Hernández R, Sousa JM, Romero-Gómez M, Ampuero J. Impact of COVID-19 on liver disease: From the experimental to the clinic perspective. World J Virol 2021; 10(6): 301-311
- URL: https://www.wjgnet.com/2220-3249/full/v10/i6/301.htm
- DOI: https://dx.doi.org/10.5501/wjv.v10.i6.301
Coronaviruses are enveloped single-stranded RNA viruses belonging to the Coronaviridae family and Orthocoronavirinae subfamily[1]. They cause zoonotic infections in humans, predominantly associated with the upper respiratory tract[2]. Two coronaviruses caused relatively recent epidemics: severe acute respiratory syndrome coronavirus (SARS-CoV) in 2003 and the Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012[3].
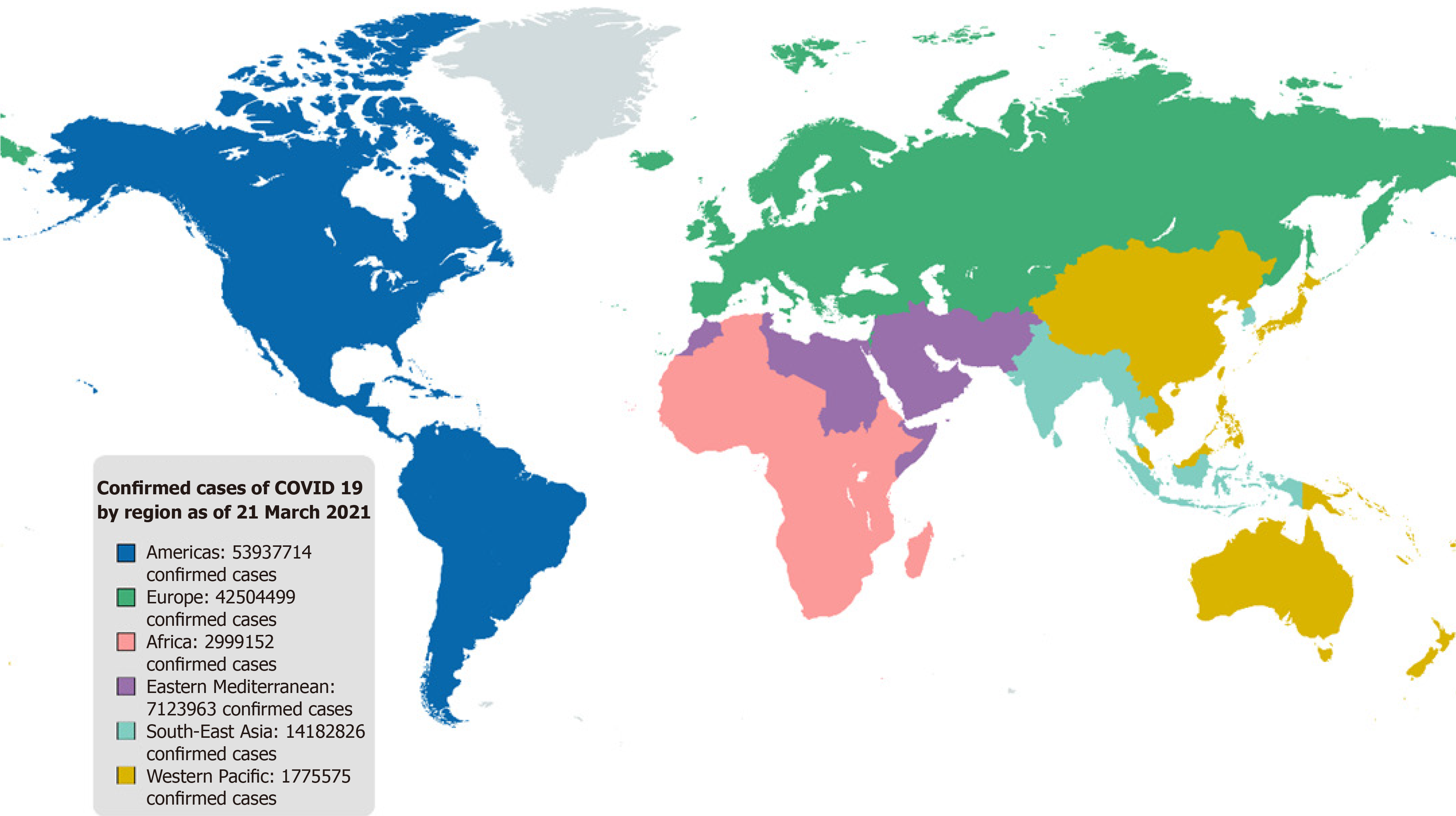
The outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first reported in China in December 2019, has posed a critical threat to global public health[4,5]. Therefore, COVID-19 has been declared an international public health emergency by the World Health Organization (WHO). As of 21st March 2021, more than 122 million confirmed cases and over 2.7 million deaths have been reported[6] (Figure 1).
In most cases, the infection is followed by a benign course with usual characteristics of viral pneumonia, such as fever, dry cough, and lymphopenia. A relatively low percentage of patients require hospitalization and intensive care for acute respiratory failure secondary to diffuse alveolar damage. There is also an important incidence of extrapulmonary manifestations, such as acute kidney injury, cardiovascular disease, neurological disorders, or hypercoagulation[7].
On the other hand, some patients with COVID-19 show different degrees of liver injury, showing mainly elevated serum transaminase and lactate dehydrogenase levels and hypoalbuminemia[8-10]. In this scenario, it has been suggested that there might be a specific relationship between SARS-CoV-2 infection and liver injury. Thus, this article reviews the impact of COVID-19 on liver disease from the experimental to the clinic perspective.
As with SARS-CoV, angiotensin-converting enzyme 2 (ACE2) appears to be the susceptible receptor for SARS-CoV-2 and is expressed in more than 80% of lung alveolar cells. In vitro studies from the SARS epidemic identified ACE2 as the host receptor for viral entry[11], but in this new coronavirus, a recent study showed a 10-20-fold higher receptor binding affinity[12].
The hepatic distribution of ACE2 is quirky; it is highly expressed in the endothelial layer of small blood vessels but not in the sinusoidal endothelium. Indeed, a study revealed that the ACE2 cell surface receptor was more highly expressed in cholangiocytes (59.7%) than hepatocytes (2.6%). Both the level of ACE2 expression in cholangiocytes and lung alveolar type 2 cells are similar, indicating that the liver could be a potential target for SARS-CoV-2[13].
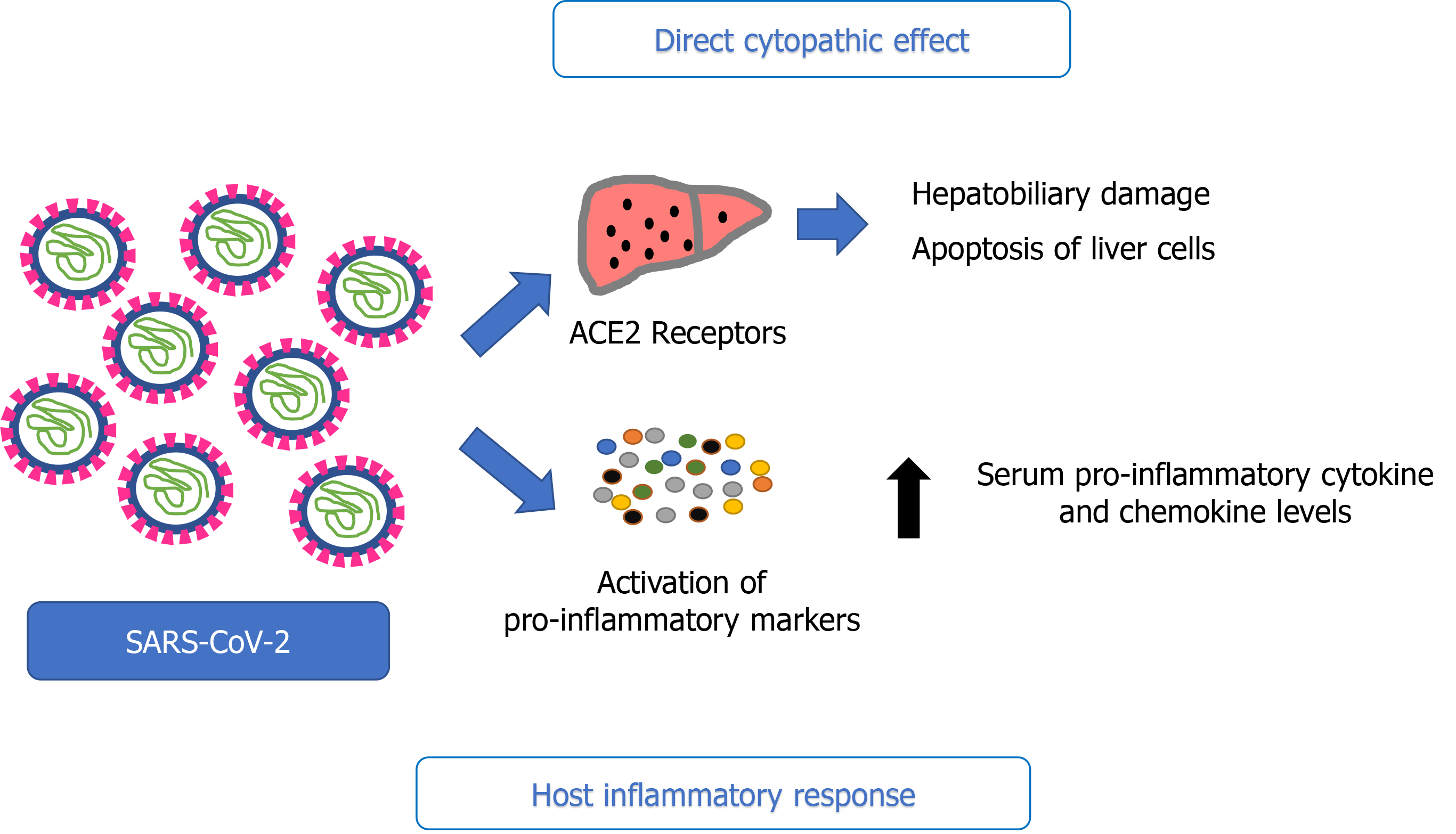
SARS-CoV-2 exerts a cytopathic effect by directly binding to ACE2 positive cholangiocytes. They are involved in liver physiology functions, including regeneration and adaptive immune response mechanisms; thus, their disruption can cause hepatobiliary damage. This is supported by cholestatic markers, including gamma-glutamyl transferase, which can be found in some case reports of COVID-19[14-16]. Permis
A recent study demonstrated that SARS-CoV-specific protein 7a induces apoptosis via a caspase-dependent pathway in cell lines of different organs, including the liver, further confirming the supposition that SARS-CoV-2 directly affects the liver tissue[19]. Nevertheless, some authors have refuted this hypothesis since the disorder of liver function is usually mild, and there is no evidence that late-onset symptoms are associated with greater liver damage[20].
As we have described previously, liver injury in patients with COVID-19 might be due to the viral infection in liver cells. However, it might also be due to other causes such as drug-induced liver injury and systemic inflammation induced by cytokine storm or pneumonia-associated hypoxia[15].
A well-established driver of liver injury is hepatic inflammation, involving the activation of innate immune cells and the release of cytokines[21] (Figure 2). A possible cause of liver injury in COVID-19 can be the dysregulation of the innate immune response. Noticeable activation of inflammatory markers, including abnormal levels of C-reactive protein (CRP), lymphocytes, neutrophils, and cytokines - particularly interleukin-6 (IL-6) - are found in patients with COVID-19[15,22-24]. In some of the available case series of COVID-19, a correlation between lymphopenia and liver injury was observed. Moreover, high levels of CRP and a low lymphocyte count were independent risk factors for liver injury. Notably, lymphopenia in COVID-19 studies was observed in 63% to 70.3% of patients, and those with lower lymphocyte counts were more susceptible to fatal outcomes[22]. These impairments have also been reported in some systemic viral infections, such as cytomegalovirus, herpes simplex virus, Epstein-Barr virus, parvovirus, and adenovirus, in which we can also observe the immune activation and inflammation caused by circulating cytokines[25]. Furthermore, some studies have reported higher serum pro-inflammatory cytokines and chemokine levels in patients with abnormal liver function than those with normal liver function[22]. Hence, these data point to a relationship between liver damage and the inflammatory response induced by SARS-CoV-2 infection.
The liver is involved in the metabolism of many drugs, and some therapeutic agents used to treat SARS-CoV-2 show potential hepatotoxicity. For example, alanine transaminase (ALT) and aspartate aminotransferase (AST) elevations were reported in 4%-6% of patients treated with remdesivir[26], and tocilizumab can also cause mild elevations in liver transaminases[27]. However, it seems unlikely that the leading cause of liver injury is the treatment as alterations in liver transaminases are usually reported at the time of hospital admission.
The prevalence of elevated liver enzymes occurs between 15% and 53% of patients with COVID-19[28,29]. The difference in the prevalence may be related to the exclusion of patients with a previous liver disease[30]. The most common disorder includes elevated aminotransferases (AST and ALT) up to 1-2 times the upper limit of normal, while the elevation of total bilirubin (TB) and alkaline phosphatase is less common. A recent study of 2073 patients with SARS-CoV-2 infection documented liver abnormalities in 1282 (61.8%) of these patients. This study observed liver impairment more frequently in patients with severe COVID-19. Besides, they described cholestasis and mixed types of liver abnormalities as independent variables associated with death[31]. Another recent meta-analysis that included more than 5000 patients from 26 studies also demonstrated that liver function (AST, ALT, and TB) was related to intensive care unit (ICU) admission and non-fatal severe complications[32]. The findings of these studies make us consider incorporating the liver profile to the routine inflammatory markers at the time of hospital admission in patients with SARS-CoV-2 infection to improve their management and anticipate the prognosis.
Several studies have analyzed the impact of chronic liver disease on SARS-CoV-2 infection. First, the prevalence of underlying liver disease in hospitalized patients for COVID-19 ranges between 0.6% to 1.4%[33]. A recent international registry of 745 patients with chronic liver disease (CLD) and SARS-CoV-2 has observed an increased risk of major adverse outcomes and death in cirrhotic patients according to the Child-Pugh class[34]. In this study, a significant increase in ICU requirement, renal repla
The preexisting liver disease most often associated with COVID-19 is metabolic-associated fatty liver disease (MAFLD)[39]. A multicenter retrospective study by Zheng et al[40] demonstrated that the severity of SARS-CoV-2 infection was greater in patients with MAFLD and obesity[40,41].
There is disparity in the data on chronic hepatitis infection prevalence in COVID-19, with percentages ranging from 0.1% to more than 10% in relation to the prevalence of hepatotropic viruses in the area[42,43]. In China, a country with an intermediate-to-high prevalence of chronic hepatitis B (HBV) infection, a surprisingly low prevalence of chronic HBV in COVID-19 patients has been observed. Anugwom et al[44] have reported an incidence of HBV of 1.36%, while the corresponding rates of HBV ranged from 7% to 11% in patients without SARS-CoV-2. This may be explained by "immune exhaustion", as HBV infection provides an inadequate immune response during SARS-CoV-2 infection. Furthermore, chronic hepatitis infection does not appear to lead to a worse prognosis in patients with COVID-19[45]. This fact could be explained by the potential in vitro antiviral effect of the drugs used for chronic infection with hepatotropic viruses (inhibitors of the NS5A protein or nucleotide analogs)[46-48]. However, this has not been demonstrated in patients under active in vivo treatment[49,50].
Finally, the role of SARS-CoV-2 on autoimmune liver diseases has not been adequately evaluated. However, some studies have not observed a higher incidence of SARS-CoV-2 infection and severe complications than in the general population[51,52]. To date, there is no evidence to support or recommend a decrease or change in the immunosuppressive therapy in these patients.
In liver transplant (LT) recipients, immunosuppression following LT may increase the likelihood of SARS-CoV-2 infection[53,54]. Once a transplant recipient is infected with SARS-CoV-2, the virus may remain to infect for a longer duration due to higher viral titers and a prolonged replication period[55]. On the other hand, immunosuppressive agents could ameliorate the systemic inflammation induced by the cytokine storm[56].
Some of the available case series in LT patients with COVID-19 show a higher hospitalization rate (40%-86.5%)[54,57-60], as well as an increase in ICU admission requirements and invasive ventilation[59,61] in these patients. Despite the fact that mortality in LT recipients by COVID-19 is approximately 20% (8%-30.6%)[54,57-60], several studies have not shown that COVID-19-related mortality could be greater in hospitalized LT patients than in the general population[59,61]. Risk factors associated with poor prognosis in LT patients with COVID-19 are older age[53,57,60,62], diabetes mellitus[57-60], chronic kidney disease[60], and liver injury (ALT > 2 times ULN)[58].
On the other hand, it has not been clearly established how the immunosuppressive treatment influences the prognosis of LT patients with COVID-19. For instance, a study showed that mycophenolate might increase the risk of severe COVID-19 in a dose-dependent manner[54], while tacrolimus use has had a positive independent effect on survival[60]. Therefore, it could be concluded that increased disease severity and mortality in LT patients with COVID-19 is caused by the higher prevalence associated with comorbidities than by the effect of immunosuppressive treatment. In fact, in LT recipients without COVID-19, international guidelines recommend against reducing immunosuppression. However, in patients diagnosed with COVID-19, a reduction of immunosuppression should be considered.
Since the beginning of the SARS-CoV-2 pandemic, the healthcare system has supported a substantial impact, and the hepatology units have suffered notable changes in the organization. The access to medical consultations has been limited due to the hospital overload and strict orders to stay at home, the resources and staff reallocation have caused a decrease in the care of non-COVID pathologies. After a year of pandemic, the epidemiology of COVID-19 has proven to be unpredictable, however, it is urgent to anticipate and plan to mitigate the consequences of the pandemic and achieve a dynamic balance of resources.
The prevalence of hepatocellular carcinoma (HCC) has increased globally in the last few years. Significant efforts have been made to decrease HCC-related mortality. For this reason, HCC screening using imaging tests at regular intervals has been implemented and standardized, and is strongly recommended by the international clinical guidelines[63,64].
A recent retrospective study comprising 127 hospitals showed a significant diminution of HCC control during the pandemic, showing screening rates below 50% compared to 2019[65]. Other studies have also found similar results with a decreased HCC surveillance by ultrasound and, more important than this, a decrease in diagnostic tests such as computed tomography or magnetic resonance imaging[66]. Thus, a significant increase in HCC-related mortality could be observed in the next months.
On the other hand, the COVID-19 pandemic has also impacted the management of HCC patients. A recent French multicenter study of 670 patients described a significant decrease in the rate of patients with HCC referred for specific treatment. The rate of patients with a treatment delay of more than one month was higher in 2020 compared to 2019 (21.5% vs 9.5%, P < 0.001)[67].
There were 1.7 million incident cases and 400000 deaths attributable to hepatitis C virus (HCV) in 2015; thus, this viral hepatitis has been recognized as a major cause of death[68]. A breakthrough in HCV treatment occurred in 2013 with the introduction of direct-acting antivirals. For this reason, the WHO approved some ambitious aims to eliminate HCV by 2030, including the reduction of new HCV cases by 80% and HCV-related deaths by 65% for 2030.
The pandemic has caused a slowing or even the halt of HCV elimination programs. The impact of COVID-19 on viral hepatitis in a recent survey has shown that only 47 (36%) of 132 responders could access viral hepatitis testing, and 28 people on treatment for hepatitis were unable to access their medication at this time[69]. Although the real impact is far from being seen, different studies have been carried out to measure the future consequences. Blach et al[70] using a previously validated Markov model, compared a “no delay” vs “one-year delay” scenario in elimination programs and evaluated changes in HCV liver-related deaths and liver cancer. Over the next ten years, the authors estimated that a single-year delay scenario could result in over 72300 liver-related deaths and 44800 excess cases of HCC[71].
To avoid the delay in HCV elimination programs, integrated circuits for massive and combined HCV, HBV, and SARS-CoV-2 diagnosis have been proposed[72]. Giacomelli et al[73] have developed a screening program using rapid immunochromatographic testing (RICT) for SARS-CoV-2 antibodies and a rapid HCV test in a single visit in three Italian cities. The results demonstrated that 2.9% of the tests were positive for HCV antibodies, and 54% of them did not know their serological status.
During the SARS-CoV-2 pandemic, there has been an initial worldwide decline in the number of LTs for several reasons. Firstly, there has been a drastic decrease in liver donors, as well as in the availability of ICU beds for both donors and recipients. Secondly, testing organ donors for the presence of the virus is recommended, and those that are positive should be ineligible for donation. Thirdly, the evaluation of potential candidates for LT has been temporarily limited due to the lower availability of hospital resources, as well as to prevent exposure to SARS-CoV-2 in patients with advanced CLD. Finally, at the beginning of the pandemic, there was a temporary decrease in LT recommendations for patients at greater risk of worsening and mortality due to transplant delay: patients with acute liver failure, high MELD score, and HCC at upper limits of the Milan criteria[74-76].
In the United States (US), the impact on LT between March and August 2020 was evaluated using historical trends between 2016 and 2020. Within the first ten weeks of the pandemic, a dramatic decrease in new listings for LT (11%-21%), deceased donor LT (9%-13%), and living donor LT (42%-49%) was found. Besides, there was a reduction of 59% in patients included in the waiting list for LT. Despite these initial data, the mortality risk of LT waitlist candidates was not significantly different before and after COVID-19[77]. On the other hand, a national survey conducted in the US between March 24th and 31st 2020 showed that 67.7% of LT centers had stopped performing live donor LT[78]. A similar evolution in LT was observed in Italy. Considering the period of the first outbreak (March 1st–March 31st), a decrease of around 35% in LT was recorded due to the decrease in the number of donations[79]. In France, there was a 28% decrease in the number of organ donations in 2020 (543 in 2020 vs 752 organ donations in 2019) and a 22% decrease in the number of liver transplantations (435 in 2020 vs 556 in 2019)[80], comparing two similar periods (January 1st-May 31st 2019 vs. January 1st-May 31st 2020). In Spain, during the first COVID-19 wave (between March 13th-April 23rd), the mean number of donors decreased from 7.2 to 1.2 per day, and the weekly mean number of LTs decreased from 23.6 to 5.7[81]. Throughout the year 2020, the number of donors and LTs reduced by 22.8% and 15.7% (1034 vs 1227), respectively, compared to 2019[82].
It is accepted that SARS-CoV-2 infection can cause liver damage, representing a relevant outcome that affects the prognosis of COVID-19. A direct pathogenic effect on the liver, systemic inflammation, and immune dysfunction appear to play a relevant role in this association. In this scenario, liver function tests such as AST, ALT, and bilirubin levels at admission have been related to a poor COVID-19-related prognosis, including more ICU admission requirements and deaths. Finally, we must pay attention to maintaining an adequate monitoring and follow-up of patients with liver diseases, focusing on the risk of cirrhosis decompensation and HCC screening.
| 1. | Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5202] [Cited by in RCA: 4706] [Article Influence: 784.3] [Reference Citation Analysis (11)] |
| 2. | Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY; SARS study group. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2097] [Cited by in RCA: 2170] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 3. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14335] [Article Influence: 2389.2] [Reference Citation Analysis (10)] |
| 4. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17888] [Article Influence: 2981.3] [Reference Citation Analysis (2)] |
| 5. | Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: An overview. J Chin Med Assoc. 2020;83:217-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 727] [Article Influence: 121.2] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. Weekly Operational Update on COVID-19 – 22 March 2021. Available from: https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19---22-march--2021. |
| 7. | Wang FS, Zhang C. What to do next to control the 2019-nCoV epidemic? Lancet. 2020;395:391-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 8. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19018] [Article Influence: 3169.7] [Reference Citation Analysis (9)] |
| 9. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18390] [Article Influence: 3065.0] [Reference Citation Analysis (13)] |
| 10. | Hu LL, Wang WJ, Zhu QJ, Yang L. [Novel coronavirus pneumonia-related liver injury: etiological analysis and treatment strategy]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 11. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4113] [Cited by in RCA: 4642] [Article Influence: 201.8] [Reference Citation Analysis (0)] |
| 12. | Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1854] [Cited by in RCA: 2029] [Article Influence: 338.2] [Reference Citation Analysis (0)] |
| 13. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. 2020 Preprint. Available from: bioRxiv:931766. [DOI] [Full Text] |
| 14. | Fan Z, Chen L, Li J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical features of COVID-19-related liver damage. 2020 Preprint. Available from: medRxiv:20026971. [DOI] [Full Text] |
| 15. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1308] [Article Influence: 218.0] [Reference Citation Analysis (8)] |
| 16. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 577] [Article Influence: 96.2] [Reference Citation Analysis (1)] |
| 17. | Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 18. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 305] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 19. | Tan YJ, Fielding BC, Goh PY, Shen S, Tan TH, Lim SG, Hong W. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J Virol. 2004;78:14043-14047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 359] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 21. | McDonald B, Kubes P. Innate Immune Cell Trafficking and Function During Sterile Inflammation of the Liver. Gastroenterology. 2016;151:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Li L, Li S, Xu M, Zheng S, Duan Z, Liu J, Chen Y, Li J. Risk factors related to hepatic injury in patients with corona virus disease 2019. 2020 Preprint. Available from: medRxiv:20028514. [DOI] [Full Text] |
| 23. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30461] [Article Influence: 5076.8] [Reference Citation Analysis (12)] |
| 24. | Cui Y, Tian M, Huang D, Wang X, Huang Y, Fan L, Wang L, Chen Y, Liu W, Zhang K, Wu Y, Yang Z, Tao J, Feng J, Liu K, Ye X, Wang R, Zhang X, Zha Y. A 55-Day-Old Female Infant Infected With 2019 Novel Coronavirus Disease: Presenting With Pneumonia, Liver Injury, and Heart Damage. J Infect Dis. 2020;221:1775-1781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 25. | Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 987] [Cited by in RCA: 995] [Article Influence: 165.8] [Reference Citation Analysis (0)] |
| 27. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases, 2012 . [PubMed] |
| 28. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 313] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 29. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2319] [Article Influence: 386.5] [Reference Citation Analysis (0)] |
| 30. | Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 31. | Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, Wang YW, Chen Q, Jin GN, Liu TT, Liang JN, Zhu P, Zhu W, Li Y, Zhang BH, Feng H, Zhang WG, Yin ZY, Yu WK, Yang Y, Zhang HQ, Tang ZP, Wang H, Hu JB, Liu JH, Yin P, Chen XP, Zhang B; Tongji Multidisciplinary Team for Treating COVID-19 (TTTC). Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 32. | Ampuero J, Sánchez Y, García-Lozano MR, Maya-Miles D, Romero Gómez M. Impact of liver injury on the severity of COVID-19: a systematic review with meta-analysis. Rev Esp Enferm Dig. 2021;113:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Téllez L, Martín Mateos RM. COVID-19 and liver disease: An update. Gastroenterol Hepatol. 2020;43:472-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 389] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 35. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 36. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 37. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 281] [Article Influence: 46.8] [Reference Citation Analysis (2)] |
| 38. | Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 39. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (2)] |
| 40. | Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (9)] |
| 41. | Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: a Pooled Analysis. SN Compr Clin Med. 2020;1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 42. | Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, Mo P, Yao L, Yang R, Gao S, Gui X, Hou W, Xiong Y, Li J, Zhang Y. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection. Virol Sin. 2020;35:842-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 43. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6572] [Article Influence: 1095.3] [Reference Citation Analysis (0)] |
| 44. | Anugwom CM, Aby ES, Debes JD. Inverse Association Between Chronic Hepatitis B Infection and Coronavirus Disease 2019 (COVID-19): Immune Exhaustion or Coincidence? Clin Infect Dis. 2021;72:180-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 13058] [Article Influence: 2176.3] [Reference Citation Analysis (4)] |
| 46. | Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;253:117592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 540] [Cited by in RCA: 646] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 47. | Jácome R, Campillo-Balderas JA, Ponce de León S, Becerra A, Lazcano A. Sofosbuvir as a potential alternative to treat the SARS-CoV-2 epidemic. Sci Rep. 2020;10:9294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 48. | Chien M, Anderson TK, Jockusch S, Tao C, Li X, Kumar S, Russo JJ, Kirchdoerfer RN, Ju J. Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19. J Proteome Res. 2020;19:4690-4697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 49. | Lens S, Miquel M, Mateos-Muñoz B, García-Samaniego J, Forns X. SARS-CoV-2 in patients on antiviral HBV and HCV therapy in Spain. J Hepatol. 2020;73:1262-1263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Vizcarra P, Pérez-Elías MJ, Quereda C, Moreno A, Vivancos MJ, Dronda F, Casado JL; COVID-19 ID Team. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7:e554-e564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 51. | Di Giorgio A, Nicastro E, Speziani C, De Giorgio M, Pasulo L, Magro B, Fagiuoli S, D' Antiga L. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020;73:702-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Verhelst X, Somers N, Geerts A, Degroote H, Van Vlierberghe H. Health status of patients with autoimmune hepatitis is not affected by the SARS-CoV-2 outbreak in Flanders, Belgium. J Hepatol. 2021;74:240-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Trapani S, Masiero L, Puoti F, Rota MC, Del Manso M, Lombardini L, Riccardo F, Amoroso A, Pezzotti P, Grossi PA, Brusaferro S, Cardillo M; Italian Network of Regional Transplant Coordinating Centers Collaborating group; Italian Surveillance System of Covid-19, Italian Society for Organ Transplantation (SITO), The Italian Board of Experts in Liver Transplantation (I-BELT) Study Group, Italian Association for the Study of the Liver (AISF), Italian Society of Nephrology (SIN), SIN-SITO Study Group. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: A nationwide population-based study. Am J Transplant. 2021;21:2509-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 54. | Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (1)] |
| 55. | Centers for Disease Control and Prevention. COVID-19 (Coronavirus): FAQs for Organ Donation and Transplantation. 11 March 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/infection-control.html. |
| 56. | Mohammed A, Paranji N, Chen PH, Niu B. COVID-19 in Chronic Liver Disease and Liver Transplantation: A Clinical Review. J Clin Gastroenterol. 2021;55:187-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Fraser J, Mousley J, Testro A, Smibert OC, Koshy AN. Clinical Presentation, Treatment, and Mortality Rate in Liver Transplant Recipients With Coronavirus Disease 2019: A Systematic Review and Quantitative Analysis. Transplant Proc. 2020;52:2676-2683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V, Moghe A, Latt NL, Kumar S, Aloman C, Catana AM, Bloom PP, Chavin KD, Carr RM, Dunn W, Chen VL, Aby ES, Debes JD, Dhanasekaran R; COLD Consortium. Liver Injury in Liver Transplant Recipients With Coronavirus Disease 2019 (COVID-19): U.S. Multicenter Experience. Hepatology. 2020;72:1900-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 59. | Mansoor E, Perez A, Abou-Saleh M, Sclair SN, Cohen S, Cooper GS, Mills A, Schlick K, Khan A. Clinical Characteristics, Hospitalization, and Mortality Rates of Coronavirus Disease 2019 Among Liver Transplant Patients in the United States: A Multicenter Research Network Study. Gastroenterology. 2021;160:459-462.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 60. | Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, Coilly A, Ericzon BG, Loinaz C, Cuervas-Mons V, Zambelli M, Llado L, Diaz-Fontenla F, Invernizzi F, Patrono D, Faitot F, Bhooori S, Pirenne J, Perricone G, Magini G, Castells L, Detry O, Cruchaga PM, Colmenero J, Berrevoet F, Rodriguez G, Ysebaert D, Radenne S, Metselaar H, Morelli C, De Carlis LG, Polak WG, Duvoux C; ELITA-ELTR COVID-19 Registry. Protective Role of Tacrolimus, Deleterious Role of Age and Comorbidities in Liver Transplant Recipients With Covid-19: Results From the ELITA/ELTR Multi-center European Study. Gastroenterology. 2021;160:1151-1163.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 61. | Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 62. | Jayant K, Reccia I, Virdis F, Pyda JS, Bachul PJ, di Sabato D, Barth RN, Fung J, Baker T, Witkowski P. COVID-19 in hospitalized liver transplant recipients: An early systematic review and meta-analysis. Clin Transplant. 2021;35:e14246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3430] [Article Influence: 428.8] [Reference Citation Analysis (3)] |
| 64. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6404] [Article Influence: 800.5] [Reference Citation Analysis (9)] |
| 65. | Mahmud N, Kaplan DE, Goldberg DS, Taddei TH, Serper M. Changes in Hepatocellular Carcinoma Surveillance and Risk Factors for Noncompletion in the Veterans Health Administration Cohort During the Coronavirus Disease 2019 Pandemic. Gastroenterology. 2021;160:2162-2164.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Toyoda H, Huang DQ, Le MH, Nguyen MH. Liver Care and Surveillance: The Global Impact of the COVID-19 Pandemic. Hepatol Commun. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 67. | Amaddeo G, Brustia R, Allaire M, Lequoy M, Hollande C, Regnault H, Blaise L, Ganne-Carrié N, Séror O, Larrey E, Lim C, Scatton O, El Mouhadi S, Ozenne V, Paye F, Balladur P, Dohan A, Massault PP, Pol S, Dioguardi Burgio M, Vilgrain V, Sepulveda A, Cauchy F, Luciani A, Sommacale D, Leroy V, Roudot-Thoraval F, Bouattour M, Nault JC; Paris Liver Cancer Group. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep. 2021;3:100199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 68. | World Health Organization. Web Annex B. WHO Estimates of the Prevalence and Incidence of Hepatitis C Virus Infection by WHO Region, 2015. Centre for Disease Analysis, 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/277005/WHO-CDS-HIV-18.46-eng.pdf?ua=1. |
| 69. | Wingrove C, Ferrier L, James C, Wang S. The impact of COVID-19 on hepatitis elimination. Lancet Gastroenterol Hepatol. 2020;5:792-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 70. | Blach S, Kondili LA, Aghemo A, Cai Z, Dugan E, Estes C, Gamkrelidze I, Ma S, Pawlotsky JM, Razavi-Shearer D, Razavi H, Waked I, Zeuzem S, Craxi A. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021;74:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 71. | Turnes J, Domínguez-Hernández R, Casado MÁ. Cost-effectiveness analysis of two treatment strategies for chronic hepatitis C before and after access to direct-acting antivirals in Spain. Gastroenterol Hepatol. 2017;40:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Crespo J, Díaz-González Á, Iruzubieta P, Llerena S, Cabezas J. SARS-CoV-2 massive testing: A window of opportunity to catch up with HCV elimination. J Hepatol. 2021;74:966-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Giacomelli A, Pagani G, Conti F, Bassoli C, Galli M. Detecting HCV infection by means of mass population SARS-CoV-2 screening: A pilot experience in Northern Italy. J Hepatol. 2021;75:484-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 75. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 340] [Article Influence: 56.7] [Reference Citation Analysis (2)] |
| 76. | APASL Covid-19 Task Force, Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14:415-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 77. | Miller J, Wey A, Musgrove D, Son Ahn Y, Hart A, Kasiske BL, Hirose R, Israni AK, Snyder JJ. Mortality among solid organ waitlist candidates during COVID-19 in the United States. Am J Transplant. 2021;21:2262-2268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Boyarsky BJ, Po-Yu Chiang T, Werbel WA, Durand CM, Avery RK, Getsin SN, Jackson KR, Kernodle AB, Van Pilsum Rasmussen SE, Massie AB, Segev DL, Garonzik-Wang JM. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809-1818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 79. | Di Sandro S, Magistri P, Bagnardi V, Catellani B, Guerrini GP, Di Benedetto F. The COVID-19 second wave risk and liver transplantation: lesson from the recent past and the unavoidable need of living donors. Transpl Int. 2021;34:585-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Turco C, Lim C, Soubrane O, Malaquin G, Kerbaul F, Bastien O, Conti F, Scatton O. Impact of the first Covid-19 outbreak on liver transplantation activity in France: A snapshot. Clin Res Hepatol Gastroenterol. 2021;45:101560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Domínguez-Gil B, Coll E, Fernández-Ruiz M, Corral E, Del Río F, Zaragoza R, Rubio JJ, Hernández D. COVID-19 in Spain: Transplantation in the midst of the pandemic. Am J Transplant. 2020;20:2593-2598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 82. | Ministerio de Sanidad. Nota de Prensa: La Organización Nacional de Trasplantes Presenta Su Balance de Actividad En 2020. Available from: http://www.ont.es/Documents/Nota%20de%20Prensa%20BALANCE%20ONT%202020.pdf. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fallatah H S-Editor: Wang JL L-Editor: Webster JR P-Editor: Xing YX