©2013 Baishideng Publishing Group Co.
World J Virol. Nov 12, 2013; 2(4): 152-159
Published online Nov 12, 2013. doi: 10.5501/wjv.v2.i4.152
Published online Nov 12, 2013. doi: 10.5501/wjv.v2.i4.152
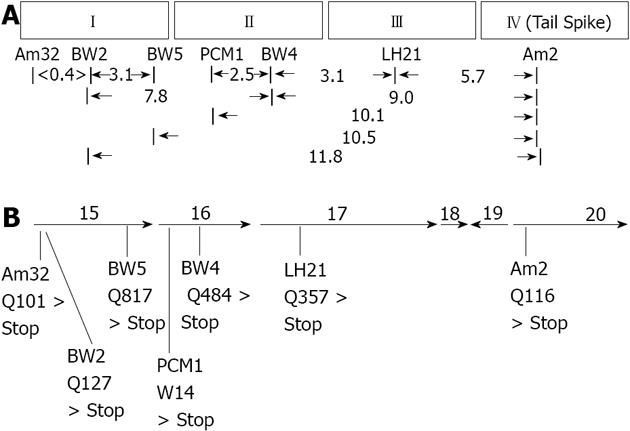
Figure 1 Genetic mapping and sequencing data showing positions of nonsense mutations that affect the protein composition of the epsilon 15 adsorption apparatus.
A: Two-factor recombination values for nonsense mutations falling within in vivo complementation groups I through IV; B: Gene sequencing data. PCM1: Pericentriolar material 1; LH: Luteinizing hormone.
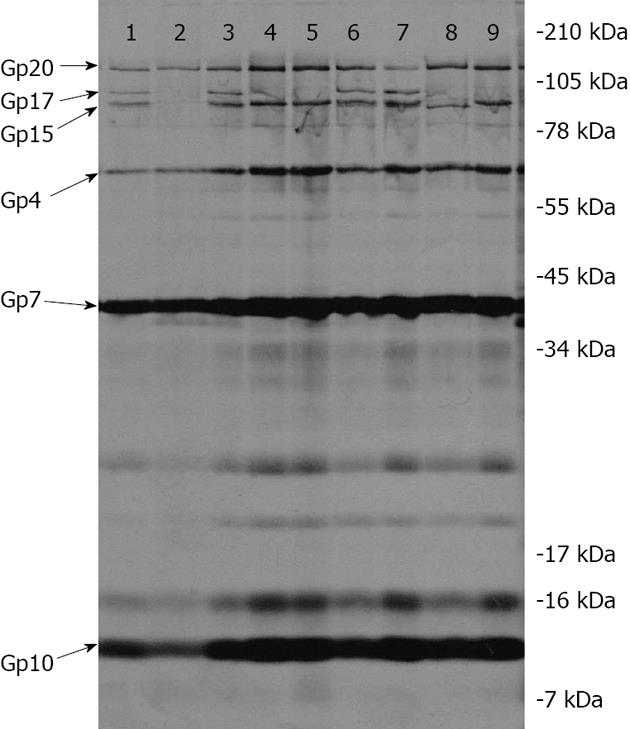
Figure 2 Autoradiogram showing compositions of non-infectious epsilon 15Vir particles.
Lanes 1, 3 and 6, E15vir; Lane 2, gene 15 mutant am32 (BW2 is not shown but gives an identical pattern); Lanes 4 and 5, gene 16 mutants pericentriolar material 1 and BW4; Lane 7, partially suppressed am2 (gp20-) particles; Lane 8, gene 15 mutant BW5; Lane 9, gene 17 mutant luteinizing hormone21. molecular weight markers are depicted to the right.
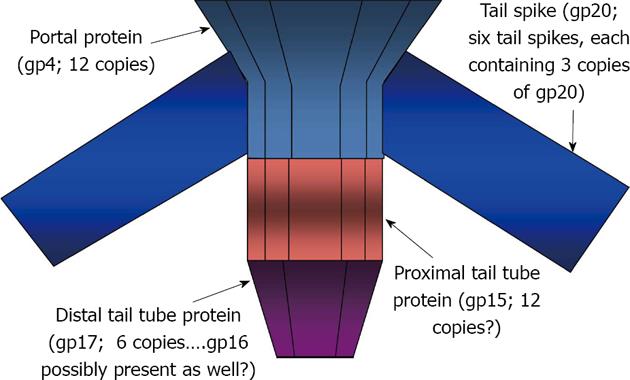
Figure 3 Schematic model for protein positions and interactions within the adsorption apparatus of bacteriophage Epsilon 15.
The estimates of 12 and 6 copies for gp15 and gp17, respectively, are based upon stoichiometric measurements made relative to the numbers of capsid and tail spike proteins present in epsilon 15[13]; tail spike attachment to portal protein may be further stabilized by interactions with gp15 and/or capsid proteins.
- Citation: Guichard JA, Middleton PC, McConnell MR. Genetic analysis of structural proteins in the adsorption apparatus of bacteriophage epsilon 15. World J Virol 2013; 2(4): 152-159
- URL: https://www.wjgnet.com/2220-3249/full/v2/i4/152.htm
- DOI: https://dx.doi.org/10.5501/wjv.v2.i4.152