Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.107462
Revised: May 14, 2025
Accepted: August 29, 2025
Published online: December 18, 2025
Processing time: 240 Days and 10.7 Hours
Pediatric liver transplantation (LT) is the definitive treatment for end-stage liver disease and acute liver failure in children. However, graft size mismatch poses significant challenges, particularly in infants weighing less than 10 kg. Large-for-size grafts can lead to severe complications, including vascular thrombosis and impaired graft perfusion. Surgical innovations, such as hyper-reduced left lateral segment (HRLLS) grafts and monosegmental grafts (MSG), offer viable solutions by tailoring graft size without compromising vascular or biliary integrity.
To analyze the techniques and outcomes of HRLLS and MSG grafts in pediatric liver trabsplantation.
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, a comprehensive literature search was conducted across PubMed, Scopus, and Google Scholar, including studies up to February 2025. Eligible stud
Eighteen studies involving various graft reduction techniques were included. Both HRLLS and MSG demonstrated comparable one-year survival rates exc
HRLLS and MSG techniques enable successful liver transplantation in small pediatric recipients, achieving long-term outcomes comparable to standard approaches. These graft modification strategies expand donor pool uti
Core Tip: Tailored graft reduction techniques—hyper-reduced, reduced, and monosegmental—effectively address graft size mismatch in pediatric liver transplantation. Optimal outcomes depend on careful donor selection, graft thickness adjustment, and individualized surgical planning. Vigilant attention to graft-to-recipient weight ratio and vascular anatomy minimizes complications. Long-term data confirm these techniques are safe, durable solutions for small pediatric recipients.
- Citation: Sain S, Pahari H, Tripathi S, Singhvi SK, Dhir U. Hyper-reduced grafts in living donor liver transplant: Techniques and outcomes. World J Transplant 2025; 15(4): 107462
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/107462.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.107462
Liver transplantation (LT) is recognized as the definitive treatment for pediatric patients suffering from end-stage liver disease and acute liver failure[1]. A key determinant of successful outcomes in pediatric LT is the appropriate matching of graft size to the recipient's abdominal cavity. Implantation of an oversized graft may lead to significant vascular complications and hinder primary abdominal closure, particularly in infants and small children[2,3].
Historically, the evolution of pediatric LT has witnessed several landmark advancements aimed at addressing these challenges. In 1963, Starzl et al[4] performed the first-ever pediatric liver transplant on a child diagnosed with biliary atresia. Subsequent innovations included Bismuth and Houssin’s introduction of graft reduction techniques in 1984[5], followed by Pichlmayr et al’s development of split LT in 1988, further advancing segmental transplantation in pediatric recipients[6].
Due to the scarcity of deceased donor organs and high mortality rates among children awaiting transplants, particularly in regions such as the Indian subcontinent, living donor liver transplantation (LDLT) has gained widespread acceptance[7]. In this context, small, high-quality left lateral segment grafts from living related donors are considered optimal for pediatric recipients. However, even these grafts may be disproportionately large for infants weighing less than 10 kg, necessitating graft size modification techniques such as reduction or hyper-reduction[8,9].
Effective graft size reduction is essential to avoid large-for-size syndrome—a significant challenge in pediatric LT. Oversized grafts are associated with complications such as vascular thrombosis, biliary strictures, and abdominal compartment syndrome, all of which may impair graft function and patient survival[10]. To mitigate these risks, surgical strategies such as delayed abdominal closure or the use of prosthetic meshes have been employed, though these app
Further graft reduction can be achieved anatomically by resecting specific segments, resulting in monosegmental grafts (MSGs), typically involving segment II or III. However, these procedures are technically demanding and necessitate thorough preoperative assessment of the donor’s biliary and intrahepatic vascular anatomy to ensure feasibility and safety[12-15].
Alternatively, hyper-reduced left lateral segment grafts (HRLLSGs) offer a more flexible and technically feasible approach. These grafts are created via non-anatomical excision of excess peripheral parenchyma, preserving vasculo-biliary continuity while customizing graft dimensions to fit the recipient’s anteroposterior abdominal diameter[16-19].
Despite the potential advantages, global experience with HRLLSGs and MSGs remains relatively limited. Concerns persist regarding the possibility of increased biliary and vascular complications, prolonged ischemia times, and overall technical complexity[20,21].
This systematic review and meta-analysis aims to comprehensively examine the surgical techniques, existing literature, and clinical outcomes associated with MSGs, reduced left lateral segment grafts, and HRLLSGs in small pediatric recipients. Through this evaluation, we aim to provide evidence-based insights to guide surgical decision-making and optimize outcomes in pediatric LDLT.
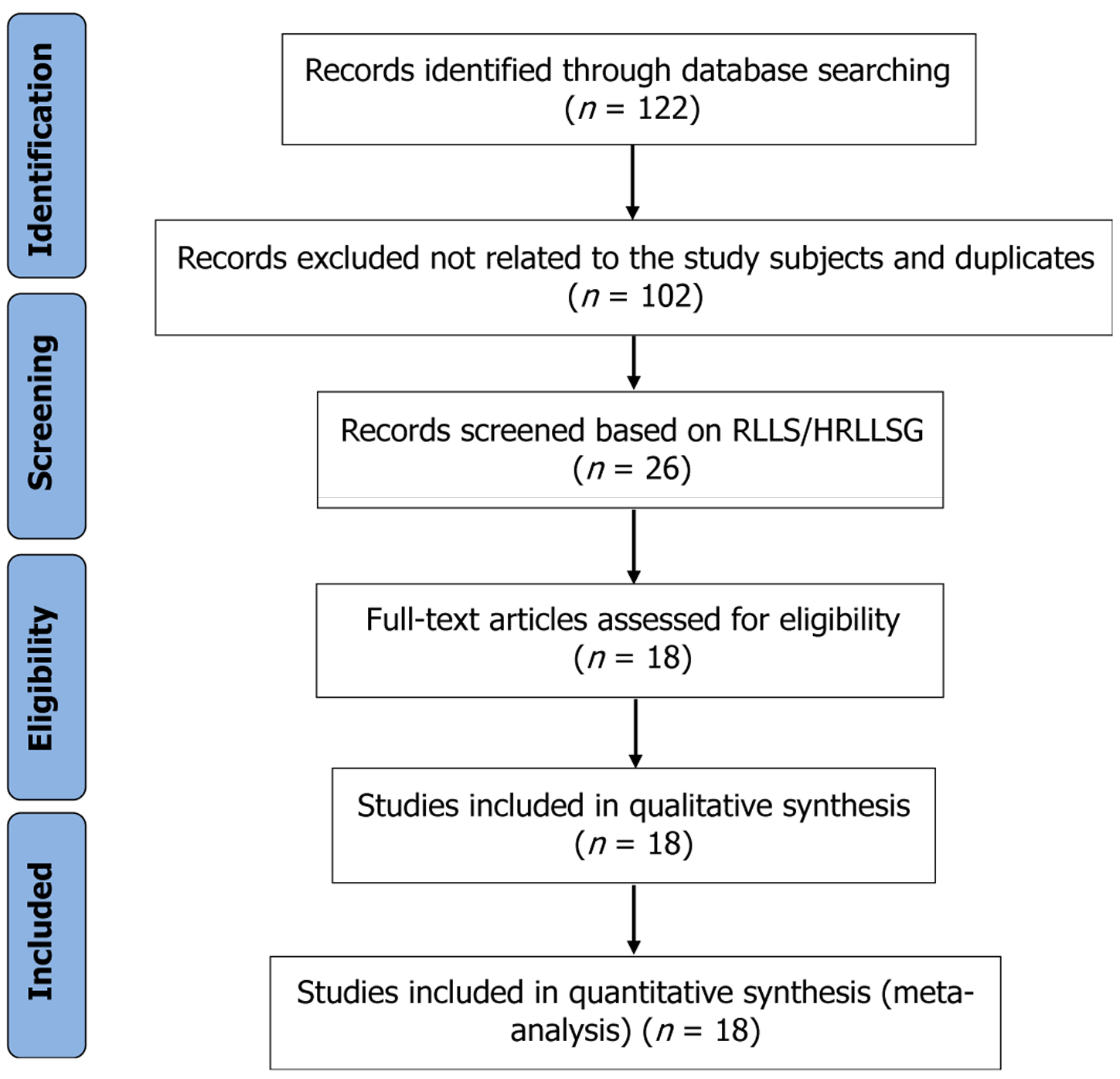
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure methodological rigor and transparency[15]. Additionally, quality assessment of the selected studies was performed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist, specifically designed for evaluating interventional studies. A PRISMA flow diagram summarizing the study selection process is presented in Figure 1.
A comprehensive and systematic literature search was undertaken across three major databases: PubMed, Scopus, and Google Scholar. The final search was conducted on February 20, 2025. Author SS was responsible for developing and executing the search strategy. The search incorporated a combination of free-text terms and Medical Subject Headings (MeSH), using key phrases such as: “liver transplantation” AND “paediatric cases,” “liver transplantation” AND “children,” “liver transplantation” AND “infants,” “liver transplantation” AND “left lateral segmental grafts,” and “hyper-reduced and monosegmental graft.” These keywords were applied in various permutations to maximize the sensitivity and scope of the search. Additionally, references from relevant articles were manually screened to identify any further eligible studies. All citations were managed using the RefWorks Add-Ons tool.
Inclusion criteria: Original human studies (case-control, observational, or randomized controlled trials). Studies reporting outcomes related to HRLLS, MSG, or reduced left lateral segment grafts (RLLS) in pediatric LT. Studies providing detailed surgical descriptions, complication rates, and survival outcomes. English-language articles published up to February 2025.
Exclusion criteria: Non-human studies, reviews, systematic reviews, editorials, letters, or case reports. Articles without relevant clinical outcome data or surgical detail. Studies not focused on pediatric LT.
All identified titles and abstracts were initially screened by one reviewer (SS) to remove duplicates and irrelevant results. The remaining abstracts were independently reviewed by four team members (HP and three others) based on the inclusion/exclusion criteria. Full-text review was conducted for all studies deemed potentially eligible. During this phase, each article was assessed for methodological rigor, relevance to graft reduction techniques, and completeness of outcome data. Disagreements in study inclusion were resolved through open discussion and consensus among three reviewers (SS, HP, and a third team member). The final list of studies included only those meeting all criteria and providing sufficient data for meta-analysis. Methodological quality was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist, ensuring reliability and minimizing bias.
Data extraction was performed independently by SS and HP using a standardized data extraction form. Extracted variables included: (1) Author names; (2) Year of publication; (3) Study location; (4) Age group of recipients; (5) Specific surgical techniques employed, and (6) Clinical outcomes, such as complication rates and overall survival, in pediatric LT recipients.
The methodological rigor of the included studies was appraised using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for studies reporting interventional data. This assessment ensured the reliability and validity of the included evidence. Seven studies of sufficient quality were selected for final analysis.
To synthesize the data, forest plots were utilized to calculate pooled rate ratios (RRs) and corresponding weight percentages for each study, alongside 95% confidence intervals (CIs) to assess the relationship between graft reduction techniques and clinical outcomes in pediatric recipients. Meta-analysis was performed using MetaXL software (Version 5.3) developed by Doi et al[16,17]. Heterogeneity across studies was assessed using the Cochran’s Q test, the I² statistic, and χ2P values. An I² value of 0% indicated negligible heterogeneity, while values above 40% were considered moderate. These heterogeneity metrics guided our interpretation of pooled outcomes. Additionally, a fixed-effect model was employed as a secondary validation step.
Appropriate donor selection is pivotal to achieving favorable outcomes when employing HRLLS grafts. The morphology of the donor’s left lateral segment (LLS) critically influences the feasibility of non-anatomical graft reduction. A thin, flat LLS configuration is ideal for non-anatomical excision, facilitating effective volume reduction without compromising vascular or biliary integrity. Conversely, a bulky or thick LLS—often described as resembling a “puffy fish”—is not suitable for such reduction due to insufficient decrease in graft thickness, which risks large-for-size complications[18].
The graft-to-recipient weight ratio (GRWR) remains a well-established predictor of graft viability. Evidence indicates that a GRWR exceeding 4% is associated with heightened risk of vascular complications and abdominal compartment syndrome, owing to spatial constraints within the recipient’s abdominal cavity[19,20]. Donors with LLS volumes corresponding to GRWR values between 4% and 8%, as assessed via computed tomography volumetry, are typically selected for volume reduction. Non-anatomical resection in such cases can reduce graft volume by approximately 50%, making these grafts more suitable for small pediatric recipients[18,20].
Beyond GRWR, the ratio of graft thickness to the recipient’s anteroposterior abdominal diameter is crucial in determining the need for graft reduction. A graft thickness exceeding the abdominal diameter necessitates reduction to facilitate primary closure of the abdominal wall and to avert complications such as abdominal compartment syndrome. This approach also reduces the necessity for prosthetic mesh closure, which introduces additional risks[19,20].
Recipient abdominal diameter varies based on factors like ascites volume, sarcopenia, and overall growth parameters. Additionally, the indication for LT—such as fulminant hepatic failure—affects abdominal girth, as such cases typically present with minimal ascites compared to chronic liver disease[21]. Hence, sole reliance on GRWR thresholds is insu
The surgical procedure begins with surface marking medial to the falciform ligament, followed by careful isolation of the left portal vein, left hepatic artery, and left hepatic vein. The left hepatic duct is encircled. In LDLT, graft reduction is typically performed in situ, though this approach carries increased risks of blood loss and air embolism[23].
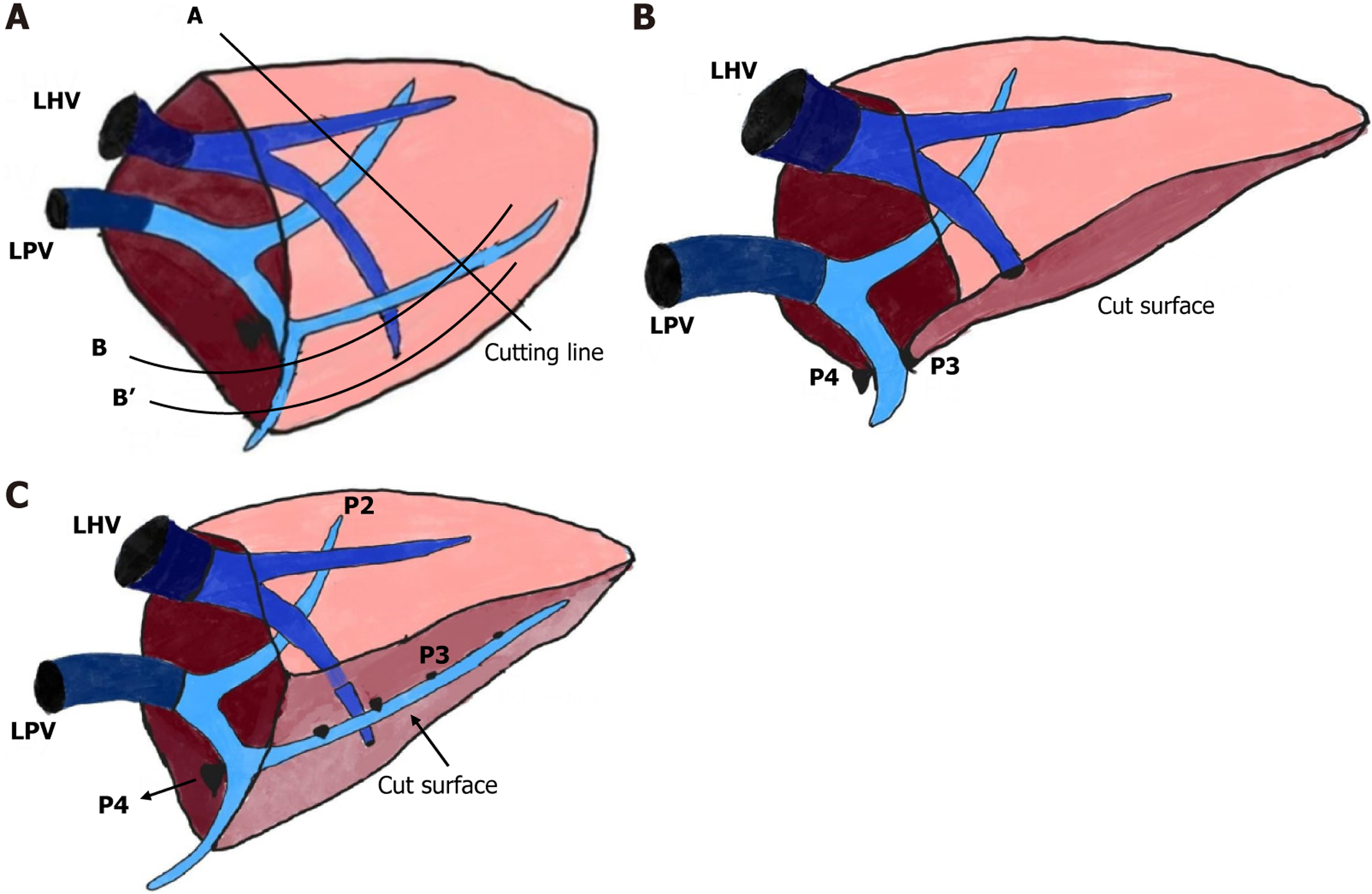
A reduced LLS graft involves excision of the lateral portion of segment II, with the transection line marked vertically along segment II (Figure 2A, Line A). For hyper-reduced LLS grafts, further reduction involves excising both lateral and caudal portions of the LLS. The marking line extends caudally while preserving the Glisson’s capsule of segment III. The cutting margin follows the ventral surface of segment III, along Line B or B’, depending on the desired weight reduction post initial excision along Line A. The transection plane is meticulously aligned perpendicular to intrahepatic Glissonian branches to minimize ischemia[18,19].
While portal vein anatomy guides segmental reduction, anatomical variations in hepatic veins primarily dictate transection lines. Special care is taken to preserve the medial branch of the left hepatic vein while excising the caudal and lateral portions of the LLS in situ[19,23]. Despite careful technique, some grafts may retain excessive thickness, nece
For anatomical reduction resulting in a monosegmental segment II graft, parenchymal transection follows the falciform ligament. Portal vein branches supplying segment II (P2) and segment III (P3) are meticulously dissected. Preoperative imaging and intraoperative evaluation are essential to identify segment III branches, which are then occluded to demarcate segments II and III (Figure 2B)[19,24]. Parenchymal transection follows these lines to preserve segment II while excising segment III. Intraoperative Doppler ultrasonography is employed to confirm portal venous flow post-resection. Care is warranted, as the left hepatic vein typically courses between segments II and III, posing a risk to venous drainage in monosegmental grafts[18,19].
A modified segment II graft technique may be used when the left portal vein bifurcates into P2 and P3 within the parenchyma. In such cases, the anterior surface of segment III is resected without extensive dissection at the umbilical fissure base. The transection line is horizontally placed at the P3 Level (Figure 2C). Ventral branches of P3 emerging at the transection plane are ligated, preserving segment III parenchyma and drainage veins, thus improving graft viability[23,24].
During reconstruction of HRLLS grafts, the left hepatic vein orifice is often narrow, necessitating venoplasty with a patch to enlarge the outflow tract. This narrowing is primarily due to the non-anatomical resection involved in graft hyper-reduction, which can distort or truncate the peripheral portion of the left hepatic vein. Additionally, flat or thin configurations of the donor's left lateral segment often have naturally tapering hepatic veins, further limiting the outflow diameter. In pediatric recipients, the challenge is compounded by the small abdominal cavity; after closure, increased intra-abdominal pressure can compress the already narrow hepatic vein orifice, risking outflow obstruction and graft congestion. To prevent this complication, surgeons create a large venous opening in the recipient's inferior vena cava, often by unifying all three hepatic vein orifices, to ensure an unimpeded outflow from the graft. This is complemented by venoplasty on the graft side to expand the hepatic vein diameter. For portal vein reconstruction, a branch patch technique is employed when the native portal vein is normal. In cases of portal vein hypoplasia, an external iliac vein homograft is used to secure adequate portal inflow[18,19,22].
From an initial pool of 122 studies, eighteen studies met the inclusion criteria and were analyzed to evaluate the efficacy of LLS, hyper-reduced left lateral segmental grafts (HRLLS), and MSG in pediatric LT. The key characteristics of these studies, including donor types, donor and recipient demographics, and surgical techniques employed, are summarized in Table 1. Donors were predominantly living donors (LD), although several studies included deceased donors (DD) as well. The donor mean age ranged between 5.0 to 46.5 years, while the mean donor body weight varied considerably, reflecting the diversity in donor profiles. Various graft reduction techniques were applied across the studies, including MSGs (segment II or III), RLLS, HRLLS (both in situ and back-table), and split LLS grafts. The mean recipient age ranged from 0.5 months to 14 years, with corresponding body weights as low as 2.6 kg, indicating the significant challenge of graft size matching in smaller pediatric patients.
| Ref. | Patients number, donor status | Mean age of donor (years) | Mean body weight of donor (kg) | Reduction technique and segment employed | Mean age of recipient (months) | Mean body weight of recipients (kg) |
| Strong et al[24] | 1, DD | NR | 65.0 | MSG: III (BT) | 4.0 | 4.7 |
| Mentha et al[25] | 1, DD | 22 | 78.0 | MSG: II (BT) | 11.0 | 6.9 |
| Srinivasan et al[14] | 6, DD | 8.8 | 33.7 | RLLS: III (BT) | 1.0 | 3.45 |
| Noujaim et al[26] | 2, DD | 10.0 | 65.0 | MSG: II (BT) | NR | 2.60 |
| Kasahara et al[8] | 14, LD | 32.0 | 61.1 | HRLLS in-situ S: III, HRLLS in-situ S: II | 7.0 | 5.95 |
| Attia et al[27] | 4, DD | 46.5 | 64.5 | HRLLS: III (BT) | 2.8 | 4.90 |
| Grabhorn et al[32] | 10, DD | 5.0 | 25.0 | RLLS: 5, HRLLS: 4, (BT) | 0.5 | 3.25 |
| Enne et al[28] | 12, LD | NR | NR | MSG: III | 12.0 | 6.20 |
| Thomas et al[30] | 8, DD 1, LD | NR | NR | HRLLS: 9 (BT) | 10.0 | 7.50 |
| Shehata et al[3] | 44, LD | 34.0 | NR | MSG III: 26 Reduced MS III: 18 | 7.0 | 5.45 |
| Kanazawa et al[2] | 31, LD | NR | NR | HRLLS (in-situ) | 7.0 | 5.80 |
| Sakamoto et al[20] | 5, LD | 36.0 | 66.6 | MSG: II | 5.0 | 6.20 |
| Sanada et al[31] | 13, LD | NR | NR | MSG II: 12 MSG III: 1 | 1.0 | 3.30 |
| Kitajima et al[29] | 89, LD | 33.0 | NR | HRLLS 47 MSG II: 42 | 7.3 | 5.90 |
| Raices et al[33] | 59, LD | 31.0 | 69.0 | HRLLS (BT) | 14.0 | 8.00 |
| Hirata et al[34] | 25, LD | NR | NR | Medial reduction (in-situ) | 10.0 | 7.10 |
| Zakaria et al[35] | 68, LD 6, DD | 30.0 | NR | LLS Split LLS | 7.4 | 5.60 |
| Malla et al[36] | 7, LD | 28.0 | 76.0 | MSG | 6.11 | 5.60 |
Table 2 outlines the indications for LT among the included studies. The majority of cases were indicated for biliary atresia, followed by fulminant hepatic failure. Other notable indications included progressive familial intrahepatic cholestasis (types I–IV), Alagille syndrome, sclerosing cholangitis, Crigler-Najjar syndrome, methyl malonic acidaemia, and neonatal hemochromatosis. Biliary atresia and fulminant hepatic failure consistently appeared as the leading causes for transplantation in nearly all studies, emphasizing their clinical significance in pediatric LT.
| Ref. | Indications |
| Strong et al[24] | Biliary atresia, re-transplantation and hepatic artery thrombosis (n = 1) |
| Mentha et al[25] | Biliary atresia and re-transplantation (n = 1) |
| Srinivasan et al[14] | Fulminant hepatic failure (n = 5), re-transplantation and hepatic artery thrombosis (n = 1) |
| Noujaim et al[26] | Fulminant hepatic failure (n = 2) |
| Kasahara et al[8] | Biliary atresia (n = 8), fulminant hepatic failure (n = 4) and hepatic artery thrombosis (n = 2) |
| Attia et al[27] | Fulminant hepatic failure (n = 1), biliary atresia (n = 1), medial reduction (n = 1) and re-transplantation (n = 1) |
| Grabhorn et al[32] | Fulminant hepatic failure (n = 10) |
| Enne et al[28] | Biliary atresia (n = 12) |
| Thomas et al[30] | Biliary atresia (n = 9) |
| Shehata et al[3] | Biliary atresia (n = 24), fulminant hepatic failure (n = 16) and medial reduction (n = 4) |
| Kanazawa et al[2] | Biliary atresia (n = 18), fulminant hepatic failure (n = 7) and medial reduction (n = 4) |
| Sakamoto et al[20] | Biliary atresia (n = 4) and methyl malonic acidaemia (n = 1) |
| Sanada et al[31] | Fulminant hepatic failure (n = 8), biliary atresia (n = 2) and others (n = 3) |
| Kitajima et al[29] | Biliary atresia (n = 50), fulminant hepatic failure (n = 17) and others (n = 22) |
| Raices et al[33] | Biliary atresia (n = 46), Alagille syndrome (n = 6) and others (n = 7) |
| Hirata et al[34] | Biliary atresia (n = 25) |
| Zakaria et al[35] | Biliary atresia (n = 28), progressive familial intrahepatic cholestasis (type I, II, III, IV) (n = 15), sclerosing cholangitis (n = 4), Alagille syndrome (n = 3), Crigler-Najjar syndrome (n = 2), Neonatal hemochromatosis (n = 3), Tyrosinaemia (n = 2) Congenital hepatic fibrosis (n = 1) Argininosuccinic aciduria (n = 1), Caroli disease (n = 4), Mitochondrial hepatopathy (n = 2) Fragile X syndrome (n = 1) and Cryptogenic cirrhosis (n = 4) |
| Malla et al[36] | Alloimmune hepatitis (n = 2), biliary atresia (n = 4) and acute liver failure (n = 1) |
The comparative clinical outcomes of MSG vs HRLLS or RLLS grafts are detailed in Table 3. The mean recipient age was observed to be nearly similar in both groups, with no significant differences. However, the mean body weight and the mean donor-to-recipient weight ratio were higher in the HRLLS or RLLS group compared to the MSG group, indicating the necessity for more aggressive graft size reduction in these cases. Similarly, the mean proportion of LD/DD and the mean graft weight were higher in the HRLLS or RLLS group, highlighting the relatively larger initial graft sizes. Regarding complications, an increasing trend was observed in the HRLLS or RLLS group. Specifically, incidences of thrombosis—both hepatic artery thrombosis (HAT) and portal vein thrombosis (PVT)—were marginally higher in this group, averaging between 5%–12%, which aligns with the upper range of published complication rates for pediatric LT. Additionally, occurrences of sepsis and mortality were more frequent among HRLLS or RLLS recipients compared to those who received MSGs. However, these differences did not reach statistical significance in most studies included in the analysis. It is noteworthy that despite the slightly elevated incidence, the overall complication rates in both groups remained within the range reported in the literature (typically 10%–20% for major complications in pediatric recipients), indicating that these surgical approaches maintain an acceptable safety profile when performed in experienced centers.
| Outcome parameters | MSG (n = 152) | HRLLS or RLLS (n = 245) |
| Mean age of recipients (years) | 6.44 | 6.33 |
| Mean body weight of recipients (kg) | 5.39 | 13.92 |
| Mean value of donor to recipient weight ratio | 11.86 | 16.40 |
| Mean proportion LD/DD | 148/4 | 217/34 |
| Mean graft weight (gm) | 167.15 | 277.57 |
| Complications | ||
| Thrombosis as HAT | 1 | 2 |
| Thrombosis as PVT | 1 | 3 |
| Sepsis | 6 | 11 |
| Death | 15 | 22 |
One-year survivability rates across the included studies are summarized in Table 4. The maximum reported survival rate was 96.7%, followed by 94.6%, 82.0%, 81.4%, and 74.0%, with the lowest survival rate recorded at 60.0%. These findings demonstrate that both graft techniques—MSG and HRLLS/RLLS—yield promising long-term outcomes, although some variability exists depending on graft type and institutional experience.
Meta-analysis was conducted to synthesize the one-year survivability outcomes, as illustrated in Figure 3. The forest plot displays the RR, 95%CI, and weight (%) assigned to each of the six studies included in the analysis. The maximum weight contribution was 91.6%, reflecting a large sample size in specific studies. The overall analysis revealed a pooled rate ratio with a 99%CI of 0.91 to 1.08, indicating no statistically significant difference between MSG and HRLLS/RLLS techniques. Additionally, the overall heterogeneity was low, as evidenced by an I² value of 0%, χ2P value of 0.99, and Cochran’s Q value of 8.28. A secondary analysis based on the fixed-effect model showed an I² value of 40% with a χ2P = 0.14 and a 95%CI ranging from -0.04 to 1.05, confirming that both graft techniques are efficacious for pediatric LT.
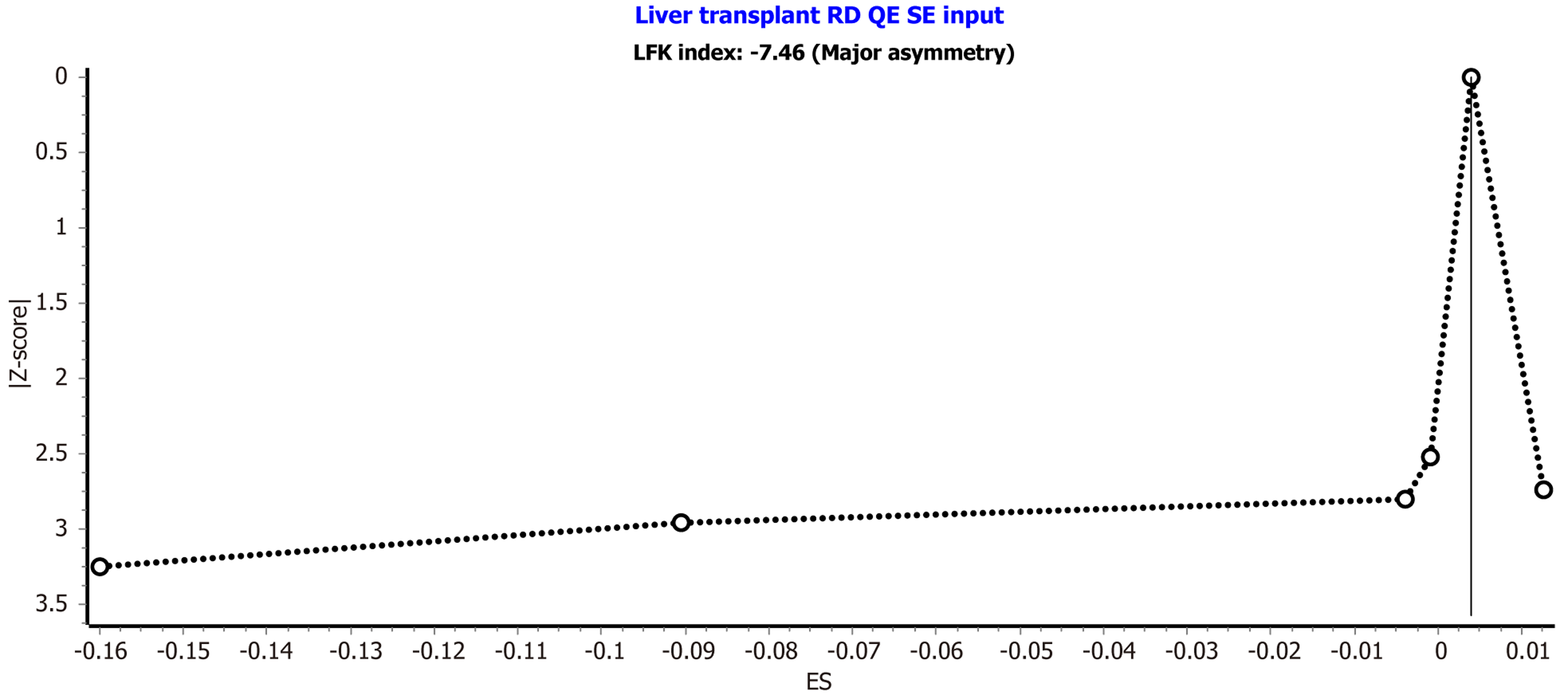
Publication bias was evaluated using the Doi plot presented in Figure 4. The analysis revealed an LFK index of -7.46, indicating major asymmetry in the distribution of studies. This suggests the presence of potential publication bias or small-study effects that should be considered when interpreting the pooled outcomes.
Pediatric LT (pLT) has evolved remarkably over recent decades, providing life-saving treatment for children with end-stage liver diseases. A key challenge in pLT remains the graft size mismatch between adult donor grafts and small pediatric recipients, necessitating specialized graft reduction techniques such as MSG, HRLLS grafts, and RLLS grafts. This discussion synthesizes the current literature on these techniques, focusing on efficacy, surgical considerations, complications, and long-term outcomes.
The primary objective of graft reduction is to mitigate large-for-size syndrome, wherein the graft's volume exceeds the recipient's abdominal cavity, leading to complications such as abdominal compartment syndrome, vascular compression, and impaired graft perfusion. MSG, HRLLS, and RLLS techniques aim to tailor graft volume to recipient anatomy.
Gavriilidis and Hidalgo reviewed 16 studies involving 330 pediatric liver transplants using MSG, HRLLS, and RLLS grafts. They reported an overall patient survival rate of 89% and graft survival rate of 84%, underscoring the viability of these reduction strategies. The median recipient age and weight were 7 months and 5.8 kg, respectively, highlighting their applicability in small infants[23]. Similarly, Shehata et al[3] analyzed 49 LDLT cases involving reduced and hyper-reduced LLS grafts, reporting patient survival rates of 83.7%, 81.4%, and 78.9% at 1, 3, and 10 years, respectively, con
Selection of an appropriate graft reduction strategy depends on factors such as donor anatomy, recipient size, and GRWR. A GRWR exceeding 4% increases the risk of large-for-size complications, necessitating reduction[8]. In com
MSG: Anatomical resection of segment II or III results in a smaller graft optimized for the recipient's size. Kasahara et al[19] were pioneers in demonstrating the feasibility of monosegmental LDLT in small infants. However, MSG requires meticulous preoperative assessment of vascular and biliary anatomy to ensure graft viability and safety[25,26].
HRLLS grafts: HRLLS involves non-anatomical excision of excess liver parenchyma, preserving vasculo-biliary continuity. Namgoong et al[18] reported favorable outcomes with HRLLS grafts in small infants, emphasizing the need for careful donor evaluation and surgical precision to minimize complications. Compared to MSG, HRLLS offers more flexibility in size reduction, especially when anatomical limitations restrict monosegmental resection.
RLLS grafts: RLLS grafts involve non-anatomical reduction of LLS to address size mismatch[27]. Sakamoto et al[20] discussed essential technical considerations for living donor hepatectomy in infants, emphasizing vascular and biliary preservation during RLLS graft preparation.
While these graft reduction techniques have broadened the donor pool and improved access to transplantation in small recipients, they are not without complications.
HAT and PVT are primary concerns in pLT[28]. Shehata et al[3] reported HAT and PVT incidences of 4.1% and 16.3%, respectively, in patients undergoing reduced and hyper-reduced LLS transplantation. To minimize these risks, meti
Biliary leaks and strictures are significant postoperative challenges. Although incidence varies, biliary complications can adversely impact graft function and patient recovery. Techniques like meticulous biliary reconstruction and intraoperative cholangiography are essential to minimize these complications.
Postoperative infections, notably sepsis, significantly affect outcomes. Kitajima et al[29] observed higher bacteremia rates in recipients of non-anatomically reduced LLS grafts compared to reduced-thickness LLS grafts. Reducing operative time, optimizing graft fit, and minimizing blood loss are crucial to mitigating infection risks.
Long-term graft durability has been a key focus in evaluating the efficacy of graft reduction strategies. Kitajima et al[29] reported a 97.6% one-year patient survival rate in reduced-thickness LLS graft recipients, compared to 83% in non-anatomically reduced LLS graft recipients. Thomas et al[30] corroborated these findings, demonstrating favorable outcomes with hyper-reduced grafts in small recipients. Sanada et al[31] further highlighted the utility of monosegmental grafts using their dorsal approach and branch patch technique, particularly beneficial for neonates and infants. In comparison, our review found comparable long-term survivability trends, affirming that both MSG and HRLLS grafts provide durable outcomes, especially when graft tailoring techniques are meticulously applied.
These results emphasize that both HRLLS and MSG techniques can deliver sustainable, long-term outcomes. However, the choice between these graft types should be individualized based on recipient characteristics, donor anatomy, and institutional expertise[29-33]. Raices et al[33] observed a 10-year survival rate of 74% in recipients of hyper-reduced grafts, underscoring their durability.
Advancements in surgical technique have played a pivotal role in improving graft reduction outcomes. Development of non-anatomical HRLLS resection has expanded donor pool utilization and improved size matching for small infants[17,19]. MSG procedures require careful preoperative imaging and intraoperative navigation to preserve vital vasculo-biliary structures. Sanada et al’s dorsal approach to monosegmental grafting underscores the importance of vascular integrity preservation[31].
Kitajima et al[29] stressed the significance of graft thickness reduction, correlating lower complication rates and superior survivability with tailored resection strategies. Biliary complications represent a significant source of morbidity following pediatric LT, particularly in the context of graft reduction techniques. These include bile leaks, strictures, and anastomotic disruptions, all of which can necessitate reoperation or long-term biliary drainage. The technical challenges associated with reduced-size and hyper-reduced grafts, such as limited ductal length and small-caliber anastomoses, increase the risk of such complications. Reported incidence rates of biliary complications range from 10% to 25% in pediatric liver transplants using reduced or monosegmental grafts[3,32]. Although our data synthesis did not permit a uniform calculation of biliary complication rates across all included studies, qualitative analysis indicated a trend toward higher biliary complications in HRLLS and RLLS grafts compared to MSGs. Despite these higher complication rates, the lack of statistically significant differences in survival outcomes (RR: 0.91–1.08; I² = 0%) suggests that, when managed in experienced centers, these complications may not substantially compromise long-term graft viability. MSG, while tech
Recipient selection and preoperative optimization remain critical for successful outcomes. Factors such as nutritional status, liver disease severity, and comorbid conditions must be thoroughly assessed. Hirata et al[34] described how medial reduction of LLS facilitates primary abdominal closure, reducing postoperative morbidity in small recipients. Recipient-specific factors such as nutritional status, underlying liver disease severity, and growth parameters play a pivotal role in graft selection and transplantation outcomes. Malnutrition and sarcopenia are common in pediatric end-stage liver disease and may reduce abdominal cavity capacity, increasing the risk of large-for-size complications[11,34]. Similarly, recipients with acute liver failure typically have reduced ascitic volume and lower abdominal girth, nece
Timely intervention is equally vital, as demonstrated by Zakaria et al[35], who reported that large-for-size grafts can be safely used with appropriate modifications. In comparison, we similarly found that careful recipient selection, pre
Postoperative management is fundamental to maintaining graft function and patient survival. Malla et al[36] recently reported excellent outcomes with monosegmental grafts in small infants, emphasizing stringent postoperative care protocols. Regular follow-up, patient education, and long-term surveillance are essential to early identification of complications. Enne et al’s meta-analysis reinforced the necessity of multidisciplinary, long-term follow-up strategies to sustain positive outcomes[37].
Graft reduction techniques, including HRLLS and MSG, have revolutionized pediatric LT by enabling successful transplantation in infants and small children who would otherwise face significant challenges due to size mismatches. This is supported by our meta-analysis, which found one-year survival rates consistently exceeding 80% for both graft types, with some studies reporting rates as high as 96.7%. The comparable long-term survival rates associated with these techniques underscore their efficacy and durability. Additionally, the meta-analytic pooled rate ratio (RR: 0.91-1.08, 99%CI) indicated no statistically significant difference in one-year survival between MSG and HRLLS/RLLS groups, reinforcing the viability of both approaches. However, the choice of technique should be individualized, taking into account donor and recipient factors, surgical expertise, and institutional experience. Ongoing advancements in surgical techniques, preoperative imaging, and postoperative care are expected to further enhance the outcomes of graft reduction procedures. While complication rates such as hepatic artery thrombosis and sepsis were marginally higher in HRLLS/RLLS recipients, they remained within clinically acceptable limits and did not adversely impact overall survival. Con
| 1. | Borenstein S, Diamond IR, Grant DR, Greig PD, Jones N, Ng V, Roberts E, Fecteau A. Outcome of pediatric live-donor liver transplantation-the Toronto experience. J Pediatr Surg. 2003;38:668-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Kanazawa H, Sakamoto S, Fukuda A, Uchida H, Hamano I, Shigeta T, Kobayashi M, Karaki C, Tanaka H, Kasahara M. Living-donor liver transplantation with hyperreduced left lateral segment grafts: a single-center experience. Transplantation. 2013;95:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Shehata MR, Yagi S, Okamura Y, Iida T, Hori T, Yoshizawa A, Hata K, Fujimoto Y, Ogawa K, Okamoto S, Ogura Y, Mori A, Teramukai S, Kaido T, Uemoto S. Pediatric liver transplantation using reduced and hyper-reduced left lateral segment grafts: a 10-year single-center experience. Am J Transplant. 2012;12:3406-3413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [PubMed] |
| 5. | Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;367-370. [PubMed] |
| 6. | Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. [Transplantation of a donor liver to 2 recipients (splitting transplantation)--a new method in the further development of segmental liver transplantation]. Langenbecks Arch Chir. 1988;373:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 350] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Narasimhan G. Living donor liver transplantation in India. Hepatobiliary Surg Nutr. 2016;5:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 8. | Kasahara M, Sakamoto S, Shigeta T, Uchida H, Hamano I, Kanazawa H, Kobayashi M, Kitajima T, Fukuda A, Rela M. Reducing the thickness of left lateral segment grafts in neonatal living donor liver transplantation. Liver Transpl. 2013;19:226-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Yang SC, Huang CJ, Chen CL, Wang CH, Wu SC, Shih TH, Juang SE, Lee YE, Jawan B, Cheng YF, Cheng KW. Living donor liver transplantation with body-weight more or less than 10 kilograms. World J Gastroenterol. 2015;21:7248-7253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Gül-Klein S, Dziodzio T, Martin F, Kästner A, Witzel C, Globke B, Jara M, Ritschl PV, Henning S, Gratopp A, Bufler P, Schöning W, Schmelzle M, Pratschke J, Öllinger R. Outcome after pediatric liver transplantation for staged abdominal wall closure with use of biological mesh-Study with long-term follow-up. Pediatr Transplant. 2020;24:e13683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Yamada N, Sanada Y, Hirata Y, Okada N, Wakiya T, Ihara Y, Miki A, Kaneda Y, Sasanuma H, Urahashi T, Sakuma Y, Yasuda Y, Mizuta K. Selection of living donor liver grafts for patients weighing 6kg or less. Liver Transpl. 2015;21:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Sakuma Y, Sasanuma H, Miki A, Shimizu A, Sata N, Yasuda Y, Lefor AK, Hirata Y, Yamada N, Okada N, Sanada Y, Ihara Y, Urahashi T, Mizuta K. Living-Donor Liver Transplantation Using Segment 2 Monosegment Graft: A Single-Center Experience. Transplant Proc. 2016;48:1110-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Hong SK, Suh KS, Kim HS, Yoon KC, Ahn SW, Kim H, Yi NJ, Lee KW. Pediatric Living Donor Liver Transplantation Using a Monosegment Procured by Pure 3D Laparoscopic Left Lateral Sectionectomy and In situ Reduction. J Gastrointest Surg. 2018;22:1135-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Srinivasan P, Vilca-Melendez H, Muiesan P, Prachalias A, Heaton ND, Rela M. Liver transplantation with monosegments. Surgery. 1999;126:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 18038] [Article Influence: 1061.1] [Reference Citation Analysis (1)] |
| 16. | Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp Clin Trials. 2015;45:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 443] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 17. | Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials II: The quality effects model. Contemp Clin Trials. 2015;45:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Namgoong JM, Hwang S, Song GW, Kim DY, Ha TY, Jung DH, Park GC, Ahn CS, Kim KM, Oh SH, Kwon H, Kwon YJ. Pediatric liver transplantation with hyperreduced left lateral segment graft. Ann Hepatobiliary Pancreat Surg. 2020;24:503-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Kasahara M, Uryuhara K, Kaihara S, Kozaki K, Fujimoto Y, Ogura Y, Ogawa K, Oike F, Ueda M, Egawa H, Tanaka K. Monosegmental living donor liver transplantation. Transplant Proc. 2003;35:1425-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Sakamoto S, Kanazawa H, Shigeta T, Uchida H, Sasaki K, Hamano I, Fukuda A, Nosaka S, Egawa H, Kasahara M. Technical considerations of living donor hepatectomy of segment 2 grafts for infants. Surgery. 2014;156:1232-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Park CS, Chung YK. Pediatric liver transplantation using hyper-reduced left lateral section and monosegment graft for infant patients: A collective review of Korean experience. Ann Liver Transplant. 2022;2:121-126. [DOI] [Full Text] |
| 22. | Kasahara M, Sakamoto S, Fukuda A. Pediatric living-donor liver transplantation. Semin Pediatr Surg. 2017;26:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Gavriilidis P, Hidalgo E. Alternatives to left lateral sector in paediatric liver transplantation-a systematic review on monosegmental and reduced grafts. Hepatobiliary Surg Nutr. 2022;11:567-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Strong R, Lynch S, Yamanaka J, Kawamoto S, Pillay P, Ong TH. Monosegmental liver transplantation. Surgery. 1995;118:904-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Mentha G, Belli D, Berner M, Rouge JC, Bugmann P, Morel P, Le Coultre C. Monosegmental liver transplantation from an adult to an infant. Transplantation. 1996;62:1176-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Noujaim HM, Mayer DA, Buckles JA, Beath SV, Kelly DA, McKiernan PJ, Mirza DF, de Ville De Goyet J. Techniques for and outcome of liver transplantation in neonates and infants weighing up to 5 kilograms. J Pediatr Surg. 2002;37:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Attia MS, Stringer MD, McClean P, Prasad KR. The reduced left lateral segment in pediatric liver transplantation: an alternative to the monosegment graft. Pediatr Transplant. 2008;12:696-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Enne M, Pacheco-Moreira L, Balbi E, Cerqueira A, Alves J, Valladares MA, Santalucia G, Martinho JM. Hepatic artery reconstruction in pediatric living donor liver transplantation under 10 kg, without microscope use. Pediatr Transplant. 2010;14:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Kitajima T, Sakamoto S, Sasaki K, Narumoto S, Kazemi K, Hirata Y, Fukuda A, Imai R, Miyazaki O, Irie R, Teramukai S, Uemoto S, Kasahara M. Impact of graft thickness reduction of left lateral segment on outcomes following pediatric living donor liver transplantation. Am J Transplant. 2018;18:2208-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Thomas N, Thomas G, Verran D, Stormon M, O'Loughlin E, Shun A. Liver transplantation in children with hyper-reduced grafts - a single-center experience. Pediatr Transplant. 2010;14:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Sanada Y, Hishikawa S, Okada N, Yamada N, Katano T, Hirata Y, Ihara Y, Urahashi T, Mizuta K. Dorsal approach plus branch patch technique is the preferred method for liver transplanting small babies with monosegmental grafts. Langenbecks Arch Surg. 2017;402:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Grabhorn E, Richter A, Fischer L, Ganschow R. Emergency liver transplantation in neonates with acute liver failure: long-term follow-up. Transplantation. 2008;86:932-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Raices M, Czerwonko ME, Ardiles V, Boldrini G, D'Agostino D, Marcó Del Pont J, Pekolj J, Mattera J, Brandi C, Ciardullo M, de Santibañes E, de Santibañes M. Short- and Long-Term Outcomes After Live-Donor Transplantation with Hyper-Reduced Liver Grafts in Low-Weight Pediatric Recipients. J Gastrointest Surg. 2019;23:2411-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Hirata Y, Agarwal S, Varma S, Balradja I, Verma S, Naganathan S, Gupta S. Impact of Medial Reduction of the Left Lateral Segment: A Novel Technique for Living Donor Liver Transplantation for Small Pediatric Recipients. Liver Transpl. 2020;26:1534-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Zakaria HM, Alobthani S, Elsarawy A, Saleh Y, Zidan A, Alabbad S, Elsheikh Y, Algoufi T, Shagrani M, Troisi RI, Broering D. Large for size in pediatrics liver transplant using left lateral segment grafts: A single center experience. Pediatr Transplant. 2021;25:e14044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Malla I, Campaña AG, Podestá G, Selzer E, Baña MT, Iolster T, Panattieri N, Fauda M. Monosegment liver transplantation in small infants. J Liver Transpl. 2024;14:100204. [DOI] [Full Text] |
| 37. | Enne M, Pacheco-Moreira L, Balbi E, Cerqueira A, Santalucia G, Martinho JM. Liver transplantation with monosegments. Technical aspects and outcome: a meta-analysis. Liver Transpl. 2005;11:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/