Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.111031
Revised: July 14, 2025
Accepted: October 30, 2025
Published online: December 18, 2025
Processing time: 150 Days and 21.2 Hours
Living donor kidney transplantation (LDKT) is considered the gold standard for treating end-stage kidney disease. Previous studies have highlighted the impact of donor and recipient demographics in influencing post-transplant outcomes. We believe that patient and graft outcomes in a tertiary university hospital setting will have no difference between pairs of standard criteria vs pairs of extended criteria (EC) donors and recipients in LDKT.
To investigate the outcomes of allocating EC donation (ECD) kidneys to EC recipients (ECR) in LDKT and compare them to standard and mixed standard and EC pair counterparts.
We collected data from adult LDKTs conducted between April 2017 and April 2022. Donor-recipient pairs were grouped based on criteria as follows: (1) Group 1: Standard criteria donor (SCD) to standard criteria recipient (SCR); (2) Group 2: SCD to ECR; (3) Group 3: ECD to SCR; and (4) Group 4: ECD to ECR.
A total of 149 living donor transplants were analysed over a 5-year period. Graft survival, patient survival, and graft function were similar across all four groups. The incidence of common postoperative complications was as follows: (1) Perioperative bleeding (5.6%); (2) Surgical site infection (6.8%); and (3) Incisional hernia (7.4%). No statistically significant differences were found in patient or graft outcomes amongst the four groups. Multivariate analysis showed that group 4 recipients might experience inferior 5-year graft function (β = -11.8, P = 0.037) when compared with group 1.
In LDKT, long-term patient and graft outcomes are comparable amongst different combinations of standard vs EC donors and recipients. These findings show the primary potential of living donor ECD to ECR kidney transplan
Core Tip: This retrospective cohort study aims to showcase the equivalent patient and graft outcomes following living donor kidney transplantation when pairs of standard criteria donors and recipients are compared to their extended criteria counterparts. This could serve as an important extension to the living donor pool in kidney transplantation, allowing easier access to the golden standard in treating end-stage kidney disease.
- Citation: Anastasopoulos NA, Charif R, Loucaidou M, Herbert PE, Muthusamy ASRE, Dor FJMF, Papalois VE. Outcomes of living donor kidney transplantation from extended criteria donors to extended criteria recipients: A retrospective cohort study. World J Transplant 2025; 15(4): 111031
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/111031.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.111031
Global prevalence and incidence of end-stage kidney disease (ESKD) are rising, and evidence shows that living donor kidney transplantation (LDKT) remains the best option for prolonging survival and improving quality of life[1-4]. A recent national study in the United Kingdom highlights the superior outcomes of LDKT[5], confirming its status as the gold standard in kidney replacement therapy (KRT) due to its low incidence of delayed graft function (DGF) and the highest long-term survival rates for both recipients and grafts[3,4,6], with minimal donor morbidity[7,8]. Interestingly, even more favourable outcomes in terms of patient and graft survival as well as quality of life have been reported when pre-emptive LDKT is undertaken[9,10].
The increasing need for a larger organ donor pool is highlighted in the 2022-2023 United Kingdom statistics, which show that of the 5655 patients on the transplant waitlist, 3272 received a kidney transplantation. Of these, 71.8% came from deceased donors and 45.9% from donation after circulatory death[11]. These data underscore that, even in a country with relatively high transplant rates, the number of kidney allografts is insufficient to meet the demands of the growing waitlist[11].
In response to this demand, extended criteria donation (ECD) has emerged as a potential strategy to mitigate the organ shortage. First explored in deceased organ donation, ECD has been supported by various technologies aimed at increasing organ availability and improving both short-term and long-term transplant outcomes[12]. A recent United Kingdom national analysis shows that deceased donor ECD kidneys offer improved survival and lower morbidity rates compared to remaining on the waitlist and provide similar outcomes to standard criteria donor (SCD) kidneys[5].
This well-established practice in deceased kidney donation needs to be explored in depth in LDKT programs. Early literature, from over a decade ago, attempts to define living kidney ECD, albeit with a degree of heterogeneity in per
If proven beneficial, the use of EC living donor kidneys could help further bridge the gap between organ supply and demand while maintaining outcomes comparable to those of standard criteria LDKT. Previous studies have begun to explore this potential[15,16]. Recent meta-analyses and systematic reviews that have also identified risk factors influencing outcomes in LDKT[17-19], provide guidance for decision-making in donor and recipient selection for EC LDKT. An even bolder concept, that has yet to be explored, is how allocation of ECD kidneys can increase accessibility to LDKT for EC recipients (ECR) as well as the potential impact this may have on long-term patient and graft outcomes.
In this retrospective, single centre study, we aim to compare outcomes and investigate the safety and long-term results of LDKT using ECD kidneys allocated to ECR, in comparison to SCD kidneys transplanted into standard criteria reci
This retrospective cohort study analysed 149 LDKTs performed at Imperial College Renal and Transplant Centre (ICRTC) between April 2017 and April 2022. All transplants included a minimum of a one-year follow-up analysis. All data collected were anonymised and patients consented to their medical data being used for research as part of the surgical procedure consent. Patients were stratified into three categories based on follow-up duration: (1) One-year; (2) Three-year; and (3) Five-year. Data were collected for both donors and recipients.
Recipient data included age, cause of ESKD, pre-transplant diabetes mellitus (DM), hypertension (HTN), body mass index (BMI), presence of atherosclerotic disease, defined as one of the following: (1) Myocardial infarction or pre-emptive coronary intervention; (2) Cerebrovascular accident; and (3) Chronic peripheral arteriopathy as well as pre-emptive tran
For donors, data included age, baseline DM, HTN, presence of atherosclerotic disease, BMI, modality of donor nephrectomy, and human leukocyte antigen mismatch category, adjusted as per the National Health Service Blood and Transplant guidelines[20] and genetic relationship to the recipient, defined as living related or living unrelated (LURD) donation.
We also documented cold ischaemic time, defined as time from the kidney perfused with hypothermic perfusion solution to the time out of ice, and vascular anatomy of the kidney allograft. Vascular anatomy was characterised as single (one renal artery and one renal vein), multiple (more than one renal artery or vein), or reconstructed (when vas
EC donors or recipients were defined as individuals who fulfilled at least one of the following criteria: (1) Age older than 60 years; (2) History of HTN; (3) History of DM; (4) History of atherosclerotic disease (including myocardial infarction or pre-emptive coronary intervention, cerebrovascular accident, chronic peripheral arteriopathy); and (5) BMI above 30 kg/m2[21].
The standard steroid-sparing immunosuppressive regimen included Alemtuzumab as induction, unless contra-indicated. Maintenance therapy consisted of tacrolimus, short-term steroids (7 days) and optionally mycophenolate. Mycophenolate was included when calculated reaction frequency exceeded 85% or for specific donor-recipient scenarios (male donor donating to female recipient that has had or will have children with this male donor, or children donating to mother). If Alemtuzumab was contra-indicated, Basiliximab was used as alternative induction immunosuppressive agent, with mycophenolate included in the maintenance regimen as well.
Peri-operative bleeding was defined as significant haemorrhage requiring graft re-exploration and haematoma eva
Subgroup analyses were performed based on donor and recipient criteria: (1) Group 1: SCD to SCR; (2) Group 2: SCD to ECR; (3) Group 3: ECD to SCR; and (4) Group 4: ECD to ECR. Estimated glomerular filtration rate (eGFR) was cal
Statistical analyses were performed using Jamovi 2.3.28, a biostatistics free software package. We considered a P value below 0.05 to be statistically significant. For univariate categorical analysis, χ2 and Fisher’s exact tests were used. Numerical variables were initially assessed for normality using Shapiro-Wilk test and accordingly analysed using a one-way analysis of variance (ANOVA) analysis for data that were following normal distribution, whereas when data would not follow normal distribution, the Kruskal Wallis test was used. Correlation matrices with Pearson’s r were generated for exploratory analysis. Multivariate regression was used to identify risk factors for subgroup differences in survival or function, utilising linear and logistic regression where appropriate. There were 12 missing entries in the donor BMI and 8 recipient BMI data columns, without any impact on subgroup classification and analysis. There were no other missing data in the baseline donor and recipient characteristics as well as recipient outcomes. Missing donor follow-up data are reported and not included in the analysis.
This retrospective cohort study analysed 149 pairs of LDKT performed at the ICRTC between April 2017 and April 2022. Table 1 outlines the baseline donor and recipient characteristics of this cohort.
| Mean | SD | Median | Interquartile range | Frequency | |
| Recipient age | 47.3 | 13.7 | 48 | 19 | |
| Recipient HTN | 4 (2.7) | ||||
| Recipient atheroma | 12 (8.1) | ||||
| Recipient DM | 21 (14.1) | ||||
| Recipient BMI (kg/m2) | 27.1 | 2.95 | 27 | 4 | |
| Donor age | 47.7 | 12.3 | 48 | 19 | |
| Donor HTN | 1 (0.7) | ||||
| Donor atheroma | 2 (1.3) | ||||
| Donor DM | 9 (6) | ||||
| Donor BMI (kg/m2) | 27.7 | 4.57 | 27 | 4.57 | |
| Donor baseline estimated glomerular filtration rate | 90 | 0 |
The primary cause of ESKD among recipients: (1) HTN: 4 (2.7%); (2) Congenital disease: 17 (11.4%); (3) Glomerulonephritis and/or vasculitis: 61 (40.9%); (4) Polycystic kidney disease: 17 (11.4%); (5) DM: 15 (10.1%); and (6) Unknown aetiology: 11 (7.4%). A total of 65 (43.6%) of LDKTs were pre-emptive, whereas haemodialysis was the main modality of KRT in our cohort (44.3%), with only 12.1% of our recipients being on peritoneal dialysis pre-transplant. In terms of pre-emptive transplantation, 27 (41.6%) transplants from ECD were carried out pre-emptively. The distribution of pre-emptive transplantation across our subgroups (1-4) was as follows: (1) Group 1: 25 (38.5%); (2) Group 2: 13 (20%); (3) Group 3: 16 (24.6%); and (4) Group 4: 11 (17%).
Donor-recipient genetic relationships were evenly distributed, with 51% of the performed transplants being from LURD. Table 2 presents perioperative characteristics and immunosuppression regimens.
| Maintenance immunosuppression | |
| Tacrolimus | 78 (53) |
| Tacrolimus + mycophenolate | 32 (21.4) |
| Tacrolimus + prednisolone + mycophenolate | 25 (16.8) |
| Tacrolimus + prednisolone | 12 (8.1) |
| Mismatch category (National Health Service Blood and Transplant) | |
| 1 | 17 (11.4) |
| 2 | 18 (12.1) |
| 3 | 67 (45) |
| 4 | 47 (31.5) |
| Cold ischaemia time (minutes) (mean ± SD) | 251 ± 104 |
| Vascular anatomy | |
| Single | 118 (79.2) |
| Multiple | 24 (16.1) |
| Reconstruction | 7 (4.7) |
| Modality of donor nephrectomy | |
| Mini open | 24 (16.1) |
| Hand-assisted retroperitoneoscopic | 42 (28.1) |
| Laparoscopic (including, hand-assisted) | 83 (55.7) |
In this cohort of 149 pairs of LDKT, 5-year follow-up data are available for 116 pairs; 3-year and 1-year follow-up data are available for all 149 pairs. One-year, three-year and five-year recipient eGFR post-transplant follow normal distribution with a Shapiro-Wilk p of 0.076, 0.105 and 0.083. Table 3 summarises overall donor and recipient outcomes.
| Classification | Frequency |
| Length of stay | 7 (2) |
| Delayed graft function | 1 (0.67) |
| Perioperative bleeding | 7 (5.6) |
| Surgical site infection | 6 (4) |
| Overall incisional hernia | 11 (7.4) |
| Death with a functioning graft | 3 (2) |
| Return to dialysis | 9 (6) |
| Time to return to dialysis | 43.1 ± 12.1 |
| 1-year recipient survival | 99.3 |
| 3-year recipient survival | 96.4 |
| 5-year recipient survival | 94.6 |
| 1-year graft survival | 99.3 |
| 3-year graft survival | 97.2 |
| 5-year graft survival | 90.4 |
| 1-year recipient eGFR | 59.2 ± 16.3 |
| 3-year recipient eGFR | 54.6 ± 19.9 |
| 5-year recipient eGFR | 52.1 ± 22.5 |
| 5-year rejection | 19 (17) |
| 1-year donor eGFR | 67 (21.5) |
| 3-year donor eGFR | 68 (21) |
| 5-year donor eGFR | 70 (21) |
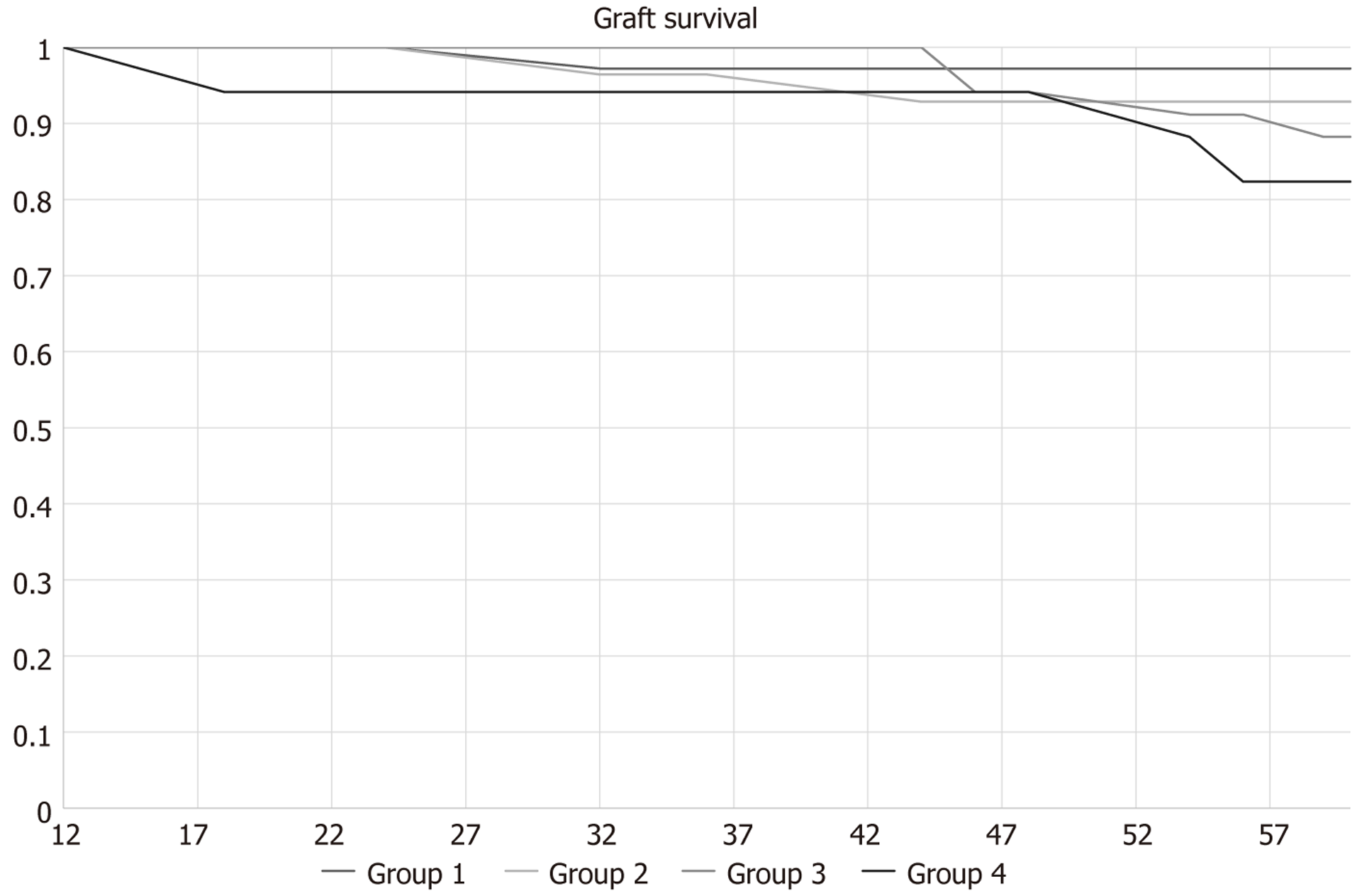
Regarding recipient mortality, 5 deaths were recorded, 3 secondaries to coronavirus disease (COVID) infection, and the remaining two from cardiovascular aetiology. Three of these patients died with a functioning graft, whereas the other two had returned to dialysis. An additional 7 patients experienced return to dialysis, totalling 9 cases of returning to dialysis in this cohort, as shown in Figure 1.
Donor follow-up rates were 82.5% for the first year, 73% for the third year and 65% for the fifth year, with most donors were lost to follow-up due to relocation. Regarding overall donor outcomes, there was no donor mortality in the five years of follow up. Post-donation eGFR values at one-year, three-year and five-year did not follow normal distribution (Shapiro-Wilk P < 0.001) as detailed in Table 3.
Based on our definitions of EC, the cohort was stratified into 4 subgroups with 54, 33, 40 and 22 donor-recipient pairs respectively.
No differences were shown in patient and graft survival as well as graft function in our subgroup analysis. Table 4 demonstrates donor and recipient outcomes of our subgroup analysis for recipient and graft survival as well as graft and donor kidney function, without any statistically significant differences in any category. One ECD was listed for a kidney transplant 5 years post donation due to an episode of acute kidney injury in intensive care.
| Group 1 | Group 2 | Group 3 | Group 4 | P value | |
| Delayed graft function | 0 | 1 | 0 | 0 | 0.398 |
| Perioperative bleeding | 2 | 2 | 1 | 2 | 0.615 |
| Surgical site infection | 3 | 1 | 1 | 1 | 0.941 |
| Incisional hernia | 0 | 2 | 3 | 2 | 0.227 |
| 1-year recipient survival | 100 | 100 | 97.5 | 100 | 0.433 |
| 1-year graft survival | 100 | 100 | 97.5 | 100 | 0.433 |
| 1-year recipient eGFR | 63.9 ± 15.3 | 55.9 ± 16.5 | 56.1 ± 14.6 | 58 ± 19.5 | 0.053 |
| 1-year donor eGFR | 67 (15.2) | 69 (14) | 67.6 (15.7) | 69.8 (14.2) | 0.834 |
| 3-year recipient survival | 98.1 | 97 | 97.4 | 89.5 | 0.451 |
| 3-year graft survival | 98.1 | 97 | 90 | 90.9 | 0.383 |
| 3-year recipient eGFR | 60 ± 19.3 | 52.3 ± 20.2 | 52 ± 16.7 | 51 ± 22.6 | 0.136 |
| 3-year donor eGFR | 71.2 (15) | 71.8 (15) | 70 (14.7) | 67.6 (15.1) | 0.801 |
| 5-year recipient survival | 97 | 92.8 | 85.3 | 82.4 | 0.170 |
| 5-year graft survival | 97 | 96 | 86.7 | 82.3 | 0.143 |
| 5-year recipient eGFR | 57.8 ± 21.6 | 51.7 ± 21.5 | 50.4 ± 22.3 | 45 ± 25.5 | 0.270 |
| 5-year rejection | 9 (26.5) | 3 (10.7) | 6 (18.2) | 1 (5.9) | 0.247 |
| 5-year donor eGFR | 70 (17.2) | 71.7 (14.3) | 72 (16) | 72 (12) | 0.568 |
Overall, the subgroup analysis suggests that EC donors and recipients can achieve comparable outcomes to standard criteria groups when carefully selected and managed. These findings contribute to the growing evidence supporting the expansion of donor and recipient criteria to address the increasing demand for LDKT.
Further analysis revealed that 5-year rejection showed statistically significant negative correlation with graft function at 5 years (r = -0.321, P < 0.001). This was confirmed in a linear regression analysis model of 5-year graft function, demonstrated in Table 5, including subgroup category, return to dialysis, 5-year recipient survival and rejection. It also showed that group 4 recipients experience inferior 5-year graft function when compared to their group 1 counterparts (β = -11.8, P = 0.037).
| Predictor | β | SE | P value |
| 5-year patient survival | 24.61 | 17.96 | 0.174 |
| Return to dialysis | -41.37 | 6.44 | 0.001 |
| Rejection | -14.31 | 4.94 | 0.005 |
| Subgroup comparisons | |||
| 1 and 2 | -6.30 | 4.77 | 0.189 |
| 1-3 | -4.25 | 4.57 | 0.354 |
| 1-4 | -11.80 | 5.58 | 0.037 |
| Intercept | 38.25 | 18.23 | 0.038 |
In this retrospective, single-centre, cohort study, we evaluated the post-operative outcomes of 149 pairs of LDKT with a focusrec on kidney donation in ECD to ECR pairs. Of note, during this period, our transplant programme halted twice, for a total duration of 9 months, due to the COVID pandemic, yet consistent outcomes were maintained across all groups. The rate of immediate post-operative complications was comparable to previously reported historical cohorts from different countries[6,22-24], featuring mostly minimally invasive donor nephrectomy procedures[25-28]. Some interesting outcomes of ECD to ECR (group 4) LDKT are highlighted. Standard criteria pairs (group 1) constitute approximately 1/3 of our cohort, but within the 5-years’ timeframe of this study, we have observed a decrease in our group 1 pair frequency, with increases in all the other groups. While our multivariate linear regression model shows that group 4 recipients experience worse graft function than group 1, the 5-year graft function ANOVA test with P = 0.27 shows that this does not apply to any comparisons amongst groups 2-4. Meanwhile, no 5-year recipient and graft survival differences are demonstrated amongst the 4 groups.
We found that donor and recipient survival, graft survival and recipient and donor eGFR were comparable between EC pairs and their standard criteria counterparts. These findings align with recent literature supporting the viability of EC LDKT (using a similar definition of ECD) under carefully managed protocols in dedicated centres[16]. Our findings are also in keeping with a historical cohort from our centre, now featuring changes in immunosuppression and nephrectomy protocols, that showcases LDKT to be safe and efficient from elderly or obese donors[15]. In our cohort, the negative impact of rejection is showcased in 5-year graft function in both correlation analysis and a linear regression model, hin
Several meta-analyses corroborate our findings. Evidence indicates that donor’s age above 60 years and BMI above 30 kg/m2 can influence post-donation renal function and increase risk of proteinuria and arterial HTN[19]. Our local data and the above metanalysis[19] showcase that a combination of modern surgical technique combined with enhanced re
Another metanalysis[17], focusing on the impact of donor characteristics on recipient outcomes in LDKT, showcases no recipient survival differences when comparing donors age below 60 vs age above 60, without any changes even when the donor age threshold changed to 50. However, in the same study, one year graft survival and serum creatinine outcomes were superior in recipients that received kidneys from donors younger than 60 years, skewed by high heterogeneity. Recipients of kidneys from donors older than 60 years were shown to be at higher risk of DGF or acute rejection. All the above can be explained by the accumulation of cellular damage and increased senescence in kidneys of older donors, triggering a more overt innate and adaptive immune response after reperfusion[30,31]. On the other hand, donor obesity was correlated with increased DGF, but not acute rejection[17], likely reflecting an adjustment to hyper filtration and changes in kidney microvascular resistance, rather than a purely immunological phenomenon. Furthermore, the proven[10] patient and graft survival benefits of pre-emptive LDKT were not confirmed in our study, as there was no correlation between pre-emptive transplant and any of the above outcome differences in the subgroup analysis.
Finally, dissecting into the relevance of recipient characteristics, a recent meta-analysis showed no difference in one year recipient and graft survival when BMI recipients above 30 kg/m2 were compared to non-obese counterparts[13,18]. However, obese recipients experienced higher incidence of DGF and acute rejection[18], compatible with the generalised pro-inflammatory state induced by obesity in conjunction with graft hyperfiltration[32]. All the above meta-analyses individually demonstrate that sole aspects of EC LDKT are safe and provide good quality outcomes for donors and recipients, but our study aimed to bridge the knowledge gap in the outcomes of ECD to ECR living kidney transpl
There are certain limitations to our study. Despite using a fully online, trust-based, electronic patient folder service, anaesthetic data were missing due to delayed integration of the anaesthetic records, resulting in missing values for anthropomorphic parameters or loss to follow-up. A limitation of our study could be considered the definition of EC, with overlying factors in some individuals, precluding precise stratification of cardiovascular risk. Thus, our study lacks the quantification of the cumulative cardiovascular risk associated with having one, more or multiple of the criteria that characterise an individual as an ECD or an ECR. More granular risk stratification associated with ECD or ECR in the context of a large-scale study could elucidate any limitations of EC kidney donation and help identify patient cohorts that could be precluded from living kidney organ donation. Finally, we would like to acknowledge the lack of reporting indu
A nation-wide scale analysis of the data from the United Kingdom Living Kidney Sharing Scheme, taking into account the variations in cold ischaemic time[33], using the same protocol to ours, could support our outcomes, providing an adequate number of events and statistical power, allowing for more robust graft and patient survival analysis. Further association of immunological data (detailed mismatch, induction and maintenance immunosuppression) with immediate and long-term post-transplant outcomes could also be achieved in larger cohort studies.
In conclusion, our study underscores the safety and efficacy of LDKT from EC donors to ECR when performed in a modern, minimally invasive donor nephrectomy programme under enhanced recovery protocols. These findings can be the basis of multicentre studies to support the expansion of donor and recipient criteria in well-resourced and dedicated tertiary LDKT programmes, addressing the growing demand for kidney transplants with careful counselling of both donors and recipients on the expected outcomes.
| 1. | Thurlow JS, Joshi M, Yan G, Norris KC, Agodoa LY, Yuan CM, Nee R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am J Nephrol. 2021;52:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 525] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 2. | Vanholder R, Domínguez-Gil B, Busic M, Cortez-Pinto H, Craig JC, Jager KJ, Mahillo B, Stel VS, Valentin MO, Zoccali C, Oniscu GC. Organ donation and transplantation: a multi-stakeholder call to action. Nat Rev Nephrol. 2021;17:554-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 3. | Traynor C, Jenkinson A, Williams Y, O'Kelly P, Hickey D, Denton M, Magee C, Conlon PJ. Twenty-year survivors of kidney transplantation. Am J Transplant. 2012;12:3289-3295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | LaPointe Rudow D, Hays R, Baliga P, Cohen DJ, Cooper M, Danovitch GM, Dew MA, Gordon EJ, Mandelbrot DA, McGuire S, Milton J, Moore DR, Morgievich M, Schold JD, Segev DL, Serur D, Steiner RW, Tan JC, Waterman AD, Zavala EY, Rodrigue JR. Consensus conference on best practices in live kidney donation: recommendations to optimize education, access, and care. Am J Transplant. 2015;15:914-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Patel K, Brotherton A, Chaudhry D, Evison F, Nieto T, Dabare D, Sharif A. All Expanded Criteria Donor Kidneys are Equal But are Some More Equal Than Others? A Population-Cohort Analysis of UK Transplant Registry Data. Transpl Int. 2023;36:11421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 6. | Lentine KL, Lam NN, Axelrod D, Schnitzler MA, Garg AX, Xiao H, Dzebisashvili N, Schold JD, Brennan DC, Randall H, King EA, Segev DL. Perioperative Complications After Living Kidney Donation: A National Study. Am J Transplant. 2016;16:1848-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | O'Keeffe LM, Ramond A, Oliver-Williams C, Willeit P, Paige E, Trotter P, Evans J, Wadström J, Nicholson M, Collett D, Di Angelantonio E. Mid- and Long-Term Health Risks in Living Kidney Donors: A Systematic Review and Meta-analysis. Ann Intern Med. 2018;168:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Kortram K, Ijzermans JN, Dor FJ. Perioperative Events and Complications in Minimally Invasive Live Donor Nephrectomy: A Systematic Review and Meta-Analysis. Transplantation. 2016;100:2264-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Kim I, Maggiore U, Knight SR, Rana Magar R, Pengel LHM, Dor FJMF. Pre-emptive living donor kidney transplantation: A public health justification to change the default. Front Public Health. 2023;11:1124453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Rana Magar R, Knight SR, Maggiore U, Lafranca JA, Dor FJMF, Pengel LHM. What are the benefits of pre-emptive versus non-pre-emptive kidney transplantation? A systematic review and meta-analysis. Transplant Rev (Orlando). 2023;37:100798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Farrell AM, Devereux J, Karpin I, Weller P. Organ and Tissue Donation and Transplantation. Health Law: Frameworks and Context. Cambridge: Cambridge University Press, 2017: 216-244. [DOI] [Full Text] |
| 12. | Tingle SJ, Figueiredo RS, Moir JA, Goodfellow M, Talbot D, Wilson CH. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. 2019;3:CD011671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 13. | Ahmadi AR, Lafranca JA, Claessens LA, Imamdi RM, IJzermans JN, Betjes MG, Dor FJ. Shifting paradigms in eligibility criteria for live kidney donation: a systematic review. Kidney Int. 2015;87:31-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Lafranca JA, Spoon EQW, van de Wetering J, IJzermans JNM, Dor FJMF. Attitudes among transplant professionals regarding shifting paradigms in eligibility criteria for live kidney donation. PLoS One. 2017;12:e0181846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | O'Brien B, Mastoridis S, Sabharwal A, Hakim N, Taube D, Papalois V. Expanding the donor pool: living donor nephrectomy in the elderly and the overweight. Transplantation. 2012;93:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Plage H, Pielka P, Liefeldt L, Budde K, Ebbing J, Sugünes N, Miller K, Cash H, Bichmann A, Sattler A, Kotsch K, Friedersdorff F. Extended Criteria Donors in Living Kidney Transplantation Including Donor Age, Smoking, Hypertension and BMI. Ther Clin Risk Manag. 2020;16:787-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Bellini MI, Nozdrin M, Pengel L, Knight S, Papalois V. How good is a living donor? Systematic review and meta-analysis of the effect of donor demographics on post kidney transplant outcomes. J Nephrol. 2022;35:807-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Bellini MI, Nozdrin M, Pengel L, Knight S, Papalois V. The Impact of Recipient Demographics on Outcomes from Living Donor Kidneys: Systematic Review and Meta-Analysis. J Clin Med. 2021;10:5556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Bellini MI, Nozdrin M, Pengel L, Knight S, Papalois V. Risks for donors associated with living kidney donation: meta-analysis. Br J Surg. 2022;109:671-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | NHSBT. POL186/11 Kidney Transplantation: Deceased Donor Organ Allocation. 2021: 1-12. Available from: https://www.odt.nhs.uk/search/?search=POL186/11%20Kidney%20Transplantation:%20Deceased%20Donor%20Organ%20Allocation. |
| 21. | Guidelines for Living Donor Kidney Transplantation Fourth Edition. United Kingdom: British Transplantation Society, 2018. |
| 22. | Ferrario M, Buckel E, Astorga C, Godoy J, Aguiló J, González G, Ormazábal J, Cámbara A, Derosas C, Herzog C, Calabrán L. Results in laparoscopic living donor nephrectomy: a multicentric experience. Transplant Proc. 2013;45:3716-3718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Khandoga A, Thomas M, Kleespies A, Kühnke L, Andrassy J, Habicht A, Stangl M, Guba M, Angele M, Werner J, Rentsch M. Surgical complications and cardiovascular comorbidity - Substantial non-immunological confounders of survival after living donor kidney transplantation. Surgeon. 2019;17:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Schamm M, Jugmohan B, Joseph C, Botha JR, Botha JF, Britz R, Loveland J. Kidney transplant outcomes following the introduction of hand-assisted laparoscopic living donor nephrectomy: a comparison of recipient groups. S Afr J Surg. 2015;53:63-66. [PubMed] |
| 25. | Sterkenburg A, Kulu Y, Mieth M, Sommerer C, Zeier M, Mehrabi A, Büchler M, Hoffmann K. Long-term Surgical Outcome and Risk Factors in Living Kidney Donors. Transplant Proc. 2020;52:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Vernadakis S, Marinaki S, Darema M, Soukouli I, Michelakis IE, Beletsioti C, Zavvos G, Bokos I, Boletis IN. The Evolution of Living Donor Nephrectomy Program at A Hellenic Transplant Center. Laparoscopic vs. Open Donor Nephrectomy: Single-Center Experience. J Clin Med. 2021;10:1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Lauridsen MB, Skov K, Øzbay LA. Short-term Outcome of Danish Kidney Donors: Postoperative Complications and Labor Affiliation. Transplant Proc. 2022;54:1763-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Nguyen NTQ, Courtney AE, Nguyen HQ, Quinn M, Maxwell AP, O'Neill C. Early clinical and economic outcomes of expanded criteria living kidney donors in the United States. J Nephrol. 2023;36:957-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Prionas A, Craddock C, Papalois V. Feasibility, Safety and Efficacy of Enhanced Recovery After Living Donor Nephrectomy: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Clin Med. 2020;10:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Huang W, Hickson LJ, Eirin A, Kirkland JL, Lerman LO. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol. 2022;18:611-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 755] [Article Influence: 188.8] [Reference Citation Analysis (0)] |
| 31. | Fang Y, Gong AY, Haller ST, Dworkin LD, Liu Z, Gong R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res Rev. 2020;63:101151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 32. | Wu D, Dawson NA, Levings MK. Obesity-Associated Adipose Tissue Inflammation and Transplantation. Am J Transplant. 2016;16:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | van de Laar SC, Robb ML, Hogg R, Burnapp L, Papalois VE, Dor FJMF. The Impact of Cold Ischaemia Time on Outcomes of Living Donor Kidney Transplantation in the UK Living Kidney Sharing Scheme. Ann Surg. 2021;274:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/