Published online Dec 24, 2017. doi: 10.5500/wjt.v7.i6.301
Peer-review started: May 10, 2017
First decision: July 20, 2017
Revised: November 8, 2017
Accepted: November 22, 2017
Article in press: November 22, 2017
Published online: December 24, 2017
Processing time: 229 Days and 14.2 Hours
In view of the availability of new immunosuppression strategies, the recurrence of allograft glomerulonephritis (GN) are reported to be increasing with time post transplantation. Recent advances in understanding the pathogenesis of the GN recurrent disease provided a better chance to develop new strategies to deal with the GN recurrence. Recurrent GN diseases manifest with a variable course, stubborn behavior, and poor response to therapy. Some types of GN lead to rapid decline of kidney function resulting in a frustrating return to maintenance dialysis. This subgroup of aggressive diseases actually requires intensive efforts to ascertain their pathogenesis so that strategy could be implemented for better allograft survival. Epidemiology of native glomerulonephritis as the cause of end-stage renal failure and subsequent recurrence of individual glomerulonephritis after renal transplantation was evaluated using data from various registries, and pathogenesis of individual glomerulonephritis is discussed. The following review is aimed to define current protocols of the recurrent primary glomerulonephritis therapy.
Core tip: Renal transplantation is the best-known therapy for end stage renal disease, with the glomerulonephritis represents a major aetiology for its prevalence. Unfortunately, recurrence of the glomerulonephritis (GN) disease after renal transplantation represents a real devastating impact on allograft survival. A clear understanding of their pathogenesis, will help not only in ameliorating GN recurrence, but also improves allograft survival.
- Citation: Abbas F, El Kossi M, Jin JK, Sharma A, Halawa A. Recurrence of primary glomerulonephritis: Review of the current evidence. World J Transplant 2017; 7(6): 301-316
- URL: https://www.wjgnet.com/2220-3230/full/v7/i6/301.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i6.301
The impact of glomerulonephritis (GN) recurrence varies widely from mild or negligible effect, e.g., IgA nephropathy (IgAN), to a real detrimental impact on graft survival, e.g., Focal Sclerosing Glomerulosclerosis (FSGS) and membranoproliferative GN (MPGN)[1]. Since it has been early recognized, the deleterious impact of the recurrent GN on allograft longevity, continuous efforts have been exerted to determine its real prevalence, clear pathogenesis and to tailor the best strategies for treatment and prevention[2]. Recently, several mechanisms have been postulated to address a clear pathogenesis of GN recurrence[1]. The prevalence of GN as an etiology of end-stage renal disease (ESRD) was reported to be exceeding 48% in China[3,4], 50% in Australian-New Zealand[2] and 30% according to USRDS 2015 report[5]. The frequent lack of kidney biopsy resulted in underestimation of the real prevalence of the GN recurrence[2]. Moreover, the distinction between recurrent GN and the de novo disease is not widely applied. Compared to an early (within the first year) post transplantation assessment of prevalence of about 4%, a value of 13% after 7.5 years[6], and 18% in other studies[7,8] have been recorded[2]. The reported wide variations in prevalence may be attributed to the variability in follow up periods of various studies[9].
The advent of the new immunosuppressive strategies in kidney transplantation have been reflected on the rates of acute and chronic rejection, but unfortunately has little (impact on the prevalence rates of GN recurrence as well as the de novo GN disease[10]). The expected improved allograft survival rate will be ultimately reflected in the future on the prevalence of the recurrent GN after kidney transplantation. It is noteworthy to mention that GN disease with a seemingly benign course, e.g., IgAN is known to recur in 40% of patients but leads to graft loss only in 10%[11,12]. The magnitude of challenge, at times, seems insurmountable despite the progress in understanding the pathogenesis of certain recurrent GN, e.g., permeability factors (suPAR in FSGS and ant-PLA2R AB in MN).
In this review, the authors have identified the most recent progress in understanding the pathogenesis of GN recurrence and its impact on the renal allograft survival. Further insights on the available strategies for treatment and prevention of GN recurrence, particularly so in the main primary GN is will be addressed.
An assumed underestimation of the real prevalence of the GN recurrence has been proved By application of the “Protocol Biopsy” that defined as: biopsy at fixed time, with no relation to a clinical guide. Protocol biopsy delineates a higher incidence of GN recurrence (5%, 18%, 21%, 35%, 42% at 1, 3, 5, 8 and 10 years respectively)[5]. Many explanations have been postulated in this concept to shed the light on the reported discrepancy in prevalence of the GN recurrence: (1) absence of clear native kidney disease diagnosis; (2) absence of valid biomarker for GN recurrence; (3) difficulty in differential diagnosis from other pathological entities, e.g., CAN and drug intoxication; (4) absence of clear stratification and characterization of GN recurrence nature in view of the advent of the new therapeutic approaches[13-15]; (5) the decision of biopsy is not always performed routinely whenever indicated (e.g., proteinuria/hematuria, renal impairment); (6) IF/EM techniques are not routinely performed after each biopsy; (7) a wide discrepancy is found in certain diseases, e.g., IgAN, between histopathologic characteristic changes and the appearance of clinical manifestations; (8) a trend to differentiate and isolate de novo disease from a true recurrent disease is usually not eventually attempted; (9) absence of basal data as regard etiology of ESRF and the native renal biopsy in many cases; and (10) data inconvenience may result in misdiagnosis of a recurrent disease as a de novo disease, which is in fact a true recurrence[2].
The detrimental impact of GN recurrence on allograft survival is irrefutable. The consideration of this impact relies on three points: (1) impact of recurrence of particular types of GN before transplantation on graft survival, e.g., FSGS and MPGN TypeIvs other types of GN. A significantly higher risk of graft failure in these types[9,16]. The proper evaluation should involve a fairly large number of patients studied and followed for an enough period of time[2]; (2) evaluation of the risk of graft failure in case of GN recurrence: The etiology of graft failure should be considered, membranous nephropathy (MN), for example, has high recurrence rate leading to hazardous effect on graft survival[17]; and (3) global allograft GN particularly recurrent disease and its relation to the death censored allograft survival: As the time of recurrence is not constant, it should be considered a time-dependent variable for a better and proper evaluation[2].
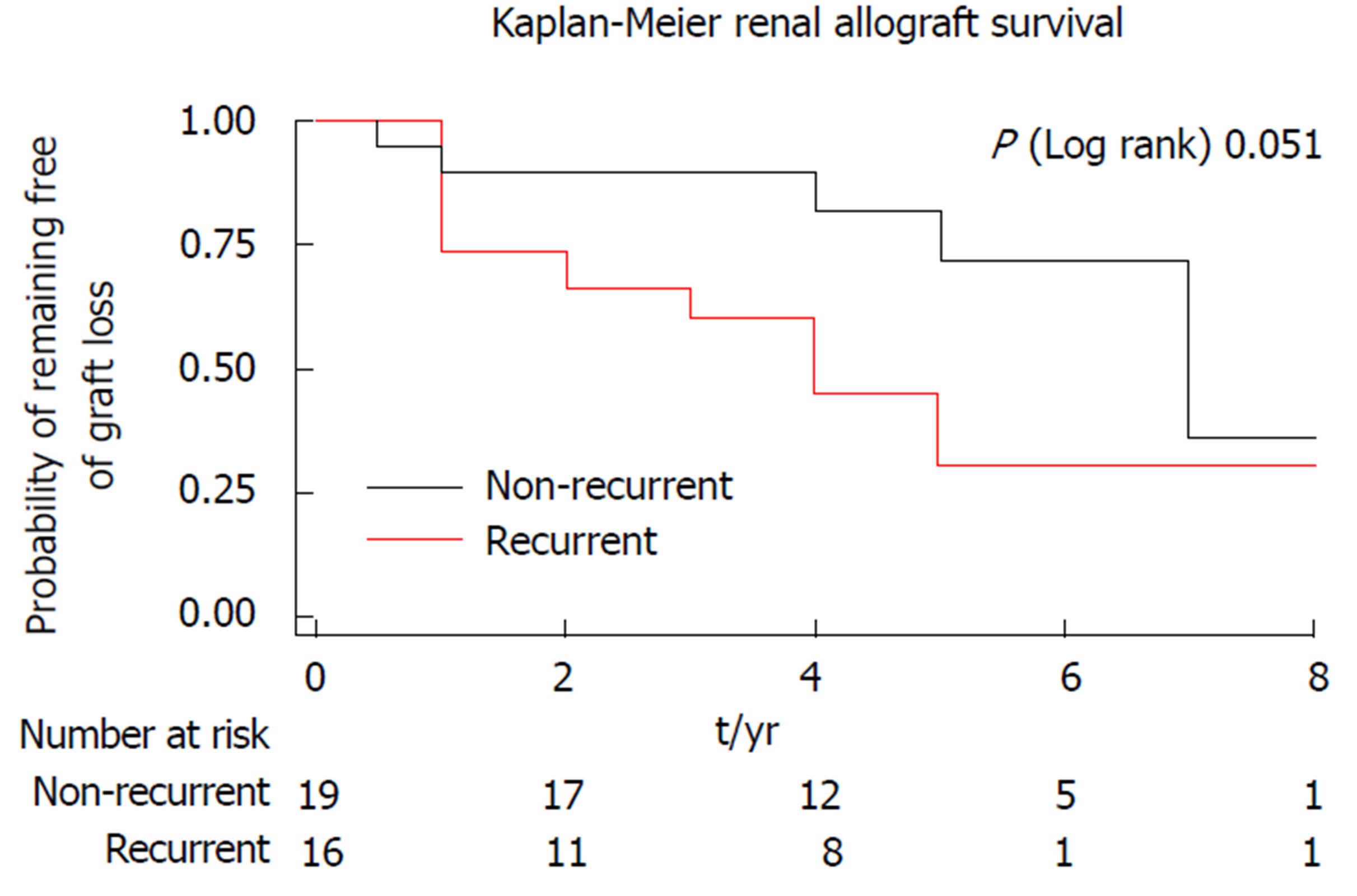
As reported by Cosio et al[2] in the American Transplant Congress, 2015, TypeIMPGN and FSGS showed the highest rate of GN recurrence with subsequent increased risk of allograft loss, followed by IgAN. These data are supported by some studies[12], but not agreed by others[6,9]. It was assumed that 18%-22% of the death-censored kidney allograft losses was attributed to allograft GN (de novo and recurrent)[7], the second most common cause of death-censored graft losses[18] and third most prevalent cause of uncensored graft losses[9,16]. However, Mashaly et al[19] observed that the best allograft survival of kidney transplantation was noted in recipients whose end stage renal failure was due to polycystic kidney disease followed by those who had urologic disease and then those who had GN as the cause of renal failure. The recurrent GN disease has a wide variety of drawbacks deranging allograft function, which made it occupy the third most common etiology of allograft loss after death with a functioning graft and chronic allograft glomerulopathy, an assumption that was agreed by Fairhead and Knoll[20] (2010) who declared that the recurrent GN disease is a major determinant of the long term graft survival (Figure 1). On the other hand, Toledo et al[21] (2011) denied the presence of any difference between GN recurrence and other causes of allograft dysfunction as regard their influence on long term allograft survival. This discrepancy could be a statistical artefact attributed to the small number of patients in their study, racial impacts and the different immunosuppression strategies.
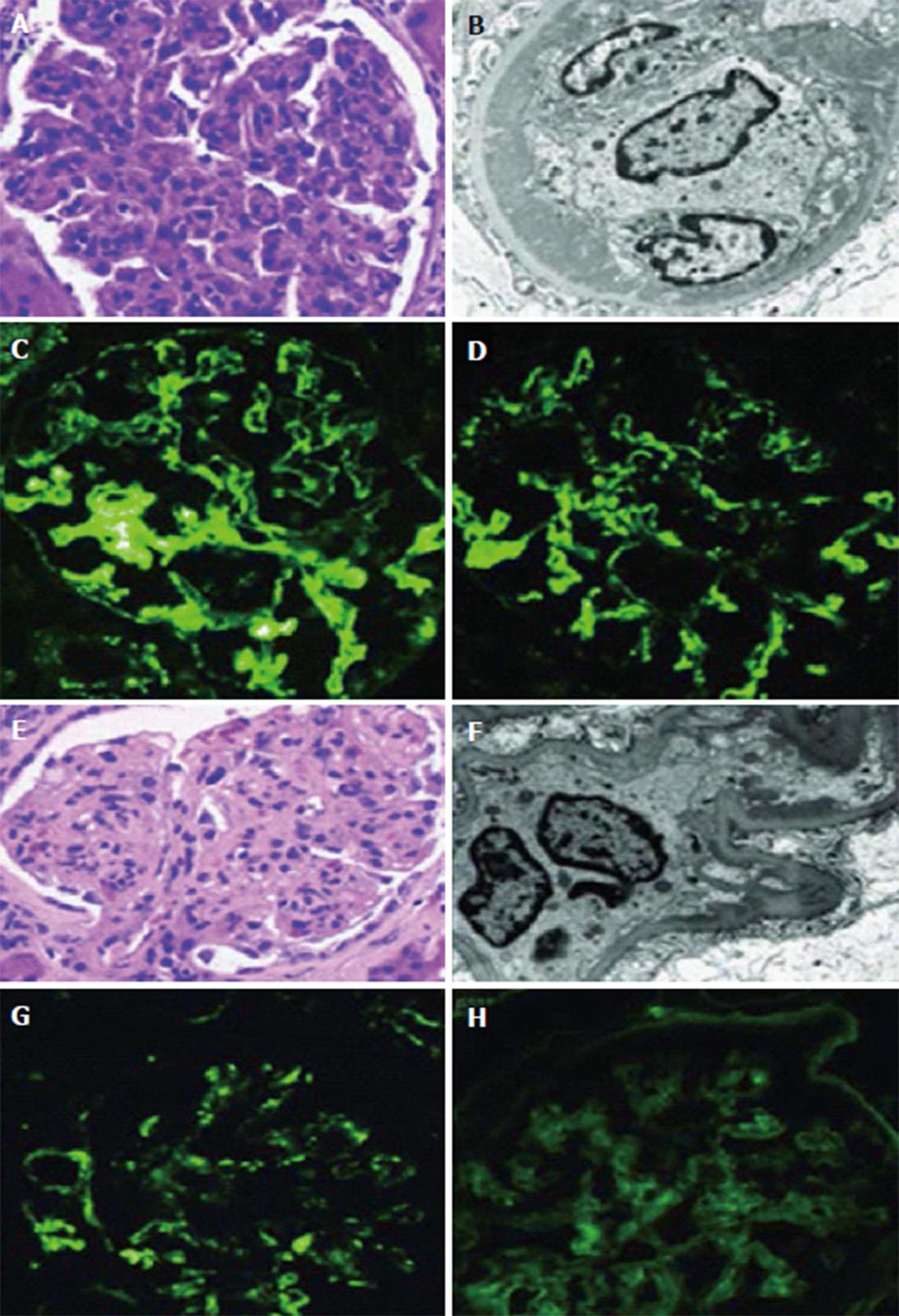
A full detailed map of allograft deterioration due to GN recurrence, can be obtained through a standard protocol biopsy, a widely applied strategy in many centers, so that the earliest changes in allograft histology can be discovered and the native GN disease recurrence can be early anticipated. An intraoperative basal kidney biopsy, at discharge, then after 3 wk, 3-6 mo, 12 mo and after 3 years biopsy is performed serially[22]. The importance of the protocol biopsy could be observed in identification of the early course changes in some transmitted GN diseases, e.g., IgAN, which accounts for more than 90% of transmitted GN[23]. Early recurrence can be detected within 1-2 mo after transplantation. At that time and after the confirmation of recurrence in the third month, no hematuria/proteinuria could be observed; only histological recurrence can be titrated with the frequent specimens[22].
Japanese pathologists pioneered protocol biopsy to understand primary and secondary GN recurrence, e.g., FSGS[24,25], IgAN[26,27], atypical HUS[28] and light chain deposition disease[29].
Green et al[23,24] (2015) reported that the risk of recurrence is higher in MPGN TypeI, with the following factors: (1) the HLA B49, HLA DR4; (2) previous transplantations; (3) acute tubular necrosis after transplantation; (4) shorter duration of dialysis before transplantation; and (5) Arab origin was all associated with decreased graft and patient survival[24].
A better allograft survival is expected in MPGN TypeI, with the following[24]: (1) unrelated living donors; and (2) absence of recurrence in the first year post transplantation.
The advent of the new concepts declaring the role of the alternative complement pathway in the pathogenesis of MPGN was addressed in appearance of the new classification of MPGN. It depends on the mechanism of glomerular injury instead of deposits distribution, which will be ultimately reflected on development of the new therapeutic policies (see therapy of GN recurrence) and its clinical interpretations[30]. So, MPGN will be immune complex mediated (ICGN), encompassing immune complexes and complement compounds, or complement-mediated (CGN) containing only complement, without immune complex (Table 1). Old studies were based on the old classification and data in this subject were very limited owing to the limited number of patients and short follow up durations. The highest prevalence rate has been observed with the previously named MPGN II[31,32].
| No | Type | Criteria | Prevalence |
| 1 | ICGN | Contains immune complexes + complement compounds | More common (most of the recurrent cases are ICGN) |
| 2 | CGN | Contains complement compounds only | Less prevalent (change from one type to another is possible) |
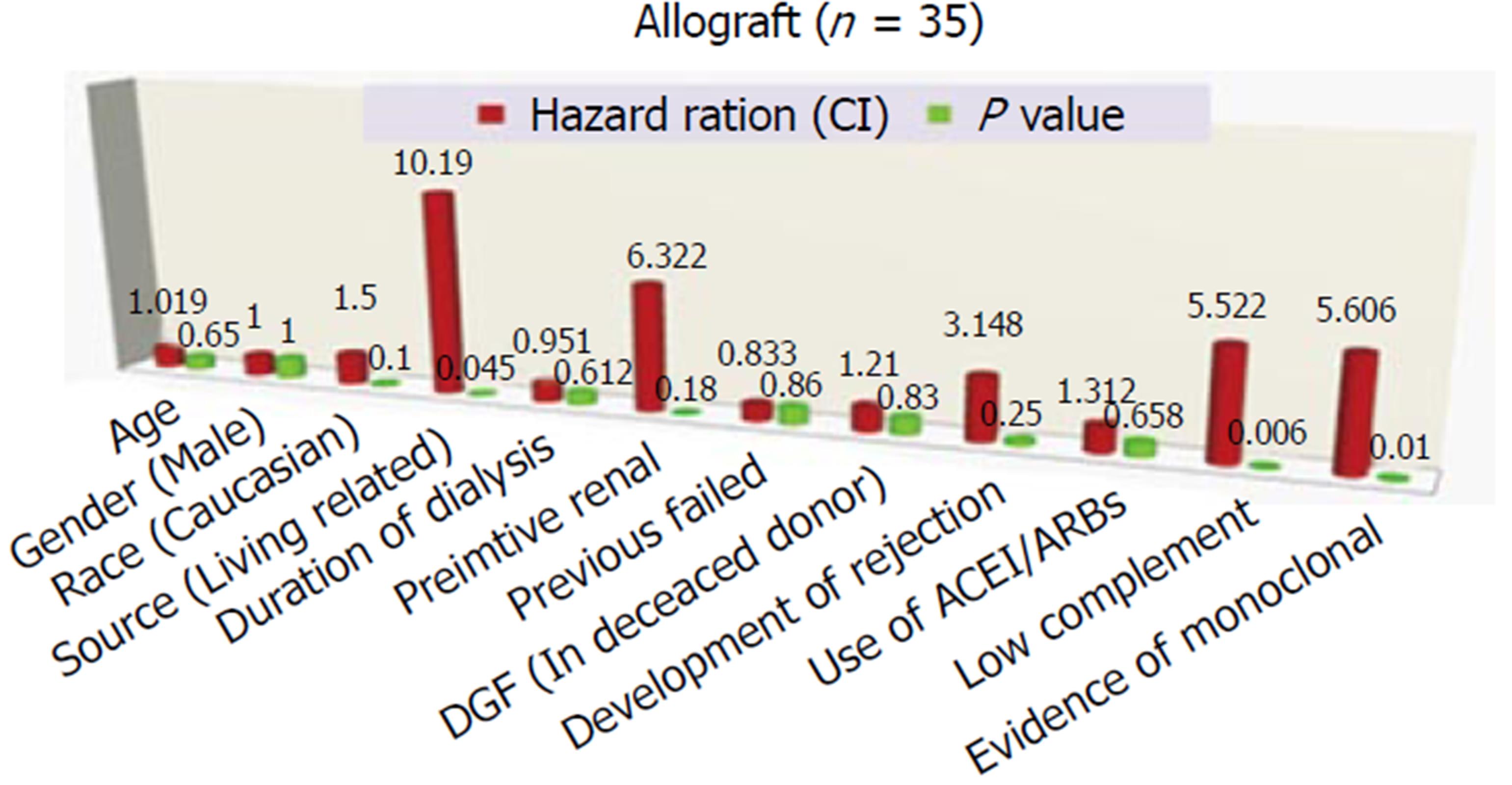
According to Alasfar et al[30] (2016) (Figure 2), the following risk factors have been proposed to be associated with more liability for MPGN recurrence: (1) preemptive renal transplantation[30]; (2) the living related donation[30]; (3) presence of monoclonal immunoglobulins[33]; (4) diminished complement levels[33]; (5) a higher level of proteinuria[32]; (6) human leukocyte antigen type: HLA B8, DR 3[34]; and (7) evidence of crescents in the original biopsy[34].
Impact of HLA typing on prevalence of MPGN recurrence: Green et al[24] (2013) concluded that the risk of recurrence is higher in MPGN TypeI, with certain human leukocyte antigen, i.e., HLA B49, HLA DR4. Andresdottir et al[34] (1997) reported an increased risk of recurrence of MBGN TypeI was observed in patients with the HLA haplotype B8 DR3.
The recurrence of primary MN after renal transplantation is obviously has deleterious impact on graft survival. For better evaluation of the death censored survival, timing of GN recurrence should be considered[17].
Anti-PLA2R autoantibodies in recurrent MN and graft survival: The pivotal role of anti-phospholipase A2 receptor (PLA2R) auto antibodies in the pathogenesis of primary MN before as well as after renal transplantation has an impressing popularity. The prevalence of anti-PLA2R antibodies in primary MN is approaching 70% and nearly the same percentage in RTR (70%-80%)[17,35,36], with about half of the patients are liable for recurrence after renal transplantation[17,37]. Patients with anti-PLA2R antibodies before transplantation have a 60%-76% chance of histologic recurrence, while absence of these autoantibodies decreases their risk of recurrence to less than 30%[17,36,38,39]. After transplantation the anti-PLA2R antibodies absorbed rapidly into the allograft and as a result of decreased antibodies production due to the immunosuppression medications leading to decline of their level in up to 50% of patients[36]. This decline is definitely associated not only by a lower risk of recurrence, but also by a slower rate of progression if MN does recur[36]. On the other hand, the significance of the anti-PLA2R post transplantation is greatly observed in their predictive value of recurrence and disease progression which is exceeding 80%[36], a high anti-PLA2R is usually accompanied by an increased risk of recurrence, rapid disease progression and probably more resistance to drug therapy[36,38].
Impact of anti-PLA2R on graft survival: Serial survey of the anti-PLA2R antibodies titer is of utmost importance for the following indications[2]: (1) evaluating the magnitude of recurrence risk; (2) determining the rate of disease progression; (3) prediction of the response to treatment[2]; and (4) differential diagnosis of proteinuria in recipients with native primary MN.
Non-anti-PLA2R MN recurrence: Not all the patients with primary MN express anti-PLA2R antibodies, 30% of these patients are negative to these antibodies. Instead, few patients have been reported to have antibodies against other types like cationic bovine serum albumin and thrombospondin type I[40] but data, however, concerned with the real significance of these mediators are still deficient[40-42].
Of note that if the anti-PLA2R antibody titer is negative, we should search for the “glomerular PLA2R” staining, in such a case there is associated anti-PLA2R MN with negative anti-PLA2R serum level, which is present in 30% of cases[36,43].
Primary focal segmental (FSGS) is proved to be one of the highest glomerulonephritis (GN) in recurrence rate after kidney transplantation (KTx), with a percentage of prevalence exceeding 30% in the most recent series[2], with an expected very poor graft survival rate[43]. It can recur immediately post-transplantation, or recur lately, where its diagnosis is usually masked by the secondary FSGS resulting from the reduced total nephron mass, or due to other causes, e.g., iatrogenic[20,44]. Of all causes of the FSGN, “genetic” subtype showed the least incidence of recurrence[19,45,46]. On the other hand, podocin mutations did not show a decreased risk of recurrence[45]. However, revising the recent series, there is consensus about certain clinical parameters that is considered the paramount risk factors for FSGS recurrence: (1) White race[43]; (2) higher level of proteinuria[46,47]; (3) rapid progression to ESRD (< 3 years); (4) younger age (< 15 years old) at time of diagnosis[46]; and (5) the most reliable risk factor for recurrence is recurrence in a previous graft[2].
By far, the most reliable risk factor for recurrence is recurrence in a failed allograft, which will be ultimately reflected on allograft survival. Losing of allograft due to recurrent FSGS is associated of an 80% liability of recurrence of the original disease[2].
The reported incidence if recurrent IgAN is quite variable according to the considered diagnosis and period of follow up. IgAN can remain silent for 5 years before it became clinically evident. So, an average incidence of 30% has been reported[48]. The histologic recurrence is by far more prevalent and discovered earlier before the disease became clinically evident. Rarely, crescentic disease with a rapidly progressing course can occur, which ultimately is associated with poor prognosis[48-50].
A growing body of evidence that three markers of an active disease indicates a great liability for recurrence: (1) galactose-deficient IgA1; (2) IgA-IgG immune complexes; and (3) lower levels of IgA-soluble CD89 circulating complexes, the myeloid cell receptor for IgA[51]. The only defect in considering these components is that they were considered on a clinically evident base of IgAN recurrence, therefore, silent disease - a quite common IgAN behavior - will be definitely missed, which means an easily missed diagnosis of IgAN recurrence[2].
Risk factors of IgAN recurrence include: (1) young RTR; (2) aggressive course of the disease before transplantation; (3) living vs deceased donation[52,53]; (4) polymorphisms in IL-10[54,55]; (5) HLA-B8-DR3 haplotype[56]; (6) steroid-free regimen[57,58]; and (7) impact of histological classification: could have prognostic implications[18,59,60].
Despite the reported excellent outcome after renal transplantation and the better graft survival in comparison with other GN diseases[61-63], recurrent IgA disease - on the other hand - have been proved to be detrimental to the allograft. So, definitely, patient with recurrent IgAN have a higher risk of losing their grafts in comparison with patients without recurrence[18,48,64,65].
A definite role of immunosuppression on recurrent GN prevalence was previously denied by the early reports[13]. However, recently, some explanations were given to argue that immunosuppressive therapy could cure or at least modulate the recurrent GN course[2]: (1) certain GN recurrences show a diminished rate of recurrence[20,66-68]; (2) an increased rate of recurrence has been observed with steroid free regimen in pediatrics as well as in IgAN[57,58,64], but not in FSGS patients[69,70]; and (3) an observed decline in antibody level (anti-PLA2R), one of the essential effects that observed once the immunosuppressive agents have been commenced[36].
On behalf of the EDTA database, Floege et al[10] tried to shed the light on the most vital recommendations in dealing with a RTR with an underlying glomerular disease as follows: (1) defining the original native glomerular disease in RTR will help prevent its recurrence; (2) with “living-related” kidney donation, and expected familial GN such as IgAN, renal biopsy should be considered. Floege et al[10] accept living related donation for RTR with MN, MPGN TypeI, IgA and anti-GBM disease; (3) sharp limiting roles should judge the living related donation pool. A deep discussion with a patient with dense deposit disease (DDD) and a child with FSGS should be instituted; (4) the list of recipients with high risk of recurrence includes advanced mesangiocapillary alterations in renal biopsy, age of less than 15 years and short duration between established diagnosis and ESRD; (5) a benefit/risk ratio should be balanced properly between proceeding to kidney transplant and surviving on dialysis accordingly; (6) etiology of graft loss in a previously failed transplant is better to be elucidated; (7) avoid living donation in case of a previously failed transplant due to GN recurrence, the risk of recurrence and subsequent allograft loss will be enhanced in presence of the recurrence risk factors[71]; (8) the impact of modification of immunosuppression protocols still questionable by some authors; and (9) robust battery of investigations is required including renal biopsy with its related studies, e.g., LM, IF, E/M and immune-studies should be accomplished with every renal biopsy, so that a perfect differential diagnosis from other possible lesions, e.g., chronic allograft glomerulopathy could be established.
The advent of a new classification of MPGN including the classic morphology as well as the other features enables not only a better understanding of the course of this disease, but also delineates the best tools of prevention and therapy of recurrence, which will be ultimately reflected on the allograft survival[72,73]. This fact is evolved from the observed wide discrepancy in the behavior of each subtype (see below) as regard the incidence and the intensity of recurrence as well as its impact on allograft survival[2].
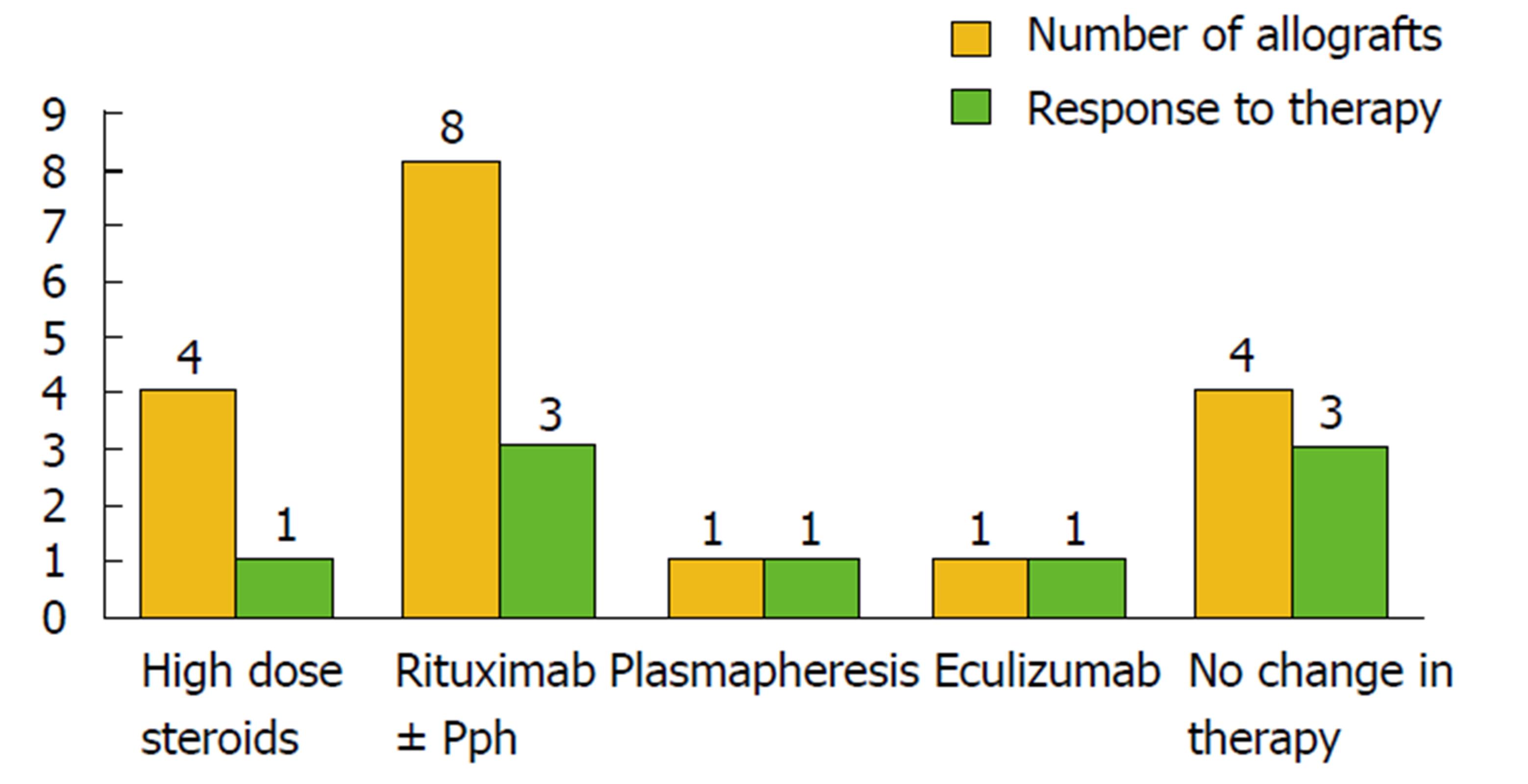
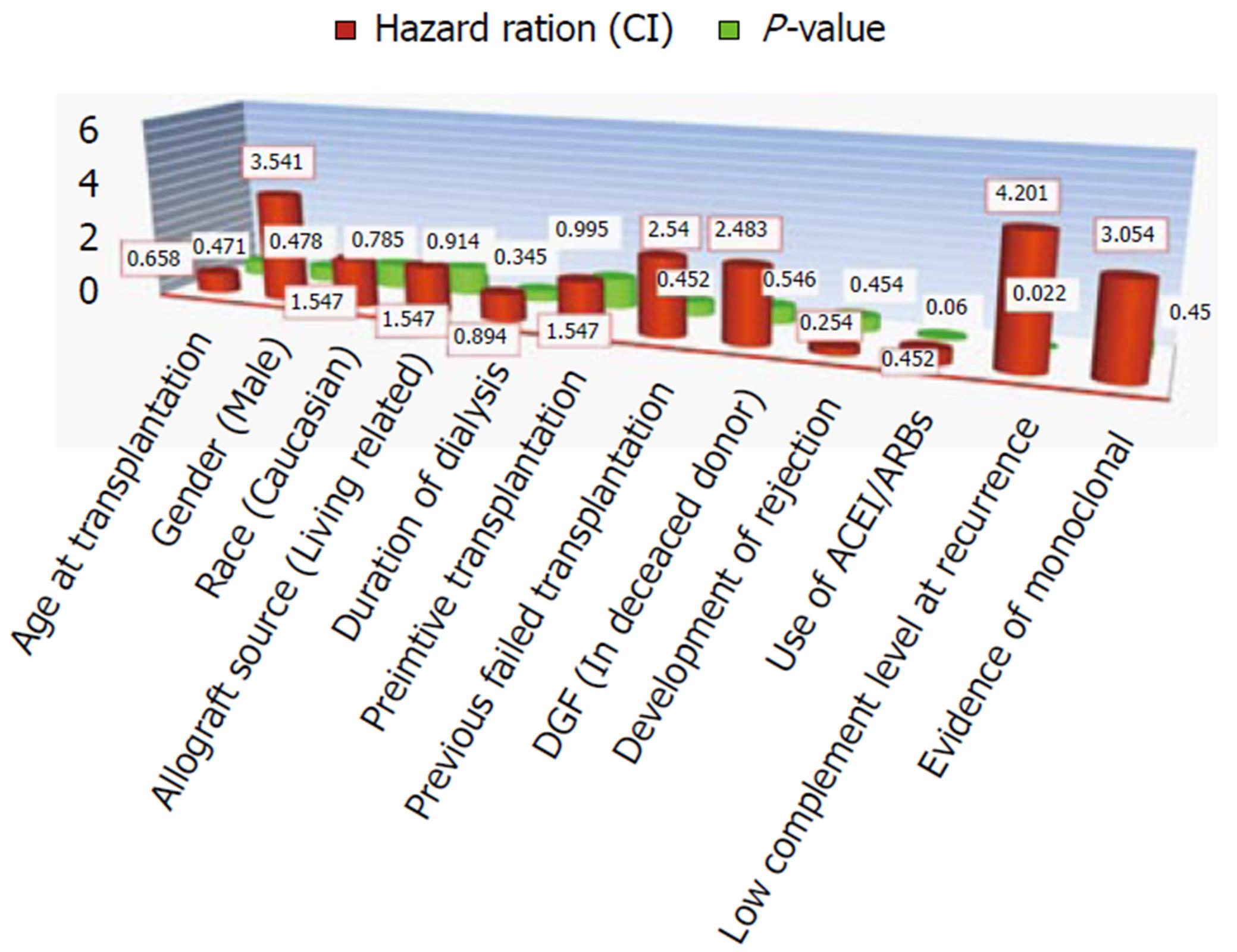
One of the largest series about post-transplant MPGN recurrence in the literature was admitted by Alasfar et al[30], it was the first study that applied the new MPGN classification in evaluating post-transplant MPGN recurrence (Table 1, Figure 3). Despite the absence of worse survival in the recurrent cohort of Alasfar et al[30], the rate of allograft loss was higher (Figure 1). They explained this discrepancy by the small sample size. Unfortunately, the response to immunosuppressive therapy in this study was poor, as less than 50% of their patients treated by high dose steroid therapy, rituximab and/or plasmapheresis, or eculizumab could attain allograft function stability and prevent graft loss (Figure 4). An assumed benefit of ACEi/ARBs therapy in prevention of graft loss was suggested by this study, which should be considered cautiously regarding the small number of cases[30]. Alasfar et al[30], however, showed that 43% of their patients who developed MPGN recurrence were of the immune complex-mediated GN (ICGN) type and were complicated by graft loss. On the other hand, one of the two patients with GN recurrence and subtyped as complement-mediated GN (CGN) developed graft loss. The average time of graft loss was 6.5 mo (2-18 mo). Interpreting these results showed non-significant results between recurrent and non-recurrent groups, despite the presence of tendency to worse survival[30]. Also, no significance could be detected with other factors, e.g., (age, race, gender, mismatching degree, graft source, pre-emptive transplantation and degree of proteinuria). In contrary to other factors and despite of non-significance, ACEi/ARB therapy could ameliorate the tendency of graft loss (Figure 4). For more specified specific therapy, all the old biopsies before the advent of the new classification, should be reclassified. The CGN is generally less prevalent, on the other hand, ICGN is more common (Table 1) and most of the native as well as the recurrent MPGN appear to be classified as ICGN. It is noteworthy to remind that some of the reclassified cases may change their microscopy by time. Unfortunately, the latter change could be difficult to differentiate from a de novo GN disease, which will be ultimately resulted in a difficulty on choosing the mode of therapy[30].
MPGN with Ig deposits: We should focus in suppression of the antibody production, but there are no controlled trials.
MPGN with monoclonal Ig deposits: The anti-CD20 antibodies are proved to be effective in uncontrolled trials in native as well as in allograft recurrence[74]. Monoclonal deposits are proved to be associated with a higher rate of recurrence[75]. This association may suggest they have their role in the pathogenesis of MPGN, consequently, two important steps have been suggested: (1) meticulous screening for “monoclonal gammopathy” during preparation of a patient with MPGN for renal transplantation; and (2) a “hematologist consultation” may be advised with strict follow up in such situation for long periods[30].
MPGN C3GN: The use anti C5 monoclonal antibodies, eculizumab, is shown to be effective with mixed results[76-80], depending on the success of this drug in preventing the recurrence of atypical HUS, which own a similar pathogenesis[80-83].
MPGN with polyclonal Ig deposits: Usually presented late, within the first 5 years, with a relatively benign course as regard low risk of recurrence and slow progression. Interestingly, the morphology of the lately recurred MPGN with polyclonal Ig deposits is difficult to be differentiated from the de novo GN which can behave similarly as regard the late presentation post transplantation as well as the presence of polyclonal Ig deposits[18]. The former group has C4d deposits in their glomeruli, fortunately help in differential diagnosis. Also, a higher risk of recurrence could be expected with the presence of reduced complement level (C3 and C4) level[74] (Figure 5).
C3GN: C3 glomerular deposits are abundant with absence or minimal Ig deposits[84,85]. The risk of recurrence in C3GN is very high, exceeding 70%, can be presented early with a very aggressive course that ultimately ends by graft failure in nearly half of the patients[86]. There is no established treatment for C3GN. For complement dysregulation in the pathogenesis of this disease, a supply of “normal plasma” has been suggested[87]. Recently, a new therapy targeting an alternative complement pathway using the anti-C5 AB[88-90] and soluble CR1 (a potent regulator of complement activity) has been reported[91]. However, controlled trials regarding the efficacy of these therapies have not yet been conducted.
Dense deposit disease subtype: The rate of recurrence of this subtype is extremely high (80%-90%), leading to reduced graft survival[32,92]. Two criteria characterize this subtype: It is usually slowly progressive with minimal or absent clinical manifestation, and the timing of recurrence is mostly delayed[92,93]. Both DDD and C3GN usually express an alteration in the alternative pathway with resultant overproduction of the activated C3[94,95]. Recently, polymorphism of the complement regulating proteins, especially in alternative pathway are found to be propagated mostly in all subtypes of MPGN, with a possible alterations related to renal outcome were assumed[96]. In DDD and other C3 glomerulopathies: Eculizumab or anti-auto antibodies activating complement cascade therapy have been suggested[97].
“Monoclonal gammopathy with renal significance”: Both C3 GN and DDD lack C4d, indicating alternative pathway activation[98]. Any MPGN subtype associated with monoclonal Ig deposits usually complicated by GN recurrence in 66% of cases and expressing a very aggressive course often complicated by allograft failure[99]. Interestingly, 70% of these cases do not express monoclonal IG either in serum or in urine, without any evidence of plasma cell dyscrasia in bone marrow and with low risk of progress into multiple myeloma[100,101].
Monoclonal proteins: Monoclonal proteins are present in 30% of cases with MPGN with monoclonal Ig deposits have serum monoclonal proteins[100] despite absence of any evidence of multiple myeloma. A subtype name of this group of patients called “monoclonal gammopathy with renal significance”[102,103], which obviously will express a very high risk of recurrence[104].
Stem cell transplantation: It is noteworthy to declare that in monoclonal gammopathy, stem cell transplantation can reverse the renal dysfunction through elimination of the light chain and immunoglobulins, with an expected general improvement. The observed link between C3GN and monoclonal and the complement (alternative pathway) activation by λ-light chain has been recorded in previous reports[105-107].
Extrapolating the aggressive behavior of these recurrent diseases, especially in the presence of monoclonal deposits and C3GN, rigorous precautions should be considered to strive against its activity. A prophylactic protocol to guard against MPGN with monoclonal deposits recurrence utilizing an anti-CD20 AB before transplantation is currently under evaluation by Cosio et al[2], with promising preliminary results. It is assumed that the C3GN remains silent until they exposed to a certain event, e.g., ischemia/reperfusion injury of transplantation that results in dysregulation of complement activation with evolution of the pathological events associated to its aggressive course[108-110]. So, it is essential to reclassify the MPGN based on the recent MPGN classification, which will help not only in designing a therapeutic protocol, but also in instituting a prophylactic policy. It is noteworthy mentioning that the clinical course of MPGN pre- and post-transplantation are not the same, i.e., slow preoperative course is not necessarily applied to the post-transplantation behavior[2].
RTR with recurrent MN are better to be under RAAS-blockade as well as symptomatic therapy in the form of diuretics, statins and anticoagulants. Other lines include were listed below.
Referring to its efficacy in MN in the native kidney disease, many RTR with recurrent MN are utilizing CNI therapy relevant to the recent advances in understanding the pathogenesis of MN recurrence[111].
Again effective in both native and recurrent MN disease[112]. Unfortunately, leukopenia could be quite troublesome, so, holding MMF while commencing the alkylating agents’ therapy is advised[112].
Rituximab is also successful in treating the native as well as the recurrent MN disease[113-117]. More than 80% of cases could achieve partial or complete remission, while 40% of cases could express subendothelial deposits resolution[17,117]. Despite the increased risk of infection with anti-CD20 therapy[17,113], rituximab is generally safe, effective, simpler to utilize and more tolerated as compared to alkylating agents. So, the anti-CD20, rituximab, is recommended as a primary line in treating MN recurrence, without alterations in the immunosuppression protocol and regardless the anti-PLA2R antibody level[2].
Alternative therapy between rituximab and alkylating agents is suggested, once one of them failed, then shift to the other line[17,115]. As the level change of anti-PLA2R antibodies titre precedes the decline of proteinuria after rituximab therapy, serial follow up of the antibody titre can be used to anticipate the magnitude of response to therapy as well as the possibility of relapse[113].
Early intervention-in contrary to native MN[116] - with anti-CD20 therapy is recommended, exactly when the proteinuria approaching one gram per 24 h. A very high rate of success would be expected[17], which will be ultimately reflected on reduction of the rate of death censored allograft failure related to MN recurrence (45%).
The anti-CD20 was used effectively by Cosio et al[2], to prevent MN recurrence in two patients with a previous allograft loss due to MN recurrence, with serial follow up through a protocol biopsy.
The use of ant-CD20 few months pre-transplantation may be applied in an attempt to prevent recurrence through the reduction of the ant-PLA2R antibody titer. However, two reasons may prevent the application of this maneuver in a wider scale: (1) the ant-PLA2R antibody titer already declines soon after transplantation, which will decrease the chance of recurrence[36]; and (2) the expected high rate of success achieved by the anti-CD20 in case of early recurrence has been documented[17,117,118].
The recent progress in understanding the pathogenesis of FSGS recurrence was unfortunately not supported by evidence-based controlled trials.
In 1985 treating FSGS with plasmapheresis (PE) sessions has been commenced with variable success[119]. Plasmapheresis has the ability to induce remission in 70% of children and 63% of adults as reported by Ponticelli et al[120]. An overestimation of these reports is postulated due to retrospective nature of the study, short follow up period and lack of controlled design. Once the disease recurrence become clinically evident, we can extrapolate a satisfactory response with commencing the PE sessions early after transplantation. PE is usually prescribed as one to two times plasma volume exchanges, three times per week, with total 8-12 treatments until remission has been established. An intensified course for longer period was suggested by other researchers[121].
Gohh et al[122] has admitted preoperative PE for eight sessions in ten patients. In case of living donation, the recipient received PE one week before and one week after the operation. In case of deceased donation, PE was only given 24 h preoperatively. No one case of FSGS recurrence has been diagnosed in the high risk group and only half of his patients has had their allograft failed. They concluded these results were less than previous reports[122], while others denied any benefits for the prophylactic PE[121,123]. A combination of PE and immunosuppressive agents has been proposed with limited data[124,125].
Only the intensified dose of CyA can reduce the proteinuria level, in contrary to the standard dose that can do nothing for FSGS recurrence[126]. Relevant to its lipophilic criteria, CyA has the ability to bind the LDL receptors on the cell surface of the peripheral lymphocytes. As a result of the rich lipid content (LDL cholesterol) in the nervous system, blood level of the drug is reduced, which could only be overcome through a higher dose augmentation. At this base, i.v. CyA 3 mg/kg/d for 3-4 wk, followed by oral route aiming at preserving the blood level at 250-350 ng/mL, have been successful in induction of remission[127]. However, this policy has been hampered by the multiple untoward effects of the high dosage.
An anti-CD20 chimeric monoclonal antibody depleting the B cells with a direct protective effect on the podocytes. It has the ability to abort the downregulation of sphingomyelin phosphodiesterase acid-like 3b (SMPDL-3b) protein and the acid sphingomyelinase (ASMase), both of them were documented to be present in the podocyte exposed to the sera of recipients with recurrent FSGS[128]. In 2006, beneficial benefits of rituximab in treating the recurrent FSGS post transplantation was suggested[126]. A remission rate of 64%, either partial or complete, has been reported with rituximab therapy[129]. A better response is expected with a normal albumin serum level, and fewer administered infusions as well as in young age recipients[130]. It is not well-proved if titrating rituximab dosage will be the best policy to deplete the B-cell or not. The typical published dosage of rituximab is 375 mg/m2/dose/2-6 doses, with 1-2 wk apart.
An augmented benefit was assumed to be expected with the combined therapy including PE in addition to rituximab[131,132]. Tsagalis et al[131] utilized one gram rituximab per dose, in two doses with two wk apart with PE not performed before 72 h. Two of his patients commenced complete remission and the other two have a partial remission with a stable renal profile and absence of severe complications for 18-60 mo of follow up.
While the resolution of recurrent FSGS was assumed to be possible[2] through the use of the anti-CD 20 AB, rituximab[133], this efficacy, unfortunately, is not consistent but rather limited to certain subtypes. The use PE proved to be effective in removing the circulating permeability factors[134]. For instance, we cannot rely only on this effect in case of recurrent FSGS disease. On the other hand, rituximab was proved in a small pediatric group with recurrent FSGS to be effective in achieving PE independence successfully. The variability in response of recurrent FSGS to both PE as well as anti-CD 20 AB (rituximab) therapy is widely spread[135-137], which indicates a variable response that varied according to different subtypes. Despite the absence of well-designed randomized prospective studies, some trials attempted to prove an effective response of removing a putative permeability factor through PE sessions to guard against FSGS recurrence, which was not confirmed by others. A new strategy has been tailored by Cosio et al[2] to evaluate the ability of the anti-CD25, rituximab, before transplantation to prevent/decrease FSGS recurrence rate has been commenced with encouraging early results.
Few case reports have proved the efficacy of renin-angiotensin system blockade on reducing proteinuria in recurrent FSGS[138,139], which shed the light on the fact that the recurrent FSGS is not completely pure immunological in origin, but additional factors including the primary as well as the adaptive form of FSGS have been incorporated.
In ameliorating the toxicity of the circulating permeability factor has been shown in one case series. Galactose therapy has been proposed by Savin et al[139] as a non-toxic agent for treatment of the FSGS-associated nephrotic syndrome. The focal segmental permeability factor (FSPF) has a high affinity to galactose. The latter has the ability to inactivate and clear FSPF from the circulation. In addition, the FSPF-galactose complex has a high liability to uptake and catabolism[139].
In addition to its untoward toxic manifestations with prolonged use, conflicting results have been determined with cyclophosphamide therapy. Kershaw et al[140] used a high dose of cyclophosphamide in three pediatric patients with recurrent FSGS, two achieved complete remission and the third one have had partial response. Cochat et al[141] reported sustained remission through a regimen composed of pulse steroid, cyclophosphamide and plasmapheresis. Cheong et al[142] reported sustained remission only in two of six patients with recurrent FSGS through a similar protocol. Dall’Amico et al[143] achieved sustained remission in seven of eleven pediatric patients through utilization of steroid pulse-free protocol composed of cyclophosphamide and PE only. Three major toxicities hampered the widespread use of cyclophosphamide, the immunosuppression burden, gonadal toxicities and the risk of malignancy[144].
There is no recommended specific therapy in treating the recurrent IgAN. Treatment of recurrent IgAN is similar to that in native disease in non-transplant patient[1]. However, the following maneuvers have been reported.
The use of ATG as induction therapy is shown to be associated with less risk of IgA recurrence[148].
A protective impact against IgAN recurrence was reported[57,58].
As advocated by the Japanese, a better prognosis post- tonsillectomy could be expected[149-151].
The use of ACEi is proved to be of no benefit in improving the allograft survival[152]. Only the anti-proteinuric effect could be beneficial to the allograft[153,154]. All patients of the study of Floege et al[152] received ACEi with graft failure occurred in more the half of them.
In of the study of Floege et al[152], only 20% of patients received steroid pulses; again more than half have had their graft lost.
No benefit could be expected with any alterations on the immunosuppressive policy in regard to improvement of graft survival[155]. However, Moroni et al[12] assumed that immunosuppressive protocols including less than three agents is an independent risk factor of recurrence, however, this theory is still debatable. The choice of immunosuppressive strategy members has nothing to do with IgAN recurrence after renal transplantation[152].
One of the most challenges for renal allograft survival is the GN recurrence after renal transplantation. With improving long-term renal allograft survival, recurrent disease has increased prominence as a significant contributor to late graft loss. Knowledge on the risk factors for recurrence, onset time and impact on graft function is prerequisite to informed decisions. There are minimal data on the risk of recurrent disease with new immunosuppressive agents. The early recognition would slow down deterioration of renal function even if it may not slow down the course of progression of GN. Each of the GN types has a very unique natural history in renal allograft. With more advancement in understanding its pathogenesis in the future, prophylactic treatment for prevention of GN recurrence might be effective.
Authors do acknowledging Dr. Sami Alasfar et al for permitting us adapting their graphs.
| 1. | Sprangers B, Kuypers DR. Recurrence of glomerulonephritis after renal transplantation. Transplant Rev (Orlando). 2013;27:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Cosio FG, Cattran DC. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int. 2017;91:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Liu ZH. Nephrology in china. Nat Rev Nephrol. 2013;9:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Li L. End-stage renal disease in China. Kidney Int. 1996;49:287-301. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Cosio FG, El Ters M, Cornell LD, Schinstock CA, Stegall MD. Changing Kidney Allograft Histology Early Posttransplant: Prognostic Implications of 1-Year Protocol Biopsies. Am J Transplant. 2016;16:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Gourishankar S, Leduc R, Connett J, Cecka JM, Cosio F, Fieberg A, Gaston R, Halloran P, Hunsicker L, Kasiske B. Pathological and clinical characterization of the ‘troubled transplant’: data from the DeKAF study. Am J Transplant. 2010;10:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1088] [Cited by in RCA: 1285] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 8. | Halloran PF, Pereira AB, Chang J, Matas A, Picton M, De Freitas D, Bromberg J, Serón D, Sellarés J, Einecke G. Microarray diagnosis of antibody-mediated rejection in kidney transplant biopsies: an international prospective study (INTERCOM). Am J Transplant. 2013;13:2865-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 361] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Floege J, Regele H, Gesualdo L; ERA-EDTA Immunonephrology Working Group. The ERA-EDTA Database on Recurrent Glomerulonephritis following renal transplantation. Nephrol Dial Transplant. 2014;29:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Ponticelli C, Glassock RJ. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol. 2010;5:2363-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Moroni G, Longhi S, Quaglini S, Gallelli B, Banfi G, Montagnino G, Messa P. The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol Dial Transplant. 2013;28:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Mulay AV, van Walraven C, Knoll GA. Impact of immunosuppressive medication on the risk of renal allograft failure due to recurrent glomerulonephritis. Am J Transplant. 2009;9:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | ameron JS. Glomerulonephritis in renal transplants. Transplantation. 1982;34:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Hariharan S, Adams MB, Brennan DC, Davis CL, First MR, Johnson CP, Ouseph R, Peddi VR, Pelz C, Roza AM. Recurrent and de novo glomerular disease after renal transplantation: a report from renal allograft disease registry. Transplant Proc. 1999;31:223-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Hariharan S, Peddi VR, Savin VJ, Johnson CP, First MR, Roza AM, Adams MB. Recurrent and de novo renal diseases after renal transplantation: a report from the renal allograft disease registry. Am J Kidney Dis. 1998;31:928-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Grupper A, Cornell LD, Fervenza FC, Beck LH Jr, Lorenz E, Cosio FG. Recurrent Membranous Nephropathy After Kidney Transplantation: Treatment and Long-Term Implications. Transplantation. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 19. | Mashaly ME, Ismail MI, Lotfy EE, Donia AF, Wafa IW, Foda MA, Denewar AA, Abbas MH, Shokeir AA. Frequency of the Original Kidney Disease and Its Effect on the Outcome of Kidney Transplant in the Urology-Nephrology Center Mansoura University. Exp Clin Transplant. 2016;14:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Fairhead T, Knoll G. Recurrent glomerular disease after kidney transplantation. Curr Opin Nephrol Hypertens. 2010;19:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Toledo K, Pérez-Sáez MJ, Navarro MD, Ortega R, Redondo MD, Agüera ML, Rodríguez-Benot A, Aljama P. Impact of recurrent glomerulonephritis on renal graft survival. Transplant Proc. 2011;43:2182-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Morozumi K, Takeda A, Otsuka Y, Horike K, Gotoh N, Watarai Y. Recurrent glomerular disease after kidney transplantation: an update of selected areas and the impact of protocol biopsy. Nephrology (Carlton). 2014;19 Suppl 3:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int. 2003;63:2286-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Green H, Rahamimov R, Rozen-Zvi B, Pertzov B, Tobar A, Lichtenberg S, Gafter U, Mor E. Recurrent membranoproliferative glomerulonephritis type I after kidney transplantation: a 17-year single-center experience. Transplantation. 2015;99:1172-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Shimizu A, Higo S, Fujita E, Mii A, Kaneko T. Focal segmental glomerulosclerosis after renal transplantation. Clin Transplant. 2011;25 Suppl 23:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Takeuchi O, Oikawa T, Usami T, Koyama K, Takeda A, Uchida K, Morozumi K. A case of IgA nephropathy after ABO-incompatible living kidney transplantation. Clin Transplant. 1999;13 Suppl 1:38-42. [PubMed] |
| 27. | Moriyama T, Nitta K, Suzuki K, Honda K, Horita S, Uchida K, Yumura W, Tanabe K, Toma H, Nihei H. Latent IgA deposition from donor kidney is the major risk factor for recurrent IgA nephropathy in renal transplantation. Clin Transplant. 2005;19 Suppl 14:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Watanabe S, Yamaguchi Y, Suzuki T, Ikezoe M, Matsumoto N, Chikamoto H, Nagafuchi H, Horita S, Hattori M, Shiraga H. Inherited factor H dysfunction and complement-associated glomerulonephritis in renal grafts of first and second transplantations. Clin Transplant. 2001;15 Suppl 5:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Horike K, Takeda A, Otsuka Y, Inaguma D, Goto N, Watarai Y, Uchida K, Morozumi K. A case of recurrent light chain deposition disease after living-related renal transplantation - detailed process of the recurrence. Clin Transplant. 2012;26 Suppl 24:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Alasfar S, Carter-Monroe N, Rosenberg AZ, Montgomery RA, Alachkar N. Membranoproliferative glomerulonephritis recurrence after kidney transplantation: using the new classification. BMC Nephrol. 2016;17:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol. 2005;16:1392-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 255] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Braun MC, Stablein DM, Hamiwka LA, Bell L, Bartosh SM, Strife CF. Recurrence of membranoproliferative glomerulonephritis type II in renal allografts: The North American Pediatric Renal Transplant Cooperative Study experience. J Am Soc Nephrol. 2005;16:2225-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Lorenz EC, Sethi S, Leung N, Dispenzieri A, Fervenza FC, Cosio FG. Recurrent membranoproliferative glomerulonephritis after kidney transplantation. Kidney Int. 2010;77:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Andresdottir MB, Assmann KJ, Hoitsma AJ, Koene RA, Wetzels JF. Recurrence of type I membranoproliferative glomerulonephritis after renal transplantation: analysis of the incidence, risk factors, and impact on graft survival. Transplantation. 1997;63:1628-1633. [PubMed] |
| 35. | Beck LH Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 356] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 36. | Kattah A, Ayalon R, Beck LH Jr, Sethi S, Sandor DG, Cosio FG, Gandhi MJ, Lorenz EC, Salant DJ, Fervenza FC. Anti-phospholipase A2 receptor antibodies in recurrent membranous nephropathy. Am J Transplant. 2015;15:1349-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Cosyns JP, Couchoud C, Pouteil-Noble C, Squifflet JP, Pirson Y. Recurrence of membranous nephropathy after renal transplantation: probability, outcome and risk factors. Clin Nephrol. 1998;50:144-153. [PubMed] |
| 38. | Quintana LF, Blasco M, Seras M, Pérez NS, López-Hoyos M, Villarroel P, Rodrigo E, Viñas O, Ercilla G, Diekmann F. Antiphospholipase A2 Receptor Antibody Levels Predict the Risk of Posttransplantation Recurrence of Membranous Nephropathy. Transplantation. 2015;99:1709-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Seitz-Polski B, Payré C, Ambrosetti D, Albano L, Cassuto-Viguier E, Berguignat M, Jeribi A, Thouret MC, Bernard G, Benzaken S. Prediction of membranous nephropathy recurrence after transplantation by monitoring of anti-PLA2R1 (M-type phospholipase A2 receptor) autoantibodies: a case series of 15 patients. Nephrol Dial Transplant. 2014;29:2334-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 693] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 41. | Ronco P, Debiec H. Pathogenesis of membranous nephropathy: recent advances and future challenges. Nat Rev Nephrol. 2012;8:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 42. | Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschênes G, Remuzzi G, Ulinski T, Ronco P. Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med. 2011;364:2101-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 43. | Rudnicki M. Focal Segmental Glomerulosclerosis Recurrence in Adults after Renal Transplantation. BioMed Research International. 2016;2016:3295618. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Cosio FG, Frankel WL, Pelletier RP, Pesavento TE, Henry ML, Ferguson RM. Focal segmental glomerulosclerosis in renal allografts with chronic nephropathy: implications for graft survival. Am J Kidney Dis. 1999;34:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Bertelli R, Ginevri F, Caridi G, Dagnino M, Sandrini S, Di Duca M, Emma F, Sanna-Cherchi S, Scolari F, Neri TM. Recurrence of focal segmental glomerulosclerosis after renal transplantation in patients with mutations of podocin. Am J Kidney Dis. 2003;41:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Pinto J, Lacerda G, Cameron JS, Turner DR, Bewick M, Ogg CS. Recurrence of focal segmental glomerulosclerosis in renal allografts. Transplantation. 1981;32:83-89. [PubMed] |
| 47. | Cameron JS. Recurrent primary disease and de novo nephritis following renal transplantation. Pediatr Nephrol. 1991;5:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Ponticelli C, Traversi L, Banfi G. Renal transplantation in patients with IgA mesangial glomerulonephritis. Pediatr Transplant. 2004;8:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Kowalewska J, Yuan S, Sustento-Reodica N, Nicosia RF, Smith KD, Davis CL, Alpers CE. IgA nephropathy with crescents in kidney transplant recipients. Am J Kidney Dis. 2005;45:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Tang Z, Ji SM, Chen DR, Wen JQ, Chen JS, Liu ZH, Li LS. Recurrent or de novo IgA nephropathy with crescent formation after renal transplantation. Ren Fail. 2008;30:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Berthelot L, Robert T, Vuiblet V, Tabary T, Braconnier A, Dramé M, Toupance O, Rieu P, Monteiro RC, Touré F. Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int. 2015;88:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 52. | Han SS, Huh W, Park SK, Ahn C, Han JS, Kim S, Kim YS. Impact of recurrent disease and chronic allograft nephropathy on the long-term allograft outcome in patients with IgA nephropathy. Transpl Int. 2010;23:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | McDonald SP, Russ GR. Recurrence of IgA nephropathy among renal allograft recipients from living donors is greater among those with zero HLA mismatches. Transplantation. 2006;82:759-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Bantis C, Heering PJ, Aker S, Schwandt C, Grabensee B, Ivens K. Influence of interleukin-10 gene G-1082A polymorphism on recurrent IgA nephropathy. J Nephrol. 2008;21:941-946. [PubMed] |
| 55. | Coppo R, Amore A, Chiesa M, Lombardo F, Cirina P, Andrulli S, Passerini P, Conti G, Peruzzi L, Giraudi R. Serological and genetic factors in early recurrence of IgA nephropathy after renal transplantation. Clin Transplant. 2007;21:728-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Andresdottir MB, Haasnoot GW, Persijn GG, Claas FH. HLA-B8, DR3: a new risk factor for graft failure after renal transplantation in patients with underlying immunoglobulin A nephropathy. Clin Transplant. 2009;23:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Sutherland S, Li L, Concepcion W, Salvatierra O, Sarwal MM. Steroid-free immunosuppression in pediatric renal transplantation: rationale for and [corrected] outcomes following conversion to steroid based therapy. Transplantation. 2009;87:1744-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Von Visger JR, Gunay Y, Andreoni KA, Bhatt UY, Nori US, Pesavento TE, Elkhammas EA, Winters HA, Nadasdy T, Singh N. The risk of recurrent IgA nephropathy in a steroid-free protocol and other modifying immunosuppression. Clin Transplant. 2014;28:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 933] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 60. | Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 794] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 61. | Soler MJ, Mir M, Rodriguez E, Orfila A, Munne A, Vázquez S, Lloveras J, Puig JM. Recurrence of IgA nephropathy and Henoch-Schönlein purpura after kidney transplantation: risk factors and graft survival. Transplant Proc. 2005;37:3705-3709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Kanaan N, Mourad G, Thervet E, Peeters P, Hourmant M, Vanrenterghem Y, De Meyer M, Mourad M, Maréchal C, Goffin E. Recurrence and graft loss after kidney transplantation for henoch-schonlein purpura nephritis: a multicenter analysis. Clin J Am Soc Nephrol. 2011;6:1768-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Samuel JP, Bell CS, Molony DA, Braun MC. Long-term outcome of renal transplantation patients with Henoch-Schonlein purpura. Clin J Am Soc Nephrol. 2011;6:2034-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Clayton P, McDonald S, Chadban S. Steroids and recurrent IgA nephropathy after kidney transplantation. Am J Transplant. 2011;11:1645-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Moroni G, Gallelli B, Quaglini S, Leoni A, Banfi G, Passerini P, Montagnino G, Messa P. Long-term outcome of renal transplantation in patients with idiopathic membranous glomerulonephritis (MN). Nephrol Dial Transplant. 2010;25:3408-3415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Choy BY, Chan TM, Lai KN. Recurrent glomerulonephritis after kidney transplantation. Am J Transplant. 2006;6:2535-2542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Lochhead KM, Pirsch JD, D’Alessandro AM, Knechtle SJ, Kalayoglu M, Sollinger HW, Belzer FO. Risk factors for renal allograft loss in patients with systemic lupus erythematosus. Kidney Int. 1996;49:512-517. [PubMed] |
| 68. | Geetha D, Eirin A, True K, Valentina Irazabal M, Specks U, Seo P, Nachman P, Fervenza FC. Renal transplantation in antineutrophil cytoplasmic antibody-associated vasculitis: a multicenter experience. Transplantation. 2011;91:1370-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Boardman R, Trofe J, Alloway R, Rogers C, Roy-Chaudhury P, Cardi M, Safdar S, Groene B, Buell J, Hanaway M. Early steroid withdrawal does not increase risk for recurrent focal segmental glomerulosclerosis. Transplant Proc. 2005;37:817-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Ibrahim H, Rogers T, Casingal V, Sturdevant M, Tan M, Humar A, Gillingham K, Matas A. Graft loss from recurrent glomerulonephritis is not increased with a rapid steroid discontinuation protocol. Transplantation. 2006;81:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Briggs JD, Jones E. Recurrence of glomerulonephritis following renal transplantation. Scientific Advisory Board of the ERA-EDTA Registry. European Renal Association-European Dialysis and Transplant Association. Nephrol Dial Transplant. 1999;14:564-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med. 2012;366:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 347] [Article Influence: 24.8] [Reference Citation Analysis (12)] |
| 73. | Sethi S, Haas M, Markowitz GS, D’Agati VD, Rennke HG, Jennette JC, Bajema IM, Alpers CE, Chang A, Cornell LD. Mayo Clinic/Renal Pathology Society Consensus Report on Pathologic Classification, Diagnosis, and Reporting of GN. J Am Soc Nephrol. 2016;27:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Guiard E, Karras A, Plaisier E, Duong Van Huyen JP, Fakhouri F, Rougier JP, Noel LH, Callard P, Delahousse M, Ronco P. Patterns of noncryoglobulinemic glomerulonephritis with monoclonal Ig deposits: correlation with IgG subclass and response to rituximab. Clin J Am Soc Nephrol. 2011;6:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 75. | Moroni G, Casati C, Quaglini S, Gallelli B, Banfi G, Montagnino G, Messa P. Membranoproliferative glomerulonephritis type I in renal transplantation patients: a single-center study of a cohort of 68 renal transplants followed up for 11 years. Transplantation. 2011;91:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, Herlitz L, Stokes MB, Markowitz GS, D’Agati VD, Canetta PA. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 77. | Herlitz LC, Bomback AS, Markowitz GS, Stokes MB, Smith RN, Colvin RB, Appel GB, D’Agati VD. Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol. 2012;23:1229-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Le Quintrec M, Lionet A, Kandel C, Bourdon F, Gnemmi V, Colombat M, Goujon JM, Frémeaux-Bacchi V, Fakhouri F. Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am J Kidney Dis. 2015;65:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 79. | McCaughan JA, O’Rourke DM, Courtney AE. Recurrent dense deposit disease after renal transplantation: an emerging role for complementary therapies. Am J Transplant. 2012;12:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 80. | Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V; French Study Group for aHUS/C3G. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 375] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 81. | Zuber J, Le Quintrec M, Sberro-Soussan R, Loirat C, Frémeaux-Bacchi V, Legendre C. New insights into postrenal transplant hemolytic uremic syndrome. Nat Rev Nephrol. 2011;7:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 82. | Krid S, Roumenina LT, Beury D, Charbit M, Boyer O, Frémeaux-Bacchi V, Niaudet P. Renal transplantation under prophylactic eculizumab in atypical hemolytic uremic syndrome with CFH/CFHR1 hybrid protein. Am J Transplant. 2012;12:1938-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 83. | Zuber J, Le Quintrec M, Krid S, Bertoye C, Gueutin V, Lahoche A, Heyne N, Ardissino G, Chatelet V, Noël LH. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am J Transplant. 2012;12:3337-3354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 84. | Servais A, Frémeaux-Bacchi V, Lequintrec M, Salomon R, Blouin J, Knebelmann B, Grünfeld JP, Lesavre P, Noël LH, Fakhouri F. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet. 2007;44:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 85. | Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 469] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 86. | Zand L, Lorenz EC, Cosio FG, Fervenza FC, Nasr SH, Gandhi MJ, Smith RJ, Sethi S. Clinical findings, pathology, and outcomes of C3GN after kidney transplantation. J Am Soc Nephrol. 2014;25:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 87. | Kakita H, Miyake T, Komiya T, Tsukamoto T, Muso E. A case report of recurrent C3 glomerulonephritis 18 mo after renal transplantation. Renal Replacement Therapy. 2016;2:38. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 88. | Daina E, Noris M, Remuzzi G. Eculizumab in a patient with dense-deposit disease. N Engl J Med. 2012;366:1161-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 89. | Vivarelli M, Pasini A, Emma F. Eculizumab for the treatment of dense-deposit disease. N Engl J Med. 2012;366:1163-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 90. | Radhakrishnan S, Lunn A, Kirschfink M, Thorner P, Hebert D, Langlois V, Pluthero F, Licht C. Eculizumab and refractory membranoproliferative glomerulonephritis. N Engl J Med. 2012;366:1165-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 91. | Athanasiou Y, Voskarides K, Gale DP, Damianou L, Patsias C, Zavros M, Maxwell PH, Cook HT, Demosthenous P, Hadjisavvas A. Familial C3 glomerulopathy associated with CFHR5 mutations: clinical characteristics of 91 patients in 16 pedigrees. Clin J Am Soc Nephrol. 2011;6:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 92. | Eddy A, Sibley R, Mauer SM, Kim Y. Renal allograft failure due to recurrent dense intramembranous deposit disease. Clin Nephrol. 1984;21:305-313. [PubMed] |
| 93. | Curtis JJ, Wyatt RJ, Bhathena D, Lucas BA, Holland NH, Luke RG. Renal transplantation for patients with type I and type II membranoproliferative glomerulonephritis: serial complement and nephritic factor measurements and the problem of recurrence of disease. Am J Med. 1979;66:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC. C3 glomerulopathy: a new classification. Nat Rev Nephrol. 2010;6:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 95. | Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJ. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012;82:465-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 96. | Iatropoulos P, Noris M, Mele C, Piras R, Valoti E, Bresin E, Curreri M, Mondo E, Zito A, Gamba S. Complement gene variants determine the risk of immunoglobulin-associated MPGN and C3 glomerulopathy and predict long-term renal outcome. Mol Immunol. 2016;71:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 97. | Chen Q, Müller D, Rudolph B, Hartmann A, Kuwertz-Bröking E, Wu K, Kirschfink M, Skerka C, Zipfel PF. Combined C3b and factor B autoantibodies and MPGN type II. N Engl J Med. 2011;365:2340-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 98. | Sethi S, Nasr SH, De Vriese AS, Fervenza FC. C4d as a Diagnostic Tool in Proliferative GN. J Am Soc Nephrol. 2015;26:2852-2859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 99. | Nasr SH, Sethi S, Cornell LD, Fidler ME, Boelkins M, Fervenza FC, Cosio FG, D’Agati VD. Proliferative glomerulonephritis with monoclonal IgG deposits recurs in the allograft. Clin J Am Soc Nephrol. 2011;6:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 100. | Nasr SH, Markowitz GS, Stokes MB, Seshan SV, Valderrama E, Appel GB, Aucouturier P, D’Agati VD. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int. 2004;65:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 101. | Nasr SH, Valeri AM, Cornell LD, Fidler ME, Sethi S, D’Agati VD, Leung N. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 102. | Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, Dispenzieri A, Song KW, Kyle RA; International Kidney and Monoclonal Gammopathy Research Group. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120:4292-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 374] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 103. | Bridoux F, Leung N, Hutchison CA, Touchard G, Sethi S, Fermand JP, Picken MM, Herrera GA, Kastritis E, Merlini G. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 104. | Czarnecki PG, Lager DJ, Leung N, Dispenzieri A, Cosio FG, Fervenza FC. Long-term outcome of kidney transplantation in patients with fibrillary glomerulonephritis or monoclonal gammopathy with fibrillary deposits. Kidney Int. 2009;75:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 105. | Bridoux F, Desport E, Frémeaux-Bacchi V, Chong CF, Gombert JM, Lacombe C, Quellard N, Touchard G. Glomerulonephritis with isolated C3 deposits and monoclonal gammopathy: a fortuitous association? Clin J Am Soc Nephrol. 2011;6:2165-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 106. | Zand L, Kattah A, Fervenza FC, Smith RJ, Nasr SH, Zhang Y, Vrana JA, Leung N, Cornell LD, Sethi S. C3 glomerulonephritis associated with monoclonal gammopathy: a case series. Am J Kidney Dis. 2013;62:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 107. | Meri S, Koistinen V, Miettinen A, Törnroth T, Seppälä IJ. Activation of the alternative pathway of complement by monoclonal lambda light chains in membranoproliferative glomerulonephritis. J Exp Med. 1992;175:939-950. [PubMed] |
| 108. | Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105:1363-1371. [PubMed] |
| 109. | Collard CD, Väkevä A, Morrissey MA, Agah A, Rollins SA, Reenstra WR, Buras JA, Meri S, Stahl GL. Complement activation after oxidative stress: role of the lectin complement pathway. Am J Pathol. 2000;156:1549-1556. [PubMed] |
| 110. | Thurman JM, Royer PA, Ljubanovic D, Dursun B, Lenderink AM, Edelstein CL, Holers VM. Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J Am Soc Nephrol. 2006;17:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 111. | Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL; North America Nephrotic Syndrome Study Group. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 112. | Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, Sasdelli M, Redaelli B, Grassi C, Pozzi C. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1995;48:1600-1604. [PubMed] |
| 113. | El-Zoghby ZM, Grande JP, Fraile MG, Norby SM, Fervenza FC, Cosio FG. Recurrent idiopathic membranous nephropathy: early diagnosis by protocol biopsies and treatment with anti-CD20 monoclonal antibodies. Am J Transplant. 2009;9:2800-2807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 114. | Ruggenenti P, Chiurchiu C, Brusegan V, Abbate M, Perna A, Filippi C, Remuzzi G. Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J Am Soc Nephrol. 2003;14:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 115. | Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 116. | Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 117. | Sprangers B, Lefkowitz GI, Cohen SD, Stokes MB, Valeri A, Appel GB, Kunis CL. Beneficial effect of rituximab in the treatment of recurrent idiopathic membranous nephropathy after kidney transplantation. Clin J Am Soc Nephrol. 2010;5:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 118. | Gallon L, Chhabra D. Anti-CD20 monoclonal antibody (rituximab) for the treatment of recurrent idiopathic membranous nephropathy in a renal transplant patient. Am J Transplant. 2006;6:3017-3021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Zimmerman SW. Plasmapheresis and dipyridamole for recurrent focal glomerular sclerosis. Nephron. 1985;40:241-245. [PubMed] |
| 120. | Ponticelli C. Recurrence of focal segmental glomerular sclerosis (FSGS) after renal transplantation. Nephrol Dial Transplant. 2010;25:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 121. | Hickson LJ, Gera M, Amer H, Iqbal CW, Moore TB, Milliner DS, Cosio FG, Larson TS, Stegall MD, Ishitani MB. Kidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrence. Transplantation. 2009;87:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 122. | Gohh RY, Yango AF, Morrissey PE, Monaco AP, Gautam A, Sharma M, McCarthy ET, Savin VJ. Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients. Am J Transplant. 2005;5:2907-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 123. | Hubsch H, Montané B, Abitbol C, Chandar J, Shariatmadar S, Ciancio G, Burke G, Miller J, Strauss J, Zilleruelo G. Recurrent focal glomerulosclerosis in pediatric renal allografts: the Miami experience. Pediatr Nephrol. 2005;20:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 124. | Fencl F, Simková E, Vondrák K, Janda J, Chadimová M, Stejskal J, Seeman T. Recurrence of nephrotic proteinuria in children with focal segmental glomerulosclerosis after renal transplantation treated with plasmapheresis and immunoadsorption: case reports. Transplant Proc. 2007;39:3488-3490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 125. | Belson A, Yorgin PD, Al-Uzri AY, Salvatierra O, Higgins J, Alexander SR. Long-term plasmapheresis and protein A column treatment of recurrent FSGS. Pediatr Nephrol. 2001;16:985-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Cravedi P, Kopp JB, Remuzzi G. Recent progress in the pathophysiology and treatment of FSGS recurrence. Am J Transplant. 2013;13:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 127. | Raafat RH, Kalia A, Travis LB, Diven SC. High-dose oral cyclosporin therapy for recurrent focal segmental glomerulosclerosis in children. Am J Kidney Dis. 2004;44:50-56. [PubMed] |
| 128. | Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 129. | Araya CE, Dharnidharka VR. The factors that may predict response to rituximab therapy in recurrent focal segmental glomerulosclerosis: a systematic review. J Transplant. 2011;2011:374213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Cravedi P, Ruggenenti P, Remuzzi G. Low-dose rituximab for posttransplant recurrent membranous nephropathy. Am J Transplant. 2010;10:1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 131. | Tsagalis G, Psimenou E, Nakopoulou L, Laggouranis A. Combination treatment with plasmapheresis and rituximab for recurrent focal segmental glomerulosclerosis after renal transplantation. Artif Organs. 2011;35:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 132. | Hristea D, Hadaya K, Marangon N, Buhler L, Villard J, Morel P, Martin PY. Successful treatment of recurrent focal segmental glomerulosclerosis after kidney transplantation by plasmapheresis and rituximab. Transpl Int. 2007;20:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 133. | Pescovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med. 2006;354:1961-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 134. | Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 501] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 135. | Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369:2416-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 136. | Yabu JM, Ho B, Scandling JD, Vincenti F. Rituximab failed to improve nephrotic syndrome in renal transplant patients with recurrent focal segmental glomerulosclerosis. Am J Transplant. 2008;8:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 137. | Canaud G, Zuber J, Sberro R, Royale V, Anglicheau D, Snanoudj R, Gaha K, Thervet E, Lefrère F, Cavazzana-Calvo M. Intensive and prolonged treatment of focal and segmental glomerulosclerosis recurrence in adult kidney transplant recipients: a pilot study. Am J Transplant. 2009;9:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 138. | Montagnino G, Tarantino A, Banfi G, Maccario M, Costamagna L, Ponticelli C. Double recurrence of FSGS after two renal transplants with complete regression after plasmapheresis and ACE inhibitors. Transpl Int. 2000;13:166-168. [PubMed] |
| 139. | Savin VJ, McCarthy ET, Sharma R, Charba D, Sharma M. Galactose binds to focal segmental glomerulosclerosis permeability factor and inhibits its activity. Transl Res. 2008;151:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 140. | Kershaw DB, Sedman AB, Kelsch RC, Bunchman TE. Recurrent focal segmental glomerulosclerosis in pediatric renal transplant recipients: successful treatment with oral cyclophosphamide. Clin Transplant. 1994;8:546-549. [PubMed] |
| 141. | Cochat P, Kassir A, Colon S, Glastre C, Tourniaire B, Parchoux B, Martin X, David L. Recurrent nephrotic syndrome after transplantation: early treatment with plasmaphaeresis and cyclophosphamide. Pediatr Nephrol. 1993;7:50-54. [PubMed] |
| 142. | Cheong HI, Han HW, Park HW, Ha IS, Han KS, Lee HS, Kim SJ, Choi Y. Early recurrent nephrotic syndrome after renal transplantation in children with focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15:78-81. [PubMed] |
| 143. | Dall’Amico R, Ghiggeri G, Carraro M, Artero M, Ghio L, Zamorani E, Zennaro C, Basile G, Montini G, Rivabella L. Prediction and treatment of recurrent focal segmental glomerulosclerosis after renal transplantation in children. Am J Kidney Dis. 1999;34:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 129] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 144. | Vinai M, Waber P, Seikaly MG. Recurrence of focal segmental glomerulosclerosis in renal allograft: an in-depth review. Pediatr Transplant. 2010;14:314-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 145. | Garin EH, Reiser J, Cara-Fuentes G, Wei C, Matar D, Wang H, Alachkar N, Johnson RJ. Case series: CTLA4-IgG1 therapy in minimal change disease and focal segmental glomerulosclerosis. Pediatr Nephrol. 2015;30:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 146. | Alachkar N, Carter-Monroe N, Reiser J. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2014;370:1263-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 147. | Alkandari O, Nampoory N, Nair P, Atta A, Zakaria Z, Mossad A, Yagan J, Al-Otaibi T. Recurrent Focal Segmental Glomerulosclerosis and Abatacept: Case Report. Exp Clin Transplant. 2016;14:456-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 148. | Berthoux F, El Deeb S, Mariat C, Diconne E, Laurent B, Thibaudin L. Antithymocyte globulin (ATG) induction therapy and disease recurrence in renal transplant recipients with primary IgA nephropathy. Transplantation. 2008;85:1505-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 149. | Hotta K, Fukasawa Y, Akimoto M, Tanabe T, Sasaki H, Fukuzawa N, Seki T, Togashi M, Harada H. Tonsillectomy ameliorates histological damage of recurrent immunoglobulin A nephropathy after kidney transplantation. Nephrology (Carlton). 2013;18:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 150. | Kennoki T, Ishida H, Yamaguchi Y, Tanabe K. Proteinuria-reducing effects of tonsillectomy alone in IgA nephropathy recurring after kidney transplantation. Transplantation. 2009;88:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 151. | Ushigome H, Suzuki T, Fujiki M, Nobori S, Sakamoto S, Okamoto M, Urasaki K, Yoshimura N. Efficacy of tonsillectomy for patients with recurrence of IgA nephropathy after kidney transplantation. Clin Transplant. 2009;23 Suppl 20:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 152. | Floege J, Gröne HJ. Recurrent IgA nephropathy in the renal allograft: not a benign condition. Nephrol Dial Transplant. 2013;28:1070-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 153. | Oka K, Imai E, Moriyama T, Akagi Y, Ando A, Hori M, Okuyama A, Toki K, Kyo M, Kokado Y. A clinicopathological study of IgA nephropathy in renal transplant recipients: beneficial effect of angiotensin-converting enzyme inhibitor. Nephrol Dial Transplant. 2000;15:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 154. | Courtney AE, McNamee PT, Nelson WE, Maxwell AP. Does angiotensin blockade influence graft outcome in renal transplant recipients with IgA nephropathy? Nephrol Dial Transplant. 2006;21:3550-3554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 155. | Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N Engl J Med. 2015;373:2225-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 530] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Kuwait
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kute VBB, Gheith O, Salvadori M S- Editor: Kong JX L- Editor: A E- Editor: Yan JL