©2013 Baishideng.
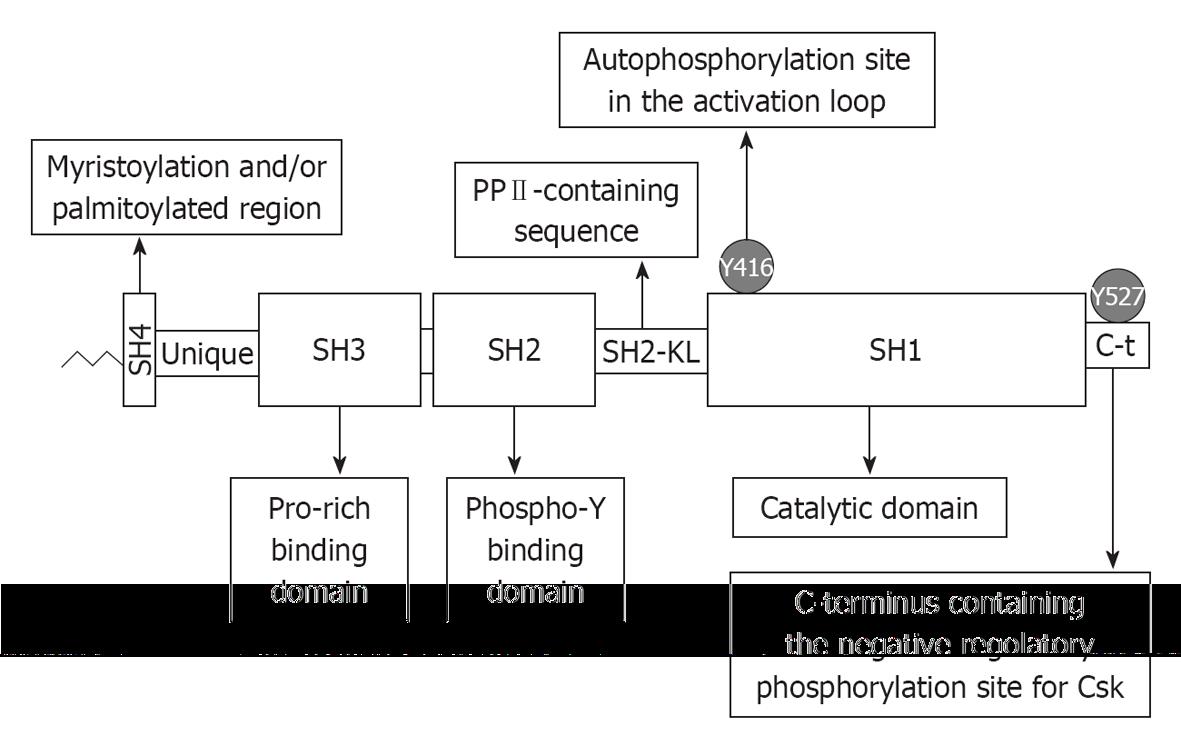
Figure 1 Diagram representing the domain organization of Src family kinases.
As reported in the text, the C-terminus (C-t in the figure) when phosphorylated at Tyr527 binds to the Src homology (SH)2 domain and the polyproline type II helical motif (PPII) motif in the SH2 kinase linker (SH2-KL in the figure) engages the SH3 domain, thus inducing an inactive conformation. Disruption of these inhibitory interactions, in the case of viruses mostly induced by proteins bearing tyrosine phosphorylated or proline-rich motifs, leads to the full activation of Src family kinases.
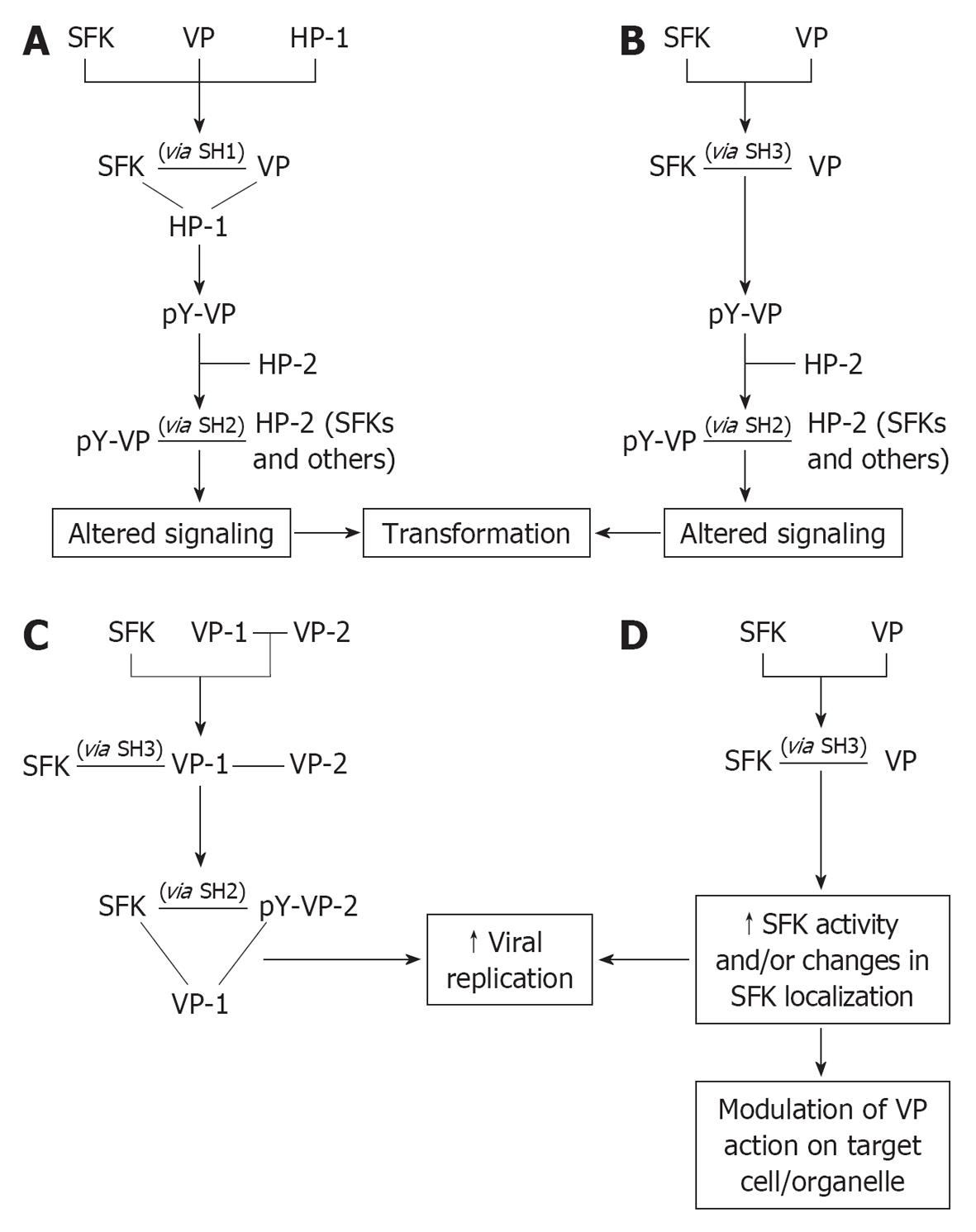
Figure 2 Models of mechanisms connecting viral proteins and Src family kinases and downstream effects.
Viral proteins bind to the various modular domains of Src family kinases (SFKs), with (A) or without (B-D) concurrent association with other host proteins, resulting in the subsequent phosphorylation of the viral proteins (VPs) by which they are engaged (A, B), ultimately conferring new functional properties to VPs, or of different VPs to stabilize multiprotein complexes (C); Moreover, VPs may delocalize SFKs to different cell compartments, where SFKs in the activated form can process local substrates or act as non-catalytic mediators of the action of VPs themselves (D) (see text for further details regarding how specific VPs fit into each model. Solid lines indicate binding, arrows indicate downstream event. HP: Host protein; pY: Phosphotyrosine.
- Citation: Pagano MA, Tibaldi E, Palù G, Brunati AM. Viral proteins and Src family kinases: Mechanisms of pathogenicity from a “liaison dangereuse”. World J Virol 2013; 2(2): 71-78
- URL: https://www.wjgnet.com/2220-3249/full/v2/i2/71.htm
- DOI: https://dx.doi.org/10.5501/wjv.v2.i2.71