Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.109609
Revised: June 19, 2025
Accepted: September 22, 2025
Published online: December 18, 2025
Processing time: 187 Days and 15.1 Hours
Liver transplant (LT) is one of the main treatment options in selected patients with hepatocellular carcinoma (HCC). Overall, macrovascular invasion has been shown to be associated with an increased risk of tumor recurrence and mortality after LT in HCC. Macrovascular invasion detected on imaging is often considered a contraindication for LT in HCC.
To investigate the effect of macrovascular invasion in explant on post-LT survival in HCC patients using a large national transplant database in the United States.
LT recipients with HCC between the years 2012 and 2022 were identified by using the United Network for Organ Sharing/Organ Procurement Transplant Network database. Patients who underwent deceased-donor LT with available liver explant pathology data were included. Kaplan-Meier curves were used for survival analysis, and multistep regression analysis was used to determine the predictors of mortality.
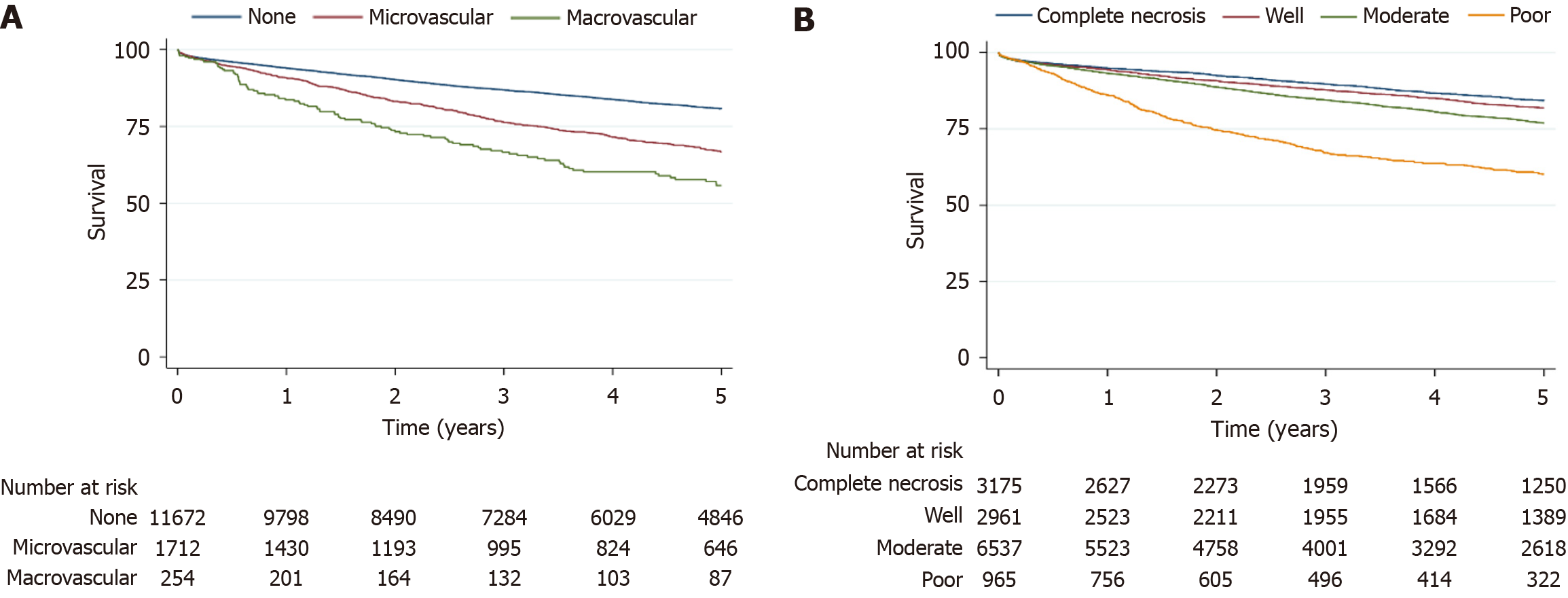
A total of 13638 LT recipients with HCC and available explant pathology were included. Of these, 254 (1.8%) showed macrovascular invasion, 1712 (12.6%) had microvascular invasion, and 11672 (85.6%) had absent invasion. Poor tumor differentiation was more common with macrovascular invasion than with microvascular or absent invasion (22.4%, 17.7%, and 5.1%, respectively, P < 0.001). Post-transplant survival at 1 year, 3 years, and 5 years was lower in the macrovascular group than in the microvascular and absent invasion cohort (83.6%, 66.6%, 55.7% vs 90.8%, 76.2%, 66.6% vs 93.9%, 86.8%, 80.7%, P < 0.001). Similarly, transplant recipients whose explants were poorly differentiated had worse 1-year, 3-year, and 5-year survival than those with well-differentiated tumors and those with complete necrosis (86.1%, 67.1%, 60.4% vs 94.3%, 87.7%, 81.9% vs 94.8%, 89.7%, 84.2%, P < 0.001). In multivariable modeling, macrovascular invasion was associated with higher mortality risk compared to absent invasion [hazard ratio (HR) = 2.3, 95%CI: 1.9–2.7], and poor differentiation carried greater mortality risk than complete necrosis (HR = 2.3, 95%CI: 2.0–2.7).
Macrovascular invasion accounted for a minority of cases at 1.8%. Macrovascular invasion and poor tumor differentiation on liver explants in patients with HCC were associated with significantly higher post-LT mortality, meaning that the extent of tumor involvement and tumor biology are important predictors of post-LT survival in HCC. However, the overall 5-year survival in patients with macrovascular invasion may still be within an acceptable range.
Core Tip: Liver transplant (LT) is one of the treatment options for hepatocellular carcinoma (HCC). Macrovascular invasion and tumor differentiation have been shown to be associated with post-LT HCC recurrence. We investigated a large national transplant database in the United States to identify post-LT survival in HCC patients between 2012 and 2022. A total of 13638 patients with HCC and available liver explant data were included. Of the cohort, 254 (1.8%) demonstrated macrovascular invasion and 1712 (12.6%) had microvascular invasion (MVI). Poor tumor differentiation was most frequent in the macrovascular invasion cohort compared to the microvascular and absent invasion cohorts. Post-transplant survival at 1 year, 3 years, and 5 years was lowest among patients with macrovascular invasion, lower than in those with MVI and lower still than in those without vascular invasion. Likewise, on explant pathology, poor differentiation was linked to worse 1-year, 3-year, and 5-year survival than either well-differentiated tumors or complete necrosis. Macrovascular invasion and poor tumor differentiation on liver explants in patients with HCC were associated with significantly higher post-LT mortality, meaning that the extent of tumor involvement and tumor biology are important predictors of post-LT survival in HCC.
- Citation: Öztürk NB, Gurakar MM, Parraga X, Alsaqa M, Sierra L, Currier E, Fakhoury B, Bonder A, Gurakar A, Saberi B. Association of vascular invasion and tumor differentiation on post-liver transplant outcomes in patients with hepatocellular carcinoma. World J Transplant 2025; 15(4): 109609
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/109609.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.109609
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and is one of the fastest-growing causes of cancer death in the United States[1]. Liver transplant (LT) is the treatment of choice for selected patients with HCC when the surgical resection is not suitable. Various selection criteria exist for determining LT candidact in patients with HCC and Milan criteria is considered the standard criteria[2].
Patients with HCC with lymph node involvement or metastasis are not LT candidates due to the high rate of post-LT recurrence and death. The presence of macrovascular invasion, particularly involving the main portal vein, is associated with a high rate of post-LT recurrence and is a contraindication for LT[3]. Macrovascular invasion with HCC usually presents as a tumor thrombus adjacent to a liver lesion, showing typical imaging features of HCC. The diagnosis of macrovascular invasion includes the presence of arterial phase hyperenhancement and restricted diffusion within the portal thrombus on contrast-enhanced imaging[4]. Radiologically, microvascular invasion (MVI) appears as an intraluminal filling defect contiguous with the primary tumor that shows arterial-phase enhancement and portal/delayed washout. Macrovascular invasion is distinct from MVI which is identifiable only on histopathology. Macrovascular invasion of the main portal vein or hepatic vein is an absolute contraindication for LT, due to very high rate of HCC recurrence following transplant. However, portal vein branch invasion in HCC may not necessarily be a contraindication for LT. This later group may not receive a model for end-stage liver disease (MELD) exception at the time of listing. However, some patients who remain stable for a minimum of 12 months interval after locoregional therapy may be suitable for consideration[5].
The majority of macrovascular invasions are seen in the setting of larger HCC lesions (> 5 cm)[6,7]. Imaging findings may be subtle for macrovascular invasion, particularly with lesions with infiltrative appearance[6]. Histopathological evaluation of the thrombus is the gold standard for the diagnosis of macrovascular invasion; however, it is not always known until after LT, when reviewing the explant pathology. In addition, most patients with HCC do not undergo biopsy of the lesion prior to transplant to confirm this finding[4]. The presence of macrovascular invasion in the main portal vein will limit the therapeutic options, such as locoregional therapies[6].
Unlike macrovascular invasion, MVI cannot be as reliably detected on conventional imaging and is best assessed by histopathological evaluation of the liver explant[8]. In addition, poor tumor differentiation is another prognostic factor that is associated with higher post-LT tumor recurrence and mortality[9]. Radiologically, MVI appears as an intraluminal filling defect contiguous with the primary tumor that shows arterial-phase enhancement and portal/delayed washout. MVI is distinct from MVI, which is identifiable only on histopathology[9].
A study conducted on the European Liver Transplant Registry, including 23124 patients transplanted for HCC (9324 with cirrhosis and available liver explant histology data) reported vascular invasion to be a superior prognosticator compared with the traditional selection criteria (Milan criteria and up-to-seven criteria)[10]. In addition, the same study also showed that the presence of macrovascular invasion was associated with the worst prognosis. Several smaller studies also reported the presence of vascular invasion as a prognostic marker for HCC[11-13].
Using a large, nationwide United States database, we evaluated post-LT outcomes in HCC patients with macrovascular invasion and poor tumor differentiation identified on explant pathology.
The data reported were supplied by the United Network for Organ Sharing (UNOS), as the contractor for the Organ Procurement Transplant Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and should not be seen as an official policy or interpretation by the OPTN or the United States government. Because UNOS database is publicly available and deidentified, informed consent and ethical approval were not required according to the policies of UNOS and the institutional review board at Beth Israel Deaconess Medical Center.
This was a retrospective cohort study using the UNOS/OPTN database. We identified adult patients with HCC between 2012 and 2022 who underwent deceased donor LT and with available liver explant pathology data. Living donor LTs and patients with cholangiocarcinoma were excluded.
Patient selection included adult candidates according to center-specific policies that follow Milan/University of California San Francisco (UCSF) criteria. Notably, as gross MVI on imaging is an absolute contraindication, any MVI recorded in our cohort reflects pathology discovered post-transplant on explant review rather than a pre-operative selection.
Our analyses used the national OPTN/UNOS registry accessed via the SRTR. Per OPTN/UNOS policy, individual-level data are released to investigators only under a signed Data Use Agreement, so we cannot publicly upload the raw dataset; instead in the present manuscript, we have added the raw real outcomes of our data. The underlying de-identified datasets are available directly from OPTN/SRTR and are publicly accessible.
A total of 13638 patients with HCC and liver explant data were included in the analysis. Among these, 254 (1.8%) had macrovascular invasion, 1712 (12.6%) had MVI, and 11672 (85.6%) had absent vascular invasion. Tumor differentiation was classified as well in 2961 (21.7%), moderate in 6537 (47.9%), poor differentiation in 965 (7.1%), and complete tumor necrosis in 2175 (23.3%) of the patients. Poorly-differentiated tumors were most frequent in the macrovascular invasion cohort, compared to the microvascular and absent vascular invasion cohorts (22.4%, 17.7%, and 5.1%, respectively, P < 0.001).
Table 1 shows the recipient characteristics in patients with no vascular invasion, MVI, and macrovascular invasion. Overall, age, gender, MELD score and alpha fetoprotein (AFP) were comparable between groups. Chronic hepatitis C was the common etiology of liver disease in all groups, followed by metabolic dysfunction-associated steatotic liver disease, and alcoholic liver disease (P < 0.07).
| Recipient characteristics | None (n = 11672, 85.6%) | Microvascular invasion | Macrovascular invasion | P value |
| Age (years), mean (SD) | 61.5 (6.8) | 61.2 (6.2) | 61.6 (6.5) | 0.97 |
| Gender, female | 2679 (22.9) | 322 (18.8) | 37 (14.5) | 0.10 |
| Model for end-stage liver disease | 12.2 (4.9) | 12.2 (5.0) | 12.2 (4.8) | 0.89 |
| AFP | ||||
| < 100 | 10,776 (92.2) | 1534 (89.6) | 220 (86.6) | 0.15 |
| 100 ≤ AFP < 1000 | 828 (7.0) | 168 (9.8) | 31 (12.2) | |
| ≥ 1000 | 68 (0.5) | 10 (0.5) | 3 (1.1) | |
| Etiology | ||||
| Hepatitis C virus | 7024 (60.1) | 1107 (64.6) | 152 (59.8) | 0.07 |
| Alcohol-associated liver disease | 1152 (9.8) | 155 (9.0) | 17 (6.6) | |
| Metabolic dysfunction-associated steatotic liver disease | 1486 (12.7) | 195 (11.3) | 37 (14.5) | |
| Hepatitis B virus | 732 (6.2) | 95 (5.5) | 13 (5.1) | |
| Other | 1278 (10.9) | 160 (9.3) | 35 (13.7) | |
| Tumor differentiation | ||||
| Complete necrosis | 3125 (26.7) | 0 (0) | 0 (0) | < 0.001 |
| Well-differentiated | 2731 (23.4) | 203 (11.8) | 27 (10.6) | |
| Moderately-differentiated | 5211 (44.6) | 1201 (68.4) | 170 (61.0) | |
| Poorly-differentiated | 605 (5.1) | 303 (17.7) | 57 (22.4) | |
| Locoregional therapy | 7966 (68.2) | 1142 (66.7) | 183 (72.0) | 0.09 |
| Death | 2326 (19.9) | 572 (33.4) | 114 (44.8) | < 0.001 |
Post-LT survival at 1 year, 3 years, and 5 years was lowest in the macrovascular invasion cohort compared to the microvascular and absent invasion cohorts (83.6%, 66.6%, 55.7% vs 90.8%, 76.2%, 66.6% vs 93.9%, 86.8%, 80.7%, P < 0.001; Figure 1A). Likewise, on explant pathology, poor tumor differentiation was linked to reduced 1-year, 3-year, and 5-year post-LT survival relative to well-differentiated tumors and complete necrosis (86.1%, 67.1%, 60.4% vs 94.3%, 87.7%, 81.9% vs 94.8%, 89.7%, 84.2%, P < 0.001; Figure 1B).
In multistep Cox regression analysis, macrovascular invasion independently predicted greater mortality relative to no vascular invasion [hazard ratio (HR) = 2.18, 95%CI: 1.80-2.66, P < 0.001]. Patients with poor tumor differentiation on explant had a highest hazard of mortality (HR = 2.35, 95%CI: 2.04-2.70, P < 0.001; Table 2).
| Variable | Hazard ratio | 95%CI (lower) | 95%CI (upper) | P value |
| Tumor differentiation | ||||
| Complete necrosis | Reference | |||
| Well-differentiated | 1.159 | 1.027 | 1.307 | 0.016 |
| Moderately-differentiated | 1.343 | 1.2089 | 1.492 | < 0.001 |
| Poorly-differentiated | 2.346 | 2.037 | 2.703 | < 0.001 |
| Age (recipient) | 1.326 | 1.249 | 1.408 | < 0.001 |
| Vascular invasion | ||||
| None | Reference | |||
| Microvascular invasion | 1.459 | 1.323 | 1.607 | < 0.001 |
| Macrovascular invasion | 2.183 | 1.798 | 2.652 | < 0.001 |
| Largest tumor size | 1.029 | 1.021 | 1.037 | < 0.001 |
| Creatinine | 1.113 | 1.0729 | 1.155 | < 0.001 |
| Age (donor) | 1.063 | 1.040 | 1.086 | < 0.001 |
| Encephalopathy | 1.123 | 1.0286 | 1.227 | 0.01 |
| Recipient’s race | ||||
| White | Reference | |||
| Black | 1.153 | 1.021 | 1.300 | 0.02 |
| Asian | 0.757 | 0.644 | 0.891 | 0.001 |
| Male gender | 1.137 | 1.038 | 1.247 | 0.006 |
| Ascites | 1.109 | 1.018 | 1.209 | 0.02 |
In this study, we evaluated explant data from LT recipients with HCC within UNOS database between 2012 and 2022. The proportion of patients with macrovascular invasion and MVI on explant were 1.8% and 12.6%, respectively. The majority of tumors (47.9%) were moderately differentiated. Post-LT survival was significantly lower in patients with macrovascular invasion and those with poorly differentiated tumors, with 5-year post-LT survival at 55.7% and 60.4%, respectively.
Milan criteria and UCSF criteria have become the standard selection criteria in HCC patients undergoing LT[14]. Centers have expanded patient selection beyond standard criteria by downstaging protocols using locoregional therapies. This approach has allowed more patients with HCC to benefit from this life-saving operation. This has led to higher rate of tumor recurrence Post-LT in HCC[15,16]. HCC recurs in approximately 10%-15% of patients within Milan criteria who undergo LT[17-19]. Although LT is curative and provides a highly successful long-term survival in selected patients with HCC, risk stratification for HCC recurrence and a specific post-LT surveillance strategy can be challenging[17,20].
Macrovascular invasion usually can be detected on pre-LT imaging, which can determine the LT candidacy[17]. To reduce the rates of under staging of vascular invasion, a revised UNOS/OPTN imaging policy was implemented for standardized imaging protocol and reporting, along with equipment specifications for the LT centers[17,21]. Macrovascular invasion often presents hypointensities on T1-weighted images, the presence of arterial phase hyperenhancement within the thrombus, and washout on portal venous and delayed phases; and on T2-weighted images, it presents as moderate to high T2 hyperintensity[6,22]. Despite this, discrepancy exists for the presence of vascular invasion between the imaging and liver histology. Liver explant histopathological evaluation is the gold standard, and imaging sometimes may not accurately detect macrovascular invasions in branch veins[23].
In a study involving 479 patients, only MVI, macrovascular invasion, and tumor differentiation grade remained significant in the multivariable model, including the number of nodules, size of the largest nodule, and the sum of nodule diameters[24]. In addition, poorly differentiated tumors have been shown to be a significant risk factor for HCC recurrence and survival post-LT in several other studies[12,25]. In another study conducted on 10 European centers on 1854 patients with HCC, the presence of histopathologic confirmation of macrovascular invasion [odds ratio (OR) = 2.71, P = 0.001] and poorly-differentiated tumor (OR = 1.56, P = 0.001), hepatitis C virus status (OR = 1.39, P = 0.001), diameter of the target lesion (OR = 1.09, P = 0.001), AFP slope (OR = 1.63, P = 0.006), and age (OR = 0.99, P = 0.01) were identified as risk factors for 10-year survival[26]. Among patients with HCC, the presence of macrovascular invasion on liver explants correlated with inferior survival after transplantation, with this group exhibiting the highest mortality. Explant tumor grade likewise showed a significant relationship with post-LT survival, particularly for poorly differentiated tumor biology. Pretransplant imaging may not reliably reflect explant pathology and can miss MVI. It is important to note that adjuvant therapy after LT remains investigational; in high-risk cases clinicians may switch to sirolimus-based or everolimus-based immunosuppression and, rarely, TKIs such as sorafenib or lenvatinib, however these therapies are yet to be studied[27].
The strengths of our study include the use of a large national database of LT candidates and a large sample size. Limitations of our study include its retrospective nature, reporting all-cause mortality, the presence of heterogeneous data within the database, and having no HCC recurrence information. Nonetheless, the present study contributes to the literature by highlighting the survival rates for patients with vascular invasion and poor tumor differentiation.
In conclusion, macrovascular invasion and poor tumor differentiation are important predictors of survival post-LT in patients with HCC. The overall 5-year survival in patients with macrovascular invasion may still be within an acceptable range. However, the decision to offer LT to patients with smaller branch macrovascular invasion may be center dependent and limited based on allograft supply and demand. Our study highlights the importance of pre-LT detection of tumor extent in veins and tumor biology in determining post-LT outcomes in HCC. Future studies, investigating novel biomarker and imaging technologies that could detect macrovascular involvement and tumor differentiation, will be helpful in candidate selection in HCC patients undergoing LT.
| 1. | Zhuo Y, Chen Q, Chhatwal J. Changing Epidemiology of Hepatocellular Carcinoma and Role of Surveillance. 2019 Aug 6. In: Hepatocellular Carcinoma: Translational Precision Medicine Approaches [Internet]. Cham (CH): Humana Press; 2019–. [PubMed] |
| 2. | Horwitz JK, Agopian VG. Indication of Liver Transplant for HCC: Current Status and Future Directions. Curr Hepatology Rep. 2024;23:185-192. [RCA] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 3. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 798] [Article Influence: 57.0] [Reference Citation Analysis (2)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6420] [Article Influence: 802.5] [Reference Citation Analysis (9)] |
| 5. | OPTN Liver and Intestinal Organ Transplantation Committee. Guidance to Liver Transplant Programs and the National Liver Review Board for: Adult MELD Exceptions for Hepatocellular Carcinoma (HCC). OPTN. 2017;. |
| 6. | Cannella R, Taibbi A, Porrello G, Dioguardi Burgio M, Cabibbo G, Bartolotta TV. Hepatocellular carcinoma with macrovascular invasion: multimodality imaging features for the diagnosis. Diagn Interv Radiol. 2020;26:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Kneuertz PJ, Demirjian A, Firoozmand A, Corona-Villalobos C, Bhagat N, Herman J, Cameron A, Gurakar A, Cosgrove D, Choti MA, Geschwind JF, Kamel IR, Pawlik TM. Diffuse infiltrative hepatocellular carcinoma: assessment of presentation, treatment, and outcomes. Ann Surg Oncol. 2012;19:2897-2907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Umar Garzali İ, I Carr B, Ince V, Işık B, Nur Akatlı A, Yılmaz S. Microvascular Invasion in Hepatocellular Carcinoma: Some Puzzling Facets. Turk J Gastroenterol. 2024;35:143-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Zavaglia C, De Carlis L, Alberti AB, Minola E, Belli LS, Slim AO, Airoldi A, Giacomoni A, Rondinara G, Tinelli C, Forti D, Pinzello G. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol. 2005;100:2708-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Pommergaard HC, Rostved AA, Adam R, Thygesen LC, Salizzoni M, Gómez Bravo MA, Cherqui D, Filipponi F, Boudjema K, Mazzaferro V, Soubrane O, García-Valdecasas JC, Prous JF, Pinna AD, O'Grady J, Karam V, Duvoux C, Rasmussen A; European Liver and Intestine Transplant Association (ELITA). Vascular invasion and survival after liver transplantation for hepatocellular carcinoma: a study from the European Liver Transplant Registry. HPB (Oxford). 2018;20:768-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Shah SA, Tan JC, McGilvray ID, Cattral MS, Cleary SP, Levy GA, Greig PD, Grant DR. Accuracy of staging as a predictor for recurrence after liver transplantation for hepatocellular carcinoma. Transplantation. 2006;81:1633-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 296] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 13. | Parfitt JR, Marotta P, Alghamdi M, Wall W, Khakhar A, Suskin NG, Quan D, McAllister V, Ghent C, Levstik M, McLean C, Chakrabarti S, Garcia B, Driman DK. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl. 2007;13:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Patel SS, Arrington AK, McKenzie S, Mailey B, Ding M, Lee W, Artinyan A, Nissen N, Colquhoun SD, Kim J. Milan Criteria and UCSF Criteria: A Preliminary Comparative Study of Liver Transplantation Outcomes in the United States. Int J Hepatol. 2012;2012:253517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1619] [Article Influence: 89.9] [Reference Citation Analysis (1)] |
| 16. | Degroote H, Geerts A, Verhelst X, Van Vlierberghe H. Different Models to Predict the Risk of Recurrent Hepatocellular Carcinoma in the Setting of Liver Transplantation. Cancers (Basel). 2022;14:2973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Verna EC, Patel YA, Aggarwal A, Desai AP, Frenette C, Pillai AA, Salgia R, Seetharam A, Sharma P, Sherman C, Tsoulfas G, Yao FY. Liver transplantation for hepatocellular carcinoma: Management after the transplant. Am J Transplant. 2020;20:333-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 18. | Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, Vibert E, Cherqui D, Grant DR, Hernandez-Alejandro R, Dale CH, Cucchetti A, Pinna A, Hwang S, Lee SG, Agopian VG, Busuttil RW, Rizvi S, Heimbach JK, Montenovo M, Reyes J, Cesaretti M, Soubrane O, Reichman T, Seal J, Kim PT, Klintmalm G, Sposito C, Mazzaferro V, Dutkowski P, Clavien PA, Toso C, Majno P, Kneteman N, Saunders C, Bruix J; iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 271] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 19. | Goldaracena N, Mehta N, Scalera I, Sposito C, Atenafu EG, Yao FY, Muiesan P, Mazzaferro V, Sapisochin G. Multicenter validation of a score to predict prognosis after the development of HCC recurrence following liver transplantation. HPB (Oxford). 2019;21:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Muhammad H, Tehreem A, Ting PS, Gurakar M, Li SY, Simsek C, Alqahtani SA, Kim AK, Kohli R, Gurakar A. Hepatocellular Carcinoma and the Role of Liver Transplantation: A Review. J Clin Transl Hepatol. 2021;9:738-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 22. | Catalano OA, Choy G, Zhu A, Hahn PF, Sahani DV. Differentiation of malignant thrombus from bland thrombus of the portal vein in patients with hepatocellular carcinoma: application of diffusion-weighted MR imaging. Radiology. 2010;254:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Bhattacharjya S, Bhattacharjya T, Quaglia A, Dhillon AP, Burroughs AK, Patch DW, Tibballs JM, Watkinson AF, Rolles K, Davidson BR. Liver transplantation in cirrhotic patients with small hepatocellular carcinoma: an analysis of pre-operative imaging, explant histology and prognostic histologic indicators. Dig Surg. 2004;21:152-159; discussion 159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | D'Amico F, Schwartz M, Vitale A, Tabrizian P, Roayaie S, Thung S, Guido M, del Rio Martin J, Schiano T, Cillo U. Predicting recurrence after liver transplantation in patients with hepatocellular carcinoma exceeding the up-to-seven criteria. Liver Transpl. 2009;15:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (2)] |
| 25. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 711] [Article Influence: 28.4] [Reference Citation Analysis (1)] |
| 26. | Lai Q, Viveiros A, Iesari S, Vitale A, Mennini G, Onali S, Hoppe-Lotichius M, Colasanti M, Manzia TM, Mocchegiani F, Spoletini G, Agnes S, Vivarelli M, Tisone G, Ettorre GM, Mittler J, Tsochatzis E, Rossi M, Cillo U, Schaefer B, Lerut JP. Prognostic Factors for 10-Year Survival in Patients With Hepatocellular Cancer Receiving Liver Transplantation. Front Oncol. 2022;12:877107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Magyar CTJ, Perera S, Rajendran L, Li Z, Almugbel FA, Feng S, Choi WJ, Aceituno L, Vogel A, Grant RC, Selzner N, Jaeckel E, Falla-Rad N, Knox JJ, Chen EX, Sapisochin G, O'Kane GM. Comparative Outcome Analysis of Lenvatinib Versus Sorafenib for Recurrence of Hepatocellular Carcinoma After Liver Transplantation. Transplantation. 2025;109:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/