©The Author(s) 2015.
World J Virology. Aug 12, 2015; 4(3): 188-197
Published online Aug 12, 2015. doi: 10.5501/wjv.v4.i3.188
Published online Aug 12, 2015. doi: 10.5501/wjv.v4.i3.188
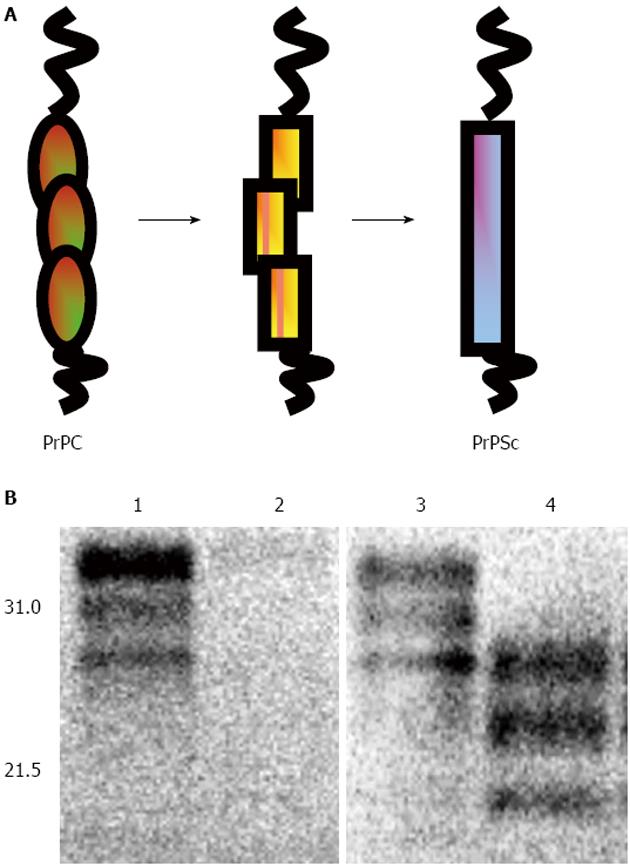
Figure 1 Conversion of PrPC into PrPSc.
A: Schematic diagram of the conversion of PrPC into PrPSc. A major structural event occurs in the C-terminal domain of PrPC as it converts from a predominantly α-helical form into one enriched for β-sheet. This conformational change may involve the formation of intermediate structures of the protein; B: Western blot detection of ovine PrP. VRQ/VRQ sheep brain homogenate from animals that were scrapie-free (tracks 1 and 2) or scrapie-infected (tracks 3 and 4) were pre-treated with (tracks 2 and 4) or without (tracks 1 and 3) PK at 32 μg/mL at 37 °C for 30 min and the products analysed by SDS/PAGE, and Western blot probed with anti-PrP monoclonal antibody 683. Molecular weight markers (in kDa) are shown on the left (Reproduced by kind permission of CAB Reviews).
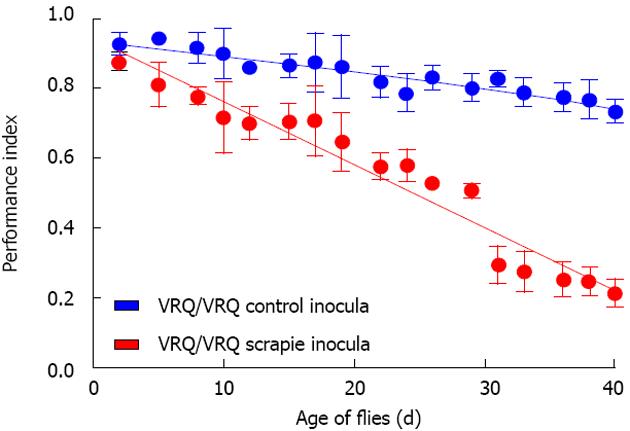
Figure 2 Prion-exposed ovine PrP transgenic Drosophila show enhanced locomotor defect.
Drosophila with pan neuronal expression of ovine VRQ(cyt) were fed VRQ/VRQ scrapie-free (blue circles, blue line) or scrapie-infected (red circles, red line) sheep brain homogenate at the larval stage of development. The locomotor activity of adult flies was assessed by a negative geotaxis climbing assay. The performance index is shown for each genotype of fly per time point (Reproduced with permission, from Thackray et al[100] 2014. © the Biochemical Society).
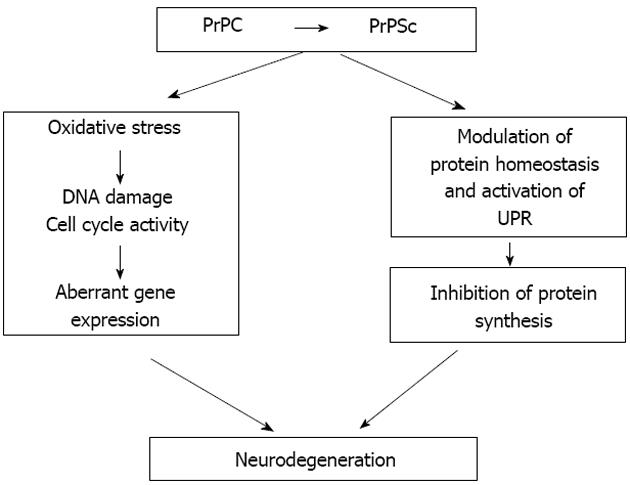
Figure 3 Hypothetical model for prion-induced neurodegeneration.
The conversion of PrPC into PrPSc is an essential requirement for the neurotoxicity that occurs during prion disease. Neurodegeneration in post mitotic neurons, stressed by prion replication, may arise through various cellular events including aberrant cell cycle re-entry and sustained inhibition of protein synthesis. These two processes may operate in parallel or may potentially represent temporally linked events. In both cases, aberrant cell cycle re-entry may contribute to the effect of sustained inhibition of protein synthesis evident in prion-induced neurotoxicity.
- Citation: Bujdoso R, Landgraf M, Jackson WS, Thackray AM. Prion-induced neurotoxicity: Possible role for cell cycle activity and DNA damage response. World J Virology 2015; 4(3): 188-197
- URL: https://www.wjgnet.com/2220-3249/full/v4/i3/188.htm
- DOI: https://dx.doi.org/10.5501/wjv.v4.i3.188