Published online Dec 24, 2015. doi: 10.5500/wjt.v5.i4.292
Peer-review started: June 11, 2015
First decision: August 25, 2015
Revised: October 14, 2015
Accepted: November 10, 2015
Article in press: November 11, 2015
Published online: December 24, 2015
Processing time: 196 Days and 1.8 Hours
AIM: To describe the clinicopathologic features of concurrent polyomavirus nephropathy (PVN) and endarteritis due to rejection in renal allografts.
METHODS: We searched our electronic records database for cases with transplant kidney biopsies demonstrating features of both PVN and acute rejection (AR). PVN was defined by the presence of typical viral cytopathic effect on routine sections and positive polyomavirus SV40 large-T antigen immunohistochemistry. AR was identified by endarteritis (v1 by Banff criteria). All cases were subjected to chart review in order to determine clinical presentation, treatment course and outcomes. Outcomes were recorded with a length of follow-up of at least one year or time to nephrectomy.
RESULTS: Of 94 renal allograft recipients who developed PVN over an 11-year period at our institution, we identified 7 (7.4%) with viral cytopathic changes, SV40 large T antigen staining, and endarteritis in the same biopsy specimen, indicative of concurrent PVN and AR. Four arose after reduction of immunosuppression (IS) (for treatment of PVN in 3 and tuberculosis in 1), and 3 patients had no decrease of IS before developing simultaneous concurrent disease. Treatment consisted of reduced oral IS and leflunomide for PVN, and anti-rejection therapy. Three of 4 patients who developed endarteritis in the setting of reduced IS lost their grafts to rejection. All 3 patients with simultaneous PVN and endarteritis cleared viremia and were stable at 1 year of follow up. Patients with endarteritis and PVN arising in a background of reduced IS had more severe rejection and poorer outcome.
CONCLUSION: Concurrent PVN and endarteritis may be more frequent than is currently appreciated and may occur with or without prior reduction of IS.
Core tip: Here we report the clinical and pathologic features of 7 cases of concurrent polyomavirus nephropathy (PVN) and endarteritis identified out of 94 renal allograft recipients who developed PVN over an 11-year period (7.4%). These cases arose both in the setting of a prior reduction in immunosuppression (IS) and without such a change. Therefore, concurrent PVN and endarteritis appears more frequent than currently reported in the literature and may occur with or without prior reduction of IS.
- Citation: McGregor SM, Chon WJ, Kim L, Chang A, Meehan SM. Clinical and pathological features of kidney transplant patients with concurrent polyomavirus nephropathy and rejection-associated endarteritis. World J Transplant 2015; 5(4): 292-299
- URL: https://www.wjgnet.com/2220-3230/full/v5/i4/292.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i4.292
Many disease processes can limit the success of kidney transplantation, including cellular (T cell-mediated) rejection, antibody-mediated rejection (AMR), and polyoma virus nephropathy (PVN)[1,2]. The pathologic distinction between acute rejection (AR) and PVN may not be straightforward, as tubulointerstitial inflammation is a feature of both processes[1-9]. Intimal arteritis or endarteritis is a pathognomonic lesion of AR and is diagnostic of this disorder[10,11]. Classically considered a manifestation of T cell-mediated rejection, recent reports suggest that endarteritis can also be seen in association with donor specific antibodies, and may be indicative of mixed T cell-mediated and AMR[1,2,10-13]. Peritubular capillary C4d staining is a feature strongly suggestive of AMR, and like endarteritis, is not a feature of PVN[1-9,14-18]. Interstitial hemorrhage, plasma cells and neutrophils are more common in PVN than in AR but are not diagnostically specific[15]. Viral cytopathic changes are characteristic of PVN and identification of polyomavirus large T antigen (TAg) in renal tubular epithelial nuclei indicates active viral replication[13,16,19].
Therapeutic or compliance-related reduction of immunosuppression (IS) significantly increases the risk of development of renal allograft rejection[20,21]. Allograft rejection in these circumstances may be a manifestation of immune recovery from cessation of IS therapy. One study of PVN in patients with resolving viremia after months of lowered IS has described the development of interstitial nephritis indistinguishable from Banff type 1 AR in serial follow-up biopsies[3]. Another study has reported increased severity of tubulitis in serial biopsies with PVN treated by reduced IS, and AR with endarteritis has been described in a patient who underwent reduction of IS therapy for PVN[4,22]. Together these studies suggest that reduction of IS, a widely used treatment of PVN, facilitates immune recovery in graft recipients and may increase the risk of graft rejection[1,3,9,22]. We have encountered 7 renal allograft biopsies with concurrent PVN and endarteritis over an 11-year period. Four arose after reduction of oral dosage of calcineurin inhibitors and discontinuation of mycophenolate maintenance immunosuppressive agents, and 3 arose without any apparent prior change of IS therapy.
For the purpose of our study PVN was defined by the presence of typical viral cytopathic effect on routine sections stained by hematoxylin and eosin (H and E) and periodic acid-Schiff (PAS) methods and positive Polyomavirus SV40 large-T antigen (TAg) expression in tubular epithelial nuclei by standard immunohistochemistry (Ab-2, Oncogene Research Products, Cambridge, Massachusetts)[6-9,19]. AR was identified by intimal arteritis (v1 or more by Banff criteria) with or without staining of the peritubular capillaries for C4d by indirect immunofluorescence (clone 10-11, Biogenesis, Burlingame, California)[10,14,23]. All renal allograft biopsies were routinely stained for C4d in the period of study. Staining methods for tubular SV40 TAg expression were performed as described previously[4,15]. Tubules were considered TAg positive if 1 or more nuclei in a given profile was positive. A numeric score for quantification of TAg expression in tubular profiles was devised as follows: 0 = no detectable TAg, 1 = 1%-10%, 2 = 11%-20%, and so forth to a maximum score of 10 when 91%-100% of tubules had TAg staining. The average across all fields at 200 × magnification was converted to a percentage to reflect the extent of tubular infection. Two separate pathologists reviewed all cases; inter-rater agreement for TAg scoring was assessed using the intraclass correlation coefficient (ICC)[24]. Cases were also scored according to the Drachenberg system[25].
Chart review was performed in compliance with the University of Chicago Institutional Review Board (IRB14-0052). Details tabulated included serum creatinine, urinary and blood BK polyomavirus (BKPyV) viral load, IS regimen, and changes in management preceding and following the index biopsy with concurrent disease. Graft loss was defined as a prolonged increase of serum creatinine to > 5 mg/dL or allograft nephrectomy. Measurements of BKPyV polymerase chain reaction (PCR) in urine were performed monthly for the first three months and then every three months for the first year and yearly thereafter. Patients with high-grade viruria (> 25 × 106/uL) were then assessed for viremia. Quantitative PCR analysis for BKPyV was performed using the MagNA Pure LC DNA isolation kit (Roche Applied Science) and LightMix kit for the detection of polyomaviruses (Roche Applied Science). The BKPyV quantitative PCR assay is an institutionally developed multiplex assay that detects both BKPyV and JC polyomavirus (JCPyV) DNA. DNA extraction was performed using the MagNA Pure LC (Roche Diagnostic, Indianapolis, IN). A 219 bp fragment of the BKPyV and a 174 bp fragment of the JCPyV genome were amplified with specific primers and detected with probes labeled with LightCycler Red 705 (JCPyV) or with LightCycler Red 640 (BKPyV). An additional PCR product of 278 bp was formed from the internal positive control DNA (IPC) to verify the absence of amplification inhibitors in negative samples. The target is the gene for TAg. Primers and probes were purchased from TIB MOLBIOL, Berlin, Germany and were composed of the following: BKfor - acagcaaagcaggcaagg, BKrev - ggagtcctggtggagttcc, JCfor - ctgaggaatgcatgcagatcta, JCrev - ggaatcctggtggataca, Anchor - ttttgccatgaagaaatgtttgccagtagatga-FL, BKV LC 640 - aagcaacagcagattctcaacactcaaca-PH, JCV LC 705 - aaaacacaggatcccaacactctacccc-PH, IPC F - atgccacgtaagcgaaaca, IPC R - gcataaacgaagcagtcgagt, IPC SS - cacttcccgaataac-FL, and IPC 705 LC 705 - cggatatttttgatctgaccgaagcg-PH. Master mix was prepared using LightCycler FastStartPLUS DNA Master Hybridization Probes from Roche. The upper and lower limits of quantification of this PCR assay for BKPyV are 25 × 106 and 2.5 × 103 copies/mL, respectively.
Between 2002 and 2012, 907 kidney transplants were performed at our institution. Of these, 94 developed PVN (10.4%) and 111 developed intimal arteritis (12.2%). Within this population, we observed 7 biopsies from 7 patients with concurrent PVN and endarteritis (7.4% of PVN cases, 6.3% of cases with intimal arteritis). The incidence of concurrent PVN and endarteritis was 0.8% in the kidney transplant population during the study period (approximately 60 times the expected frequency due to chance). All 7 recipients were male with a mean age of 48.3 years (range: 15-68 years). In comparison, there was a male:female ratio of 2.2 among patients with PVN (51 male, 23 female) as a whole and of 2.3 among all patients with intimal arteritis (77 male, 34 female), indicating a preponderance of males in our study population. All patients received transplants from deceased donors, with an average donor age of 31.4 years (range: 17-57 years). Following the transplant the mean baseline serum creatinine was 1.4 mg/dL (range: 1.1-1.8 mg/dL), although 1 biopsy was performed in the early transplant period before a stable serum creatinine was established. One patient had a simultaneous pancreas transplant. No patients had pretransplant donor specific antibodies (DSA). Patient demographics are depicted in Table 1.
| Known prior change of IS (n = 4) | No known prior change of IS (n = 3) | All cases (n = 7) | |
| Age, years (range) | 55.5 (43-68) | 38.7 (15-58) | 48.3 (15-68) |
| Sex, n | |||
| Male | 4 | 3 | 7 |
| Female | 0 | 0 | 0 |
| Cause of end stage renal disease, n | |||
| DM ± HTN | 4 | 1 | 5 |
| PCKD | 0 | 1 | 1 |
| CON | 0 | 1 | 1 |
| No. of HLA matches, average (range) | |||
| Class I (HLA-A, HLA-B) | 0.25 (0-1) | 0 | 0.14 (0-1) |
| Class II (HLA-DR) | 0.50 (0-2) | 0.33 (0-1) | 0.43 (0-2) |
| Donor age, years (range) | 38.5 (17-57) | 22.0 (18-30) | 31.4 (17-57) |
| Cold ischemia time, hours (range) | 22.4 (15.0-37.5) | 17.8 (14.1-21.2) | 20.4 (14.1-37.5) |
| Delayed graft function, n | 1 | 0 | 1 |
| Baseline creatinine, mg/dL (range) | 1.4 (1.1-1.8) | 1.6 (1.2-1.8) | 1.4 (1.1-1.8) |
| Time of index biopsy, months after | 231 (135-317) | 507 (45-1293) | 349 (45-1293) |
| transplant (range) | |||
| Creatinine at index biopsy, mg/dL | 3.3 (1.8-6.2) | 1.9 (1.7-2.2) | 2.7 (1.7-6.2) |
| (range) | |||
| Donor-specific antibodies prior to | 0 | 0 | 0 |
| transplant |
Induction IS consisted of basiliximab in 6 patients and anti-thymocyte globulin (ATG) in 1 patient. Six patients were maintained on prednisone, tacrolimus and mycophenolate mofetil (MMF), and 1 patient was maintained on tacrolimus, sirolimus and prednisone (patient #7). Four patients had reduction of IS prior to the index biopsy, three for BK-related disease and one for pulmonary tuberculosis. For those with BK-related disease, two had biopsy-verified PVN, and one had BK viremia without confirmation of PVN on biopsy. MMF had been discontinued in 3 patients and tacrolimus dosage was reduced in 2 of the patients. Antiviral agents, leflunomide and cidofovir, were also given to these 3 patients. One patient also received 3 doses of pulsed steroids and 2 doses of intravenous immunoglobulin (IVIG) for pancreatic rejection that occurred 1 mo prior to the index kidney biopsy. Three patients had no known change of IS prior to the index biopsy. A detailed summary of IS for each patient is depicted in Table 2.
| Baseline Cr | Serum Cr at index biopsy (mg/dL) | BKV DNA copies/mL at index biopsy | Maintenance IS | Change of IS after diagnosis of PVN | Antirejection therapy | Antiviral | Creatinine trend (mo; mg/dL) | BK viremia trend (mo; × 103 copies/mL) | ||||||||||||
| Serum (× 103) | Urine (× 106) | Disc. MMF | Reduced Tacrolimus | Steroids | IVIG | Rapamycin | Thymoglobulin | Arava | Cidofovir | 1 | 3 | 6 | 12 | 3 | 12 | Time to clear | ||||
| 1 | 1.2 | 3.4 | 33002 | > 13002 | MTP | Yes1 | Yes1 | Yes | No | No | No | Yes | Yes | 1 | 2 | 3 | 3.933 | Pos4 | Pos4 | Not cleared4 |
| 2 | 1.1 | 1.8 | 0 | 1.3 | MTP | Yes1 | Yes1 | No | Yes | No | No | No | Yes | 1 | 3 | 2 | 2 | 0 | 0 | NA |
| 3 | 1.3 | 6.2 | ND | ND | MTP | Yes1 | No | ND | ND | ND | ND | ND | ND | 4 | ND | 3 | GL | ND | ND | ND |
| 4 | 1.8 | 1.9 | 0 | ND | MTP | Yes1 | No | Yes | No | No | Yes | Yes | No | 2 | 3 | 4 | GL | 0 | 0 | NA |
| 5 | 1.8 | 2.2 | 2102 | > 13002 | MTP | Yes | No | No | Yes | No | No | Yes | No | 2 | 2 | 2 | 2 | < 2.5 | 0 | 9 |
| 6 | 1.7 | 1.7 | 7 | > 25 | MTP | Yes | No | Yes | No | No | Yes | Yes | No | 2 | 2 | 2 | 1 | 0 | 0 | 2 |
| 7 | 1.2 | 1.7 | 1342 | 17002 | STP | NA | Yes | No | No | No | No | Yes | No | 2 | 2 | 2 | 1 | 0.6 | 0 | 9 |
The mean serum creatinine was 2.7 mg/dL (range: 1.7-6.2 mg/dL) at the time of the index biopsy overall. The average time elapsed from transplantation to the index biopsy was 11.6 mo (range: 1.5-43.1 mo). The average time from reduction of IS to the index biopsy was 116 d (range: 21-236 d) for the patients who underwent reduced IS. Of note, patients with a reduction in IS prior to the index biopsy had higher average creatinine (3.3 mg/dL, range: 1.8-6.2 mg/dL) than those without (1.9 mg/dL, range: 1.7-2.2 mg/dL), had a higher frequency of diabetes mellitus (4/4 compared to 1/3) and higher donor age (38.5 years compared to 22.0 years). The clinical presentations are depicted in Table 2.
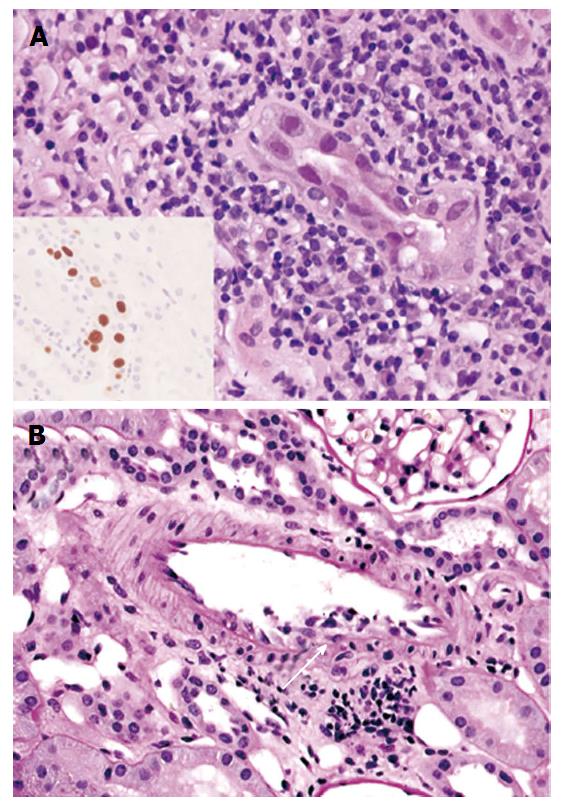
Biopsy specimens consisted of cortex only in 3 cases (43%) and both cortex and medulla in 4 (57%) cases. Index biopsies contained 23.2 glomeruli on average (range: 9-67). The average global glomerulosclerosis was 13.5% (range: 0%-70%). All cases demonstrated viral cytopathic effect and TAg expression by immunohistochemistry (Figure 1A). The average extent of TAg expression was 5.7% (range: 0.7%-11.5%, ICC = 0.8789). Endarteritis, with v1 lesions by Banff criteria, was evident in all 7 cases (Figure 1B). Three cases in the group with reduced IS also had C4d staining of the peritubular capillaries, diffuse in 2 and focal in 1. One of these patients had negative assays for DSA around the time of the index biopsy, and 2 had no DSA data. Peritubular capillaritis was focal (Banff ptc score 0) and one had glomerulitis.
Two patients who had undergone IS reduction had prior biopsies showing PVN. Three of 4 patients who had undergone IS reduction developed graft loss. Index biopsies from allografts that subsequently underwent graft loss had diffuse tubulointerstitial inflammatory infiltrates (i + t score = 6) and abundant interstitial plasma cell infiltrates. Two of three had peritubular capillary C4d staining. A breakdown of the pathologic indices is given in Table 3.
| Patient ID | AR type | C4d | % SV40-T antigen + tubules | DS | I | T | CI | CT | V | HMX | G | MM | CV | AH | Plasma cells1 |
| 1 | 2A | - | 8.4 | B3 | 3 | 3 | 1 | 3 | 1 | 1 | 0 | 1 | 2 | 2 | Yes |
| 2 | 2A | + | 0.7 | B2 | 3 | 1 | 3 | 3 | 1 | 3 | 0 | 0 | 1 | 0 | No |
| 3 | 2A | + | 8 | B2 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | Yes |
| 4 | 2A | + | 0.9 | B3 | 3 | 3 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | Yes |
| 5 | 2A | - | 11.5 | B3 | 1 | 3 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | No |
| 6 | 2A | - | 1.2 | B2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | No |
| 7 | 2A | - | 11.3 | B3 | 3 | 2 | 3 | 3 | 1 | 0 | 1 | 0 | 1 | 1 | No |
Two of 4 patients who had undergone reduced IS had follow up biopsies demonstrating PVN without AR at 20 d, and tubulointerstitial rejection (Banff type IA) at 35 d. Endarteritis was absent. All 3 allograft nephrectomy specimens had lesions of severe transmural arteritis (AR type III) with focal evidence of PVN (SV40-T antigen expression in collecting ducts) in 1. One of the 3 patients with simultaneous PVN and endarteritis had a follow up biopsy 13 d later demonstrating PVN with no apparent AR.
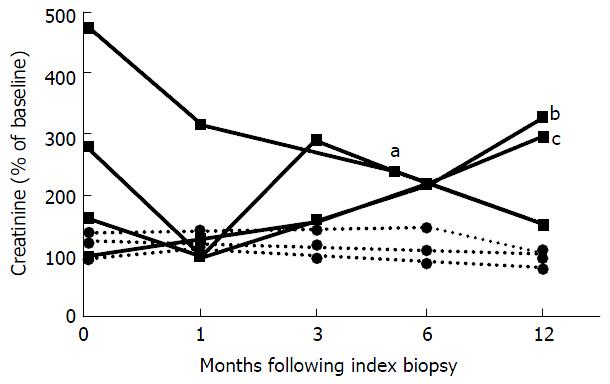
Reduced oral maintenance IS was continued after the index biopsy for all patients with prior PVN or viremia (n = 3). Two of 3 patients received pulsed steroids either alone (n = 1) or with ATG (n = 1); another received IVIG without steroids. The recipient of IVIG had a stable serum creatinine at 155% of the baseline serum creatinine value at 12 mo follow up. The remaining 2 patients developed end-stage allograft failure due to rejection at 144 and 483 d after the index biopsy (Figure 2). One patient had persistent viremia at 3 and 12 mo after the index biopsy, 1 patient had resolution of viremia prior to the index biopsy, and 1 patient never had detectable viremia. No data on viral copy numbers were available for 1 patient with pulmonary tuberculosis and the patient underwent allograft nephrectomy at 336 d after the index biopsy (Table 2).
Patients with spontaneous PVN and AR without a previous change of IS received leflunomide for PVN, pulsed steroids and ATG (n = 1), IVIG (n = 1) or no additional therapy (n = 1) for AR. MMF was discontinued in 2 and calcineurin inhibitor dosage reduced in 1. Two cleared viremia at 9 mo and 1 at 2 mo of follow-up. All 3 had stable serum creatinine at 80%, 100% and 108% of the baseline value at 12 mo after the index biopsy (Figure 2). None had detectable viremia at 1 year of follow-up.
This study describes the clinical and pathologic findings in a group of patients with compelling evidence of concurrent viral infection and rejection, as determined by polyomavirus cytopathic changes and TAg expression combined with endarteritis in the same biopsy. In 4 patients lesions concurred after therapeutic reduction of oral dosage of calcineurin inhibitors and discontinuation of mycophenolate maintenance IS for treatment of PV infection or pulmonary tuberculosis; three occurred without any apparent change of IS therapy. These cases comprised 7.4% of allografts with PVN presenting over an 11-year period, and 0.8% of all kidney transplants over the same time period. Concurrent PVN and rejection is probably uncommon, and while recommendations on treatment of these disorders have been made, the available literature on PVN and endarteritis consists primarily of case reports[4,9,16,26,27]. Hirsch et al[28] described simultaneous tubulointerstitial rejection (Banff type 1) and PVN in 4 of 78 transplant patients (5.1%). However, these 4 patients had received antirejection therapy before the onset of PVN, in contrast to our patients, and none had endarteritis when PVN was identified on biopsy[3,9,15,28,29]. In our study, we have included only cases with endarteritis, a defining feature of rejection, from 94 patients with biopsy-proven PVN out of over 900 renal transplants performed in the study period. We realize that a substantial proportion of patients with PVN may also have interstitial rejection, but given the difficulty of distinguishing tubulointerstitial rejection from viral tubulointerstitial nephritis and the lack of agreement on criteria for doing so, it is not possible to make an accurate assessment of the frequency of concurrent interstitial rejection and PVN[3,9,15,28,29].
It is of interest that there were differences between PVN and AR arising with lowered IS and those arising spontaneously without change of IS regimens. PVN and AR in the setting of lowered IS was associated with higher serum creatinine levels at time of the index biopsy, and higher Banff interstitial inflammation and tubulitis scores, with diffuse interstitial mononuclear inflammation, severe tubulitis and plasma cell infiltrates. Most had peritubular capillary C4d staining suggestive of AMR, however, assays for DSA were negative or unavailable and the diagnosis of AMR was not clearly established. Nonetheless, these findings identified a more severe rejection reaction compared to the group with no IS changes. Rejection, a likely consequence of immune recovery from reduced IS, demonstrated more severe patterns of tubulointerstitial and microvascular inflammation in the index biopsies obtained from grafts eventually lost to rejection. Plasma cells were prominent in cases with reduced IS but not in those with spontaneous concurrent diseases. Plasma cell infiltrates have been associated with poorer outcomes in the setting of rejection, but are also abundant in PVN, making determination of whether these are part of a rejection or interstitial nephritis, or both, difficult to resolve[15,16,30,31].
Prior changes of immunosuppressive therapy were not clinically apparent in 3 patients and the concurrence of PVN and AR in this setting of stable, reduced immune function seems paradoxical. PVN and endarteritis were identified at the same instance and hence determination of whether AR was preceded by PVN is difficult. However, lesions of endarteritis were not accompanied by foam cells, neointima or fibrosis, and were therefore interpretable as lesions of recent onset. Sites of PV infection were accompanied by interstitial fibrosis and tubular atrophy indicative of a chronic inflammatory lesion that we strongly suspect predated the lesions of rejection. It is thus possible that these cases are also examples of rejection superimposed on PV infection. Renal dysfunction and rejection was milder and each had a good outcome. Two patients were treated with antirejection therapy that may have helped stabilize graft function. One was treated by reduction of maintenance IS without antirejection therapy and had graft dysfunction for more than 6 mo after diagnosis, with eventual return of creatinine levels to baseline and clearance of viremia similar to the patients described by Menter et al[3], even though their patients only had tubulointerstitial and not arterial inflammation. Our three patients had stable graft function, at < 110% of baseline creatinine, with clearance of viremia by 9 mo of follow up, and no evidence of rejection in follow-up biopsies. Although trends from this small and somewhat heterogeneous group of patients must be interpreted with caution, our observations suggest that renal allografts with PVN and endarteritis arising with reduced IS may potentially have more severe rejection and be at greater risk of allograft loss from rejection.
This small series clearly shows that AR may arise during the course of PVN treated by reduced IS, and perhaps surprisingly, that these lesions may present simultaneously without such a change in treatment. Concurrent PVN and AR also appears to be more frequent than currently appreciated in the literature, as these findings were evident in 7.4% of allografts in patients with PVN and 0.8% of all renal allografts performed in the study period.
Kidney transplants are at constant risk of acute rejection (AR) for which recipients receive immunosuppression (IS). IS increases the risk of infection. Here the authors report the concurrence of both polyomavirus infection and rejection-associated endarteritis in renal allografts and describe the clinical and pathologic features of these lesions.
Both polyomavirus nephropathy (PVN) and AR are characterized by tubulointerstitial inflammation and distinction of these processes, although essential, is difficult. Endarteritis is pathognomonic of AR and its identification in the context of PVN indicates that both AR and viral infection are present in the allograft.
Concurrent AR and polyomavirus infection is not well characterized in renal allografts. This biopsy series has diagnostic features of both processes allowing observation of the clinical course of allografts with these lesions.
Concurrent polyomavirus infection and endarteritis arose in 7.4% of our patients with PVN, suggesting a higher frequency than is currently appreciated. The authors also noted that when endarteritis arose after reduction of IS, graft loss from rejection occurred in 3 of 4 patients. Three of 3 allograft recipients with simultaneous PVN and endarteritis had stable function at 1 year follow up.
Endarteritis is arterial intimal mononuclear inflammation found specifically in acute rejection. Polyomavirus nephropathy is viral infection of the allograft manifested by cytopathic changes in tubular epithelium, detectable large T antigen by immunohistochemistry, viremia and viruria.
The manuscript by McGregor et al studies the concurrency between polyomavirus nephropathy and endarteritis in 94 kidney transplant patients. They found 7 patients (all male) that developed both PVN and endarteritis. In four of them endarteritis arose after reduction of immunosuppression, and three of them lost their grafts. Patients that got PVN and endarteritis after lowered immunosuppression had high serum creatinine levels and Banff interstitial inflammation and tubulitis scores.
| 1. | Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation. 2009;87:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (4)] |
| 3. | Menter T, Mayr M, Schaub S, Mihatsch MJ, Hirsch HH, Hopfer H. Pathology of resolving polyomavirus-associated nephropathy. Am J Transplant. 2013;13:1474-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | McGilvray ID, Lajoie G, Humar A, Cattral MS. Polyomavirus infection and acute vascular rejection in a kidney allograft: coincidence or mimicry? Am J Transplant. 2003;3:501-504. [PubMed] |
| 5. | Ramos E, Drachenberg CB, Papadimitriou JC, Hamze O, Fink JC, Klassen DK, Drachenberg RC, Wiland A, Wali R, Cangro CB. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002;13:2145-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 342] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Nickeleit V, Hirsch HH, Binet IF, Gudat F, Prince O, Dalquen P, Thiel G, Mihatsch MJ. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J Am Soc Nephrol. 1999;10:1080-1089. [PubMed] |
| 7. | Randhawa PS, Finkelstein S, Scantlebury V, Shapiro R, Vivas C, Jordan M, Picken MM, Demetris AJ. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67:103-109. [PubMed] |
| 8. | Howell DN, Smith SR, Butterly DW, Klassen PS, Krigman HR, Burchette JL, Miller SE. Diagnosis and management of BK polyomavirus interstitial nephritis in renal transplant recipients. Transplantation. 1999;68:1279-1288. [PubMed] |
| 9. | Nickeleit V, Mihatsch MJ. Polyomavirus allograft nephropathy and concurrent acute rejection: a diagnostic and therapeutic challenge. Am J Transplant. 2004;4:838-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Nickeleit V, Vamvakas EC, Pascual M, Poletti BJ, Colvin RB. The prognostic significance of specific arterial lesions in acute renal allograft rejection. J Am Soc Nephrol. 1998;9:1301-1308. [PubMed] |
| 11. | Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2522] [Cited by in RCA: 2514] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 12. | Haas M. An updated Banff schema for diagnosis of antibody-mediated rejection in renal allografts. Curr Opin Organ Transplant. 2014;19:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Broecker V, Mengel M. The significance of histological diagnosis in renal allograft biopsies in 2014. Transpl Int. 2015;28:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, Cosimi AB, Colvin RB. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208-2214. [PubMed] |
| 15. | Meehan SM, Kadambi PV, Manaligod JR, Williams JW, Josephson MA, Javaid B. Polyoma virus infection of renal allografts: relationships of the distribution of viral infection, tubulointerstitial inflammation, and fibrosis suggesting viral interstitial nephritis in untreated disease. Hum Pathol. 2005;36:1256-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Nickeleit V, Steiger J, Mihatsch MJ. BK Virus Infection after Kidney Transplantation. Graft. 2002;5:S46-S57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 17. | Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708-714. [PubMed] |
| 18. | Nickeleit V, Zeiler M, Gudat F, Thiel G, Mihatsch MJ. Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol. 2002;13:242-251. [PubMed] |
| 19. | Ahuja M, Cohen EP, Dayer AM, Kampalath B, Chang CC, Bresnahan BA, Hariharan S. Polyoma virus infection after renal transplantation. Use of immunostaining as a guide to diagnosis. Transplantation. 2001;71:896-899. [PubMed] |
| 20. | Vlaminck H, Maes B, Evers G, Verbeke G, Lerut E, Van Damme B, Vanrenterghem Y. Prospective study on late consequences of subclinical non-compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant. 2004;4:1509-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 170] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Lerut E, Kuypers DR, Verbeken E, Cleutjens J, Vlaminck H, Vanrenterghem Y, Van Damme B. Acute rejection in non-compliant renal allograft recipients: a distinct morphology. Clin Transplant. 2007;21:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Celik B, Shapiro R, Vats A, Randhawa PS. Polyomavirus allograft nephropathy: sequential assessment of histologic viral load, tubulitis, and graft function following changes in immunosuppression. Am J Transplant. 2003;3:1378-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1126] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 24. | Banerjee M, Capozzoli M, McSweeney L, Sinha D. Beyond kappa: A review of interrater agreement measures. Can J Stat [Internet]. 1999;27:3-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 551] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 25. | Drachenberg CB, Papadimitriou JC, Hirsch HH, Wali R, Crowder C, Nogueira J, Cangro CB, Mendley S, Mian A, Ramos E. Histological patterns of polyomavirus nephropathy: correlation with graft outcome and viral load. Am J Transplant. 2004;4:2082-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 26. | Nickeleit V, Hirsch HH, Zeiler M, Gudat F, Prince O, Thiel G, Mihatsch MJ. BK-virus nephropathy in renal transplants-tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant. 2000;15:324-332. [PubMed] |
| 27. | Mayr M, Nickeleit V, Hirsch HH, Dickenmann M, Mihatsch MJ, Steiger J. Polyomavirus BK nephropathy in a kidney transplant recipient: critical issues of diagnosis and management. Am J Kidney Dis. 2001;38:E13. [PubMed] |
| 28. | Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 936] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 29. | Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 417] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 30. | Chang A, Moore JM, Cowan ML, Josephson MA, Chon WJ, Sciammas R, Du Z, Marino SR, Meehan SM, Millis M. Plasma cell densities and glomerular filtration rates predict renal allograft outcomes following acute rejection. Transpl Int. 2012;25:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Kemény E, Hirsch HH, Eller J, Dürmüller U, Hopfer H, Mihatsch MJ. Plasma cell infiltrates in polyomavirus nephropathy. Transpl Int. 2010;23:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Du C, Moens U, Randhawa P
S- Editor: Ji FF L- Editor: A E- Editor: Li D