Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.104111
Revised: April 13, 2025
Accepted: May 10, 2025
Published online: December 18, 2025
Processing time: 341 Days and 19.3 Hours
Normothermic machine perfusion (NMP) utilizing OCS Liver is becoming increasingly common in adult liver transplantation (LT), but not in pediatric transplantation. OCS Liver has been shown to decrease post-reperfusion synd
Three pediatric patients, all with different etiologies of liver disease, were successfully transplanted with livers preserved with NMP. All patients are currently doing well with follow-ups of 7 to 16 months post-transplant.
The use of OCS Liver in pediatric LT is feasible and can be performed even in the sickest recipients with excellent outcomes. Utilization of OCS Liver can optimize donor and recipient factors to allow for optimal outcomes.
Core Tip: We report the first published use of normothermic machine perfusion in pediatric liver transplant recipients.
- Citation: Hwang CS, Shubin AD, Aqul A, Sanchez-Vivaldi JA, Colvill KM, MacConmara MP, Kadakia Y, Johansen C, Shah JA, Hanish SI, Vagefi PA, Patel MS. Utilization of normothermic machine perfusion in pediatric liver transplantation: Three case reports. World J Transplant 2025; 15(4): 104111
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/104111.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.104111
Waitlist mortality amongst children listed for pediatric liver transplantation (LT) has decreased over the past decade but remains unacceptably high. Despite being granted the highest allocation priority, 1 in 10 will die while waiting for a suitable donor liver[1].
Normothermic machine perfusion (NMP) maintains the ex-vivo donor liver in a normal physiologic state by perfusing the organ with warm, oxygenated blood and nutrients[2]. This results in superior recipient outcomes compared to static cold storage[3,4]. NMP also allows for longer preservation of the liver when compared to cold storage[5], assessment of organ function prior to transplantation, and minimization of ischemia reperfusion injury. In 2021 the United States Food and Drug Administration approved the first commercially available NMP device, known as Organ Care System (OCS, TransMedics, Andover, MA, United States) for use in the United States. NMP has been shown to significantly increase the number of livers utilized in adult LT[6].
Although there has been significant interest expressed in utilization of NMP in pediatric LT, all existing experience has been exclusive to adult recipients[7]. We report the first case series where pediatric patients were transplanted with livers preserved using NMP.
The first patient had fulminant hepatic failure. The second patient had liver cirrhosis from Progressive Familial Intrahepatic Cholestasis Type 3. The third patient had cirrhosis secondary to Fontan associated liver disease. All three were listed for LT with the third patient being jointly listed for liver and heart transplantation.
The first patient is a previously healthy 13 year-old who presented with a 3-day history of abdominal pain, nausea, and emesis. He was found to have fulminant hepatic failure and was listed for LT. Over the next 24 hours he was transferred to the intensive care unit, intubated, and started on molecular adsorbent recirculating system treatment. The second patient is an 8 year-old male with a history of liver disease secondary to Progressive Familial Intrahepatic Cholestasis Type 3 and listed for LT at 4 years of age. The third patient is a 19-year-old female with a past medical history significant for hypoplastic left heart syndrome status postFontan and cirrhosis secondary to Fontan associated liver disease. She was listed for combined heart-liver transplant. With her cardiac and liver disease, she was referred to a pediatric hospital prior to the age of 18 years for evaluation of combined heart and LT.
Prior to the presentation the first patient had no significant past medical history. The second patient. The second patient is an 8 year-old male with a history Progressive Familial Intrahepatic Cholestasis Type 3 with cirrhosis and portal hyper
The first patient had a family history of a non-specified autoimmune disease in his paternal grandmother. The 2nd patient has a brother who has cirrhosis due to Progressive Familial Intrahepatic Cholestasis Type 3 as well as two uncles with an unspecified type of liver disease. The third patient had no relevant family history.
The first patient had right upper quadrant tenderness with palpable hepatomegaly. The second patient had hepatosplenomegaly on exam. The third patient had no abnormal physical exam findings and was normal size for her age.
The first patient had laboratory values consistent with acute liver failure including a total bilirubin of 8.0 mg/dL and an international ratio (INR) of 8.0. The second patient had an elevated alkaline phosphatase of 510 units/L, aspartate transaminase of 78 units/L and alanine transaminase of 52 units/L and a total bilirubin of 2.2 mg/dL, however INR was normal (1.0). The third patient had no significant laboratory abnormalities. Her alkaline phosphatase was mildly elevated to 162 units/L, she had normal synthetic function of her liver with an INR of 1.1 and a total bilirubin of 1.0 mg/dL.
The first patient had a heterogenous and coarsened appearance of hepatic parenchyma on abdominal ultrasound. Computed topography of the second patient showed nodular contour of the liver, massive splenomegaly and gastric and esophageal varices. For the third patient pre-transplant abdominal magnetic resonance imaging showed cirrhosis of the liver and splenomegaly and a splenorenal shunt
The first patient had an explanted liver with diffuse acute lobular injury with cholestasis, mixed inflammation. The pathology of the explant of the second patient’s liver showed cirrhosis and cholestasis. The third patient’s pathology of the explanted liver was consistent with Fontan associated liver disease with grade 3 fibrosis
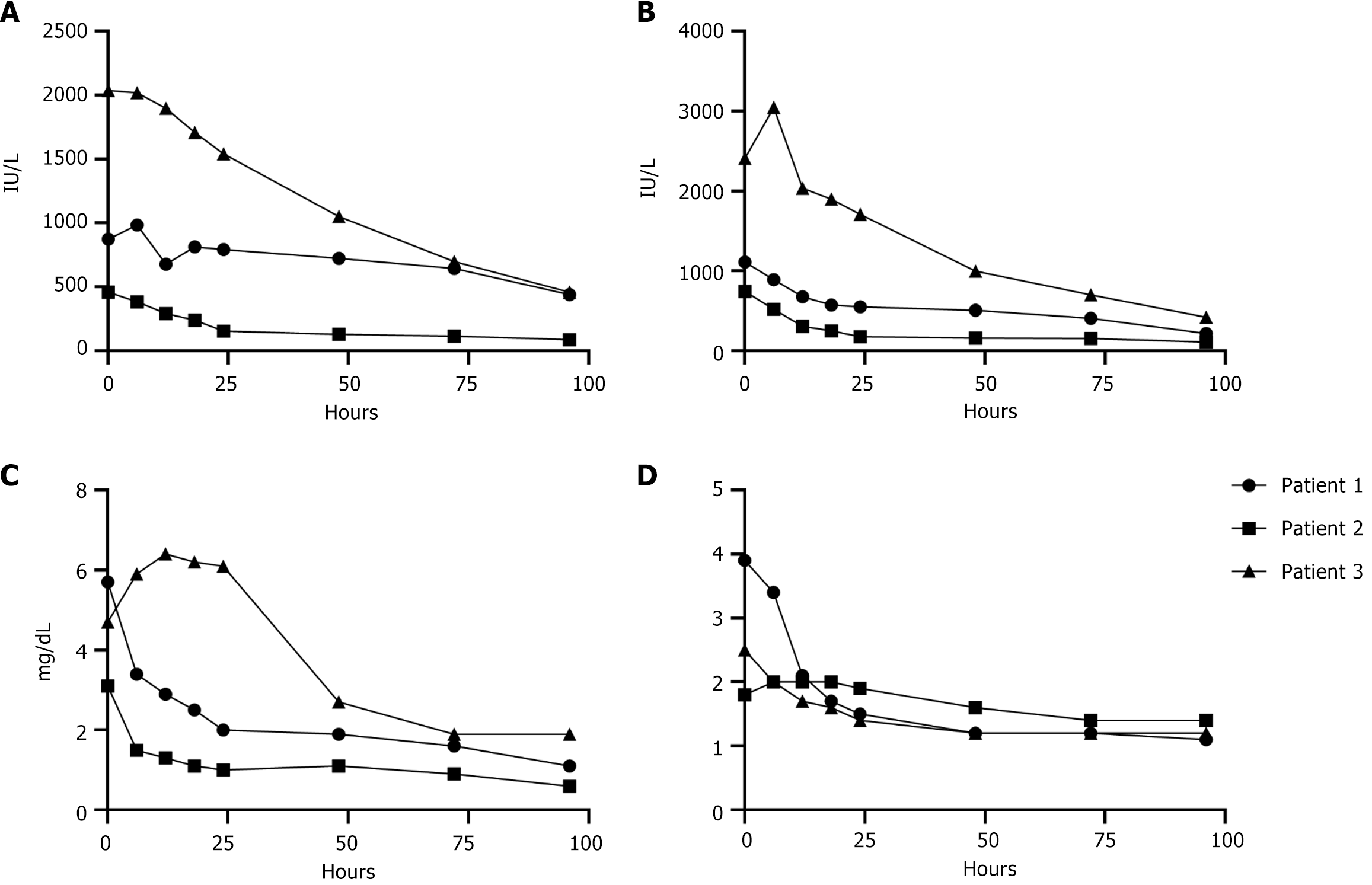
For the first patient, the donor was a 25 year-old female who died of anoxia secondary to drug intoxication. NMP was utilized in order to minimize post-reperfusion syndrome (PRS) in this unstable recipient. The recipient operation was performed using the piggyback technique. The liver reperfused well and functioned immediately. Transaminases began decreasing within the first 24 hours after transplant and the patient had normal bilirubin and INR by post-operative day 4 (Figure 1). The patient was discharged home on post-operative day 7.
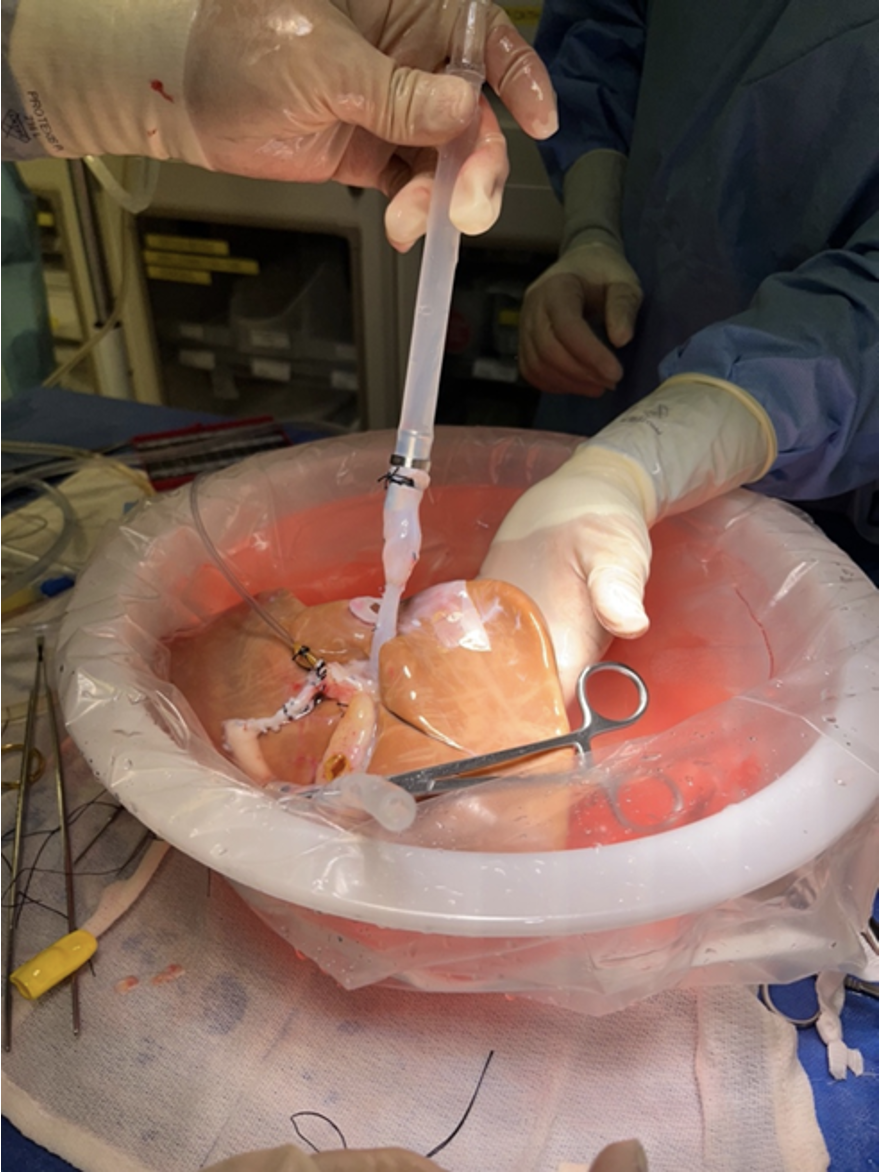
For the second patient. The donor was a 7-year-old female who died of anoxia secondary to drowning. NMP was utilized due to logistical issues with timing of the donor and recipient cases. Given the small size of the donor, cannulation was performed in the following fashion: Arterial cannulation was performed via the thoracic aorta; portal cannulation was performed after sewing an interposition graft of donor IVC to the portal vein (Figure 2). The recipient surgery was performed in the piggyback fashion, and the liver functioned immediately with clearance of lactate immediately after reperfusion. Transaminases decreased consistently starting 12 hours after transplant with normalization of Total-Bilirubin and INR 72 hours after transplantation (Figure 1). The patient recovered from the surgery and was discharged on post-operative day 12.
The donor for the third patient was a 22-year-old male who died of head trauma. Both the liver and heart were procured and placed on their respective OCS devices, with the indication for NMP use in the liver being the prolonged waiting time needed for the patient to finish their heart transplant. The liver transplant was performed sequentially following the completion of the heart transplant in the bicaval fashion given friable recipient tissue. The total time between donor cross clamp and liver implant was 13 hours and 31 minutes, the majority of the time the liver allograft was on the OCS device. Transaminases peaked in the 2000s within the first 24 hours and subsequently decreased (Figure 1A and B). Total-Bilirubin peaked at 6.4 mg/dL in the first 24 hours after transplant and decreased to 1.8 at 96 hours post-transplant (Figure 1C). INR peaked at 2.5 immediately post-op and decreased to 1.2 in first 48 hours. The patient returned to the operating room on post-operative day seven for an abdominal washout of a hematoma. No surgical cause of bleeding was found and the source was determined to be heparin anticoagulation while patient was on continuous veno-venous hemofiltration. Allograft function was maintained and she experienced renal recovery. She was discharged post-operative day 34 to home.
The first patients post-transplant course has been significant for one occurrence of moderate to severe acute cellular rejection that occurred 24 months after transplant which was successfully treated with pulse-dosed steroids. He is now 28 months post-transplant is doing well with normal liver function and has returned to age-appropriate activities like soccer. Other than treatment for rejection he has not had any readmissions after transplant.
The patient is now 17 months post-transplant who is doing well with normal liver function. He has not had any readmissions and has fully returned to school.
The third patient is now 19 months post-transplant and is doing well. She was readmitted one month post-transplant for management of a biliary anastomotic stricture and underwent an endoscopic retrograde cholangiopancreatography with sphincterotomy and stent placement, the stricture has since resolved and the stent has been removed.
Normothermic machine perfusion has caused a paradigm shift in donor practices. Despite its rapid dissemination in the adult liver transplant community, adoption in the pediatric community has been slow. We report the first case series of a pediatric patient transplanted with a liver preserved with OCS liver; a pediatric patient transplanted with a pediatric donor liver preserved with OCS liver; and a combined heart liver transplant performed using OCS for preservation of both organs.
Utilization of NMP has the potential to increase opportunities for children on the pediatric liver transplant waiting list in numerous ways. NMP mitigates the barrier of preservation time and distance. Portability of OCS liver permits transport of livers by air while being preserved with NMP. This offers significantly broader access to livers for sick children. Since the liver is preserved under physiologic conditions during travel, the risks that result from prolonged cold storage and reperfusion are substantially decreased. In addition, there is the potential to split livers in the ex vivo setting after preservation on OCS liver, which can also increase livers available to pediatric patients.
NMP allows for optimization of the liver, minimizing ischemia-reperfusion injury and PRS. This facilitates use of organs with potentially marginal characteristics, such as higher macrosteatosis, donation after circulatory death status, or high transaminases to be considered and successfully transplanted even in critically ill patients. This allows for a valuable expansion of donor options. None of the recipients experienced post-reperfusion syndrome in our cohort. The PROTECT trial recently demonstrated that the incidence of early allograft dysfunction (EAD) was lowered with NMP[3]. There was no EAD in any of our recipients despite having risk factors associated with its development.
Importantly, NMP allowed the team to have greater control of both the donor and recipient procedures, as we did for the heart-liver transplant. In the future, these high-risk cases could be converted from overnight to elective hours, offsetting the potential burden on OR teams. It should be noted that given the ability for longer preservation times, in the case of last-minute turndowns due to recipient issues, OCS allows for continued preservation and reallocation without substantial increased risk associated with increased static cold preservation times.
We acknowledge the small number of cases in this report limits the implications of our findings. This study serves as proof of feasibility of NMP use with pediatric donors and recipients and future studies should focus on comparisons with cold static storage to better determine recipient and logistical benefits of NMP.
In summary, we describe the first series of liver transplants in pediatric patients with livers preserved with NMP. The multiple advantages of NMP contributed to optimized recipient care. As death on the pediatric liver transplant waitlist persists, NMP offers promise as a feasible way to increase the pool of livers available for even the sickest of potential recipients.
| 1. | Kwong AJ, Ebel NH, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Foutz J, Gauntt K, Cafarella M, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2020 Annual Data Report: Liver. Am J Transplant. 2022;22 Suppl 2:204-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 287] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 2. | Brockmann J, Reddy S, Coussios C, Pigott D, Guirriero D, Hughes D, Morovat A, Roy D, Winter L, Friend PJ. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Markmann JF, Abouljoud MS, Ghobrial RM, Bhati CS, Pelletier SJ, Lu AD, Ottmann S, Klair T, Eymard C, Roll GR, Magliocca J, Pruett TL, Reyes J, Black SM, Marsh CL, Schnickel G, Kinkhabwala M, Florman SS, Merani S, Demetris AJ, Kimura S, Rizzari M, Saharia A, Levy M, Agarwal A, Cigarroa FG, Eason JD, Syed S, Washburn WK, Parekh J, Moon J, Maskin A, Yeh H, Vagefi PA, MacConmara MP. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022;157:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 313] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 4. | Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, García-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malagò M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera MTPR, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 914] [Article Influence: 114.3] [Reference Citation Analysis (2)] |
| 5. | Eshmuminov D, Becker D, Bautista Borrego L, Hefti M, Schuler MJ, Hagedorn C, Muller X, Mueller M, Onder C, Graf R, Weber A, Dutkowski P, Rudolf von Rohr P, Clavien PA. An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol. 2020;38:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 6. | MacConmara M, Hanish SI, Hwang CS, De Gregorio L, Desai DM, Feizpour CA, Tanriover B, Markmann JF, Zeh H 3rd, Vagefi PA. Making Every Liver Count: Increased Transplant Yield of Donor Livers Through Normothermic Machine Perfusion. Ann Surg. 2020;272:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Kadakia Y, MacConmara M, Patel MS, Shah JA, de Gregorio Muniz L, Desai DM, Hanish S, Vagefi PA, Hwang CS. Normothermic Machine Perfusion in pediatric liver transplantation: A survey of attitudes and barriers. Pediatr Transplant. 2022;26:e14282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/