Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.107461
Revised: April 13, 2025
Accepted: May 21, 2025
Published online: December 18, 2025
Processing time: 240 Days and 11 Hours
Liver transplantation (LT) is the preferred treatment for end-stage liver diseases. Early allograft failure (EAF) can result in death or retransplantation. One of the key factors predicting EAF is the degree of graft injury, which is typically assessed by elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Aminotransferase levels exceeding 5000 U/L within 48 hours of LT are indicative of poor short-term graft survival.
To investigate outcomes in liver transplant recipients with peak aminotransferase levels exceeding 5000 U/L and to identify predictors of EAF.
Adult patients who underwent LT from a deceased (brain-dead) donor between 2011 and 2024 at Hospital de Clínicas de Porto Alegre were screened. Patients with peak AST or ALT levels > 5000 U/L post-LT were included, excluding those with vascular thrombosis. EAF was defined as death or re-transplantation within 90 days. A receiver operating characteristic curve were generated for each EAF predictor to determine the area under the curve (AUC). Sensitivity, specificity, negative predictive value, and positive pre
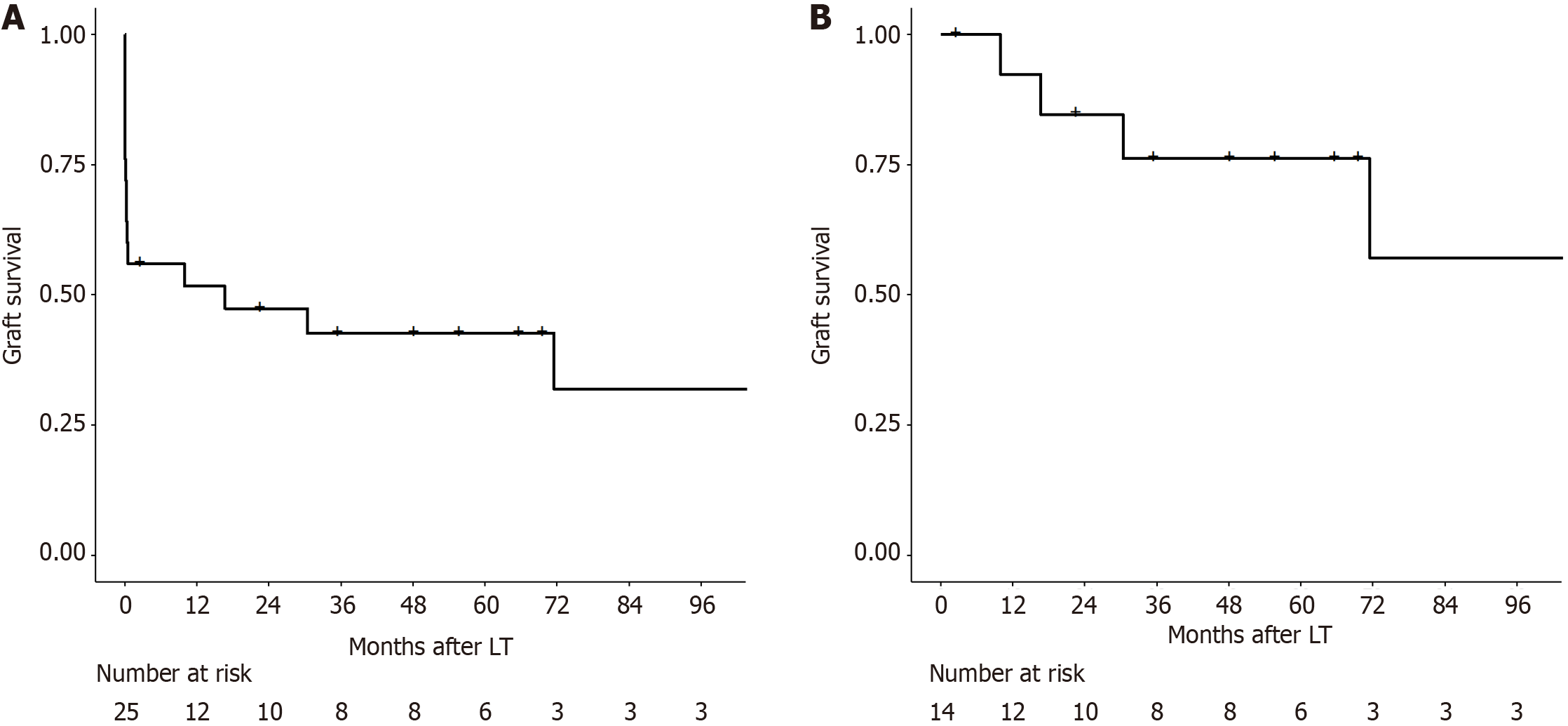
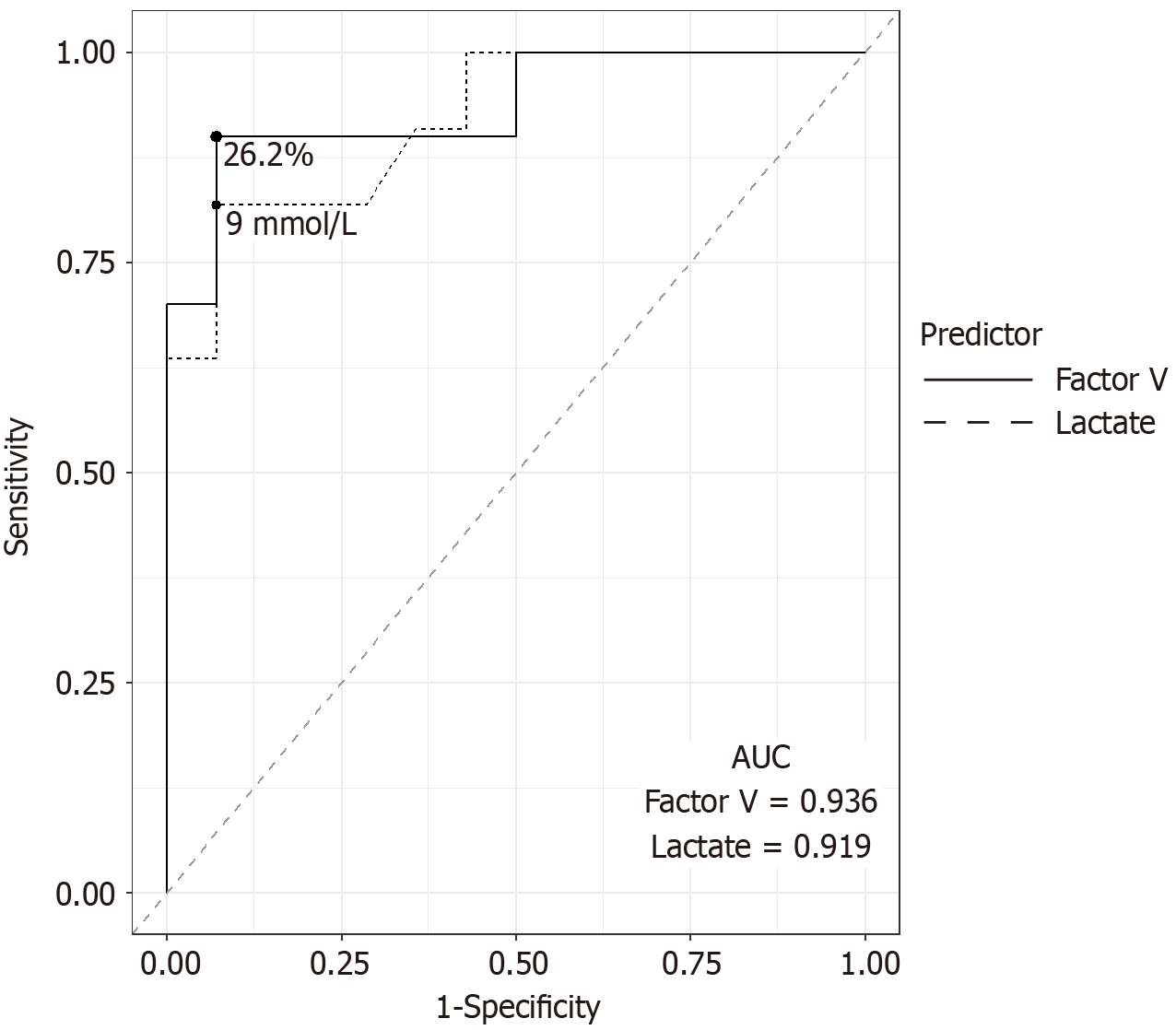
Between 2011 and 2024, 341 patients underwent LT. Of these, 29 (8.5%) patients had AST and/or ALT levels exceeding 5000 U/L within the first 48 hours post-LT. Four patients were excluded due to vascular thrombosis, resulting in a study cohort of 25 patients. EAF were also observed in 11 patients. One-year and five-year graft survival rates were 51.7% and 42.6%, respectively. For patients without EAF, one-year and five-year graft survivals were 92.3% and 76.2%, respectively. The key predictors of EAF included serum factor V and arterial lactate levels on postoperative day (POD) 1, with AUCs of 0.936 and 0.919, respectively. The optimal cutoff for EAF prediction were 26.2% for serum factor V and 9 mmol/L for arterial lactate.
Aminotransferase levels > 5000 U/L were associated with high EAF risk. However, favorable graft function indicators on POD 1 were associated with long-term survival comparable to that of general LT recipients. Serum factor V and arterial lactate levels emerged as valuable prognostic markers.
Core Tip: Among 341 liver transplant recipients, 25 had an aminotransferase level > 5000 U/L. Early allograft failure (death or re-transplantation within 90 days) occurred in 11 patients. One-year and five-year graft survival rates were 51.7% and 42.6%, respectively. However, approximately 50% of these patients withstood initial graft injury and had a satisfactory long-term prognosis. Therefore, early identification of patients who will recover graft function or undergo graft loss is paramount. Serum factor V and arterial lactate levels can aid in deciding whether to relist patients to retransplantation early in the postoperative period.
- Citation: Lazzarotto-da-Silva G, Chaves BM, Feier FH, Rodrigues PD, Grezzana-Filho TJM, de Araujo A, Alvares-da-Silva MR, Marchiori RC, Chedid MF, Kruel CRP. Serum factor V and arterial lactate levels predict graft survival in liver transplant recipients with aminotransferase above five thousand. World J Transplant 2025; 15(4): 107461
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/107461.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.107461
Liver transplantation (LT) is the preferred treatment for end-stage liver disease[1]. Over the past few decades, LT outcomes have significantly improved, with 1-year and 5-year patient survival rates reaching 92% and 81%, respectively[2]. However, early allograft failure (EAF) remains a critical issue, often resulting in death or retransplantation[3-5].
A key predictor of EAF is the extent of graft injury, which is typically assessed using alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels[6-8]. Extreme liver graft injury, defined as ALT and/or AST levels exceeding 5000 U/L, is associated with poor short-term graft survival[9]. Thus, early relisting of patients predicted to lose their graft may optimize outcomes[10,11]. However, as some patients with peak aminotransferase levels exceeding 5000 U/L manage to survive the initial post-LT phase, approximately 50% according to previous studies[7,12], rendering retransplantation unnecessary, early distinction between patients who will and will not necessitate retransplantation is paramount.
This study aimed to characterize outcomes in liver transplant recipients with peak aminotransferase levels > 5000 U/L and identify predictors of EAF.
This retrospective analysis utilized a prospectively collected dataset from the Hospital de Clínicas de Porto Alegre Liver Transplant Program. Adult patients (aged > 18 years) who underwent LT from a brain-dead donor between 2011 and 2024 were screened. Only those with peak aminotransferase (ALT and/or AST) levels > 5000 U/L within the first 48 hours after LT were included in the study. Patients with hepatic artery thrombosis or postoperative portal vein thrombosis were excluded.
Pre-transplant model for end stage liver disease (MELD)-Na score[13] and Child-Pugh-Turcotte classification[14] were calculated based on laboratory and clinical parameters. The etiology of liver disease was evaluated based on clinical features. Pathological reports of liver explants were reviewed for patients with an unknown etiology on clinical grounds. The following laboratory data were obtained on postoperative day (POD) 1: (1) AST; (2) ALT; (3) Total bilirubin; (4) Serum factor V; (5) Arterial lactate; (6) Prothrombin time (international normalized ratio); and (7) Serum creatinine levels. The laboratory parameters on POD 1 were generally obtained between 12 hours and 24 hours after the end of surgery. AST and ALT levels were measured on POD 2. Peak ALT and AST levels were defined as the highest levels of ALT and AST within the first 48 hours post-transplantation. The laboratory MELD score on POD 1 was calculated as described by Malinchoc et al[15]. Doppler ultrasound was used to screen for vascular complications in the first week after LT. Patients with abnormal Doppler ultrasound findings, such as absent arterial or portal flow or an arterial resistive index below 0.5 or above 0.9, underwent computed tomography angiography.
Graft offers were evaluated by consensus between transplant surgeons and hepatologists. Graft biopsy was performed selectively when the donor surgeon judged the liver allograft to have a steatotic appearance. In general, only grafts with < 30% macrovesicular steatosis were used.
EAF was defined as graft failure (patient death or need for retransplantation) on POD 90[16,17]. The cause of graft failure and the most likely cause of peak aminotransferase levels > 5000 U/L were independently reviewed by two investigators (Lazzarotto-da-Silva G and Chaves BM). A receiver operating characteristic (ROC) curve was plotted for each EAF predictor to calculate the area under the curve (AUC). Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated for each predictor’s best cutoff, as defined by the Youden Index. Survival curves were plotted using the Kaplan-Meier method. The day of LT was considered day zero.
This study complied with the ethical standards and was approved by the Institutional Review Board of the Hospital de Clínicas de Porto Alegre. The requirement for informed consent was waived by the ethics committee because of its observational and retrospective design.
Continuous data were expressed as mean and SD or median and interquartile range (IQR) and compared using Student’s t-test or the Mann-Whitney U test, as appropriate. Categorical data were expressed as proportions. All comparisons were two-sided with a significance level of 0.05. All analyses were performed using R for MacOS (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
Between 2011 and 2024, 341 patients underwent LT. Of these, 29 (8.5%) patients had AST and/or ALT levels exceeding 5000 U/L within the first 48 hours post-LT. Four patients were excluded because of hepatic arterial thrombosis (n = 2) and postoperative portal vein thrombosis (n = 2), leaving 25 patients for the study. The detailed characteristics of the patients are presented in Table 1. Thirteen patients (52%) were male. The median patient age was 54 years (IQR: 45–60 years). The most common etiology of liver disease was hepatitis C virus viral infection (14 patients, 56%). Three patients (12%) underwent retransplantation. The median MELD-Na score was 13 (IQR: 10–17).
| Number | 25 |
| Age (years), median (IQR) | 54 (45-60) |
| Male sex | 13 (52) |
| Etiology of liver disease | |
| Hepatitis C virus | 14 (56) |
| Hepatitis B virus | 2 (8) |
| Alcohol | 2 (8) |
| Biliary | 2 (8) |
| Acute liver failure | 2 (8) |
| Polycystic liver disease | 1 (4) |
| Metabolic-dysfunction-associated fatty liver disease | 1 (4) |
| Autoimmune hepatitis | 1 (4) |
| Child-Pugh-Turcotte classification | |
| A | 9 (36) |
| B | 9 (36) |
| C | 7 (28) |
| Model for end stage liver disease-Na, median (IQR) | 13 (10-17) |
| Aspartate aminotransferase | 8053 (6730-11340) |
| Alanine aminotransferase | 4135 (2980-6414) |
| Serum factor V | 28 (14-46) |
| Arterial lactate | 4.4 (2.6-11.6) |
The graft survival rates at one year and five years were 51.7% and 42.6%, respectively (Figure 1A). EAF were also observed in 11 patients. Only 1 of these patients underwent retransplantation within 90 days post-LT and was alive after one year of follow-up. The cause of EAF was primary non-function in 6 patients, hemorrhagic shock due to intraoperative or postoperative bleeding in 3 patients, and abdominal compartment syndrome due to large-for-size graft syndrome in 1 patient. One patient experienced postoperative bleeding and was found to have biliary necrosis and extensive ischemic areas in the liver during laparotomy, in the absence of arterial complications. Median peak AST was higher in patients who suffered EAF in comparison to patients who did not (10390 U/L vs 7246 U/L, P = 0.05). The median peak ALT level was comparable between the groups (4217 U/L vs 3852 U/L, P = 0.256).
Among the 14 patients who did not develop EAF, one-year and five-year graft survival rates were 92.3% and 76.2%, respectively (Figure 1B). Only 1 patient in this subgroup underwent retransplantation 302 days after LT.
The prognostic yields of the EAF predictors on POD 1 are shown in Table 2. Serum factor V had the highest AUC (0.936). The optimal cut-off value for serum factor V was 26.2%. The sensitivity, specificity, NPV, and PPV of the cut-off were 90%, 92.8%, 92.8%, and 90%, respectively. Arterial lactate levels also displayed a similar AUC (0.919). The optimal cut-off value was 9 mmol/L. The sensitivity, specificity, NPV, and PPV were 81.8%, 92.8%, 86.7%, and 90%, respectively. All eight patients with both serum factor V below 26.2% and lactate levels above 9 mmol/L had EAF (Figure 2). The ROC curves for serum factor V and arterial lactate levels are shown in Figure 3.
| Predictor | Area under the curve | Cutoff | Sensitivity | Specificity | Negative predictive value | Positive predictive value |
| Factor V | 0.936 | 26.2% | 90 | 92.8 | 92.8 | 90 |
| Lactate | 0.919 | 9 mmol/L | 81.8 | 92.8 | 86.7 | 90 |
| Bilirubin | 0.672 | 4.4 mg/dL | 45 | 92.8 | 68.4 | 83.3 |
| International normalized ratio | 0.825 | 2.7 | 90.1 | 64.3 | 90 | 66.7 |
| Laboratory model for end stage liver disease | 0.870 | 27 | 81.8 | 78.6 | 84.6 | 75 |
| Aspartate aminotransferase | 0.669 | 6947 U/L | 90.1 | 57.1 | 88.9 | 62.5 |
| Alanine aminotranferase | 0.575 | 7235 U/L | 36.4 | 100 | 66.7 | 100 |
Although statistically higher in patients with EAF, the AUC of the peak AST level was 0.559, which was considerably lower than that of the aforementioned predictors. However, none of the seven patients with an AST peak of < 6800 U/L had EAF. The most likely cause of peak aminotransferase > 5000 U/L in each patient is listed in Table 3, along with peak AST, ALT, factor V, and serum lactate levels.
| Peak aspartate aminotransferase (U/L) | Peak alanine aminotransferase (U/L) | Factor V POD 1 | Lactate POD 1 | Early allograft failure | Most likely cause of peak aminotransferase > 5000 U/L |
| 14256 | 4440 | 33.9 | 2.3 | No | Long cold ischemia time (540 minutes) and warm ischemia time (56 minutes) |
| 6491 | 2980 | 27.3 | 4.1 | No | Arterio-portal fistula secondary to core needle biopsy of the allograft |
| 7763 | 4479 | 67.9 | 2.58 | No | Intraoperative and/or postoperative bleeding |
| 8511 | 5545 | 14.6 | 8.9 | No | Intraoperative and/or postoperative bleeding |
| 8053 | 3030 | 46.3 | 4.4 | No | Unclear/liver with ischemic areas at relaparotomy |
| 5151 | 1736 | 35.6 | 11.5 | No | Intraoperative and/or postoperative bleeding |
| 5415 | 4135 | 27.6 | 1.41 | No | Unclear |
| 6730 | 2740 | 46.3 | 5.8 | No | Long warm ischemia time (60 minutes) |
| 5138 | 2661 | 35.8 | 1.6 | No | Biopsy-proven graft steatosis |
| 11340 | 5188 | 28.5 | 1.96 | No | Low initial portal inflow |
| 10120 | 6860 | 57.4 | 3.5 | No | Right hepatic vein thrombosis causes ischemia in right posterior liver section |
| 6166 | 3568 | 66 | 1.1 | No | Long cold ischemia time (645 minutes) |
| 6450 | 2830 | 54 | 1.2 | No | Severe postoperative hemodynamic instability |
| 13394 | 6414 | 63.2 | 3.7 | No | Abdominal compartment syndrome due to large-for-size-graft |
| 10995 | 8971 | Not applicable | 11.1 | Yes | Primary nonfunction |
| 7164 | 4135 | 10 | 17.4 | Yes | Primary nonfunction |
| 10390 | 1655 | 9.9 | 18.1 | Yes | Intraoperative and/or postoperative bleeding |
| 27840 | 10320 | 37.5 | 3.61 | Yes | Abdominal compartment syndrome due to large-for-size-graft |
| 12628 | 8166 | 17 | 14.8 | Yes | Intraoperative and/or postoperative bleeding |
| 8276 | 3997 | 6.8 | 14.7 | Yes | Primary nonfunction |
| 7543 | 3355 | 13.3 | 11.6 | Yes | Primary nonfunction |
| 13900 | 6589 | 3.7 | 16 | Yes | Primary nonfunction |
| 7857 | 4217 | 13 | 9.2 | Yes | Primary nonfunction |
| 7708 | 2681 | 25.2 | 4.1 | Yes | Intraoperative and/or postoperative bleeding |
| 15115 | 7610 | 14.2 | 18 | Yes | Intraoperative and/or postoperative bleeding |
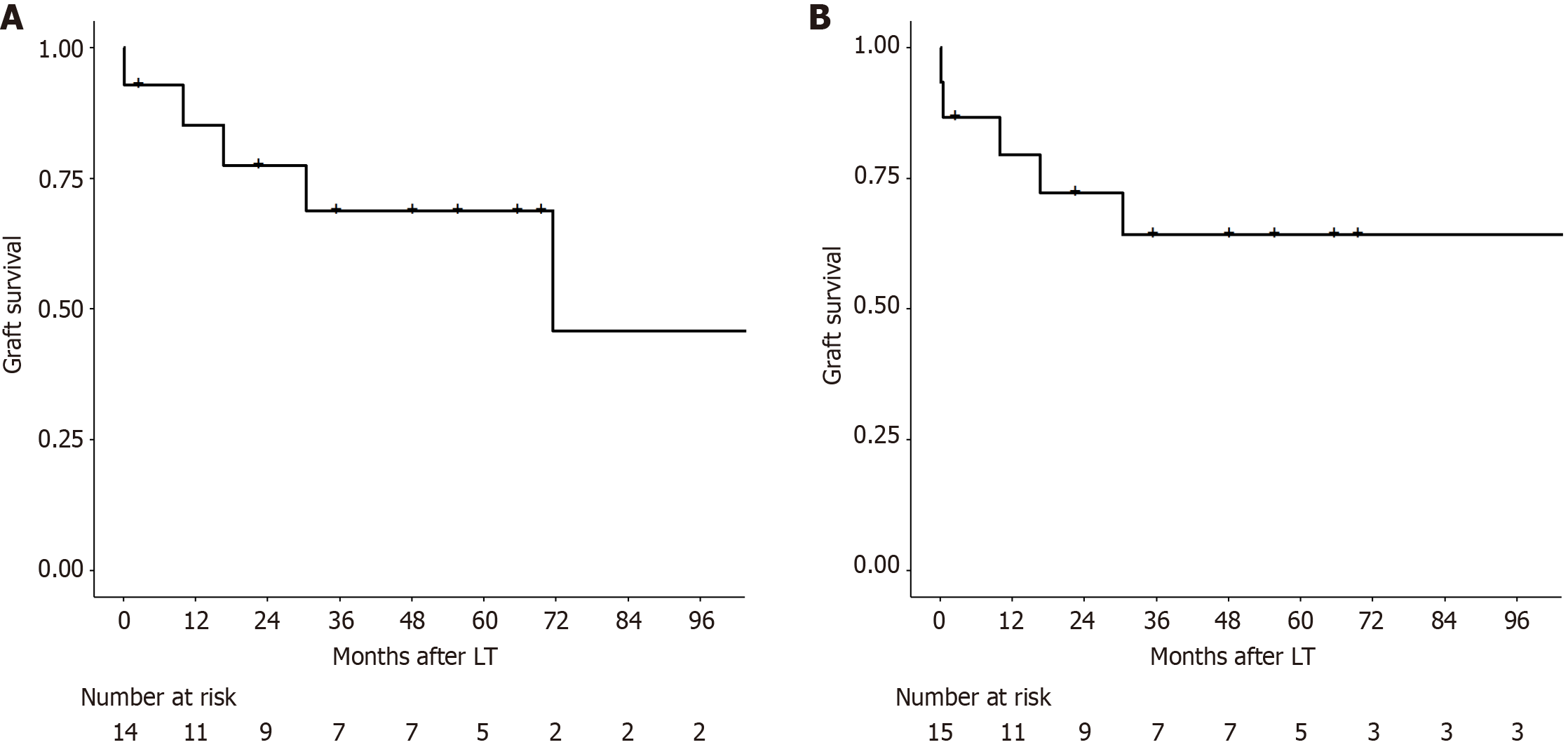
Fourteen patients had serum factor V > 26.2% on POD 1. Only one those patient had EAF, which occurred on POD 3. One-year and 5-year graft survival rates in patients with factor V levels above the cutoff were 85.1% and 68.8%, respectively. Conversely, 15 patients had arterial lactate levels < 9 mmoL on POD 1. Two of these patients developed EAF on POD 3 and 15. One-year and 5-year graft survival rates were 79.4% and 64.2%, respectively.
Elevated aminotransferase levels in the early postoperative period are considered an ominous sign in LT. Most definitions of early graft dysfunction or poor initial graft function include AST and/or ALT in their criteria[3,6,18,19]. Additionally, an aminotransferase level elevation above 5000 U/L has been suggested as an indication for early retransplantation due to the poor prognosis of these patients[9].
In the present study, 25 patients with aminostransferase exceeding 5000 U/L within the first 48 hours after LT in the absence of vascular inflow thrombosis were evaluated for short-term and long-term outcomes. Forty-four percent of the patients died or underwent retransplantation by POD 90, which is consistent with previous reports on graft survival in this population[7,9,12]. Despite the initial poor outcome, our findings indicate that patients who withstand the initial insult and survive beyond POD 90 exhibit 1-year and 5-year graft survival rates similar to those of patients who are not exposed to severe hepatocellular injury. Our findings also showed that serum factor V and arterial lactate levels are adequate tools for predicting EAF in this population.
Given the inferior results of liver retransplantation compared to primary LT[20-22], accurately identifying recipients who are likely to recover graft function, not only to ensure judicious utilization of liver grafts, but also to prevent unnecessary risks to the patient. However, once the inevitable evolution to graft failure is identified, retransplantation should be attempted before irreversible shock and multi-organ failure ensue. Therefore, it is crucial to correctly identify which patients are at an increased risk of EAF and which are likely to recover their graft function as soon as possible. In this study, we explored the performance of several laboratory parameters of liver function on POD 1 in predicting EAF. Serum factor V and arterial lactate levels on POD 1 were the two best predictors of EAF. Two previous publications from our group have shown an association between serum factor V on POD 1, mortality and graft failure[23,24]. Additionally, a high post-LT arterial lactate concentration has been correlated with poor graft survival[25-28], although a recent large cohort study did not corroborate these findings[29].
When confronted with a condition for which the only available effective treatment is a high-risk procedure, such as retransplantation, clinicians should strive to minimize overtreatment. In other words, they should avoid indicating the treatment to patients who do not require it. Therefore, a test with adequate specificity and NPV is necessary to minimize the number of false positives. Importantly, both serum factor V and arterial lactate levels on POD 1 showed a specificity > 90%. Furthermore, patients with serum factor V levels > 26.2% and lactate levels < 9 mmol/L on POD 1 had 1-year graft survival rates of 85% and 79.4%, respectively, which were significantly better than those of liver retransplantation[21,30]. Thus, we suggest that these tests should be incorporated into the decision-making process for patients with aminotransferase levels > 5000 U/L.
In previous publications as well as in the present study, an aminotransferase cutoff of 5000 U/L was set arbitrarily[7,9,12]. In our findings, although peak AST level was not among the best predictors of EAF, no patient with peak AST between 5000 U/L and 6800 U/L had EAF. A similar trend was not observed for ALT levels. Additionally, some patients achieved long-term graft survival despite a peak AST over 10000 U/L. Therefore, our findings suggest that although aminotransferase above 5000 U/L in the early posttransplant period should be regarded as a “red flag”, the elevation per se is insufficient to determine which patients will suffer graft loss. The decision on whether a patient needs to undergo retransplantation should rely on liver function parameters instead of hepatocellular injury findings.
Heterogeneity in the cause of elevated aminotransferase levels may be considered a confounding factor in our study. In at least seven patients, peak aminotransferase levels > 5000 U/L were attributed to either intraoperative or postoperative bleeding. In these cases, excessive bleeding was likely a manifestation of coagulopathy that could be ascribed to graft dysfunction or delayed graft function, both of which are closely related to the degree of graft injury. Thus, we believe that the cause of aminotransferase elevation in most cases was ultimately graft injury in some of the following phases: (1) Pre-preservation (e.g., steatosis); (2) Preservation (e.g., cold-preservation injury and rewarming injury); and (3) Reperfusion
Our study had some limitations. First, this was a retrospective study with a small sample size. Second, we did not have data on whether the patients received fresh frozen plasma, which could potentially influence serum factor V levels. However, in a previous study by our group, patients with low serum factor V levels received more fresh frozen plasma intraoperatively, demonstrating that the transfusion of plasma clotting factors had little impact on the interpretation of serum factor V results[24]. Third, as few centers use serum factor V in the post-transplant period, extrapolating our results to other transplant services might be difficult. Ideally, the results of this study should be evaluated in a large prospective multicenter study.
In conclusion, aminotransferase levels exceeding 5000 U/L in LT recipients was associated with a high rate of graft loss within 90 days. However, patients demonstrating adequate graft function on POD 1 exhibited long-term graft survival comparable to general LT recipients, despite severe hepatocellular injury. Both serum factor V and arterial lactate concentrations appear to be effective indicators for evaluating prognosis in the early phase of LT.
| 1. | Lucey MR, Furuya KN, Foley DP. Liver Transplantation. N Engl J Med. 2023;389:1888-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 2. | Kwong AJ, Ebel NH, Kim WR, Lake JR, Smith JM, Schladt DP, Schnellinger EM, Handarova D, Weiss S, Cafarella M, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2021 Annual Data Report: Liver. Am J Transplant. 2023;23:S178-S263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 200] [Reference Citation Analysis (0)] |
| 3. | Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 809] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 4. | González FX, Rimola A, Grande L, Antolin M, Garcia-Valdecasas JC, Fuster J, Lacy AM, Cugat E, Visa J, Rodés J. Predictive factors of early postoperative graft function in human liver transplantation. Hepatology. 1994;20:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 208] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Deschênes M, Belle SH, Krom RA, Zetterman RK, Lake JR. Early allograft dysfunction after liver transplantation: a definition and predictors of outcome. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Transplantation. 1998;66:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 185] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Nanashima A, Pillay P, Verran DJ, Painter D, Nakasuji M, Crawford M, Shi L, Ross AG. Analysis of initial poor graft function after orthotopic liver transplantation: experience of an australian single liver transplantation center. Transplant Proc. 2002;34:1231-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Glanemann M, Langrehr JM, Stange BJ, Neumann U, Settmacher U, Steinmüller T, Neuhaus P. Clinical implications of hepatic preservation injury after adult liver transplantation. Am J Transplant. 2003;3:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Pokorny H, Gruenberger T, Soliman T, Rockenschaub S, Längle F, Steininger R. Organ survival after primary dysfunction of liver grafts in clinical orthotopic liver transplantation. Transpl Int. 2000;13 Suppl 1:S154-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Hoyer DP, Sotiropoulos GC, Saner FH, Treckmann JW, Paul A, Mathé Z. MELD at POD 1 as a predictor of outcome in liver allografts with peak AST >5000 U/l. Transpl Int. 2014;27:1285-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Doyle HR, Morelli F, McMichael J, Doria C, Aldrighetti L, Starzl TE, Marino IR. Hepatic Retransplantation--an analysis of risk factors associated with outcome. Transplantation. 1996;61:1499-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Powelson JA, Cosimi AB, Lewis WD, Rohrer RJ, Freeman RB, Vacanti JP, Jonas M, Lorber MI, Marks WH, Bradley J. Hepatic retransplantation in New England--a regional experience and survival model. Transplantation. 1993;55:802-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Rosen HR, Martin P, Goss J, Donovan J, Melinek J, Rudich S, Imagawa DK, Kinkhabwala M, Seu P, Busuttil RW, Shackleton CR. Significance of early aminotransferase elevation after liver transplantation. Transplantation. 1998;65:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1104] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 14. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5826] [Article Influence: 109.9] [Reference Citation Analysis (2)] |
| 15. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2107] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 16. | Agopian VG, Harlander-Locke MP, Markovic D, Dumronggittigule W, Xia V, Kaldas FM, Zarrinpar A, Yersiz H, Farmer DG, Hiatt JR, Busuttil RW. Evaluation of Early Allograft Function Using the Liver Graft Assessment Following Transplantation Risk Score Model. JAMA Surg. 2018;153:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 17. | Avolio AW, Franco A, Schlegel A, Lai Q, Meli S, Burra P, Patrono D, Ravaioli M, Bassi D, Ferla F, Pagano D, Violi P, Camagni S, Dondossola D, Montalti R, Alrawashdeh W, Vitale A, Teofili L, Spoletini G, Magistri P, Bongini M, Rossi M, Mazzaferro V, Di Benedetto F, Hammond J, Vivarelli M, Agnes S, Colledan M, Carraro A, Cescon M, De Carlis L, Caccamo L, Gruttadauria S, Muiesan P, Cillo U, Romagnoli R, De Simone P. Development and Validation of a Comprehensive Model to Estimate Early Allograft Failure Among Patients Requiring Early Liver Retransplant. JAMA Surg. 2020;155:e204095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Ardite E, Ramos C, Rimola A, Grande L, Fernández-Checa JC. Hepatocellular oxidative stress and initial graft injury in human liver transplantation. J Hepatol. 1999;31:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 933] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 20. | Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A; All contributing centers (www. eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 663] [Article Influence: 47.4] [Reference Citation Analysis (5)] |
| 21. | Kumar N, Wall WJ, Grant DR, Bloch M, Ghent CN, Adams PC, Marotta P. Liver retransplantation. Transplant Proc. 1999;31:541-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Mora NP, Klintmalm GB, Cofer JB, Poplawski SS, Goldstein RM, Gonwa TA, Husberg BS. Results after liver retransplantation (RETx): a comparative study between "elective" vs "nonelective" RETx. Transplant Proc. 1990;22:1509-1511. [PubMed] |
| 23. | Zulian MC, Chedid MF, Chedid AD, Grezzana Filho TJ, Leipnitz I, de Araujo A, Alvares-da-Silva MR, Cardoni MG, Guimaraes LS, Kruel CD, Kruel CR. Low serum factor V level: early predictor of allograft failure and death following liver transplantation. Langenbecks Arch Surg. 2015;400:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 24. | Gorgen A, Prediger C, Prediger JE, Chedid MF, Backes AN, de Araujo A, Grezzana-Filho TJM, Leipnitz I, Chedid AD, Alvares-da-Silva MR, Sapisochin G, Kruel CRP. Serum Factor V Is a Continuous Biomarker of Graft Dysfunction and a Predictor of Graft Loss After Liver Transplantation. Transplantation. 2019;103:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Kim S, Zerillo J, Tabrizian P, Wax D, Lin HM, Evans A, Florman S, DeMaria S Jr. Postoperative Meld-Lactate and Isolated Lactate Values As Outcome Predictors Following Orthotopic Liver Transplantation. Shock. 2017;48:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Cardoso N, Silva T, Cagnolati D, Freitas T, Mente ED, Basile-Filho A, Castro e Silva O. Can joint analysis of postoperative MELD, base excess and blood lactate levels be used as an index of postoperative outcome for patients submitted to liver transplantation? Acta Cir Bras. 2013;28 Suppl 1:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Coeckelenbergh S, Drouard L, Ickx B, Lucidi V, Germanova D, Desebbe O, Duhaut L, Moussa M, Naili S, Vibert E, Samuel D, Duranteau J, Vincent JL, Rinehart J, Van der Linden P, Joosten A. Arterial Lactate Concentration at the End of Liver Transplantation is Independently Associated With One-Year Mortality. Transplant Proc. 2023;55:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Golse N, Guglielmo N, El Metni A, Frosio F, Cosse C, Naili S, Ichaï P, Ciacio O, Pittau G, Allard MA, Castaing D, S A Cunha A, Cherqui D, Adam R, Vibert E. Arterial Lactate Concentration at the End of Liver Transplantation Is an Early Predictor of Primary Graft Dysfunction. Ann Surg. 2019;270:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Galli AM, Kothari R, Adelmann D, Holm Z, Bokoch MP, De Gasperi A, Niemann CU, Kolodzie K. Lactate concentration at the end of liver transplant: Early predictor of graft function or just one piece of the puzzle? Clin Transplant. 2023;37:e15057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | Dakroub A, Anouti A, Cotter TG, Lee WM. Mortality and Morbidity Among Adult Liver Retransplant Recipients. Dig Dis Sci. 2023;68:4039-4049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/