Published online Dec 24, 2017. doi: 10.5500/wjt.v7.i6.285
Peer-review started: September 15, 2017
First decision: October 24, 2017
Revised: November 2, 2017
Accepted: November 10, 2017
Article in press: November 10, 2017
Published online: December 24, 2017
Processing time: 101 Days and 10.6 Hours
The glomerular diseases after renal transplantation can occur de novo, i.e., with no relation to the native kidney disease, or more frequently occur as a recurrence of the original disease in the native kidney. There may not be any difference in clinical features and histological pattern between de novo glomerular disease and recurrence of original glomerular disease. However, structural alterations in transplanted kidney add to dilemma in diagnosis. These changes in architecture of histopathology can happen due to: (1) exposure to the immunosuppression specifically the calcineurin inhibitors (CNI); (2) in vascular and tubulointerstitial alterations as a result of antibody mediated or cell-mediated immunological onslaught; (3) post-transplant viral infections; (4) ischemia-reperfusion injury; and (5) hyperfiltration injury. The pathogenesis of the de novo glomerular diseases differs with each type. Stimulation of B-cell clones with subsequent production of the monoclonal IgG, particularly IgG3 subtype that has higher affinity to the negatively charged glomerular tissue, is suggested to be included in PGNMID pathogenesis. De novo membranous nephropathy can be seen after exposure to the cryptogenic podocyte antigens. The role of the toxic effects of CNI including tissue fibrosis and the hemodynamic alterations may be involved in the de novo FSGS pathophysiology. The well-known deleterious effects of HCV infection and its relation to MPGN disease are frequently reported. The new concepts have emerged that demonstrate the role of dysregulation of alternative complement pathway in evolution of MPGN that led to classifying into two subgroups, immune complex mediated MPGN and complement-mediated MPGN. The latter comprises of the dense deposit disease and the C3 GN disease. De novo C3 disease is rather rare. Prognosis of de novo diseases varies with each type and their management continues to be empirical to a large extent.
Core tip: The role of post-transplant glomerulonephritis in affecting both patient and allograft survival is well documented. For decades recurrent glomerular diseases after renal transplantation have been thoroughly investigated. On the other hand a group of a newly classified de novo glomerular diseases attained an increasing interest. However, the paucity of data concerned with de novo glomerular diseases after renal transplantation have been shown to be a great obstacle necessitating more active cooperation between transplant centers. A thorough work up is clearly warranted to declare not only their pathogenesis, but also to draw the proper therapeutic plan.
- Citation: Abbas F, El Kossi M, Jin JK, Sharma A, Halawa A. De novo glomerular diseases after renal transplantation: How is it different from recurrent glomerular diseases? World J Transplant 2017; 7(6): 285-300
- URL: https://www.wjgnet.com/2220-3230/full/v7/i6/285.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i6.285
De novo glomerular disease is a glomerular disease that damages the renal allograft and it is totally different from the native renal disease. The most common types of de novo glomerulonephritis (GN) are: Membranous nephropathy (MN), focal segmental glomerulosclerosis (FSGS), membranoproliferative glomerulonephritis (MPGN) and TMA secondary to drug intake[1,2]. Since immunofluorescence technique (IF) and electron microscopy (EM) are not used that often when assessing histopathology of a biopsy specimen in early post-transplant period, and the possibility of a range of renal diseases of unknown etiology, make it difficult to evaluate the real prevalence of de novo GN diseases[3]. De novo GN disease is reportedly uncommon[4-9]. In this review we shall discuss the most common de novo GN after renal transplantation in addition to the recently presented de novo proliferative GN with monoclonal IgG deposits (PGNMID). The de novo GN disease presents late, usually one year after renal transplantation, on the other hand recurrent GN might present earlier, sometimes within the first few weeks of renal transplantation. Unfortunately, both types of patterns of GN, whether de novo or recurrent, do have a lower graft survival as compared to patients without glomerular involvement[3].
Definition:De novo MN, is rather uncommon etiology among causes of allograft failure, can be defined as a MN lesion that is developed in the renal allograft of a patient originally suffered from another renal disease in native kidney[10].
De novo or recurrent MN: The type of IgG subclass deposition is different in recurrent MN when compared to de novo MN, where IF is of immense use. Kearney et al[11] (2011) reported that IgG4 was dominant in glomerular deposits of recurrent MN, IgG1 was the dominant subtype in de novo MN. Honda et al[12] (2011) and others reported a clear predominance of IgG4 in idiopathic MN in comparison with the de novo type[13]. Another vital difference is the lack of phospholipase A2 receptor (PLA2R) staining in de novo MN, in contrast to the recurrent MN that is characterized by positive glomerular PLA2R staining[14,15].
Incidence: Of 1000 allograft biopsy, 19 cases of de novo MN were reported in a large French series[16], while the incidence was 1.8% in another French study[17], which means that 2% of renal transplant recipients can develop de novo MN[14]. In United Kingdom, de novo MN is considered to be the second most common cause of nephrotic syndrome after kidney transplantation[18]. The disease was reported to be 9% in a pediatric series[19]. De novo MN can be associated with: Alport’s syndrome, ureteral obstruction, newly diagnosed HCV and recurrent IgA[10].
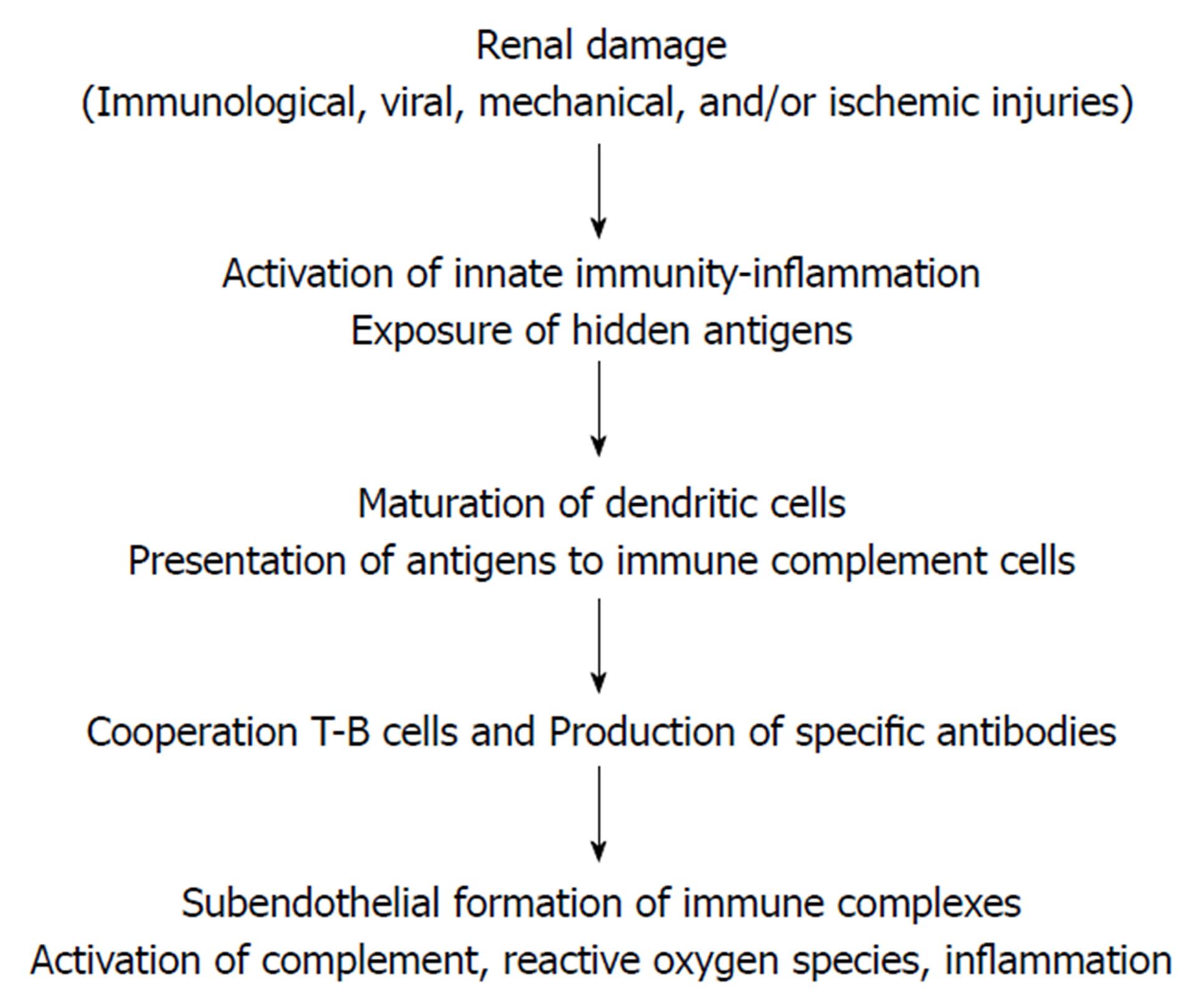
Pathogenesis: The new autoimmune disease IgG-related lesions have been recently shown to affect the renal allograft in several ways including de novo MN[20]. A novel regulatory protein (named: Pdlim2) has been recognized, with an observed decline of this protein in the podocytes of MN patients. A possible role of this protein in de novo MN pathogenesis has been suggested[21]. Various types of injury, e.g., viral, ischemic, immunological and mechanical can induce podocyte damage, exposing the hidden or cryptogenic antigens, which could be different from that of the idiopathic MN. This is quite evident, for example, in allogenic hematopoietic stem cell transplantation[22]. Consequently, these damaged cells will generate danger signals that intercepted by toll-like receptors and other receptors, which in turn initiate a cascade of signals activating transcription factors encoding the inflammatory gene[23]. Finally, the inflammatory cells of the innate system (PNLC, monocytes, macrophages and natural killers’ cells) eventually release cytokines, inflammatory mediators and other mediators. Dendritic cells present the antigen to immunocompetent CD4 T cells that trigger B-cell induced antibody production. The end result is subepithelial immune complex deposition, complement activation and glomerular effector cell induced injury[24] (Figure 1).
No one single antigen can be “blamed” to be responsible of evolution of de novo MN, but rather a wide array of various antigens. An alloimmune response, viral infection and may be mechanical injury can create an environment that lead to release of various cryptogenic (hidden) autologous podocyte-antigen with subsequent production of auto- and alloantibodies (namely IgG1 subtype) that ultimately results in “in situ” immune complex formation, subepithelial deposits and eventually histological form of MN[10]. A thorough search for underlying malignancy and a hidden viral infection should be performed in view of the clear general association between MN and both cancer and infection[14]. Honda et al[12] (2011) reported frequent association of the AMR with de novo MN. El Kossi et al[25] (2008) suggested a possible evolutional role of DSA in development of de novo MN.
Role of HCV: HCV is a small RNA virus (30-38 nm) with lipid envelope and related to the Flaviviridae family. A robust relation to many glomerular (FSGS, immunotactoid, IgA, post-infectious and fibrillary GN)[26-31] and non-glomerular (tubulointerstitial and TMA) diseases has been reported[30,32]. Prevalence of HCV exceeds 8%-10% in many dialysis centers. HCV is known to be related to a range of renal diseases, such as MPGN TypeIassociated type II mixed cryoglobulinemia being the most common, other less common pathologies include MNGN and non-cryoglobulinemic MPGN[3]. Thereby, chronic HCV infection is a serious risk factor for development of de novo MN[27]. Another series reported an incidence of 3.6% in patients with positive HCV infection as compared to those with lack of HCV infection (0.36%)[13]. Genesis of de novo GN can be influenced by several factors on long-run including impact of immunosuppressive agents, HCV-induced modulation of lymphocyte response and the production of antibodies, so that an imbalance between antigens and antibodies will be created and the subjective allograft susceptibility of the allograft itself[33]. For patients with chronic HCV and post-transplant AMR, a particular focus of attention should be directed to de novo MN, with the IgG subtype staining being much helpful to differentiate recurrent from de novo MN[13].
The LM features of allograft biopsy of de novo lesions are similar to that in idiopathic MN, but with more foam cells in arterial intima and possibly with signs of AMR[34]. IF shows diffuse granular deposits of IgG in the subepithelial side of the glomerular basement membrane, with the IgG1 subclass being dominant in de novo MN, while the IgG4 is usually seen in recurrent type[11].
Clinical presentation: Clinical features vary much from no symptoms up to nephrotic range proteinuria[7,35,36], with some 25% of them would present with allograft dysfunction[19]. De novo MN usually presents a few years after renal transplantation[11,12,37,38].
Prognosis: There are no established risk factors for poor prognosis. In pediatric patients, 60% of Antignac et al[19] (1988) patients, for example, lost their grafts in 6 years after diagnosis of de novo MN, despite 20% have no proteinuria. In another series, 4 of 7 patients who received a second transplant, developed de novo MN for the second time[11]. Prognosis of de novo MN in adults is different. De novo MN was reported to have no impact on allograft function in a large French series as well as in Schwarz et al[39] (1991) study that reported similar 5 year survival in 21 patients with de novo MN to other RTR. On the other hand, Monga et al[34] (1993) reported a progression of the pathological stage and deposit extension to more glomeruli in serial biopsies. Accelerated allograft loss was also reported by Dische et al[40] (1981). Of note, most cases with deleterious outcome showed signs of chronic rejection in allograft biopsy[10]. The observed poor prognosis of de novo MN may be attributed by some authors to the associated AMR[41]. The latter is responsible of 20%-30% of allograft losses in the literature[37]. The impact of de novo MN on allograft survival continues to be debatable. While a higher rate of allograft loss associated with signs of chronic rejection was reported in some series[37], no impact on allograft survival has been shown by others[14,37].
The role of vascular endothelial growth factor (VEGF) in angiogenesis is well documented[42,43]. Local (intravitreal) and systemic (IV) anti-VEGF therapy have been recently introduced in many diseases. Systemic (IV) therapy has been used in the management of advanced cancer therapy. Unfortunately, this type of therapy has been associated with several untoward effects, e.g., hypertension, hemorrhage, proteinuria and thromboembolic events[44]. On the other hands, local (intravitreal) route is usually well tolerated[45], due to its low administrated dose and the localized nature of injection. However, clearance of these agents has individual variations that may be reflected as systemic insults[46].
Recently, Wisit Cheungpasitporn et al[46] (2015) reported two cases of allograft dysfunction that are related to the administration of intravitreal anti-VEGF therapy. First case developed MN with spherular deposits after one year of initiation of therapy[47]. Moreover, PLA2R antibodies were reported to be negative in biopsy and no anti-PLA2R antibodies were detected in serum[48,49], which favours the de novo nature of MN, as only one third of idiopathic MN can express the lack of anti-PLA2R antibodies[48,49]. The increased level of proteinuria was not due to MN, as no evidence of immune complex GN in subsequent biopsy (4 mo), which is in agreement with other reports[49,50]. The second case has long standing decline but stable renal function, it showed progressing proteinuria observed few months after initiation of therapy with a clear evidence of both acute and chronic AMR in allograft biopsy[51].
Relation to proteinuria: Appearance of proteinuria in anti-VEGF treated patients is reported to be related to the start of anti-VEGF therapy, a finding that is supported by allograft biopsy findings (microspherule substructure variant of MN) properly due to a new antibody formation or unmasking of already present anti-HLA antibodies. The appearance of proteinuria is clearly related to the systemic use of anti-VEGF in cancer patients[44]. An observation that can be explained by the well documented effect of VEGF on preserving the glomerular filtration barrier integrity[42,52]. Moreover, an altered VEGF activity has been proposed to be a potential aetiology of mTOR inhibitors-induced proteinuria[53]. On the other hand local (intravitreal) administration of anti-VEGF may lack this effect[45]. A given explanation may be due to its different formulation and the local route of administration. However, clearance of these agents is ultimately systemic[45]. Furthermore, a recent report recorded a precipitous decline of allograft function (GFR < 25 mL/min per 1.73 m2) in a group of anti-VEGF treated patients[54].
Mechanism of renal injury: The following mechanisms have been postulated as a given explanations for allograft injury: (1) Disruption of the normal survival signals mediated by VEGF leading to creation of alloreactive antibodies or exaggerated renal allograft injury induced by the already present antibodies; (2) Loss of the mitigating effect exerted by VEGF on CyA toxicity[55]; (3) Unmasking action on the already present anti-HLA antibodies; (4) Renal allograft susceptibility to anti-VEGF-induced injury leading to an increased tissue marker expression, including HLA and non-HLA antibodies; and (5) Evolution of antibody-mediated rejection through anti-HLA antibodies production[46]. The exact role of anti-VEGF agents’ interference in allograft biology is complex, necessitating more extensive investigations[56-58].
Recommendations: Two recommendations have been proposed in the context of anti-VEGF therapy: (1) RTR should be strictly monitored through at least monthly determination of urinary proteins; and (2) The threshold index for allograft biopsy should be lowered, with application of both IF and EM studies[46].
The recent classification of MPGN relies primarily on the immunofluorescence (IF) findings. While cases with only capillary and mesangial complement deposition with lack of the Ig deposits are categorized as C3 glomerulopathy (C3 GN or DDD) or complement-mediated GN (CGN)[14,59], other cases with Ig mesangial and capillary deposits can be classified as immune complex-mediated GN (ICGN) (Table 1) (monoclonal, oligoclonal and polyclonal). Recurrence of MPGN post renal transplantation is frequent (mostly ICGN)[59]. On the other hand, de novo C3 glomerulopathy has not been reported[14]. In regards to de novo IC-mediated MPGN, it can be seen, but not frequently after renal transplantation, usually in association with HCV infection in about 50% of patients[60].
| No. | MPGN subtype | Pathological criteria | Recurrent MPGN | De novo MPGN |
| 1 | ICGN (immune complex-mediated GN) | Contains immune complexes + complement compounds | More common (most of the recurrent cases are ICGN) | Reported (3.25%) |
| 2 | CGN (complement-mediated GN) | Contains complement compounds only | Less prevalent (change from one type to another) | Not reported (Ponticelli et al[14], 2014) |
Incidence: In a large French study, only 13 of 399 (3.25%) patients develop de novo MPGN[60]. According to Ponticelli et al[14] (2014), de novo C3 glomerulopathy (CGN) subtype has not been reported, however, some case reports appear thereafter (see below section “VI”).
Allograft biopsy: Typical pattern of hypercellularity with broad capillary loops due to reduplication of the glomerular basement membrane. In IF study, mesangial and subendothelial deposition of Ig as well as complement glomerular subendothelial electronic dense deposits (EDD), while fibrillary pattern is usually seen with cryoglobulinemia, most probably as a result of the associated HCV infection[61]. The impact of the associated systemic diseases is usually responsible of the de novo pattern of allograft biopsy[14].
Pathogenesis: The pathogenesis is not completely understood. However, the glomerular deposits of the hepatitis C virus as well as the anti-HCV antibodies may be responsible of the histological patterns in HCV positive patients[60]. Presence of cryoglobulin is seen in some patients[62]. Evolution of the clinical and histological pattern associated with de novo MPGN may be also triggered by the stress of rejection, calcineurin inhibitors (CNI) toxicity as well as viral infection[14].
Clinical features: Nearly, about 50% of cases presents with nephrotic syndrome, but the majority usually show non-nephrotic range proteinuria (i.e., < one gram). Presence of signs of thrombotic microangiopathy in allograft biopsy is usually associated with the clinical and laboratory manifestations of hemolytic uremic syndrome. Some patients with normal kidney function and non-nephrotic range proteinuria usually experience a slow and silent course, while in others the evolution of de novo MPGN can trigger rapid graft loss[33].
De novo proliferative GN with monoclonal IgG deposits (PGNMID) is an extremely rare disease[63-68]. PGNMID is a unique type of GN that was first presented in the literature for the first time in 2004[69], 5 years later the largest series (37 case) was presented in 2009[70]. PGNMID is a proteinuria/hematuria syndrome with a reported incidence of only 0.17%, usually with a normal workup for paraproteinemia[70]. While the recurrent PGNMID presents early (within the initial two years after renal transplantation), de novo PGNMID appears several years later[63,64]. A handful of cases of de novo PGNMID have been reported in the literature (Table 2), since Nasr et al[70] (2009) presented his largest series of the native PGNMID. After a 30 mo follow up of these patients, 38% had complete or partial recovery, 22% developed ESRF, and (38%) of these patients experienced persistent allograft dysfunction. Only 10% of patients expressed low complement level. No M protein bands were detected, which indicates that PGNMID disease should not be considered a precursor for multiple myeloma development[9]. However, Batal et al[68] (2014) reported that 18% of their patient with native PGNMID disease showed an evidence of low grade lymphoma. Moreover, Barbour et al[71] (2011) and others also reported two patients with native PGNMID kidney disease with evidence of chronic lymphocytic lymphoma.
| Case | Age at diagnosis | Gender | Onset time (mo) | Type of IgG deposits | C1q deposition | Native kidney disease | Pattern of glomerular injury | Monoclonal gammopathy | Ref. |
| 1 | 24 | M | 43 | IgG3κ | N/A | T1DM | MPGN | None | Albawardi et al[64] (2011) |
| 2 | 68 | F | 156 | IgG1κ | N/A | PKD | MPGN | None | Albawardi et al[64] (2011) |
| 3 | 38 | F | 72 | IgG3κ | 1+ | T1DM | MesGN or EC | N/A | Hussain et al[72] (2017) |
| 4 | 61 | F | 98 | IgG3κ | C1q | MPGN | EC | None | Al-Rabadi et al[73] (2015) |
| 5 | 40 | F | 132 | IgG3κ | N/A | MPGN | MPGN | None | Al-Rabadi et al[73] (2015) |
| 6 | 46 | M | 49 | IgG1κ | 1+ | FSGS | MesGN | N/A | Li et al[77] (2017) |
| 7 | 69 | M | 6 | IgG3κ | 1+ | Obesity (FSGS?) | MPGN | N/A | Merhi et al[75] (2017) |
Case reports of de novo PGNMID in the literature: A detailed summary of the case reports of the de novo PGNMID in the literature, as regard age, gender, time elapsed since kidney transplantation, type of the deposited IgG, presence of C1q, native kidney disease, pattern of glomerular injury as well as presence of monoclonal gammopathy have been shown in Table 2[64,72-75].
Clinical presentation: Like recurrent PGNMID, de novo PGNMID usually manifests with allograft dysfunction associated with a variable degrees of proteinuria with or without hematuria in a white female patient, the disease generally can be seen in adults above 50 years of age[72,73].
Pathogenesis: Pathogenesis of PGNMID is not clear. However, the reported recurrence of this disease suggests the presence of a circulating factor in RTR[72]. Other reports suggest deposition of a circulating non-deleted monoclonal IgG in the glomeruli, followed by complement fixation with outburst of inflammatory mediators[69]. A variety of intrinsic and extrinsic antigens would cause glomerular injury through stimulation of B-cell clones with subsequent production of the monoclonal IgG, particularly IgG3 subtype (8% of the total IgG). The latter is rapidly absorbed by the glomeruli so that it cannot be detected by immunofixation. Three criteria have been postulated to increase the avidity of IgG3 to glomerular deposition: (1) Positively charged nature; (2) The heaviest molecular weight; and (3) The greatest complement fixing capacity. So, these criteria would augment the affinity of IgG3 to the negatively charged glomerular elements, making it highly nephritogenic[63].
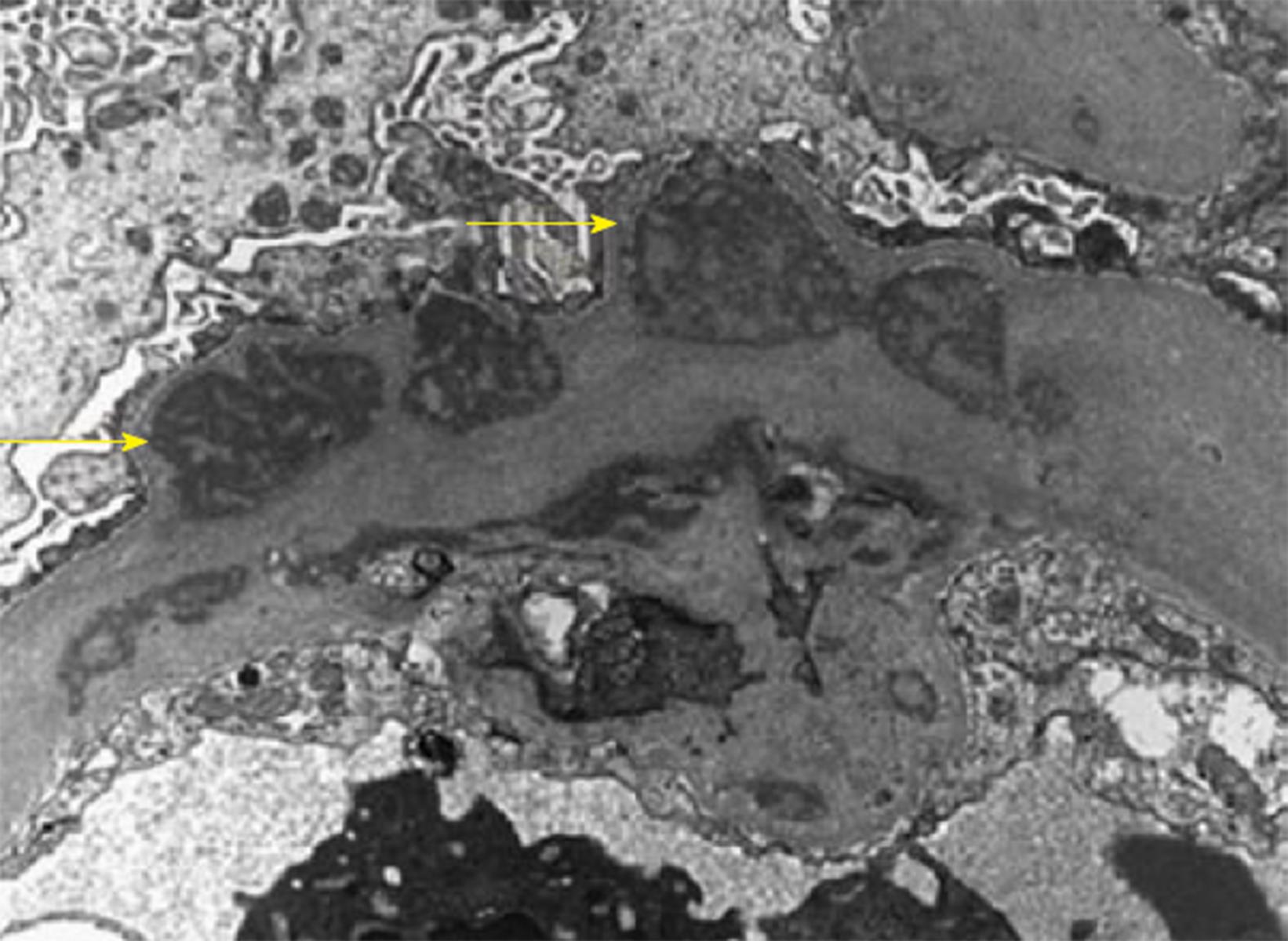
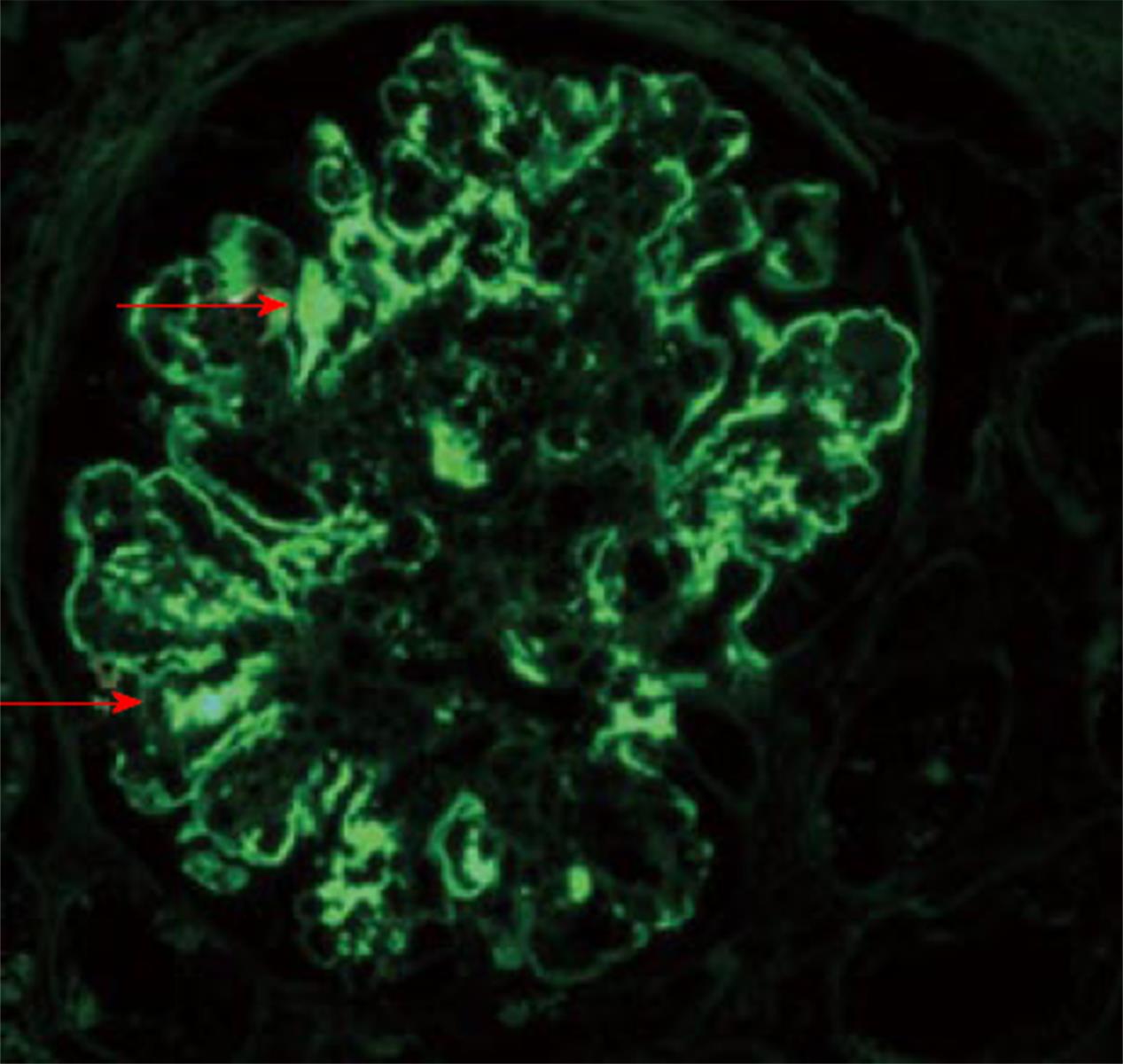
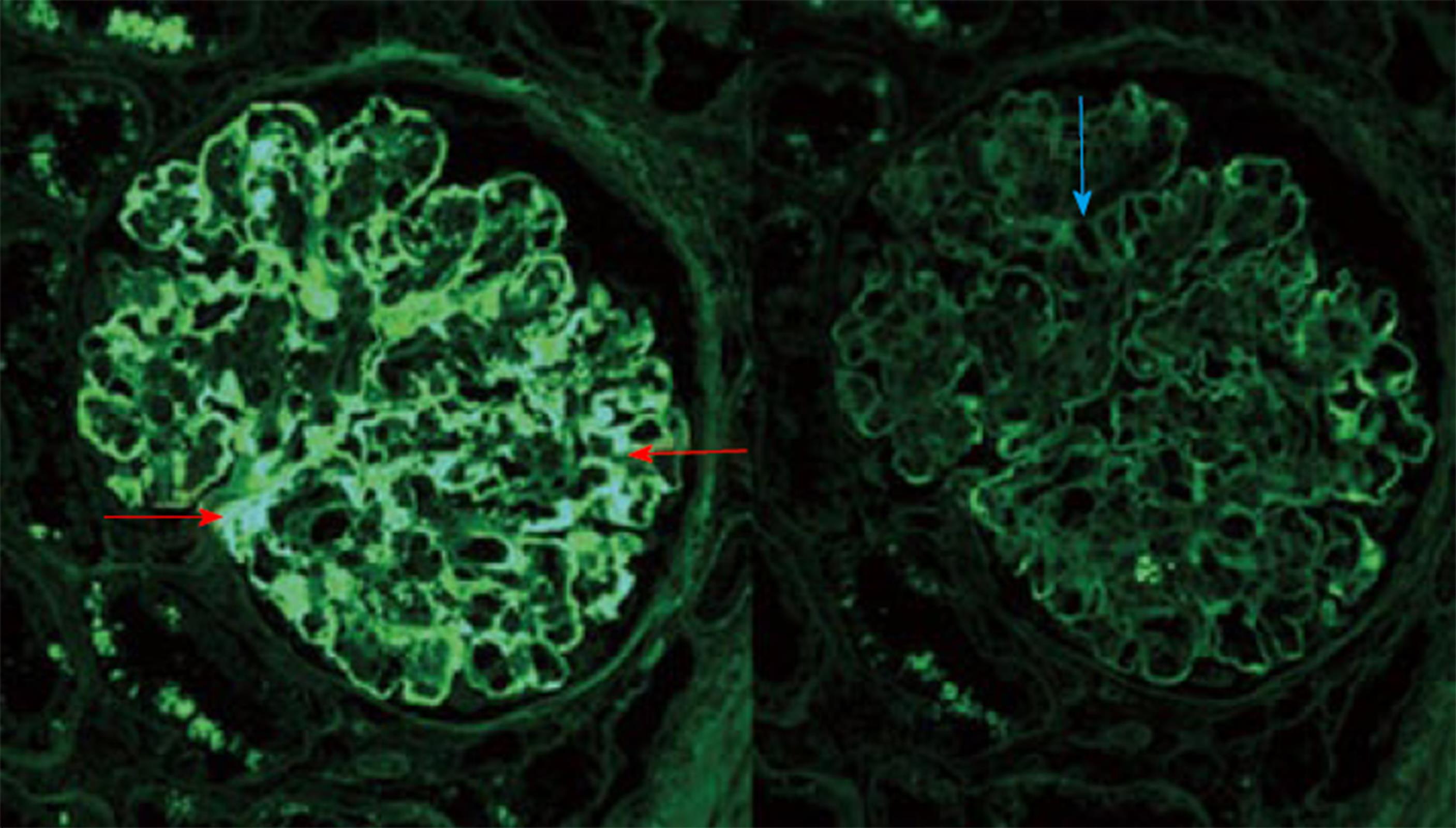
Histopathology: LM usually shows mesangioproliferative or endocapillary GN. EM shows prominent granular mesangial and subendothelial electron dense deposits (EDD) (Figure 2)[73]. Finally, IF study could ultimately establish the PGNMID diagnosis. A positive staining of one of the monoclonal IgG subtypes, with IgG3 being the most common and either kappa (most common) or the less common lambda subtype, strictly and exclusively in the glomerular constituents. C1q and complement 3 may be positive denoting complement activation (Figures 3 and 4)[73].
Differential diagnosis: PGNMID should be differentiated from other entities, e.g., TypeIcryoglobulinemic GN, transplant glomerulopathy, primary MPGN, post infectious GN, immunotactoid and fibrillary GN. In comparison to TypeIcryoglobulinemic GN, PGNMID lacks the serologic evidence of cryoglobulinemia, and also the annular-tubular as well as the fibrillary substructure by EM are absent. The microtubules of 30-40 nm in EM that characterizing immunotactoid are missed, so did the negative Congo red randomly organized fibrils of 16-24 nm diameter of the fibrillary GN. Lack of IF and EM studies can lead to a suspicion of transplant glomerulopathy, but the absence of monoclonality and the faint staining of the IgG can differentiate it from PGNMID[64]. PGNMID may simulate LHCDD in many aspects, but the pathogenesis is not alike[73]. While the heavy and the light chains deposition involve the glomerular as well as the tubular basement membrane in LHCDD, deposition of the intact monoclonal Ig is usually confined to the glomerular constituents in PGNMID. Also, the EDD are of granular nature in PGNMID as opposed to the powdery nature of LCDD deposits[73].
In the last few years, PGNMID disease attained a particular entity. Given the lack of monoclonal bands either in urine or serum, with normal appearance of bone marrow biopsy, this entity should be differentiated from other diseases with similar presentation, a challenging insight during RTR preparation[73]. Once the features of MPGN have been observed in the LM of allograft kidney biopsy, PGNMID disease should be considered among differential diagnoses. Long term monitoring of PGNMID is recommended to look for occult hematological malignancy[71].
De novo FSGS was reported to be the commonest form of de novo GN in some Canadian series[76]. While the recurrent FSGS can develop early post renal transplantation, usually in the form of nephrotic syndrome, de novo FSGS usually presents more than 12 mo after renal transplantation.
Clinically:De novo FSGS presents with a variable amounts of proteinuria up to nephrotic syndrome. Hypertension and progressive decline in allograft function can be seen[14].
Pathogenesis: The size discrepancy between the recipient’s body mass and the nephron mass of his allograft as a single kidney will induce compensatory hyperfiltration of the residual nephrons. DM, hypertension, BK polyomavirus[77], or parvovirus B19[78] can be also included in the pathogenesis of FSGS in addition to any pathological event that results in nephron loss. CNI toxicity can result in development of de novo FSGS months after transplant procedures in the form of proteinuria, hypertension and progressive decline in allograft function. The robust vasoconstrictor effect in addition to the typical microvascular lesions induced by CNI could ultimately induce arteriolar ischemic changes with subsequent characteristic histopathological lesions. A pivotal role of transforming growth factor-β (TGF-β), the multipotent protein responsible of cell growth regulation, differentiation and matrix formation can be observed. CNI can augment the expression of the podocyte TGF-β[79], leading to podocyte apoptosis and detachment from the glomerular basement membrane with synechia and glomerulosclerosis[14].
Allograft biopsy: Focal and/or global glomerular sclerosis in addition to arteriolar occlusion, tubular atrophy, interstitial nephritis and striped interstitial fibrosis could be seen. On the other hand, RTR who converted their immunosuppression protocol from CNI to a high dose of sirolimus (SRL) have developed de novo FSGS with proteinuria and similar lesion to that seen in the classic FSGS. The immunohistochemical studies show decreased or lack of expression of the podocyte-specific epitopes synaptopodin p57 with acquired expression of cytokeratin and PAX2, which reflects an immature fetal phenotype[80]. This pattern reflects a state of podocyte dysregulation, which was confirmed in human by exposure of human podocytes to sirolimus. Decreased VEGF and protein kinase B phosphorylation have been also observed. Dose-dependent decline in Wilms’ tumor 1, a transcription factor responsible of podocyte integrity was also observed[81]. De novo non-collapsing FSGS usually presents months or year after renal transplantation, with expected poor prognosis. The 5 years graft survival is only 40% after disease diagnosis in cases associated with CAN findings[82,83].
Collapsing FSGS (CG) is a distinct clinical and pathological variant from FSGS[14]. The reported incidence of de novo collapsing FSGS is about 0.6%[84]. De novo CG usually present 4-5 years after renal transplantation with heavy proteinuria and rapid decline of allograft function. Allograft biopsy usually shows segmental and/or global collapse of the glomerular capillary tuft. Prominent podocytes occupying the Bowman’s capsule with marked tubulointerstitial damage as well as obliterative vascular disease are usually seen[85]. Pathogenesis is not clear, but these histological changes could be seen with acute rejection, diabetic nephropathy and immune complex GN disease. Altered hemodynamic stability may be included in the de novo CG behavior. Certain infections, e.g., CMV and parvovirus B19 may be also associated with de novo CG disease[86,87]. Post-transplant antibody to angiotensin II TypeIreceptors, have been implicated in development of AMR[88].
A deleterious impact to the glomerular visceral and parietal epithelial cells integrity leading to cellular dedifferentiation with loss of glomerular filtration barrier function has been suggested[89-91]. Mitochondrial function disturbance has also been postulated as a deleterious mechanism in CG pathogenesis[92]. Ten cases of CG have been reported in serial biopsies in Mayo Clinic performed between 1994 and 2003[93]. CG is found to be prevalent in deceased donor kidney, presented usually with heavy proteinuria, higher serum creatinine level and poor response to plasmapheresis and ultimately allograft loss[94].
Prognosis: Outcomes of de novo CG is ultimately poor. All cases reported by Swaminathan et al[93] (2006), for example, lost their allograft within three years.
C3GN is a recently presented rare GN disease, characterized by predominant C3 glomerular deposits with similar morphology to that seen in DDD. However, in C3 GN there is lack of the ribbon-like intramembranous EDD. Recurrence of C3GN is reported, however, de novo C3GN disease is very rare[95].
In 2012, Sethi et al[96] (2012) presented the first two cases of recurrent C3GN, with subsequently reported 14 cases more in the next two years[97]. On the other hand, in 2008, Boyer et al[98] (2008) present two cases of de novo C3GN, however, these cases were presented as an aHUS or complement H deficiency. Furthermore, Nahm et al[95] (2016), reported a case of de novo C3 GN in a patient with no past history of alternative complement pathway abnormality, family history of renal disease or any symptoms related to glomerular disease. Tests related to complement factor H, complement factor H-related protein 5 genes and C3 nephritic factors were all negative[95]. They postulated an acquired complement abnormality after renal transplantation.
Histopathology: The C3GN early pathological changes usually show minimal mesangial expansion which may progress later to mesangial proliferation. EDD initially located in the mesangium, extend later to the subepithelial and subendothelial areas[97]. The EDD that present early in tubular basement membrane and in Bowman’s capsule may change to band-like simulating that present in dense deposition disease (DDD) that is characterizing and specified to its diagnosis[99]. However, C3 GN showed segmental tubular basement membrane deposits[100]. The DDD disease may experience phenotypical transformation to C3GN in the native kidney[101]. However, DDD usually shows more profound MP features as well as more intense complement abnormalities as compared to C3 GN[102]. The presence of an overlap may justify using the term “C3 glomerulopathy” instead of exerting to separate the two pathological identities, DDD and C3 GN[95]. De novo C3GN is a rare subtype of post renal transplantation GN diseases. The fundamental role observed through both IF and E/M studies in diagnosis and serial follow up is quite mandatory[95]. Of note that despite the observed decline in C3 deposition, renal function as well as histopathological changes continue to progress.
De novo minimal change disease (MCD) is a rarely reported disease in RTR. Fulfilled criteria of MCD diagnosis is not always present in some cases, which suggests a misdiagnosis of FSGS disease. While Markowitz et al[103] (1998) succeeded to report eight cases with full criteria of MCD, Truong and his associates (2002) added five more cases[104]. Furthermore, de novo MCD have been reported in incompatible ABO transplants[105]. With evolution of de novo MCD, a nephrotic range proteinuria developed rapidly after renal transplantation, however, some cases reported eight years after transplantation[106].
Histopathology: LM show typically normal appearance of the glomeruli. Some cases show hypercellularity and IgM/C3 deposition[103,105].
Pathogenesis: The pathogenesis of de novo MCD still uncertain. An activation of the innate and/or the adaptive immunity with T cell dysfunction and cytokines release, e.g., cardiotrophin-like cytokine-1[107] or the soluble urokinase-plasminogen receptor[108], leading to alteration of the glomerular capillary wall permeability has been suggested. The initial culprit agent is unknown, but certain viral-induced activity has been postulated. Another suggested factor, the costimulatory molecule B7-1 (CD80) in podocytes, has an additional impact on glomerular permselectivity. This agent [B7-1 (CD80)] has been proved to have a role in inducing an experimental nephrotic syndrome[109]. The role of this factor in inducing foot process fusion and proteinuria in the renal allograft is to be determined. The reported development of de novo MCD in a patient was on SRL therapy with clearance of the disease with drug withdrawal, has suggested a possible role of certain drugs in de novo MCD pathogenesis[110].
Prognosis:De novo MCD has a favorable prognosis in most cases[14]. Owing to its potential reversibility, de novo MCD has no deleterious impact on allograft survival on the long run. However, this disease is possibly still underestimated as a pivotal cause of nephrotic syndrome in the renal allograft (Table 3).
| Disease | Presentation | Time of onset | Difference with native GN | Treatment | Prognosis |
| MN | Proteinuria sometimes in nephrotic range | Late after transplant | Associated with trans-plant complications; IgG1 deposits instead of IgG4 | No specific treatment | Slowly progressive |
| MPGN | Proteinuria, hematuria, NS, nephritic sediment | Months or years after transplant | Often associated with HCV, or with other diseases | Steroids + cytotoxic drugs if crescentic GN (?) | Slowly progressive; poor with many crescents. |
| FSGS | Proteinuria, rarely in nephrotic range | Months or years after transplant | NS is rare; signs of rejection or CNI toxicity at biopsy | Removal of associated events | Usually poor, particularly in collapsing GN |
| MCD | NS | Early after transplant | Mild mesangial sclerosis, hypercellularity | Steroids | Good |
IgAN has been one of the most common GN worldwide. Graft loss has been frequently reported with recurrent IgAN[8]. On the other hand, this fate is rarely reported with de novo IgAN[111].
Incidence:De novo IgAN has been reported to be less common than recurrent IgAN[112]. Considering the high frequency of asymptomatic IgAN, some authors argue that de novo IgAN might be considered as “transmitted disease”, which means that recipient received an allograft that already had a “latent” form of IgAN[14], this argument is supported by the finding that a considerable percentage of mesangial IgA deposition (16.1%) has been reported in 0-hour protocol biopsy performed by a Japanese study[113].
Histopathology: Intracapillary proliferation with a possibility of crescent formation can be observed in many biopsies. IgA and C3 granular deposits in the glomerular capillary wall and mesangium are frequently seen in IF studies.
Clinical features: despite the presence of frequent IgA deposition, de novo IgA is frequently asymptomatic especially in Asian population that may be discovered only in protocol biopsy.
Course and prognosis: In case of presence of crescent formation in allograft biopsy, prognosis of de novo IgA is ultimately poor, otherwise course and prognosis is quiescent with mild mesangial hypercellularity[8]. For example, Robles et al[4] (1991) reported a case of de novo IgAN with progressive proteinuria, microscopic hematuria and rapid deterioration of allograft function after renal transplantation in a patient with ESRD due to MPGN. On the other hand, de novo Henoch-Schönlein purpura has been described post renal transplantation with a rapid graft loss[114,115].
Options of de novo MN therapy are variable, including rituximab, bortezomib, PE, and intravenous Ig[116-118]. Unfortunately, absence of randomized control prospective studies and the high cost would be an obvious obstacles[13]. Therapy of de novo MN is still unclear. There is no enough data to support the use of rituximab in de novo MN therapy and there no clear base supporting the introduction of cytotoxic therapy or the intensified immunosuppressive agents would be efficacious[37,119].
Indications for retransplantation: MN is a slowly progressive disease, there is no contraindication to retransplant (Table 4).
| Disease | Indications to retransplant |
| MN | In view of the slow progression, there is no contraindication to retransplant |
| MPGN | The risk of recurrence is high in carriers of HCV, active autoimmune disease, or monoclonal gammopathy. These risk factors should be removed or inactivated before retransplant |
| FSGS | If FSGS was caused by calcineurin inhibitor or mTOR inhibitor toxicity, there is no contraindication to retransplant, but the dosage of the offending drug should be minimized. If FSGS was associated with AMR, the risk of recurrence is increased. Circulating antibodies should be removed before retransplant |
| Collapsing nephropathy | Risk of recurrence is probably high. Antiviral and/or removal of circulating AB before retransplant are recommended according to the possible role played by virus infection or AMR in the 1st transplant |
| MCD | In view of the favorable prognosis, there is no contraindication to retransplant |
| IgAN | No contraindication to retransplant |
Therapy of de novo MPGN is still elusive. Trial of intensification of immunosuppression and the use of steroids generally showed poor and unstable results. Re-transplantation, however, is not contraindicated as long as the HCV infection as well as other risk factors have been eliminated. In this instance, the newly introduced oral anti-HCV agents, e.g., protease inhibitors and/or RNA polymerase inhibitors, should be considered before attempting renal transplantation[14].
Indications for retransplantation: The risk of recurrence is high in HCV carriers, active autoimmune disease, or in monoclonal gammopathy. Risk factors should be eliminated before retransplantation (Table 4).
There is no established therapy for de novo PGNMID[68]. However, a trial of rituximab, cyclophosphamide, plasmapheresis and high dose steroids have been introduced[63,65-67]. An observed reasonable response to rituximab and cyclophosphamide was reported with the recurrent disease, which was attributed by the authors to an early application of the protocol biopsy[63]. Multiple protocols have been tried by others including: High-dose steroids, RAS blocking agents, bortezomib, rituximab with and without steroids and plasmapheresis[78] (Table 3).
Rationale of rituximab use: B cells in PGNMID hypersecrete an abnormal IgG. The latter have the ability of self-aggregation and glomerular deposition. Rituximab, a monoclonal antibody has been widely used post renal transplantation for PTLPD, resistant antibody-mediated rejection and recurrent glomerular disease and as a prophylactic therapy for chronic antibody mediated rejection through inhibiting antibody production and hampering the B-cell immunity[120-127].
The recent advents of rituximab in PGNMID therapy have been shown to improve allograft function with better outcome[67,76,128]. Merhi et al[75] (2017), reported a unique results with the use of rituximab in two male patients one de novo (with IgG3κ restriction) and the other is recurrent (with IgG1κ restriction). They reported better allograft function with continuous stability and return to basal creatinine level that have been continued for almost two years with persistent stable clinical and pathological response (Table 3). To declare the magnitude of benefits of rituximab, a clear insight on the pathogenesis of PGNMID depending in a wide scale of prospective controlled randomized trials should be accomplished. The role of allograft protocol biopsy in PGNMID in immunosuppressed patients is to be also declared[75].
The early interference in the course of de novo FSGS by CNI withdrawal and introduction of MMF or mTOR inhibitors (mammalian target of rapamycin) may induce stabilization or even improvement of allograft function. One major drawback should be expected, i.e., the increased risk of rejection, particularly so, if there is associated proteinuria or the CrCl was below 40 mL/min[129]. Allograft loss due de novo FSGS, however, does not preclude the attempt of retransplantation as long as the factors incriminated in the pathogenesis of FSGS would be eliminated. It will be also worthy to modulate the therapeutic strategies to decrease the risk of recurrence, e.g., by CNI minimization and/or considering antiviral prophylaxis[14,129] (Table 3).
Retransplantation: In patients with de novo FSGS due to either CNI- or mTOR inhibitors-induced toxicity, there is no contraindication to retransplantation, however, the dose of the drug should be modified. If there was an associated AMR, the risk of recurrence would be high. Donors organs that are likely to trigger a repeat challenge by corresponding antigens leading to a rise in DSA should be excluded before retransplantation and, if feasible, desensitization be considered (Table 4).
There is no particular therapy for de novo CG. With the presence of evidence of viral infection, antiviral agents may be suggested. Despite the unpredicted results, an attempt to use abatacept may be tried if there is B7-1 (CD80) expression in the podocytes[130]. In view of scarce data as regard re-transplantation in patients who lost their grafts due to de novo CG, there is no specific recommendation. However, an attempt to do re-transplantation in such a situation should be preceded by meticulous screening of antibodies to angiotensin II TypeIreceptors, in addition to an intensive course of antiviral therapy[14].
Retransplantation: The risk of recurrence of FSGS is potentially high. Antiviral therapy and/or clearance of the circulating antibodies are recommended in view of the potential role of viral infection and/or AMR in the first transplant (Table 4).
Impact of therapy on glomerular morphology: Eculizumab has been reported to induce partial reduction in glomerular inflammatory activity as well as decline in deposits distribution[100]. On the other hands, other reports showed that eculizumab may be associated with EDD[131]. However, Nahm et al[95] (2016) used pulse steroids, ATG, rituximab, PE and IVIG to treat the associated AMR, with good response as regard normalization of serum creatinine and reduction of glomerular C3 deposition, but unfortunately the EDD persist. They speculate that C3 deposits may be masked at the locations that they were hard to wash out.
Follow up: Serial biopsies show more intensified tubular basement membrane deposits as compared to glomerular deposits. So, the E/M examination can declare these deposits more precisely as compared to the IF studies as shown by Hou et al[132] (2014), with IF pattern changes in about 43% of cases in repeated biopsies.
Rationale of eculizumab use: Eculizumab has been used in 11 cases of C3GN, with mostly but not always favorable results[101,102,133-141]. Eculizumab is a humanized monoclonal antibodies with a potent affinity to complement 5 and prevents the generation of serum membrane attack complex (sMAC) and release of a very potent inflammatory mediator C5a, giving an effective target of therapy[142]. So, it has been suggested that eculizumab administration could be effective in C3GN therapy if given early in cases with minimal fibrosis, short disease course and in patients with increased sMAC with accepted results[138]. These benefits were confirmed by Kersnik Levart et al[143] (2016). They reported clinical as well as laboratory improvement, in addition to normalization of the sMAC levels. Moreover, a quite evident decline in glomerular inflammatory activity was observed in the latest biopsies in the form of absent neutrophilic infiltration and necrotic lesions as well as reduced glomerular proliferation activity. Active cellular crescents get transformed into inactive fibrous crescents.
Decision to commence eculizumab therapy should not be attempted until all other differential diagnoses have been excluded and failures of other immunosuppressive measures have been proved[144]. This will work only if properly guided by serial allograft biopsies as well as the clinical features before commencing to use such an expensive drug with a prolonged-term therapeutic approach[143]. Renal function recovery and decline of proteinuria could be expected even in a patient with crescentic GN with a rapidly progressive course[140]. Furthermore, patient already commenced dialysis can quit RRT after only five months of eculizumab therapy[141]. Six months, however, should be elapsed prior to reporting the failure of eculizumab therapy[141,144]. Long-term sequalae of this drug is uncertain, however, it has been tried successfully in paroxysmal nocturnal hemoglobinuria without evidence of appearance of proteinuria or decline in renal function[145]. Serial long-term biopsies follow up declared also the new observation of eculizumab binding to the renal tissues, an evidence with no harmful impact, despite the fact that eculizumab deposits are similar to that of the monoclonal Ig deposits[143].
A sustained remission of the nephrotic syndrome is usually expected with intensification of steroid therapy and other immunosuppressive agents[14]. A good renal function can be maintained after remission with or without minimal proteinuria (Table 3).
Retransplantation: Prognosis is quite favorable, there is no contraindication to retransplantation (Table 4).
For mild and moderate de novo IgA, no specific therapy is advised. However, Shabaka et al[111] (2017) reported that potentiation of immunosuppressive therapy with CNI and augmentation of RAS blockade can lead to a complete remission and better renal function. On the other hand, Carneiro-Roza et al[146] (2006) reported a better initial response in decreasing urinary protein level with no improvement in renal function. In patients presented with crescentic IgAN and a rapidly progressive course, pulse steroid, cyclophosphamide and PE may be tried with expected poor results[14].
Retransplantation: No contraindication to retransplant (Table 4).
The management of de novo GN diseases poses unique set of challenges. For a transplanting team, it is paramount to be armed with as much information as possible about the original disease of the native kidney when proceeding with renal transplantation. A lacunae in information would raise the risk of graft loss due to recurrent GN disease. Moreover, awareness of the pathogenesis of these diseases, their clinical features as well as their potential prognosis would help in improving both allograft and patient survival. One of the greatest obstacles hampering the achievement of these targets is the scarce number of the reported de novo GN diseases after renal transplantation. A world-wide cooperation between transplantation centers through multicenter randomized controlled trials would address many questions in regards to making a clear diagnosis and defining a robust management plan.
Authors of this article do appreciate the permission offered by Professor, Dr. Ponticelli C, allowing the use of the graphs included in his valuable articles.
| 1. | Ivanyi B. A primer on recurrent and de novo glomerulonephritis in renal allografts. Nat Clin Pract Nephrol. 2008;4:446-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Denton MD, Singh AK. Recurrent and de novo glomerulonephritis in the renal allograft. Semin Nephrol. 2000;20:164-175. [PubMed] |
| 3. | Molina M, Sánchez J, Sevillano ÁM, Bengoa I, Gutiérrez E, Martínez MA, Hernández E, Praga M. Two cases of glomerulonephritis in one patient infected with the hepatitis C virus. Nefrologia. 2013;33:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Robles NR, Gomez Campdera FJ, Anaya F, Niembro De Rasche E, Galan A, Rengel MA, Valderrabano F. IgA nephropathy with rapidly progressive course after kidney transplantation. Nephron. 1991;58:487-488. [PubMed] |
| 5. | Francisco S, Wall BM, Cooke CR. Immunoglobulin A nephropathy in a renal allograft of a black transplant recipient. Am J Nephrol. 1992;12:121-125. [PubMed] |
| 6. | Jeong HJ, Kim YS, Kwon KH, Kim SI, Kim MS, Choi KH, Lee HY, Han DS, Park K. Glomerular crescents are responsible for chronic graft dysfunction in post-transplant IgA nephropathy. Pathol Int. 2004;54:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Gough J, Yilmaz A, Yilmaz S, Benediktsson H. Recurrent and de novo glomerular immune-complex deposits in renal transplant biopsies. Arch Pathol Lab Med. 2005;129:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Kowalewska J, Yuan S, Sustento-Reodica N, Nicosia RF, Smith KD, Davis CL, Alpers CE. IgA nephropathy with crescents in kidney transplant recipients. Am J Kidney Dis. 2005;45:167-175. [PubMed] |
| 9. | Tang Z, Ji SM, Chen DR, Wen JQ, Chen JS, Liu ZH, Li LS. Recurrent or de novo IgA nephropathy with crescent formation after renal transplantation. Ren Fail. 2008;30:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Ponticelli C, Glassock RJ. De novo membranous nephropathy (MN) in kidney allografts. A peculiar form of alloimmune disease? Transpl Int. 2012;25:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Kearney N, Podolak J, Matsumura L, Houghton D, Troxell M. Patterns of IgG subclass deposits in membranous glomerulonephritis in renal allografts. Transplant Proc. 2011;43:3743-3746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Honda K, Horita S, Toki D, Taneda S, Nitta K, Hattori M, Tanabe K, Teraoka S, Oda H, Yamaguchi Y. De novo membranous nephropathy and antibody-mediated rejection in transplanted kidney. Clin Transplant. 2011;25:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Doke T, Sato W, Takahashi K, Hayashi H, Koide S, Sasaki H, Kusaka M, Shiroki R, Hoshinaga K, Takeda A. Post-Transplant Membranous Nephropathy Associated with Chronic Active Antibody-Mediated Rejection and Hepatitis C Infection after Deceased Donor Renal Transplantation. Intern Med. 2016;55:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Ponticelli C, Moroni G, Glassock RJ. De novo glomerular diseases after renal transplantation. Clin J Am Soc Nephrol. 2014;9:1479-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Larsen CP, Walker PD. Phospholipase A2 receptor (PLA2R) staining is useful in the determination of de novo versus recurrent membranous glomerulopathy. Transplantation. 2013;95:1259-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Charpentier B, Lévy M. [Cooperative study of de novo extramembranous glomerulonephritis in renal allografts in humans: report of 19 new cases in 1550 renal transplant patients of the transplantation group of the Ile de France]. Nephrologie. 1982;3:158-166. [PubMed] |
| 17. | Aline-Fardin A, Rifle G, Martin L, Justrabo E, Bour JB, D’Athis P, Tanter Y, Mousson C. Recurent and de novo membranous glomerulopathy after kidney transplantation. Transplant Proc. 2009;41:669-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Davison AM, Johnston PA. Allograft membranous nephropathy. Nephrol Dial Transplant. 1992;7 Suppl 1:114-118. [PubMed] |
| 19. | Antignac C, Hinglais N, Gubler MC, Gagnadoux MF, Broyer M, Habib R. De novo membranous glomerulonephritis in renal allografts in children. Clin Nephrol. 1988;30:1-7. [PubMed] |
| 20. | Cornell LD. IgG4-related kidney disease. Curr Opin Nephrol Hypertens. 2012;21:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Sistani L, Dunér F, Udumala S, Hultenby K, Uhlen M, Betsholtz C, Tryggvason K, Wernerson A, Patrakka J. Pdlim2 is a novel actin-regulating protein of podocyte foot processes. Kidney Int. 2011;80:1045-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Stevenson WS, Nankivell BJ, Hertzberg MS. Nephrotic syndrome after stem cell transplantation. Clin Transplant. 2005;19:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2861] [Cited by in RCA: 2944] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 24. | Couser WG. Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol. 2012;23:381-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 25. | El Kossi M, Harmer A, Goodwin J, Wagner B, Shortland J, Angel C, McKane W. De novo membranous nephropathy associated with donor-specific alloantibody. Clin Transplant. 2008;22:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Ji F, Li Z, Ge H, Deng H. Successful interferon-α treatment in a patient with IgA nephropathy associated with hepatitis C virus infection. Intern Med. 2010;49:2531-2532. [PubMed] |
| 27. | Cruzado JM, Torras J, Gil-Vernet S, Grinyó JM. Glomerulonephritis associated with hepatitis C virus infection after renal transplantation. Nephrol Dial Transplant. 2000;15 Suppl 8:65-67. [PubMed] |
| 28. | Gonzalo A, Navarro J, Bárcena R, Quereda C, Ortuño J. IgA nephropathy associated with hepatitis C virus infection. Nephron. 1995;69:354. [PubMed] |
| 29. | Cabezuelo JB, Enríquez R, Andrada E, Amorós F, Sirvent AE, Reyes A. [Extracapillary IgA nephropathy associated with infection with hepatitis C virus and hepatic cirrhosis]. Nefrologia. 2000;20:379-382. [PubMed] |
| 30. | Sumida K, Ubara Y, Hoshino J, Suwabe T, Nakanishi S, Hiramatsu R, Hasegawa E, Hayami N, Yamanouchi M, Sawa N. Hepatitis C virus-related kidney disease: various histological patterns. Clin Nephrol. 2010;74:446-456. [PubMed] |
| 31. | Coccoli P, Esposito P, Cianciaruso B, Pota A, Visciano B, Annecchini R, Parrilli G. Hepatitis C and kidney disease. Dig Liver Dis. 2007;39 Suppl 1:S83-S85. [PubMed] |
| 32. | Meyers CM, Seeff LB, Stehman-Breen CO, Hoofnagle JH. Hepatitis C and renal disease: an update. Am J Kidney Dis. 2003;42:631-657. [PubMed] |
| 33. | Cruzado JM, Carrera M, Torras J, Grinyó JM. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant. 2001;1:171-178. [PubMed] |
| 34. | Monga G, Mazzucco G, Basolo B, Quaranta S, Motta M, Segoloni G, Amoroso A. Membranous glomerulonephritis (MGN) in transplanted kidneys: morphologic investigation on 256 renal allografts. Mod Pathol. 1993;6:249-258. [PubMed] |
| 35. | Heidet L, Gagnadoux ME, Beziau A, Niaudet P, Broyer M, Habib R. Recurrence of de novo membranous glomerulonephritis on renal grafts. Clin Nephrol. 1994;41:314-318. [PubMed] |
| 36. | Teixeira e Costa F, Pinto JR, Carvalho F, Galvão MJ. An early case of de novo membranous nephropathy in a renal transplant patient. Transplant Proc. 2002;34:364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Truong L, Gelfand J, D’Agati V, Tomaszewski J, Appel G, Hardy M, Pirani CL. De novo membranous glomerulonephropathy in renal allografts: a report of ten cases and review of the literature. Am J Kidney Dis. 1989;14:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Cosyns JP, Pirson Y, Squifflet JP, Alexandre GP, van Ypersele de Strihou C, Pinn VW, Sweet SJ, Shapiro KS, Cho S, Harrington JT. De novo membranous nephropathy in human renal allografts: report of nine patients. Kidney Int. 1982;22:177-183. [PubMed] |
| 39. | Schwarz A, Krause PH, Offermann G, Keller F. Recurrent and de novo renal disease after kidney transplantation with or without cyclosporine A. Am J Kidney Dis. 1991;17:524-531. [PubMed] |
| 40. | Dische FE, Herbertson BM, Melcher DH, Morley AR. Membranous glomerulonephritis in transplant kidneys: recurrent or de novo disease in four patients. Clin Nephrol. 1981;15:154-163. [PubMed] |
| 41. | Kim M, Martin ST, Townsend KR, Gabardi S. Antibody-mediated rejection in kidney transplantation: a review of pathophysiology, diagnosis, and treatment options. Pharmacotherapy. 2014;34:733-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 43. | Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2451] [Cited by in RCA: 2556] [Article Influence: 127.8] [Reference Citation Analysis (5)] |
| 44. | Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69 Suppl 3:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 45. | Macugen AMD Study Group, Apte RS, Modi M, Masonson H, Patel M, Whitfield L, Adamis AP. Pegaptanib 1-year systemic safety results from a safety-pharmacokinetic trial in patients with neovascular age-related macular degeneration. Ophthalmology. 2007;114:1702-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Cheungpasitporn W, Chebib FT, Cornell LD, Brodin ML, Nasr SH, Schinstock CA, Stegall MD, Amer H. Intravitreal Antivascular Endothelial Growth Factor Therapy May Induce Proteinuria and Antibody Mediated Injury in Renal Allografts. Transplantation. 2015;99:2382-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Kowalewska J, Smith KD, Hudkins KL, Chang A, Fogo AB, Houghton D, Leslie D, Aitchison J, Nicosia RF, Alpers CE. Membranous glomerulopathy with spherules: an uncommon variant with obscure pathogenesis. Am J Kidney Dis. 2006;47:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | El-Zoghby ZM, Grande JP, Fraile MG, Norby SM, Fervenza FC, Cosio FG. Recurrent idiopathic membranous nephropathy: early diagnosis by protocol biopsies and treatment with anti-CD20 monoclonal antibodies. Am J Transplant. 2009;9:2800-2807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Herrmann SM, Sethi S, Fervenza FC. Membranous nephropathy: the start of a paradigm shift. Curr Opin Nephrol Hypertens. 2012;21:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Myslak M, Amer H, Morales P, Fidler ME, Gloor JM, Larson TS, Stegall MD, Cosio FG. Interpreting post-transplant proteinuria in patients with proteinuria pre-transplant. Am J Transplant. 2006;6:1660-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 330] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 52. | Jiménez-Sousa MA, Fernández-Rodríguez A, Heredia M, Tamayo E, Guzmán-Fulgencio M, Lajo C, López E, Gómez-Herreras JI, Bustamante J, Bermejo-Martín JF. Genetic polymorphisms located in TGFB1, AGTR1, and VEGFA genes are associated to chronic renal allograft dysfunction. Cytokine. 2012;58:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Ko HT, Yin JL, Wyburn K, Wu H, Eris JM, Hambly BD, Chadban SJ. Sirolimus reduces vasculopathy but exacerbates proteinuria in association with inhibition of VEGF and VEGFR in a rat kidney model of chronic allograft dysfunction. Nephrol Dial Transplant. 2013;28:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Georgalas I, Papaconstantinou D, Papadopoulos K, Pagoulatos D, Karagiannis D, Koutsandrea C. Renal injury following intravitreal anti-VEGF administration in diabetic patients with proliferative diabetic retinopathy and chronic kidney disease--a possible side effect? Curr Drug Saf. 2014;9:156-158. [PubMed] |
| 55. | Alvarez Arroyo MV, Suzuki Y, Yagüe S, Lorz C, Jiménez S, Soto C, Barat A, Belda E, González-Pacheco FR, Deudero JJ. Role of endogenous vascular endothelial growth factor in tubular cell protection against acute cyclosporine toxicity. Transplantation. 2002;74:1618-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Peng W, Chen J, Jiang Y, Shou Z, Chen Y, Wang H. Acute renal allograft rejection is associated with increased levels of vascular endothelial growth factor in the urine. Nephrology (Carlton). 2008;13:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Bieri M, Oroszlan M, Farkas A, Ligeti N, Bieri J, Mohacsi P. Anti-HLA I antibodies induce VEGF production by endothelial cells, which increases proliferation and paracellular permeability. Int J Biochem Cell Biol. 2009;41:2422-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Mittal RD, Srivastava P, Singh V, Jaiswal P, Kapoor R. Association of common variants of vascular endothelial growth factor and interleukin-18 genes with allograft survival in renal transplant recipients of North India. DNA Cell Biol. 2011;30:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Alasfar S, Carter-Monroe N, Rosenberg AZ, Montgomery RA, Alachkar N. Membranoproliferative glomerulonephritis recurrence after kidney transplantation: using the new classification. BMC Nephrol. 2016;17:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Roth D, Cirocco R, Zucker K, Ruiz P, Viciana A, Burke G, Carreno M, Esquenazi V, Miller J. De novo membranoproliferative glomerulonephritis in hepatitis C virus-infected renal allograft recipients. Transplantation. 1995;59:1676-1682. [PubMed] |
| 61. | Hammoud H, Haem J, Laurent B, Alamartine E, Diab N, Defilippis JP, Berthoux P, Berthoux F. Glomerular disease during HCV infection in renal transplantation. Nephrol Dial Transplant. 1996;11 Suppl 4:54-55. [PubMed] |
| 62. | Faguer S, Kamar N, Boulestin A, Esposito L, Durand D, Blancher A, Rostaing L. Prevalence of cryoglobulinemia and autoimmunity markers in renal-transplant patients. Clin Nephrol. 2008;69:239-243. [PubMed] |
| 63. | Nasr SH, Sethi S, Cornell LD, Fidler ME, Boelkins M, Fervenza FC, Cosio FG, D’Agati VD. Proliferative glomerulonephritis with monoclonal IgG deposits recurs in the allograft. Clin J Am Soc Nephrol. 2011;6:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Albawardi A, Satoskar A, Von Visger J, Brodsky S, Nadasdy G, Nadasdy T. Proliferative glomerulonephritis with monoclonal IgG deposits recurs or may develop de novo in kidney allografts. Am J Kidney Dis. 2011;58:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Sumida K, Ubara Y, Marui Y, Nakamura M, Takaichi K, Tomikawa S, Fujii T, Ohashi K. Recurrent proliferative glomerulonephritis with monoclonal IgG deposits of IgG2λ subtype in a transplanted kidney: a case report. Am J Kidney Dis. 2013;62:587-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Ranghino A, Tamagnone M, Messina M, Barreca A, Biancone L, Basolo B, Segoloni GP, Mazzucco G. A case of recurrent proliferative glomerulonephritis with monoclonal IgG deposits after kidney transplant treated with plasmapheresis. Case Rep Nephrol Urol. 2012;2:46-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Wu D, Chen JS, Cheng DR, Chen H, Li X, Ji SM, Xie KN, Ni XF, Liu ZH, Wen JQ. Recurrent Proliferative Glomerulonephritis With Monoclonal IgG Deposits After a Renal Transplant Which Was Insensitive to Pulse Therapy Remitted by Double Filtration Plasmapheresis. Exp Clin Transplant. 2015;13:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Batal I, Bijol V, Schlossman RL, Rennke HG. Proliferative glomerulonephritis with monoclonal immunoglobulin deposits in a kidney allograft. Am J Kidney Dis. 2014;63:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Nasr SH, Markowitz GS, Stokes MB, Seshan SV, Valderrama E, Appel GB, Aucouturier P, D’Agati VD. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int. 2004;65:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 70. | Nasr SH, Satoskar A, Markowitz GS, Valeri AM, Appel GB, Stokes MB, Nadasdy T, D’Agati VD. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 322] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 71. | Barbour SJ, Beaulieu MC, Zalunardo NY, Magil AB. Proliferative glomerulonephritis with monoclonal IgG deposits secondary to chronic lymphocytic leukemia. Report of two cases. Nephrol Dial Transplant. 2011;26:2712-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Hussain SM, Sureshkumar KK. Proliferative glomerulonephritis with monoclonal IgG deposits; an unusual cause of de novo disease in kidney allograft. J Nephropathol. 2017;6:220-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 73. | Al-Rabadi L, Francis JM, Henderson J, Ghai S. Proliferative glomerulonephritis with monoclonal immunoglobulin in renal allografts. Clin Kidney J. 2015;8:722-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Tsuji T, Miura M, Yanai M, Itami H, Ishii Y, Akimoto M, Fukasawa Y. De novo proliferative glomerulonephritis with monoclonal IgG deposits of the IgG1κ subtype in a kidney allograft. Nephrology (Carlton). 2016;21 Suppl 1:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Merhi B, Patel N, Bayliss G, Henriksen KJ, Gohh R. Proliferative glomerulonephritis with monoclonal IgG deposits in two kidney allografts successfully treated with rituximab. Clin Kidney J. 2017;10:405-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Chailimpamontree W, Dmitrienko S, Li G, Balshaw R, Magil A, Shapiro RJ, Landsberg D, Gill J, Keown PA; Genome Canada Biomarkers in Transplantation Group. Probability, predictors, and prognosis of posttransplantation glomerulonephritis. J Am Soc Nephrol. 2009;20:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 77. | Li RM, Branton MH, Tanawattanacharoen S, Falk RA, Jennette JC, Kopp JB. Molecular identification of SV40 infection in human subjects and possible association with kidney disease. J Am Soc Nephrol. 2002;13:2320-2330. [PubMed] |
| 78. | Waldman M, Kopp JB. Parvovirus B19 and the kidney. Clin J Am Soc Nephrol. 2007;2 Suppl 1:S47-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Olyaei AJ, de Mattos AM, Bennett WM. Nephrotoxicity of immunosuppressive drugs: new insight and preventive strategies. Curr Opin Crit Care. 2001;7:384-389. [PubMed] |
| 80. | Letavernier E, Bruneval P, Mandet C, Duong Van Huyen JP, Péraldi MN, Helal I, Noël LH, Legendre C. High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol. 2007;2:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 81. | Letavernier E, Bruneval P, Vandermeersch S, Perez J, Mandet C, Belair MF, Haymann JP, Legendre C, Baud L. Sirolimus interacts with pathways essential for podocyte integrity. Nephrol Dial Transplant. 2009;24:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Cosio FG, Frankel WL, Pelletier RP, Pesavento TE, Henry ML, Ferguson RM. Focal segmental glomerulosclerosis in renal allografts with chronic nephropathy: implications for graft survival. Am J Kidney Dis. 1999;34:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Gupta R, Sharma A, Mahanta PJ, Agarwal SK, Dinda AK. Focal and segmental glomerulosclerosis in renal allograft recipients: a clinico-pathologic study of 37 cases. Saudi J Kidney Dis Transpl. 2013;24:8-14. [PubMed] |
| 84. | Meehan SM, Pascual M, Williams WW, Tolkoff-Rubin N, Delmonico FL, Cosimi AB, Colvin RB. De novo collapsing glomerulopathy in renal allografts. Transplantation. 1998;65:1192-1197. [PubMed] |
| 85. | Gupta R, Sharma A, Agarwal SK, Dinda AK. Collapsing glomerulopathy in renal allograft biopsies: A study of nine cases. Indian J Nephrol. 2011;21:10-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Tomlinson L, Boriskin Y, McPhee I, Holwill S, Rice P. Acute cytomegalovirus infection complicated by collapsing glomerulopathy. Nephrol Dial Transplant. 2003;18:187-189. [PubMed] |
| 87. | Moudgil A, Shidban H, Nast CC, Bagga A, Aswad S, Graham SL, Mendez R, Jordan SC. Parvovirus B19 infection-related complications in renal transplant recipients: treatment with intravenous immunoglobulin. Transplantation. 1997;64:1847-1850. [PubMed] |
| 88. | Alachkar N, Gupta G, Montgomery RA. Angiotensin antibodies and focal segmental glomerulosclerosis. N Engl J Med. 2013;368:971-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 89. | Schwimmer JA, Markowitz GS, Valeri A, Appel GB. Collapsing glomerulopathy. Semin Nephrol. 2003;23:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Albaqumi M, Soos TJ, Barisoni L, Nelson PJ. Collapsing glomerulopathy. J Am Soc Nephrol. 2006;17:2854-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 91. | Albaqumi M, Barisoni L. Current views on collapsing glomerulopathy. J Am Soc Nephrol. 2008;19:1276-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 92. | Sauter M, Jülg B, Porubsky S, Cohen C, Fischereder M, Sitter T, Schlondorff D, Gröne HJ. Nephrotic-range proteinuria following pamidronate therapy in a patient with metastatic breast cancer: mitochondrial toxicity as a pathogenetic concept? Am J Kidney Dis. 2006;47:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Swaminathan S, Lager DJ, Qian X, Stegall MD, Larson TS, Griffin MD. Collapsing and non-collapsing focal segmental glomerulosclerosis in kidney transplants. Nephrol Dial Transplant. 2006;21:2607-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Sureshkumar KK, Dosani I, Jasnosz KM, Arora S. De Novo Collapsing Glomerulopathy: An Unusual Cause of Early Graft Failure following Kidney Transplantation. Case Rep Transplant. 2011;2011:263970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 95. | Nahm JH, Song SH, Kim YS, Cheong HI, Lim BJ, Kim BS, Jeong HJ. De novo C3 glomerulonephritis in a renal allograft. Ultrastruct Pathol. 2016;40:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJ. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012;82:465-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 97. | Zand L, Lorenz EC, Cosio FG, Fervenza FC, Nasr SH, Gandhi MJ, Smith RJ, Sethi S. Clinical findings, pathology, and outcomes of C3GN after kidney transplantation. J Am Soc Nephrol. 2014;25:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 98. | Boyer O, Noël LH, Balzamo E, Guest G, Biebuyck N, Charbit M, Salomon R, Frémeaux-Bacchi V, Niaudet P. Complement factor H deficiency and posttransplantation glomerulonephritis with isolated C3 deposits. Am J Kidney Dis. 2008;51:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Medjeral-Thomas NR, O’Shaughnessy MM, O’Regan JA, Traynor C, Flanagan M, Wong L, Teoh CW, Awan A, Waldron M, Cairns T. C3 glomerulopathy: clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol. 2014;9:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 100. | Herlitz LC, Bomback AS, Markowitz GS, Stokes MB, Smith RN, Colvin RB, Appel GB, D’Agati VD. Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol. 2012;23:1229-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 101. | Gurkan S, Fyfe B, Weiss L, Xiao X, Zhang Y, Smith RJ. Eculizumab and recurrent C3 glomerulonephritis. Pediatr Nephrol. 2013;28:1975-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 102. | Servais A, Noël LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 418] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 103. | Markowitz GS, Stemmer CL, Croker BP, D’Agati VD. De novo minimal change disease. Am J Kidney Dis. 1998;32:508-513. [PubMed] |
| 104. | Zafarmand AA, Baranowska-Daca E, Ly PD, Tsao CC, Choi YJ, Suki WN, Truong LD. De novo minimal change disease associated with reversible post-transplant nephrotic syndrome. A report of five cases and review of literature. Clin Transplant. 2002;16:350-361. [PubMed] |
| 105. | Mochizuki Y, Iwata T, Nishikido M, Uramatsu T, Sakai H, Taguchi T. De novo minimal change disease after ABO-incompatible kidney transplantation. Clin Transplant. 2012;26 Suppl 24:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 106. | Madhan KK, Temple-Camp CR. Late de novo minimal change disease in a renal allograft. Saudi J Kidney Dis Transpl. 2009;20:266-269. [PubMed] |
| 107. | McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:2115-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 108. | Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 662] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 109. | Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 431] [Article Influence: 19.6] [Reference Citation Analysis (11)] |
| 110. | Mainra R, Mulay A, Bell R, Karpinski J, Hoar S, Knoll G, Robertson S, Wang D. Sirolimus use and de novo minimal change nephropathy following renal transplantation. Transplantation. 2005;80:1816. [PubMed] |
| 111. | Shabaka A, Pérez-Flores I, Cortés JA, Sánchez-Fructuoso AI. De Novo IgA Nephropathy in a Renal Allograft. Exp Clin Transplant. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 112. | Habib R, Antignac C, Hinglais N, Gagnadoux MF, Broyer M. Glomerular lesions in the transplanted kidney in children. Am J Kidney Dis. 1987;10:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 113. | Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int. 2003;63:2286-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 114. | Shimizu T, Tanabe K, Tokumoto T, Shimmura H, Koga S, Ishikawa N, Oshima T, Toma H, Yamaguchi Y. A case of rapid progressive glomerulonephritis with IgA deposits after renal transplantation. Clin Transplant. 2001;15 Suppl 5:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 115. | Nin M, Cordero R, Doufrechou L, Larre-Borges A, Coria V, Acosta N, Gadola L, Orihuela S, González F, Noboa O. Post-transplant Henoch-Schonlein purpura de novo: clinical-histological discordance. Nefrologia. 2012;32:850-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 116. | Everly MJ. An update on antibody reduction and rejection reversal following bortezomib use: a report of 52 cases across 10 centers. Clin Transpl. 2010;353-362. [PubMed] |
| 117. | Jordan SC, Reinsmoen N, Peng A, Lai CH, Cao K, Villicana R, Toyoda M, Kahwaji J, Vo AA. Advances in diagnosing and managing antibody-mediated rejection. Pediatr Nephrol. 2010;25:2035-2045; quiz 2045-2048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 118. | Kim MG, Kim YJ, Kwon HY, Park HC, Koo TY, Jeong JC, Jeon HJ, Han M, Ahn C, Yang J. Outcomes of combination therapy for chronic antibody-mediated rejection in renal transplantation. Nephrology (Carlton). 2013;18:820-826. [PubMed] |
| 119. | Poduval RD, Josephson MA, Javaid B. Treatment of de novo and recurrent membranous nephropathy in renal transplant patients. Semin Nephrol. 2003;23:392-399. [PubMed] |
| 120. | Faguer S, Kamar N, Guilbeaud-Frugier C, Fort M, Modesto A, Mari A, Ribes D, Cointault O, Lavayssière L, Guitard J. Rituximab therapy for acute humoral rejection after kidney transplantation. Transplantation. 2007;83:1277-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 121. | Kaposztas Z, Podder H, Mauiyyedi S, Illoh O, Kerman R, Reyes M, Pollard V, Kahan BD. Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant. 2009;23:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 122. | Ponticelli C, Glassock RJ. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol. 2010;5:2363-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 123. | Zimmermann H, Trappe RU. Therapeutic options in post-transplant lymphoproliferative disorders. Ther Adv Hematol. 2011;2:393-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 124. | Tydén G, Kumlien G, Fehrman I. Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003;76:730-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 216] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 125. | Kohei N, Hirai T, Omoto K, Ishida H, Tanabe K. Chronic antibody-mediated rejection is reduced by targeting B-cell immunity during an introductory period. Am J Transplant. 2012;12:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 126. | Loupy A, Suberbielle-Boissel C, Zuber J, Anglicheau D, Timsit MO, Martinez F, Thervet E, Bruneval P, Charron D, Hill GS. Combined posttransplant prophylactic IVIg/anti-CD 20/plasmapheresis in kidney recipients with preformed donor-specific antibodies: a pilot study. Transplantation. 2010;89:1403-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 127. | Becker YT, Becker BN, Pirsch JD, Sollinger HW. Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant. 2004;4:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 128. | Ranghino A, Segoloni GP, Lasaponara F, Biancone L. Lymphatic disorders after renal transplantation: new insights for an old complication. Clin Kidney J. 2015;8:615-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 129. | Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, Campistol JM, Racusen L, Polinsky MS, Goldberg-Alberts R. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation. 2009;87:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 130. | Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369:2416-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 131. | Brealey JK, Cassidy J. Electron-dense deposit in renal transplant patients on eculizumab may be drug-derived. Ultrastruct Pathol. 2016;40:2-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 132. | Hou J, Markowitz GS, Bomback AS, Appel GB, Herlitz LC, Barry Stokes M, D’Agati VD. Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int. 2014;85:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 133. | Radhakrishnan S, Lunn A, Kirschfink M, Thorner P, Hebert D, Langlois V, Pluthero F, Licht C. Eculizumab and refractory membranoproliferative glomerulonephritis. N Engl J Med. 2012;366:1165-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 134. | Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, Herlitz L, Stokes MB, Markowitz GS, D’Agati VD, Canetta PA. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 271] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 135. | Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V; French Study Group for aHUS/C3G. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 374] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 136. | Kerns E, Rozansky D, Troxell ML. Evolution of immunoglobulin deposition in C3-dominant membranoproliferative glomerulopathy. Pediatr Nephrol. 2013;28:2227-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Vivarelli M, Emma F. Treatment of C3 glomerulopathy with complement blockers. Semin Thromb Hemost. 2014;40:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 138. | Bomback AS. Eculizumab in the treatment of membranoproliferative glomerulonephritis. Nephron Clin Pract. 2014;128:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 139. | Payette A, Patey N, Dragon-Durey MA, Frémeaux-Bacchi V, Le Deist F, Lapeyraque AL. A case of C3 glomerulonephritis successfully treated with eculizumab. Pediatr Nephrol. 2015;30:1033-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 140. | Le Quintrec M, Lionet A, Kandel C, Bourdon F, Gnemmi V, Colombat M, Goujon JM, Frémeaux-Bacchi V, Fakhouri F. Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am J Kidney Dis. 2015;65:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 141. | Inman M, Prater G, Fatima H, Wallace E. Eculizumab-induced reversal of dialysis-dependent kidney failure from C3 glomerulonephritis. Clin Kidney J. 2015;8:445-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 142. | Nester CM, Brophy PD. Eculizumab in the treatment of atypical haemolytic uraemic syndrome and other complement-mediated renal diseases. Curr Opin Pediatr. 2013;25:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 143. | Kersnik Levart T, Ferluga D, Vizjak A, Mraz J, Kojc N. Severe active C3 glomerulonephritis triggered by immune complexes and inactivated after eculizumab therapy. Diagn Pathol. 2016;11:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 144. | Rodriguez-Osorio L, Ortiz A. Timing of eculizumab therapy for C3 glomerulonephritis. Clin Kidney J. 2015;8:449-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 145. | Hillmen P, Elebute M, Kelly R, Urbano-Ispizua A, Hill A, Rother RP, Khursigara G, Fu CL, Omine M, Browne P. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010;85:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 146. | Carneiro-Roza F, Medina-Pestana JO, Moscoso-Solorzano G, Franco M, Ozaki K, Mastroianni-Kirsztajn G. Initial response to immunosuppressive and renoprotective treatment in posttransplant glomerulonephritis. Transplant Proc. 2006;38:3491-3497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheungpasitporn W, Fourtounas C, Salvadori M S- Editor: Ji FF L- Editor: A E- Editor: Yan JL