Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.101926
Revised: February 28, 2025
Accepted: April 7, 2025
Published online: December 18, 2025
Processing time: 414 Days and 1.1 Hours
Type 1 diabetes (T1D) is a chronic, lifelong, autoimmune disease that is debi
Core Tip: Type 1 diabetes is a lifelong disease that is debilitating and life-threatening due to severe hypoglycaemic events or impaired hypoglycaemic awareness. β-cell replacement with either pancreas or islet allotransplantation can reverse diabetes leading to improved quality of life. The limited supply of cadaveric organ donors is a major barrier to this therapeutic option. Thus, alternative sources of islets are being explored, mainly human pluripotent stem-cell derived islets and xenogeneic porcine islets. This review will concentrate on pre-clinical and clinical studies, in addition to the latest scientific discoveries relevant to type 1 diabetes transplantation therapy using allogeneic or xenogeneic donor islet cells.
- Citation: Jiang H, Henley D, Jiang FX. Towards curing type 1 diabetes: Prospects and challenges of allogeneic or xenogeneic donor islet cell transplantation. World J Transplant 2025; 15(4): 101926
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/101926.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.101926
Diabetes mellitus (DM) is a metabolic condition of chronic hyperglycaemia due to the absolute deficiency (type 1 DM) or relative deficiency (type 2 DM) of the hormone insulin. Type 1 diabetes (T1D) is an autoimmune disease characterised by the immune mediated loss through CD4+ T cells, CD8+ T cells and autoantibodies against one’s own insulin-secreting β cells within the islets of Langerhans. The symptoms of hyperglycaemia usually occur following the loss of about 70%-90% β cells[1-3]. In contrast, type 2 diabetes is due to the combination of insulin resistance in peripheral tissues and impaired insulin secretion[4].
The initial presentation of T1D can include polydipsia, polyuria, nocturia, weight loss, lethargy, hunger and blurred vision driven by dysfunctional hyperglycaemia. The chronic hyperglycaemia in DM leads to microvascular and macrovascular complications of end organs such as retinopathy, nephropathy, neuropathy and vascular diseases. These complications can be debilitating and life-threatening, with coronary artery disease, peripheral vascular disease and ischaemic stroke being the main causes of morbidity and mortality in patients with DM.
DM is a significant global health burden that continues to grow in tandem with increasing urbanization and its associated changes in human lifestyles[5]. DM is a growing epidemic affecting 537 million adults, according to the International Diabetes Federation 2021, with that figure expected to grow to 643 million adults by the year 2030, and 783 million by the year 2045[6]. T1D is the more common form of DM in childhood, with almost one-fifth of T1D patients (> 1.2 million) being under 20 years old[6].
DM is a principal cause for end stage renal failure, lower limb amputations and loss of vision[7-9]. The burden of this chronic disease also has significant impacts on a patient’s mental health, with 20%-30% reporting elevated diabetes distress[10]. The economic costs in treating DM and its complications and the associated lost productivity is not an insignificant amount. DM is an extremely costly disease with estimated expenditures of $327 billion by the United States healthcare system in the year 2017 alone[11,12]. T1D patients also have a 3-6 times higher mortality rate compared to non-diabetics across the globe, a positive correlation of increased mortality with higher glycosylated haemoglobin (HbA1c), and a shortened life expectancy by about 12 years[13-17]. To help readers better understand this article, we will first briefly describe some key islet functions and physiology.
The adult human pancreas is about 25 cm long and 45 cm3 in volume, comprising of about 1-3 million islets of Langerhans, each with a diameter of 110 mm. Islets are made up of 57% insulin-secreting β cells (about 1.25 g), 33% α cells that produce glucagon and 10% δ cells that produce somatostatin[18,19]. These pancreatic islets secrete hormones that tightly regulate blood glucose levels (BGLs) to an average of 5.5 mmol/L (100 mg/dL) in response to fluctuating glucose levels following food intake or energy consumption such as exercise, with rapid replenishment of insulin stores.
β cells continuously sense glucose levels and secrete insulin swiftly in a glucose-dependent manner through a KATP channel-dependent mechanism[20]. It then transitions to a KATP channel-independent mechanism, resulting in biphasic glucose-stimulated insulin secretion (GSIS)[20]. Glucose is transported into β cells and phosphorylated through oxidation and glycolysis to generate ATP, along with the Krebs cycle, which leads to the closing of KATP channels and allows sodium to enter and generate membrane depolarisation. Voltage-dependent T-type calcium channels open, and the increase in calcium results in secretory granules containing insulin fuse to the plasma membrane and release insulin into the blood circulation. There are various other stimulatory signals of β cells including glucagon like peptide 1, nutrients such as free fatty acid, amino acids and parasympathetic inputs[20].
Physiologically, insulin secretion is extremely dynamic, responding to changes in glucose levels due to food and physical activities, working closely with the liver and gastrointestinal system. Insulin is secreted into the portal vein before food is swallowed during the cephalic phase from food-related stimulated (first phase) insulin release and returning to basal levels during food absorption. The Nobel prize winning discovery of insulin over a century ago was an important turning point in availing insulin therapy to T1D patients. Since then, T1D was no longer a terminal condition but instead became a chronic condition. However unfortunately for some children in lower income countries without access to insulin replacement therapy, T1D remains a life-limiting disease[21].
The current standard management of T1D, and for some insulin requiring type 2 diabetes, requires regular blood glucose monitoring and multiple daily subcutaneous insulin injections to control BGLs via either an insulin pen, syringe or pump. The daily insulin regimen can comprise of a combination of rapid-acting, short-acting, intermediate-acting and long-acting insulin; most commonly in a basal bolus regimen or twice daily injections, in a relatively crude attempt to maintain normoglycaemia. However, insulin therapy does not prevent long-term vascular complications, instead turning T1D from a fatal disease into a chronic condition.
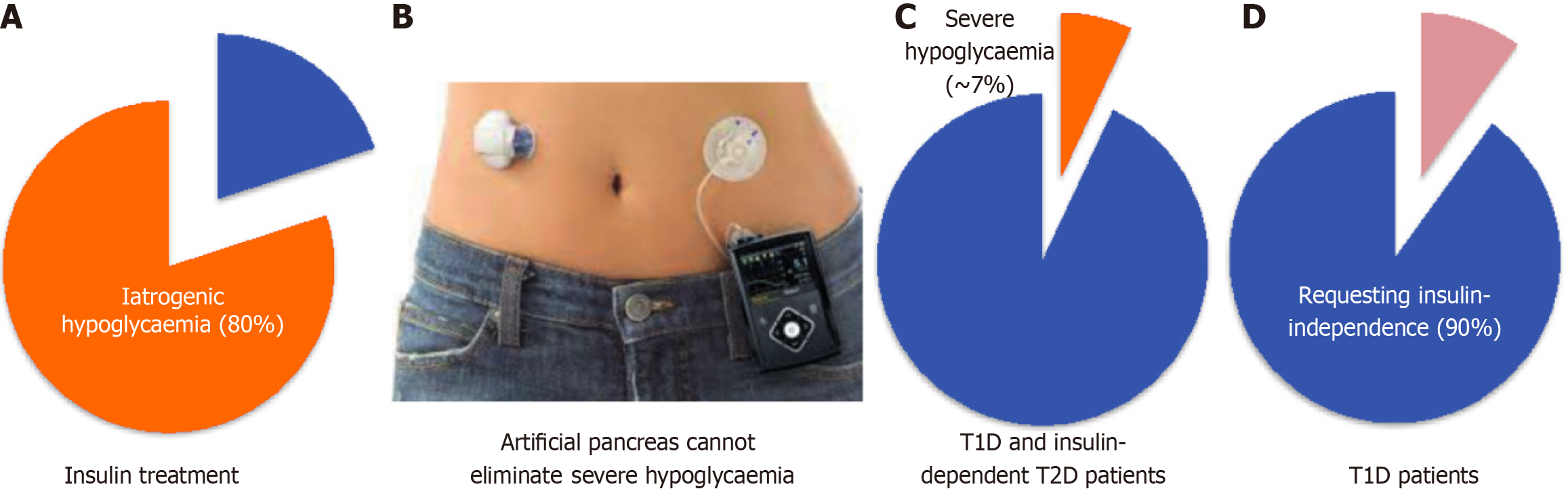
Insulin therapy has undergone many developments and iterations. Insulin was initially sourced from pigs for many years, until the production of recombinant insulin DNA in 1982[22], and the first generation of recombinant insulin analogues was mass-manufactured in 1996[23]. Since the development of long-acting and rapid-acting insulin analogues with improved stability and enabling basal and mealtime peak coverage, T1D patients were able to achieve better glucose targets as well as reduce, but not eliminate, the risk of severe hypoglycaemia. However, this also introduced the risk of nocturnal iatrogenic hypoglycaemia (Figure 1A)[24].
In addition to the chronic complications of hyperglycaemia or acute cases of diabetic ketoacidosis, T1D patients are also at risk of acute complications of iatrogenic hypoglycaemia due to inappropriately high insulin levels from insulin therapy, the delayed action of insulin and defective counterregulatory response to raised BGL (Figure 1A). Hypoglycaemia is defined by a blood glucose of < 3.9 mmol/L (70 mg/dL), warranting treatment with carbohydrate ingestion. Serious hypoglycaemia known as brittle hypoglycaemia or labile diabetes is a BGL of < 3.0 mmol/L (54 mg/dL) and can lead to cognitive impairment, loss of consciousness, cardiac arrhythmia, seizures, coma and death. The insulin pump improves quality of life but unfortunately does not significantly reduce anamnesis hypoglycaemia (about 80% compared to insulin injections) based on continuous glucose monitoring (CGM) records[25].
Impaired awareness of hypoglycaemia can develop with intense insulin therapy due to defective counterregulatory response to hypoglycaemia. The resultant reduced hypoglycaemia symptoms and inappropriate response in correcting BGL leads to increased risk of recurrent, severe hypoglycaemia and its acute life-threatening complications[26]. About 25% of people with T1D are affected by impaired awareness of hypoglycaemia[27]. Approximately 7% of T1D adults experienced severe hypoglycaemia leading to seizure or loss of consciousness in the prior 3 months, regardless of their HbA1c (Figure 1C)[28]. About 4%-10% of deaths in T1D patients is due to severe hypoglycaemia[29]. Therefore, it is not surprising that over 90% people with T1D wish to be insulin-independent (Figure 1D)[30], which cannot be achieved with current insulin therapies.
The artificial pancreas showed superior glycaemic control, with less hypoglycaemic episodes, compared to conventional multiple daily injections in diabetic patients[31,32]. However, glycaemic control remains inadequate as the artificial pancreas is a hybrid device that still requires user input with insulin boluses at mealtimes, as there is no machine cephalic phase insulin release. There is also delay in insulin absorption associated with the subcutaneous route of administration. The artificial pancreas continues to be developed with research being conducted on inclusion of other hormones into the system such as glucagon within a coupled glucagon infusion pump, or amylin. A 1.3% reduction in time in moderate hypoglycaemia has been observed with the addition of glucagon[33,34].
Despite the benefits offered by diabetes devices, they remain inconvenient to use, imprecise and expensive for some patients[28]. Only 63% uptake of insulin pumps and 30% use of CGM were recorded in a United States registry[28]. Barriers to adopting diabetes devices include 45% patients finding the technology troublesome to wear, 35% disliking devices on their body, 61% due to cost of supplies and 57% due to cost of the device itself[35]. Young T1D adults (18-25 years old) have the lowest adoption rates but the highest diabetes distress and HbA1c levels[35]. About 30% of young T1D patients discontinued the use of their artificial pancreas due to difficulties with technology such as error alerts, alarms (particularly at night time that may disturb sleep), amount of work required for calibrations, disappointment in glycaemic control results and high costs, as well as problems with skin irritation, contact dermatitis and sensor adhesiveness in young children[36,37]. Furthermore, the continuous subcutaneous insulin infusion pump may improve quality of life but does not significantly reduce anamnesis hypoglycaemia based on CGM records (Figure 1B)[25].
Although the current insulin therapy has advanced significantly since the development of CGM, only 1 in 5 T1D adults can attain the target HbA1c < 7.0% needed to prevent vascular complications[28]. Not only that, healthy non-diabetic individuals are able to maintain 5.4-5.5 mmol/L (98-99 mg/dL) BGL with little variability, as measured with CGM[38], which is much tighter than the diabetic target range of 3.9-10.0 mmol/L (70-180 mg/dL) set out by consensus guidelines[39]. Tight glycaemic control can reduce risks of microvascular complications, as demonstrated in a prospective randomised control trial comparing intensive and standard insulin therapy in T1D[40]. Although the artificial pancreas has been shown to improve glycaemic control, it is not able to achieve the physiological glycaemic control seen in non-diabetic individuals[41-43]. A significant amount of T1D patients do not even meet guideline glycaemic targets, especially young adults[44]. It is imperative to lower BGL and HbA1c towards normal as it is positively associated with reduced risks of microvascular and macrovascular complications, as well as reduced mortality rates[45,46]. In summary, exogenous insulin therapy cannot replicate the normal physiology of insulin secretion, as subcutaneous injected insulin has about a 1-hour delay to reach peak levels in the bloodstream and does not follow the physiological portal vein route. Additionally, islets continuously sense and respond to BGLs, as opposed to intermittent glucose monitoring of capillary blood self-performed every few hours by T1D patients on exogenous insulin therapy.
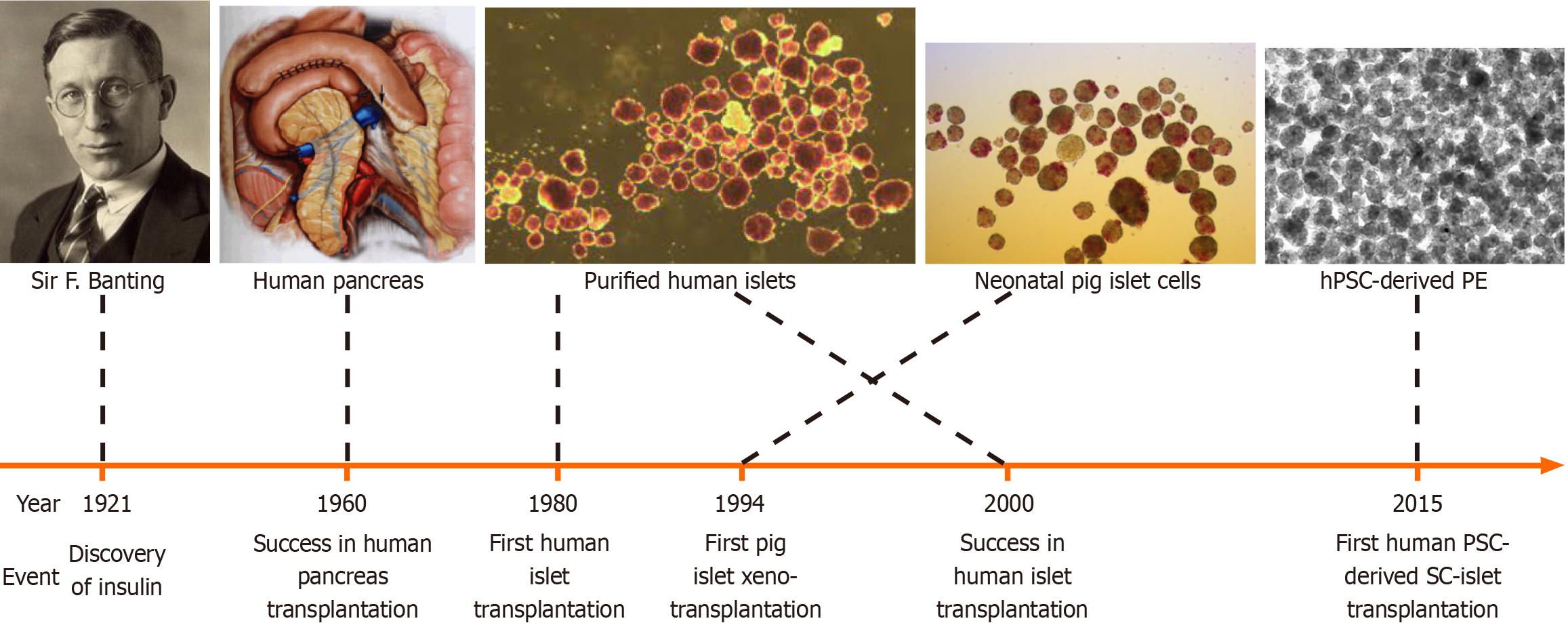
In contrast, the revolutionised β-cell replacement therapy can provide a curative solution for T1D by improving glycaemic control, quality of life and life expectancy, preventing hypoglycaemia, and even curing DM. The β-cell replacement therapy has been progressively developed over the last several decades, firstly through whole pancreas, and later through donor islet transplantation (Figure 2)[47,48]. Mechanistically, β-cell replacement can restore islet function and achieve greater physiological glycaemic control compared to exogenous insulin[48].
β-cell replacement therapies aim to re-establish physiological glycaemic control that is independent and relieves the burden of exogenous insulin therapy on patients. There are two main methods for allogeneic β-cell replacement, either through whole pancreas or islet transplantation.
Whole pancreas transplantation to treat DM was first performed in 1966 by Kelly and Lillehei (Figure 2). With im
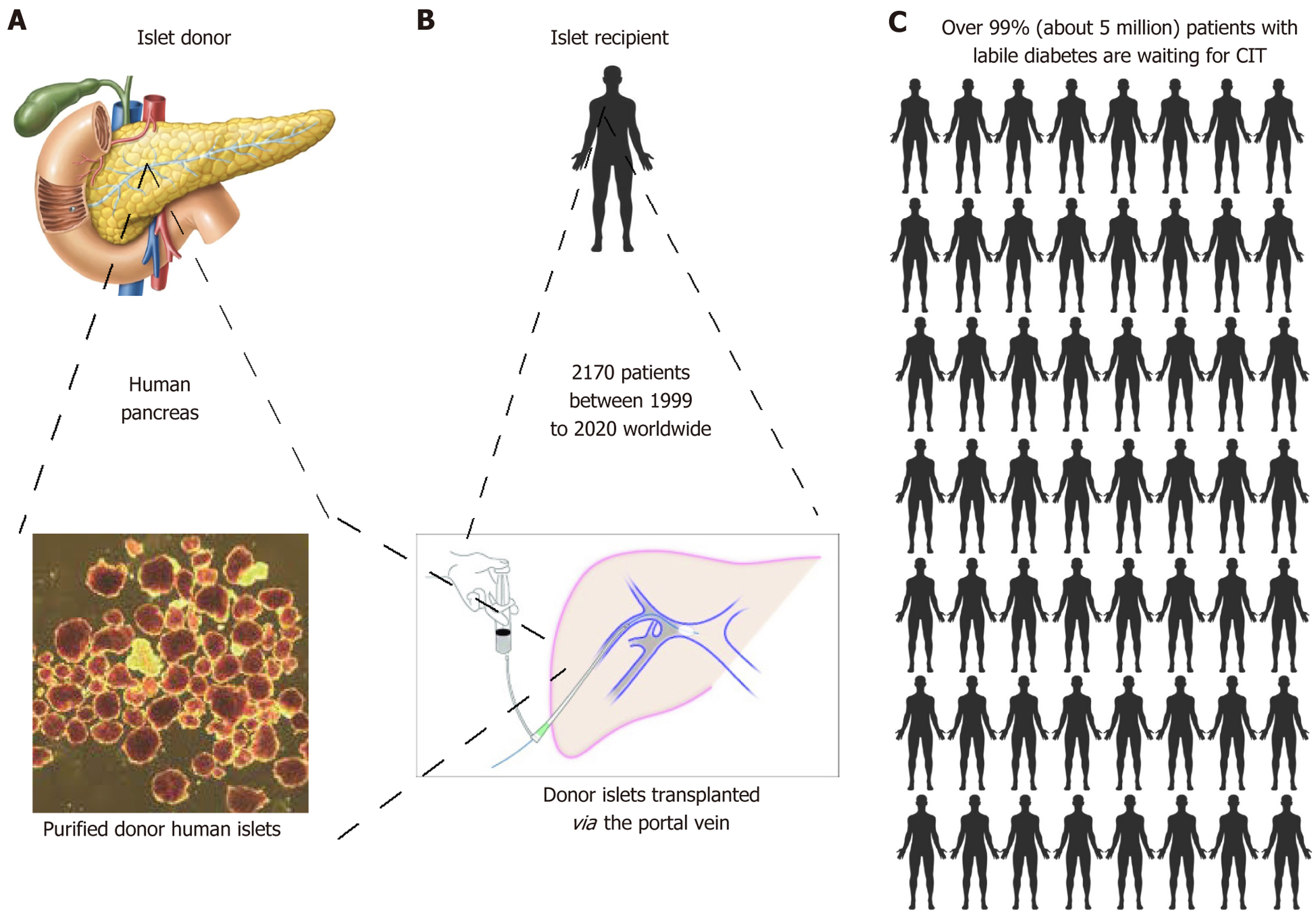
In contrast, islet transplantation (Figure 3) is a minimally invasive procedure whereby islets cells are injected intraportally under radiological guidance, avoiding the risks of major surgery and offering the convenience of being a day procedure for most patients. In addition to this, islets have the advantage for the potential to be modulated prior to transplant to reduce risks of rejection and even forgo the need for immunosuppression.
Although initially islet transplantation was not as effective as whole pancreas transplantation, the efficacy of the procedure has continuously been improved upon over the last four decades. Islet transplantation as a β-cell replacement therapy has been shown to attain insulin independence. Najarian et al[51] were able to demonstrate this through islet autotransplantation via portal vein infusion in 10 patients who underwent pancreatectomy for chronic pancreatitis in 1980. Shapiro et al[52] showed long-term glycaemic stability following cadaveric islet transplantation into the liver of T1D patients with severe hypoglycaemia unawareness in 2000, known as the Edmonton protocol (Figure 3A and B).
The Edmonton protocol uses a large islet mass of > 13000 islet equivalents (IEQ)/kg body weight, usually requiring more than one islet transplant from multiple donors[52]. The Edmonton protocol uses a corticosteroid-free immunosuppression regime consisting of an anti-CD25/anti-interleukin-2 antibody daclizumab to inhibit T-cell activation, with calcineurin inhibitor tacrolimus and mechanistic target of rapamycin inhibitor sirolimus. The development of the Edmonton protocol was a turning point for the islet transplantation procedure, and is the most widely used β-cell replacement therapy approach so far[53]. Since then, islet transplantation has been able to achieve physiological glucose control similar to non-diabetic individuals, with persistent graft function as demonstrated by 43% recipients remaining insulin independent at the 3 years follow-up, reduction in average HbA1c from 8.3% to 6.7% with mean glucose levels of 6.2 mmol/L (standard deviation 1.8)[54]. The long-term survival and function of islet grafts was 56%, 49% patients maintained HbA1c < 7.0%, > 90% were free from severe hypoglycaemia, and over half maintained insulin independence at 8 years in the CIT-08 study, a prospective trial that included 48 islet-alone transplant recipients[55]. An Italian 20-year retrospective single-centre study of 78 islet transplant patients found median graft survival of 3.9 years and 44% maintained insulin independence for median of 6 years, which increased to 9.7 years and 73% respectively in patients who received > 10000 IEQ/kg islets with basiliximab, tacrolimus and rapamycin immunosuppression, with estimated graft survival rates of 40% at 20 years[56]. In 2023, the United States Food and Drug Administration approved donislecel (trade name Lantidra) by CellTrans Inc., an intraportal allogeneic islet cell therapy from deceased donor pancreases[57].
Islet transplantation is superior to exogenous insulin therapies in maintaining tighter, physiological glucose control and thereby preventing the long-term vascular complications of DM[58,59]. Importantly, islet transplantation prevents life-threatening hypoglycaemic events associated with the insulin therapies such as severe recurrent hypoglycaemia, hypoglycaemic unawareness and glycaemic lability[52,60]. A randomised control trial showed metabolic control was superior in islet transplantation compared with intensive insulin therapy over 6 months, and one patient in the intensive insulin therapy group died due to hypoglycaemia[61]. Islet transplantation can also prevent progression of complications such as diabetic retinopathy, neuropathy and microangiopathy, and subsequently enhance quality of life[62,63]. Within 10 years of transplant, the cost of the transplant would be more economical than exogenous insulin therapy, as well as showing immediate clinical effect[64].
The current gold standard site for human islet transplantation is through the portal vein route, as it can be accessed percutaneously without need for open surgery or general anaesthesia. Another advantage is that this site allows the islets to mimic physiological insulin secretion by the pancreas to the liver, which is a primary target organ of insulin, rather than a systemic insulin supply[65]. This site remains the gold standard decades after it was first demonstrated in 1972 to ameliorate hyperglycaemia, polyuria and glycosuria when pancreatic islets were injected into the portal vein of the liver of diabetic rats[66], showing superior results compared to transplantation into the subcutaneous tissue or peritoneum[67]. The technique for the intraportal route of islet transplantation has been improved upon over the years, with the use of soluble haemostatic pastes to occlude and obliterate the catheter track to prevent intraperitoneal bleeding during the withdrawal of the catheter[68]. Additionally, therapeutic heparin is administered into the portal vein at 3-5 units/kg/hour, in conjunction with limiting packed cell volume to < 5 mL, to prevent portal vein thrombosis[69]. There is also a restriction of 15 mL of > 20000 IEQ/mL islet tissue volume to be infused, thus purification of islets is important for increasing the success of the graft, however, purification itself reduces islet yield with 18% of the islets lost in the process[70].
Nevertheless, pancreas transplantation is still apparently superior to islet transplantation, though conclusive data remains unavailable. In a large single clinical centre analysis, pancreas transplant resulted in superior results for insulin independence, graft survival and blood glucose control. However, pancreas transplantation is associated with higher rates of mortality due to procedural complexity and immunosuppression risks, procedural complications and post-surgery readmissions[71]. Although patients can become free of exogenous insulin post pancreas transplantation, their lifelong immunosuppression medications are associated with risks such as infections, malignancy, osteoporosis, hypertension, dyslipidaemia, insulin resistance and affects reproduction[50]. Thus, pancreas transplant is usually reserved for patients who already require immunosuppression for another reason such as end-stage diabetic nephr
Unfortunately, β cell replacement therapy has yet to become a standard therapy due to severe lack of supply of donor insulin producing islets for transplant and the risks associated with immunosuppression. Due to this, there is stringent criteria on eligibility for islet allotransplantation, and only 20%-30% T1D patients suffering from severe hypoglycaemic events who have already exhausted all other treatments were recommended islet allotransplantation[73]. Currently the indication for islet transplantation are brittle type 1 diabetic patients at risk of severe recurrent hypoglycaemic coma despite adherence to an optimal insulin regime, or patients with refractory chronic pancreatitis undergoing pancreatectomy - although they usually receive autologous islet transplantation rather than allogenic islets[74,75]. However, there is a higher incidence of hypoglycaemia related mortality in patients who were referred but did not receive islet transplantation (Figure 4), even taking into consideration of the risks associated with the procedure and immunosuppression[76].
Current limitations of islet transplantation include, in addition to donor scarcity, the need to take immunosuppression drugs to prevent alloimmune and xenoimmune rejection, as well as immune attack by the autoantibodies of the recipient’s immune system on graft secreted insulin and other graft antigens. Immunosuppression drugs have various side effects such as mouth ulcers, hypercholesterolaemia, nephrotoxicity, risks of infections and malignancies including lymphomas. Not only that, immunosuppressive drugs such as tacrolimus and cyclosporine are diabetogenic, directly toxic to β cells and inhibit β cell regeneration[77,78].
As islets are thrombogenic, following intraportal transplantation, the islets embolise to the portal sinusoidal capillaries, leading to islet cell death. Furthermore, the liver environment differs from the endogenous islet microenvironment in terms of oxygen content and blood supply, thus leading to further islet graft loss due to hypoxia and ischemia[79-82]. About 50%-70% donor islets are lost within hours of transplantation due to the recipient’s immune response[74,83-86]. Metabolic stress from chronic hyperglycaemia, hypoxia, ischaemia and immunosuppression further increases islet graft failure. Islets are vulnerable to damage by fatty acids[87], amyloid deposition[88,89], reactive oxygen species and oxidative stress, pro-inflammatory cytokines such as tumor necrosis factor alpha mediated apoptosis, free radicals H2O2 and peroxynitrite, and superoxide dismutases[90].
As a result of islet losses described above, large numbers of donor islet cells are required for transplantation. This leads to the other major limitation, which is severe lack of supply of donor islets for transplantation. In addition to these issues, the exorbitant cost of islet transplantation is another barrier to becoming a standard therapy. For example, the first United States Food and Drug Administration approved donor islet replacement therapy donislecel (Lantidra) was estimated to cost approximately United States $300000 per treatment per patient[91]. This is not including the cost of lifelong immunosuppression medications or treatment of complications associated with this treatment. Furthermore, the loss of islet grafts post-transplantation due to immune responses or deficient nutrition and oxygen supply to the grafts at the site of engraftment contribute to graft failure and the need for further transplants.
In summary, islet transplantation has the following three main barriers to becoming a widespread, mainstream therapy for T1D, which are: (1) A severe shortage in supply of functional insulin-producing donor islets. Less than 0.5% T1D patients could be treated with an islet allotransplantation due to the limited supply of cadaveric organs, especially when more than one donor pancreas is required per recipient, as a large number of islets are needed for successful transplantation (Figure 3C)[74]; (2) Side-effects of lifelong immunosuppression required to protect transplanted cells from immune rejection and autoantibodies that damage the function of grafted donor β cells. This topic is not the major theme of this review; and (3) Optimal transplantation site with sufficient nutritional supply to the graft whilst supporting timely insulin secretion in response to fluctuating BGL, that is additionally accessible for retrieval and biopsy if required in cases of suspected graft failure and avoids islet losses associated with the intraportal route. Alternative sources of donor islets may ensure an adequate supply, such as human pluripotent stem cell (hPSC)-derived allogeneic β-cells or xenogeneic sources such as porcine islets.
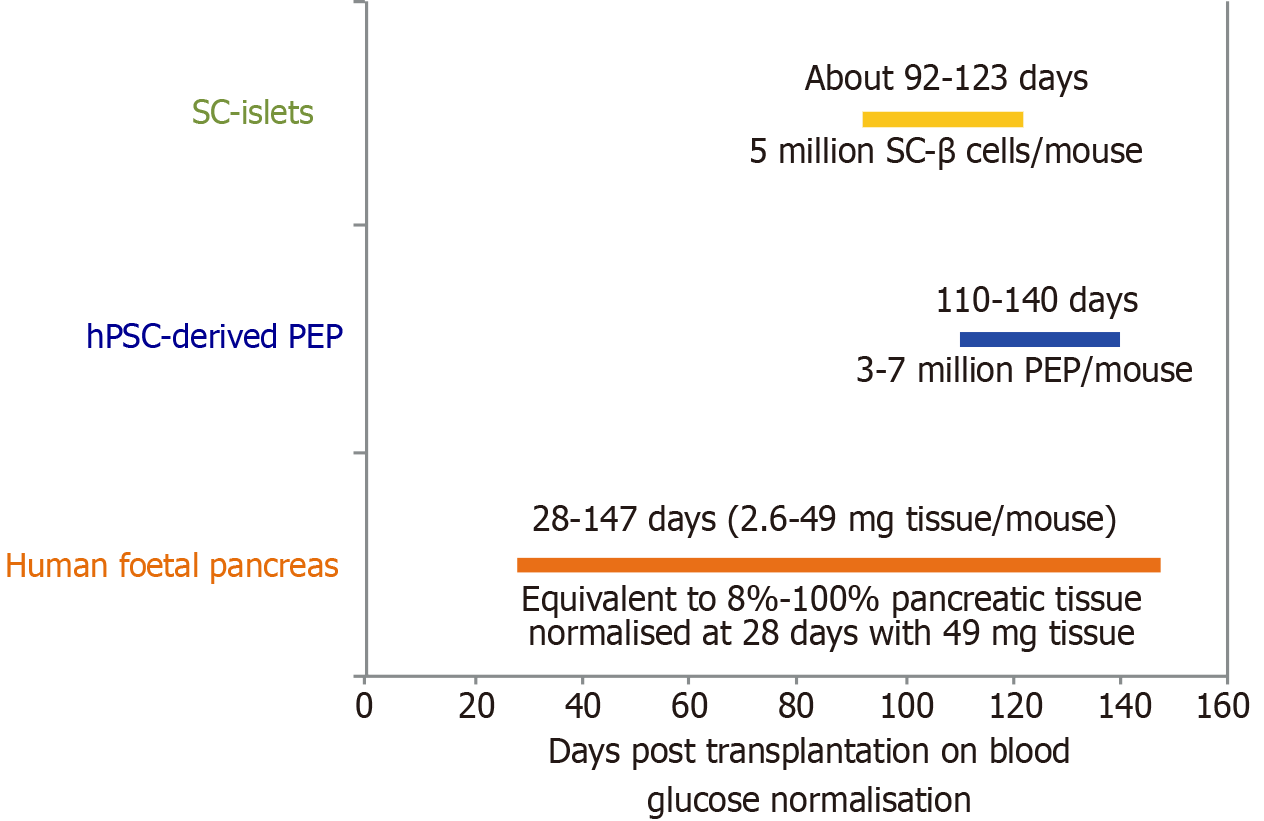
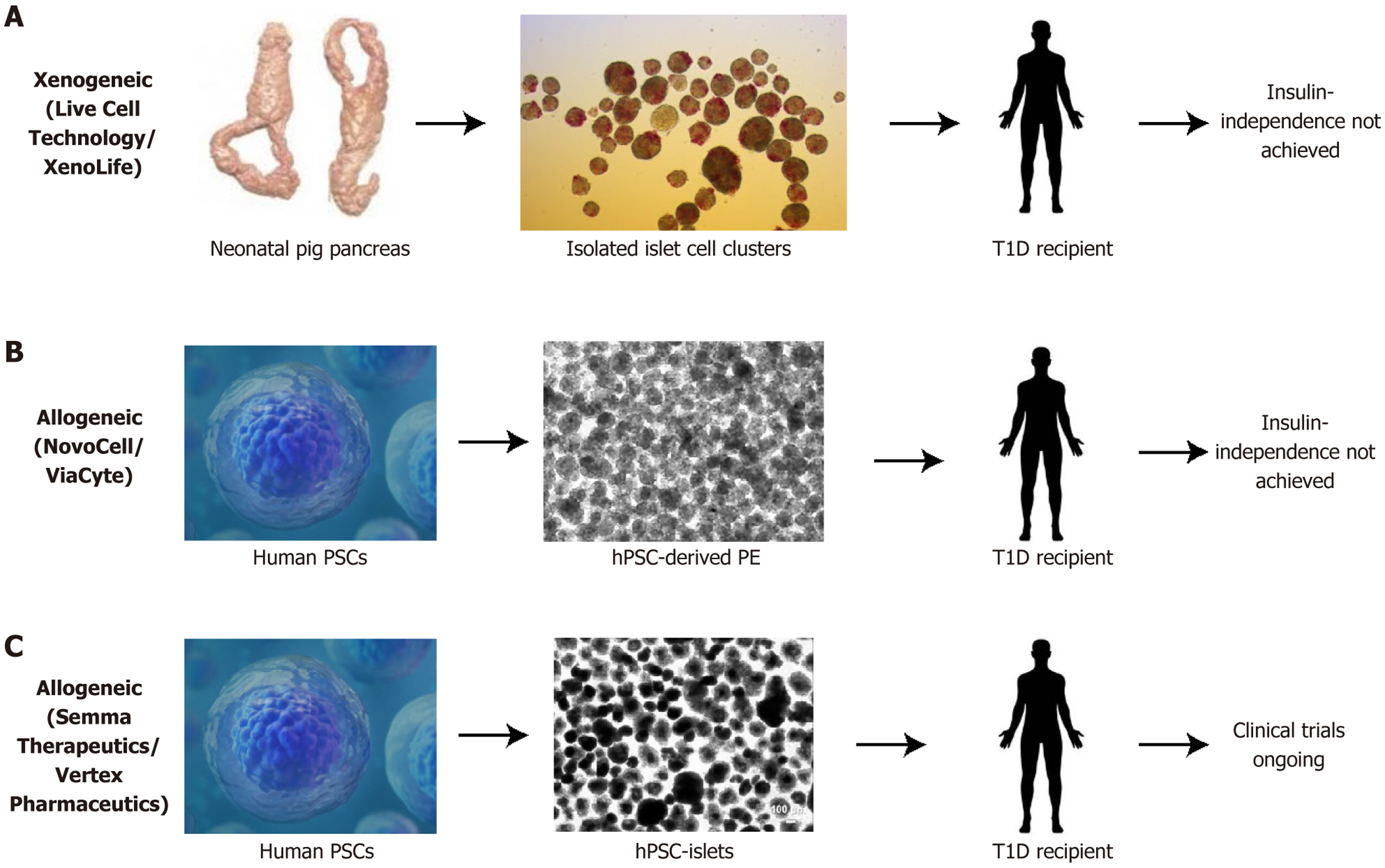
An attractive source for donor islets cells are β cells derived from hPSCs including human embryonic stem cells and human induced pluripotent stem cells, which theoretically have unlimited potential for growth and differentiation to all somatic cell lineages[92]. Pre-clinical transplantation of wide developmental stages of human foetal pancreas tissues demonstrated that experimental diabetic hyperglycaemia can be normalised as early as 28 days post-engraftment with 49 mg pancreatic tissue in mice (Figure 4)[93]. Transplantation of 3-7 million hPSC-derived pancreatic endodermal progenitors per mouse by ViaCyte normalised experimental hyperglycaemia around four months post transplantation[94]. However, post-transplantation of approximately 5 million hPSC-derived (SC)-β cells per mouse only marginally accelerated glucose normalisation to around 90-120 days[95]. However, the transplantation of human foetal pancreas tissue can only represent a partial positive control as we are unable to consider the exact differences amongst inter-laboratory variabilities, mouse strain differences, exact number of grafted β cells per mouse in each laboratory and the developmental stages of the grafted β cells from each laboratory. Nevertheless, these data support the direct comparison by single-cell RNA-seq analysis of in vivo foetal human islet cells with the hPSC derived islets (SC-islets or SC-β cells), clearly indicating that the SC-β cells are similar to foetal human islet cells[96].
SC-islets may bypass the need for islet organ donors[97]. Since 2014, the first SC-islets were demonstrated to have GSIS and normalise diabetes in mice[95,98,99]. Restoration of insulin secretion and improvement of diabetes was shown following transplant of human induced pluripotent stem cell-islets in non-human primate (NHP) macaques[100]. Stem cell-derived β cells produced by several in vitro protocols, although expressing variable maturation markers, are functionally immature both in vitro and in vivo[92]. The immaturity of differentiated cells, cell types, molecular signatures and safety concerns of SC-islets have been reviewed recently in detail[92]. Further maturation of SC-islets involves the complex interplay of various transcription factors, molecular mechanisms and metabolic conditions, with several ex vivo protocols to differentiate hPSCs into islet-like cells with variable expressions of maturation markers[92], and further research is required to develop mature, safe, genuine insulin-secreting β-like cells. In the last decade, human clinical trials of SC-islet transplantation in T1D patients have been underway.
ViaCyte Inc. was the first to generate the human embryonic stem cell-derived insulin-secreting pancreatic endoderm cells (PEC-01) macroencapsulated in a device called EncaptraTM and has conducted several rounds of clinical trials but has never achieved insulin-independence for participating patients (Figure 5)[101].
Vertex had transplanted their own SC-islets with systemic immunosuppression called VX-880 in T1D patients with impaired hypoglycaemia awareness or severe hypoglycaemic events in phase I/II clinical trials[102]. The trial was briefly put on hold after two participants died, reportedly unrelated to VX-880[102]. The first patient was insulin independent at day 270 post-transplantation, whereas it was unclear when the second patient who died became insulin-independent[103]. All 12 VX-880 participants who received the full dose had GSIS and elimination of severe hypoglycaemic events by day 90, as well as improved glycaemic control with HbA1c < 7% and time-in-range > 70% on CGM[102]. Three patients achieved the secondary endpoint of insulin independence at the one-year follow-up, and the trial expanded from 17 to 37 participants in 2024 due to the positive results[102]. Despite these positive results, a HbA1c up to 7% does not signify diabetes-free, as 5.7%-6.4% is prediabetes levels and 6.5% or higher is diabetic, as well as the time outside the target glucose range suggests there are ongoing risks of hypoglycaemic and hyperglycaemic complications. Concurrently VX-264 was developed and is in phase I/II trials, which is the encapsulated version of VX-880 without the need for immunosuppression (Figure 5)[104]. Vertex recently announced that it is collaborating with TreeFrog Therapeutics to license its C-StemTM technology to scale up and optimise stem cell production[105].
Xenotransplantation is the transplant of organs, tissues or cells from one species to another species and is a potential solution to the severe shortage of donor organs for transplant in patients with end-organ failure. Designated pathogen-free healthy, young pigs are a favourable source for clinical transplantation in humans due to their similar anatomy and insulin DNA sequence with only one amino acid difference, and suitability for genetic modification to address genetic differences[106]. Organ supply would not be an issue as pigs are easily bred, and already bred for human consumption, thus posing less of an ethical dilemma compared to primates[107]. A xenotransplantation preclinical study at Seoul National University in South Korea has been able to maintain insulin independence for over 900 days and ongoing, following porcine islet transplantation in five immunosuppressed diabetic rhesus monkeys[108].
There is no consensus on whether adult pig islets or neonatal islet cell clusters (NICC) are superior for transplant[109]. Adult pigs provide a larger islet yield with higher β cell percentage and can provide up to 720000 IEQ, an amount that can be used to transplant into a 60 kg human recipient through isolation with good manufacturing practice grade collagenase AF-1 with thermolysin or neutral protease to increase islet yield, function and viability[110]. Adult pig islets have also shown immediate insulin response and longer survival time, with weaker or no expression of the xenoantigen a-galactose-1,3-galactose that triggers antibody-mediated hyperacute rejection due to pre-formed antibodies, compared to neonatal porcine islets[111,112]. NICC has higher islet yield per gram of pancreas tissue, lower cost per islet (USD $0.02 compared to $0.09 for adult islets) due to shorter duration of pig maintenance, lower isolation cost due to ease of isolation in neonatal pigs, and greater resistance to hypoxia, scalability and proliferative ability[111,113,114]. However, an additional challenge with NICC is that it will likely take longer to ameliorate diabetes than more mature islets, as shown by more mature foetal human pancreas sources leading to shorter time for diabetes reversal in streptozotocin-treated mice[93].
A key barrier for xenotransplantation is the challenges of the immune barrier and organ rejection, of which there are three main types of rejections - hyperacute xenograft rejection, delayed xenograft rejection (either acute humoral xenograft rejection, cellular rejection or coagulation dysregulation) and chronic rejection[115,116].
However, donor pig-human recipient compatibility can now be increased following genetic editing of pigs by using targeted genome editing techniques such as CRISPR-Cas9[117]. For example, genetic editing can be used to remove the carbohydrate pig antigen a-galactose-1,3-galactose, as well as two other carbohydrate xenoantigens N-glycolylneuraminic acid and Sda, to produce triple-knockout pigs and prevent antibody-mediated rejection in human recipients[118,119]. Inclusion of human transgenes such as human complement-regulatory genes CD46, CD55, CD59 and thrombomodulin can reduce coagulation and inflammation in xenotransplantation[114]. Tissue factor pathway inhibitor (TFPI), CD39, HLA-E and programmed death-ligand 1 could increase physiological compatibility between the porcine xenograft and human recipient[120,121]. Genetic engineering of grafts to express human complement and coagulation regulatory proteins are important for protecting against coagulation dysfunction and graft survival[121,122]. There are also methods to inactivate porcine endogenous retrovirus (PERV), eliminate several xenoantigens and express human antigens to improve porcine-human immunological and coagulation compatibility through a combination of CRISPR-Cas9 and transposon technology, which is a promising advancement towards viable and safe porcine xenotransplantation in humans[123,124].
Xenotransplantation of genetic modified porcine islets was shown to function for up to 1 year in streptozotocin induced diabetic NHP[125]. The expression of the human complement-regulatory protein (hCD46) limits antibody-mediated rejection and reduced the amount of immunosuppression required to prevent loss of islet cell mass[125]. This allowed sustained normoglycaemia, although it did not reduce loss from Instant Blood Mediated Immune Reaction (IBMIR)[125]. Furthermore, the development of multi-transgenic pigs with 4 modified genes - alpha1,3-galactosyltransferase gene-knockout (GTKO), hCD46, human tissue factor pathway inhibitor (hTFPI) and CTL4-Ig - were able to achieve preserved islet mass and long-term islet engraftment up to 1 year post-transplant[126]. The addition of hTFPI helps provide anti-thrombosis and anti-inflammatory effects, whilst CTL4-Ig helps inhibit cellular immune response[126]. Thus, multi-gene donor pig islets have been shown to be an effective source of islets for NHP transplants[127].
A preclinical study on xenotransplantation of genetically modified GTKO/CD55-CD59-HT porcine NICC with costimulation blockade immunosuppression into 5 baboons showed promising results for graft survival against immune rejection[128]. Baboons share similar temperature and immune system to humans in contrast to macaques that were used in most other preclinical studies. The baboon recipients were able to achieve and maintain insulin independence for a mean of 87 ± 43, and 397 ± 174 days post-transplant respectively, and was able to avoid pig antibody response and IBMIR[128]. The immunosuppression was largely well tolerated with most recipients gaining weight, except one baboon who lost weight after developing lymphoma likely due to over-immunosuppression and genetic predisposition from the closed colony source; another recipient developed ulcerative skin lesions of undetermined cause[128].
In pioneering xenotransplantation clinical trials, NICCs were intraportally injected, or placed under the kidney capsule of T1D patients who also received a combination of immunosuppression drugs[129]. Interestingly, pig C-peptide was detectable in urine for 200-400 days, though the amount of exogenous insulin required was not reduced for glycaemic control[129]. In New Zealand, biopsies of the alginate-encapsulated porcine islets 9.5 years after they were transplanted into the peritoneum of a T1D man showed a small amount of GSIS remaining[130]. The registered clinical trials in New Zealand began in 2009, and fourteen participants who suffered from severe unaware hypoglycaemia underwent xenotransplantation with alginate-microencapsulated neonatal porcine islets (DIABECELL®) and followed for 52 weeks[131]. The results demonstrated a reduction of unaware hypoglycaemia events, positive porcine C-peptide levels and four participants achieved HbA1c < 7%, however, it lacked clinical benefit due to ongoing exogenous insulin requirements with only a small decrease in doses[131]. Further clinical trials on another 8 patients at two doses over two transplants, with the higher dose (total 20000 IEQ/kg) received by 4 of the patients showed improved HbA1c < 7% for over 600 days and reduced unaware hypoglycaemia, however the HbA1c did not normalise[132].
In Mexico, a 4-year study wherein neonatal porcine islets and Sertoli cells within a novel subcutaneous stainless steel tube encapsulation device were transplanted into twelve patients showed positive insulin staining cells at the 3 years follow-up, and 2 participants achieving insulin independence for several months[133]. At the 4 years follow-up, six participants maintained reduced insulin requirements and three participants showed porcine insulin secretion in response to glucose challenge[133]. The long-term longitudinal study of 23 participants demonstrated improved HbA1c, detection of porcine C peptide and lower incidence of chronic diabetic complications compared to those of similar age and duration of the disease[134].
Further islet xenotransplantation clinical trials are required to improve clinical outcomes, however supply of clinical-grade pigs is reported to be limited with currently only two clinical-grade facilities worldwide in China and New Zealand[135]. The Medical Porcine Development Organization in Japan was recently created to develop technologies for increased production of clinical-grade pigs[135].
Combined islet-kidney xenotransplantation for patients with renal insufficiency due to diabetic nephropathy is a future possibility with continued advancements in porcine islet xenotransplantation and by addressing each of the barriers. A preclinical study of sequential porcine kidney-then-islet transplantation into five diabetic baboons has achieved insulin independence and normal creatinine for 180 days[136]. This sequential kidney-then-islet xenotransplantation may have the additional benefit of reducing IBMIR through anti-pig antibody absorption by the kidney graft first. Adjunctive immunomodulation by vascularised thymic lobe co-transplantation could reduce the number of islets required to reverse hyperglycaemia - 12500 IEQ/kg compared to allotransplantation of the 16117 IEQ/kg purified human pancreatic islets after kidney transplant required in a phase 3 clinical trial on 24 patients[136,137].
There has not been any detection of PERV or anti-PERV antibodies thus far in preclinical and clinical studies, which could be due to low PERV expression in pig pancreas and thus low probability of viral release[138,139]. The use of good manufacturing practice and designated pathogen-free facilities also minimise pathogen transmission[140]. It would be prudent to screen for and eradicate viruses such as picornavirus and rotaviruses from donor pigs, as these viral infections can impact on islet function and insulin secretion[141].
Encapsulation devices could help overcome the challenge of high immunogenicity of xenografts, in addition to traditional immunosuppression or genetic engineering to evade the recipient’s immune system. A preclinical study of macroencapsulated porcine islet xenografts in a device called betaO2 bioartificial pancreas transplanted into three diabetic NHP without immunosuppression showed safety with no transmission of PERV or porcine cytomegalovirus, maintained graft function and avoidance of hypoglycaemic events up to 6 months before the device was retrieved[142]. However, it did not show insulin independence, likely related to the NHP model and diffusion limitations of the encapsulation device[142].
On a smaller scale, microencapsulation is another potential option for immune protection/immunoisolation. Neonatal porcine islet with alginate microencapsulation was transplanted intraperitoneally without immunosuppression in 8 T1D patients leading to improved HbA1c and unaware hypoglycaemia, however, it did not reverse diabetes[132]. This strategy may be improved with an alternate site of transplant such as the omentum, which has greater vascularisation, instead of the intraperitoneal site used[132].
Due to the severe shortage of high-quality human donor islets, there is less data on encapsulation of allogeneic islets and its survival and function compared to xenogeneic islets. In a 10-month study with one patient, allogeneic islets were macroencapsulated in an oxygenated chamber and transplanted into the subperitoneal space of a 63-year-old male patient[143]. There was maintained graft survival and function at 10 months with no graft rejection, improvement in HbA1c and modest reduction in insulin requirements from 52 ± 5.8 IU/day to 43 ± 4.9 IU/day post-transplant[143]. There was low C peptide levels, which may be due to smaller islet mass of 2100 IEQ/kg transplanted, the transplant site and diffusion limitations[143].
Various encapsulation methods such as altering biomaterial chemistry, biologics, cell co-transplantation, as well as promoting neovascularisation have been studied[144]. A challenge to encapsulation is that it lacks the vascular structures, innervation and beneficial extracellular matrix molecules of native islets, thus it is important to consider and mimic these functions[145]. Another issue is that the recipient’s immune system can detect the cytokine and damage associated membrane products released due to injured encapsulated donor islets. The damage associated membrane products attract the recipient’s innate immune macrophages, neutrophils, dendritic cells that lead to proinflammatory cytokines production and damage to the encapsulated islets and trigger the recipient’s foreign body response[146]. Currently several strategies have been studied to address local inflammation and fibrosis. These include immunomodulating biomaterials on the islet surface or microcapsules, or coencapsulation with immunomodulating cells such as regulatory T cells, mesenchymal stem cell, human amniotic epithelial cells, dendritic or Sertoli cells to improve long-term survival of grafts[53,146].
Another encapsulation method is nanoencapsulation. Nanoencapsulation is the uniform coating of polymer films such as polyethylene glycol based biomaterials onto the surface of islets or using a layer-by-layer technique using alternating positive and negative electrons to form a nanomembrane of polyamino acids, polysaccharides or synthetic polymers[147]. Nanoencapsulation has the advantage of reduced distance for passive diffusion, increasing the efficiency of nutrient and hormone exchange[148,149]. Another advantage of using nanoencapsulation is the reduced transplant volume for intraportal injection compared to microencapsulation, thus reducing the risks of thrombosis[148,149]. Furthermore, nanoencapsulation may be useful to prevent IBMIR and other functions[147]. The disadvantage of nanoencapsulation is that it does not fully shield the islet, is vulnerable to mechanical and biochemical stressors and the coating may degrade over time[150]. Both nano- and micro-encapsulation devices are also difficult to retrieve[151].
Alternative sites to the liver portal vein route have been explored to combat the issue of IBMIR and reduce the amount of donor pancreatic islet mass needed for successful transplant, accessibility for biopsy, graft retrieval and imaging. Such sites include the peritoneum, the pancreas, omental pouch, liver, spleen, skeletal muscle, renal subcapsule, subcutaneous tissue, gastric submucosa, bone marrow, thymus, testis and anterior eye chamber - with varying results[152-157].
Intramuscular grafts failed to function and developed fibrosis and necrosis at the transplantation site[153,154]. Islet transplant into the renal subcapsule space in streptozotocin-induced diabetic mice demonstrated less islets and less days needed to achieve normoglycaemia, although this is yet to be validated in non-murine models[158]. And although the renal subcapsule represents a retrievable site for transplant, it has limited capacity and thus would not be a suitable site for encapsulated islets in humans[159]. Subcutaneous transplants are simple surgical procedures with small surgical risks, free from IBMIR and would allow for monitoring and graft retrieval; however, there is inadequate nutrient and oxygen supply due to lack of blood supply, which can be further hampered by fibrosis[47]. Although the thymus, testis and anterior eye chamber sites are immunoprivileged sites, their size limits the transplantation of adequate volume of islets needed to achieve normoglycaemia, and implanting into the eye has the risk of impairing vision[160].
The retroperitoneal retrocolic space is an attractive alternative site that could accommodate a larger volume of donor islets. Encapsulated islets from rat, neonatal porcine or human transplanted into the retrocolic space of diabetic mice showed longer duration of normoglycaemia up to 275 days (rat islets) and better preserved structure on retrieval of the grafts compared to the kidney capsule or peritoneum[161]. Another advantage of this site is the access to the portal system, however, further investigation in large animal studies is required[161].
The omental pouch may represent another fair alternative, with large surface area and vascularisation supportive of oxygenation and metabolic exchange. This site is also favourable in terms of being accessible as a laparoscopic procedure, able to support large graft volumes including unpurified or encapsulated islets, and delivery of secreted insulin to the portal vein[162]. There is an ongoing phase I/II clinical trial investigating transplantation of allogeneic islet cells at this site achieving and maintaining insulin independence at 17 days and 12 months post-transplant respectively in a 43 years old woman[163]. Further research with a large cohort is required to determine if the omentum can be the preferred alternative site for human islet transplantation. Despite the advantages of alternative sites, issues with oxygen/nutrient supply, increased number of islets needed, and more invasive procedures means intraportal infusion to the liver is currently still the preferred site for islet allotransplantation and xenotransplantation.
As described in the previous sections, SC-islets and pig NICC in clinical trials are all functionally immature, reflected by a protracted time for graft maturation post-transplantation in recipients’ bodies, with limited and disappointing outcomes. This is because stem cell maturation biology has been vastly understudied and is one of the latest frontiers in regenerative medicine. Along the maturation biological pathway, a number of transcription factors have been discovered over the last two decades, some of which are involved in β cell-maturation in vivo that were reviewed recently[92]. Whether, and how, to utilise those transcription factor signaling pathways to mature immature human SC-β cells and pig NICC is a significant knowledge gap. For instance, the thyroid hormone receptors were discovered to be expressed on human mature islets[164]. The administration of thyroid hormone triiodothyronine (T3) or overexpression of the transcription factor MAF bZIP transcription factor A (MafA) was shown to increase human foetal islet-like clusters, insulin secretion at 16.8 mmol/L glucose and proinsulin-to-insulin processing[165]. Chromatin immunoprecipitation study visualised the binding of thyroid receptors to MafA promoter, thereby confirming that T3 directly regulates the expression of MafA[92].
Secondly, as there is high prevalence of acquired cancer-related mutations in hPSC lines and their differentiated derivatives[166], it would be critically important to understand whether the mutated hPSC lines and their differentiated derivatives have any unique biological, biochemical and/or cell surface markers. These markers can subsequently be used to remove the mutated cells from the transplant products. Thirdly, as SC-islets may contain various percentages of hPSC-derived enterochromaffin cells[167], discovery of methods to enrich hPSC-derived genuine islet progenitors for further differentiation would be critically important too.
Although not the focus of this review, transplantation immunology has made remarkable progress in the last few decades on the molecular and cellular mechanisms of chronic immune rejection. How to minimise and eventually elimi
The survival and functionality of transplanted donor islets in different sites require better understanding. Further long-term studies directly comparing graft survival and function in promising alternative sites of transplantation, which could include the comparison of various encapsulation methods to prevent immune rejection, would be invaluable to identify an optimal transplantation site. For example, direct comparative studies designed to assess the long-term survival and function of grafts transplanted via the current gold standard hepatic portal vein route compared with the promising alternative retroperitoneal retrocolic space and/or omental pouch sites in animal models before it proceeding to clinical trials.
Finally, it is well known that genetic engineering on GGTA1[168], NeuGc[169] and SDa[170] gene-knockout pigs may minimise up to 95% antibody-initiated xenogeneic rejection against pig cells[171,172]. However, the molecular identities of the remaining 5% xenogeneic antigens and their role in immune rejection remains largely understudied. On the other hand, the basal insulin secretion of isolated GTKO pig islets was higher than in wild type pig islets[173]. Whether this and other gene knockout and the addition of other human transgenes in as many as 69 genomic-edited pigs[174] would affect the long-term survival and function of pig islets in humans also requires further research.
The safety of hPSC-derived islet-like cells is of clear concern; a recent study discovered a high prevalence of acquired cancer-related mutations in 146 hPSC lines and their differentiated derivatives after analysing over 2200 transcriptomics[166]. In other words, the mutated differentiated SC-β cells, off-target differentiation and a low frequency of undifferentiated PSC could form teratoma in patients after transplantation. Indeed, a recent report described the development of large numbers of immature teratomas in patients after transplantation of iPSC-derived β cells[175]. Furthermore, 2 patients died in early 2024 after being transplanted with Vertex’s VX880 SC-β cells, although their deaths were reported to be unrelated to the transplanted cells. VX880 SC-β cells had been selected with the cell surface marker CD49a (also known as ITGA1) from cultured products[176]. However, it is unknown whether the hPSC line or lines that were used to give rise to VX880 SC-β cells acquired any cancer-related mutations, similar to that of any 2200 transcriptomic datasets[166].
Selection and determination of genuine islet progenitor-differentiated islet-like cells would be critically important for developing a durable stem cell therapy for T1D. SC-islets, according to single nucleus multi-omics analysis, are not only clearly different from donated human islets, but also only gradedly and not distinctively, different from hPSC-derived enterochromaffin cells[167], confirming that the current differentiation protocols for production of SC-islets have clearly generated a various percentage of off-target differentiated cells including enterochromaffin cells. This is because the embryonic pancreas and small intestine are derived from the neighbouring posterior foregut endodermal domain. Indeed, developing foetal human small intestine contains insulin-expressing enteroendocrine cells[177].
However, it is currently unknown whether the lifespan of enteroendocrine cells is similar to that of gut epithelial cells, which are approximately only 3-5 days. The high proportion of SC-derived enterochromaffin cells in VX880 SC-β cell products may partially explain the delay in achieving insulin independence for many months[102], in addition to allogeneic immune rejection, islet autoantibodies against and cytotoxicity of immunosuppressants on grafted cells. Therefore, after removing mutated hPSC and their derivatives, selection of hPSC-derived genuine islet progenitors for further differentiation and maturation into matured islet-like cells may help significantly progress this critical area for a curative allogeneic T1D therapy.
Given hPSC- and pig-derived donor islets are currently functionally immature, the discovery of maturation methods for both types of islets is another future research direction, and the research design can be based on knowledge from islet maturation in vivo. The maturation methods and their effects on insulin secretion capabilities, and duration of time to achieve insulin-independence would need to be directly compared to identify effective methods.
The key barrier for xenotransplantation of pig islets is overcoming the immune barrier and chronic rejection. Engineered donor pigs that carry as many as 69 genome modifications have been produced[174]. Eliminating swine glycan antigens and overexpressing selected human transgenes would significantly reduce immune rejection and prolong graft survival time. Inactivating pig endogenous retroviruses would significantly improve the safety profile of donor tissues[174]. This genomic-modified pig line and its further optimal lines may further help address the problem of long-term chronic xenogeneic rejection of pig islets after transplantation in human recipients. Better mechanistic understanding of the immune rejections on xenotransplantation would assist in addressing the problems of acute and chronic xenogeneic rejections. Additionally, better encapsulation devices or methods would also improve the long-term safety and function of xenogeneic islets. When these problems are addressed, pig islet xenotransplantation would be a promising treatment option for people suffering with debilitating T1D.
In conclusion, after successfully addressing the technological limitations and challenges, bridging knowledge gaps and avoiding the need for long-term immunosuppression, the cure for T1D could then be successfully realised. The widespread adoption of allogeneic and/or xenogeneic β cell replacement therapy would save the lives of millions of diabetic patients who suffer from severe hypoglycaemic events, help restore their physiological glucose homeostasis and achieve insulin independence.
| 1. | Alam U, Asghar O, Azmi S, Malik RA. General aspects of diabetes mellitus. Handb Clin Neurol. 2014;126:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Cernea S, Dobreanu M. Diabetes and beta cell function: from mechanisms to evaluation and clinical implications. Biochem Med (Zagreb). 2013;23:266-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 1057] [Article Influence: 132.1] [Reference Citation Analysis (0)] |
| 4. | DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 1456] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 5. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 2998] [Article Influence: 249.8] [Reference Citation Analysis (2)] |
| 6. | Magliano DJ, Boyko EJ; IDF Diabetes Atlas 10th edition scientific committee, editors. IDF Diabetes Atlas [Internet], 10th edition. Brussels: International Diabetes Federation, 2021. [PubMed] |
| 7. | Vinik AI. Diabetic Neuropathies. In: Atlas of Diabetes. United States: Springer, 2012. |
| 8. | Vinik AI, Nevoret ML, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. 2013;42:747-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Porta M, Bandello F. Diabetic retinopathyA clinical update. Diabetologia. 2002;45:1617-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Sturt J, Dennick K, Due-Christensen M, McCarthy K. The detection and management of diabetes distress in people with type 1 diabetes. Curr Diab Rep. 2015;15:101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Zghebi SS, Kontopantelis E, Mamas MA. Cardiovascular Risk Prediction Tools in Patients With Diabetes-Are Not There Enough? Am J Cardiol. 2024;210:306-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 1. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S10-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 13. | Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Mortality in Type 1 Diabetes in the DCCT/EDIC Versus the General Population. Diabetes Care. 2016;39:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry. Diabetes Care. 2010;33:2573-2579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992-1999. Diabetologia. 2006;49:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Huo L, Harding JL, Peeters A, Shaw JE, Magliano DJ. Life expectancy of type 1 diabetic patients during 1997-2010: a national Australian registry-based cohort study. Diabetologia. 2016;59:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Petrie D, Lung TW, Rawshani A, Palmer AJ, Svensson AM, Eliasson B, Clarke P. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia. 2016;59:1167-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Weir GC, Bonner-Weir S. Islets of Langerhans: the puzzle of intraislet interactions and their relevance to diabetes. J Clin Invest. 1990;85:983-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Ionescu-Tirgoviste C, Gagniuc PA, Gubceac E, Mardare L, Popescu I, Dima S, Militaru M. A 3D map of the islet routes throughout the healthy human pancreas. Sci Rep. 2015;5:14634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Komatsu M, Takei M, Ishii H, Sato Y. Glucose-stimulated insulin secretion: A newer perspective. J Diabetes Investig. 2013;4:511-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Beran D, Hirsch IB, Yudkin JS. Why Are We Failing to Address the Issue of Access to Insulin? A National and Global Perspective. Diabetes Care. 2018;41:1125-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Johnson IS. Human insulin from recombinant DNA technology. Science. 1983;219:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 206] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Piemonti L. Felix dies natalis, insulin… ceterum autem censeo "beta is better". Acta Diabetol. 2021;58:1287-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Mathieu C, Gillard P, Benhalima K. Insulin analogues in type 1 diabetes mellitus: getting better all the time. Nat Rev Endocrinol. 2017;13:385-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 25. | Hasanbegovic S, Obarcanin E, Hasanbegovic E, Begic N. Impact of Insulin Delivery Method on Hypoglycemia Incidence in Pediatric Type 1 Diabetes Mellitus Patients. Med Arch. 2017;71:391-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Amiel SA. Hypoglycemia: from the laboratory to the clinic. Diabetes Care. 2009;32:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Lin YK, Fisher SJ, Pop-Busui R. Hypoglycemia unawareness and autonomic dysfunction in diabetes: Lessons learned and roles of diabetes technologies. J Diabetes Investig. 2020;11:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, Maahs DM, Tamborlane WV, Bergenstal R, Smith E, Olson BA, Garg SK. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019;21:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1501] [Article Influence: 214.4] [Reference Citation Analysis (0)] |
| 29. | Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R; American Diabetes Association; Endocrine Society. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab. 2013;98:1845-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 30. | Kawabe A, Matsumoto S, Shimoda M. Patient and family expectations of beta-cell replacement therapies in type 1 diabetes. Islets. 2018;10:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Bally L, Thabit H, Hartnell S, Andereggen E, Ruan Y, Wilinska ME, Evans ML, Wertli MM, Coll AP, Stettler C, Hovorka R. Closed-Loop Insulin Delivery for Glycemic Control in Noncritical Care. N Engl J Med. 2018;379:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 32. | Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, Hermanns N. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 398] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 33. | Latres E, Finan DA, Greenstein JL, Kowalski A, Kieffer TJ. Navigating Two Roads to Glucose Normalization in Diabetes: Automated Insulin Delivery Devices and Cell Therapy. Cell Metab. 2019;29:545-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, Mondesir D, Esmaeili A, Hartigan C, Thompson MJ, Malkani S, Lock JP, Harlan DM, Clinton P, Frank E, Wilson DM, DeSalvo D, Norlander L, Ly T, Buckingham BA, Diner J, Dezube M, Young LA, Goley A, Kirkman MS, Buse JB, Zheng H, Selagamsetty RR, Damiano ER, Russell SJ. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017;389:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 35. | Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes Device Use in Adults With Type 1 Diabetes: Barriers to Uptake and Potential Intervention Targets. Diabetes Care. 2017;40:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 36. | Messer LH, Berget C, Vigers T, Pyle L, Geno C, Wadwa RP, Driscoll KA, Forlenza GP. Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2020;21:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 37. | Englert K, Ruedy K, Coffey J, Caswell K, Steffen A, Levandoski L; Diabetes Research in Children (DirecNet) Study Group. Skin and adhesive issues with continuous glucose monitors: a sticky situation. J Diabetes Sci Technol. 2014;8:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Shah VN, DuBose SN, Li Z, Beck RW, Peters AL, Weinstock RS, Kruger D, Tansey M, Sparling D, Woerner S, Vendrame F, Bergenstal R, Tamborlane WV, Watson SE, Sherr J. Continuous Glucose Monitoring Profiles in Healthy Nondiabetic Participants: A Multicenter Prospective Study. J Clin Endocrinol Metab. 2019;104:4356-4364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 39. | Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, Beck R, Bosi E, Buckingham B, Cobelli C, Dassau E, Doyle FJ 3rd, Heller S, Hovorka R, Jia W, Jones T, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Maahs D, Murphy HR, Nørgaard K, Parkin CG, Renard E, Saboo B, Scharf M, Tamborlane WV, Weinzimer SA, Phillip M. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017;40:1631-1640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1717] [Cited by in RCA: 1459] [Article Influence: 162.1] [Reference Citation Analysis (0)] |
| 40. | Lachin JM, Bebu I, Bergenstal RM, Pop-Busui R, Service FJ, Zinman B, Nathan DM; DCCT/EDIC Research Group. Association of Glycemic Variability in Type 1 Diabetes With Progression of Microvascular Outcomes in the Diabetes Control and Complications Trial. Diabetes Care. 2017;40:777-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 41. | Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:501-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 42. | Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, Haidich AB, Hovorka R, Tsapas A. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 287] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 43. | Dai X, Luo ZC, Zhai L, Zhao WP, Huang F. Artificial Pancreas as an Effective and Safe Alternative in Patients with Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Ther. 2018;9:1269-1277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | McKnight JA, Wild SH, Lamb MJ, Cooper MN, Jones TW, Davis EA, Hofer S, Fritsch M, Schober E, Svensson J, Almdal T, Young R, Warner JT, Delemer B, Souchon PF, Holl RW, Karges W, Kieninger DM, Tigas S, Bargiota A, Sampanis C, Cherubini V, Gesuita R, Strele I, Pildava S, Coppell KJ, Magee G, Cooper JG, Dinneen SF, Eeg-Olofsson K, Svensson AM, Gudbjornsdottir S, Veeze H, Aanstoot HJ, Khalangot M, Tamborlane WV, Miller KM. Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32:1036-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 45. | Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5819] [Cited by in RCA: 6091] [Article Influence: 234.3] [Reference Citation Analysis (0)] |
| 46. | Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 684] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 47. | Caldara R, Tomajer V, Monti P, Sordi V, Citro A, Chimienti R, Gremizzi C, Catarinella D, Tentori S, Paloschi V, Melzi R, Mercalli A, Nano R, Magistretti P, Partelli S, Piemonti L. Allo Beta Cell transplantation: specific features, unanswered questions, and immunological challenge. Front Immunol. 2023;14:1323439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 48. | Marzorati S, Pileggi A, Ricordi C. Allogeneic islet transplantation. Expert Opin Biol Ther. 2007;7:1627-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Vrochides D, Paraskevas S, Papanikolaou V. Transplantation for type 1 diabetes mellitus. Whole organ or islets? Hippokratia. 2009;13:6-8. [PubMed] |
| 50. | Larsen JL. Pancreas transplantation: indications and consequences. Endocr Rev. 2004;25:919-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Najarian JS, Sutherland DE, Baumgartner D, Burke B, Rynasiewicz JJ, Matas AJ, Goetz FC. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg. 1980;192:526-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3881] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 53. | Grimus S, Sarangova V, Welzel PB, Ludwig B, Seissler J, Kemter E, Wolf E, Ali A. Immunoprotection Strategies in β-Cell Replacement Therapy: A Closer Look at Porcine Islet Xenotransplantation. Adv Sci (Weinh). 2024;11:e2401385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 54. | Vantyghem MC, Raverdy V, Balavoine AS, Defrance F, Caiazzo R, Arnalsteen L, Gmyr V, Hazzan M, Noël C, Kerr-Conte J, Pattou F. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) Is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β-score greater than 3). J Clin Endocrinol Metab. 2012;97:E2078-E2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Rickels MR, Eggerman TL, Bayman L, Qidwai JC, Alejandro R, Bridges ND, Hering BJ, Markmann JF, Senior PA, Hunsicker LG; Clinical Islet Transplantation Consortium. Long-term Outcomes With Islet-Alone and Islet-After-Kidney Transplantation for Type 1 Diabetes in the Clinical Islet Transplantation Consortium: The CIT-08 Study. Diabetes Care. 2022;45:2967-2975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 56. | Catarinella D, Melzi R, Mercalli A, Magistretti P, Tentori S, Gremizzi C, Paloschi V, De Cobelli F, Esposto G, Costa S, Secchi A, Caldara R, Maffi P, Nano R, Piemonti L. Long-term outcomes of pancreatic islet transplantation alone in type 1 diabetes: a 20-year single-centre study in Italy. Lancet Diabetes Endocrinol. 2025;13:279-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 57. | Stabler CL, Russ HA. Regulatory approval of islet transplantation for treatment of type 1 diabetes: Implications and what is on the horizon. Mol Ther. 2023;31:3107-3108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Warnock GL, Thompson DM, Meloche RM, Shapiro RJ, Ao Z, Keown P, Johnson JD, Verchere CB, Partovi N, Begg IS, Fung M, Kozak SE, Tong SO, Alghofaili KM, Harris C. A multi-year analysis of islet transplantation compared with intensive medical therapy on progression of complications in type 1 diabetes. Transplantation. 2008;86:1762-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Thompson DM, Meloche M, Ao Z, Paty B, Keown P, Shapiro RJ, Ho S, Worsley D, Fung M, Meneilly G, Begg I, Al Mehthel M, Kondi J, Harris C, Fensom B, Kozak SE, Tong SO, Trinh M, Warnock GL. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 60. | Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1206] [Article Influence: 57.4] [Reference Citation Analysis (1)] |
| 61. | Lablanche S, Vantyghem MC, Kessler L, Wojtusciszyn A, Borot S, Thivolet C, Girerd S, Bosco D, Bosson JL, Colin C, Tetaz R, Logerot S, Kerr-Conte J, Renard E, Penfornis A, Morelon E, Buron F, Skaare K, Grguric G, Camillo-Brault C, Egelhofer H, Benomar K, Badet L, Berney T, Pattou F, Benhamou PY; TRIMECO trial investigators. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 62. | Bassi R, Trevisani A, Tezza S, Ben Nasr M, Gatti F, Vergani A, Farina A, Fiorina P. Regenerative therapies for diabetic microangiopathy. Exp Diabetes Res. 2012;2012:916560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8:1990-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 64. | Beckwith J, Nyman JA, Flanagan B, Schrover R, Schuurman HJ. A health economic analysis of clinical islet transplantation. Clin Transplant. 2012;26:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Rickels MR, Fuller C, Dalton-Bakes C, Markmann E, Palanjian M, Cullison K, Tiao J, Kapoor S, Liu C, Naji A, Teff KL. Restoration of Glucose Counterregulation by Islet Transplantation in Long-standing Type 1 Diabetes. Diabetes. 2015;64:1713-1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72:175-186. [PubMed] |
| 67. | Kemp CB, Knight MJ, Scharp DW, Ballinger WF, Lacy PE. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia. 1973;9:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 205] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 68. | Gamble A, Pepper AR, Bruni A, Shapiro AMJ. The journey of islet cell transplantation and future development. Islets. 2018;10:80-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 69. | Kawahara T, Kin T, Kashkoush S, Gala-Lopez B, Bigam DL, Kneteman NM, Koh A, Senior PA, Shapiro AM. Portal vein thrombosis is a potentially preventable complication in clinical islet transplantation. Am J Transplant. 2011;11:2700-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbaro B, Bridges ND, Cano J, Clarke WR, Eggerman TL, Hunsicker LG, Kaufman DB, Khan A, Lafontant DE, Linetsky E, Luo X, Markmann JF, Naji A, Korsgren O, Oberholzer J, Turgeon NA, Brandhorst D, Chen X, Friberg AS, Lei J, Wang LJ, Wilhelm JJ, Willits J, Zhang X, Hering BJ, Posselt AM, Stock PG, Shapiro AM, Chen X. National Institutes of Health-Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a Complex Cellular Product at Eight Processing Facilities. Diabetes. 2016;65:3418-3428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 71. | Marfil-Garza BA, Hefler J, Verhoeff K, Lam A, Dajani K, Anderson B, O'Gorman D, Kin T, Bello-Chavolla OY, Grynoch D, Halpin A, Campbell PM, Senior PA, Bigam D, Shapiro AMJ. Pancreas and Islet Transplantation: Comparative Outcome Analysis of a Single-centre Cohort Over 20-years. Ann Surg. 2023;277:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 72. | Cerise A, Nagaraju S, Powelson JA, Lutz A, Fridell JA. Pancreas transplantation following total pancreatectomy for chronic pancreatitis. Clin Transplant. 2019;33:e13731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AM, Stock PG, Turgeon NA; Clinical Islet Transplantation Consortium. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care. 2016;39:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 482] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 74. | Shapiro AM, Ryan EA, Lakey JR. Diabetes. Islet cell transplantation. Lancet. 2001;358 Suppl:S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Kanak MA, Takita M, Kunnathodi F, Lawrence MC, Levy MF, Naziruddin B. Inflammatory response in islet transplantation. Int J Endocrinol. 2014;2014:451035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 76. | Lee MH, Ward GM, MacIsaac RJ, Howe K, Holmes-Walker DJ, Anderson P, Radford T, Coates PT, Kay TW, O'Connell PJ, Goodman DJ. Mortality in People With Type 1 Diabetes, Severe Hypoglycemia, and Impaired Awareness of Hypoglycemia Referred for Islet Transplantation. Transplant Direct. 2018;4:e401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117:2553-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 449] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 78. | Oetjen E, Baun D, Beimesche S, Krause D, Cierny I, Blume R, Dickel C, Wehner S, Knepel W. Inhibition of human insulin gene transcription by the immunosuppressive drugs cyclosporin A and tacrolimus in primary, mature islets of transgenic mice. Mol Pharmacol. 2003;63:1289-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Menger MD, Jaeger S, Walter P, Feifel G, Hammersen F, Messmer K. Angiogenesis and hemodynamics of microvasculature of transplanted islets of Langerhans. Diabetes. 1989;38 Suppl 1:199-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 80. | Jansson L, Sandler S. Influence of hyperglycemia on blood perfusion of autotransplanted pancreatic islets in diabetic rats. Diabetes. 1989;38 Suppl 1:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 81. | Stagner JI, Samols E. Induction of angiogenesis by growth factors: relevance to pancreatic islet transplantation. EXS. 1992;61:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 82. | Stagner J, Nakagawa A, Samols E. A comparison of the angiogenic potential of the renal, splenic and pancreatic subcapsular space suggests that the pancreas may be a favorable islet transplant site. In: Diabetes. United States: American Diabetes Association, 1993. |
| 83. | Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 332] [Article Influence: 11.1] [Reference Citation Analysis (6)] |
| 84. | Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, Warnock GL, Larsen I, Shapiro AM. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 648] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 85. | Eich T, Eriksson O, Lundgren T; Nordic Network for Clinical Islet Transplantation. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N Engl J Med. 2007;356:2754-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 86. | Rickels MR, Schutta MH, Markmann JF, Barker CF, Naji A, Teff KL. {beta}-Cell function following human islet transplantation for type 1 diabetes. Diabetes. 2005;54:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 87. | Lee Y, Ravazzola M, Park BH, Bashmakov YK, Orci L, Unger RH. Metabolic mechanisms of failure of intraportally transplanted pancreatic beta-cells in rats: role of lipotoxicity and prevention by leptin. Diabetes. 2007;56:2295-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Westermark GT, Westermark P, Berne C, Korsgren O; Nordic Network for Clinical Islet Transplantation. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 89. | Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 624] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 90. | Ramkumar KM, Sekar TV, Bhakkiyalakshmi E, Foygel K, Rajaguru P, Berger F, Paulmurugan R. The impact of oxidative stress on islet transplantation and monitoring the graft survival by non-invasive imaging. Curr Med Chem. 2013;20:1127-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | United States Food and Drug Administration. FDA Approves First Cellular Therapy to Treat Patients with Type 1 Diabetes. [cited 15 November 2024]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-cellular-therapy-treat-patients-type-1-diabetes. |
| 92. | Jiang H, Jiang FX. Human pluripotent stem cell-derived β cells: Truly immature islet β cells for type 1 diabetes therapy? World J Stem Cells. 2023;15:182-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 93. | Tuch BE. Reversal of diabetes by human fetal pancreas. Optimization of requirements in the hyperglycemic nude mouse. Transplantation. 1991;51:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1317] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 95. | Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1583] [Article Influence: 143.9] [Reference Citation Analysis (1)] |
| 96. | Sean dlO, Liu Z, Sun H, Yu SK, Wong DM, Chu E, Rao SA, Eng N, Peixoto G, Bouza J, Shen Y, Knox SM, Tward AD, Gloyn AL. Single-Cell Multi-Omic Roadmap of Human Fetal Pancreatic Development. 2022 Preprint. Available from: BioRxiv:2022:2022.02.17.480942. [DOI] [Full Text] |
| 97. | Cañibano-Hernández A, Sáenz Del Burgo L, Espona-Noguera A, Ciriza J, Pedraz JL. Current advanced therapy cell-based medicinal products for type-1-diabetes treatment. Int J Pharm. 2018;543:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O'Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1203] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 99. | Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, Guo T, Puri S, Haataja L, Cirulli V, Blelloch R, Szot GL, Arvan P, Hebrok M. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34:1759-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 482] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 100. | Du Y, Liang Z, Wang S, Sun D, Wang X, Liew SY, Lu S, Wu S, Jiang Y, Wang Y, Zhang B, Yu W, Lu Z, Pu Y, Zhang Y, Long H, Xiao S, Liang R, Zhang Z, Guan J, Wang J, Ren H, Wei Y, Zhao J, Sun S, Liu T, Meng G, Wang L, Gu J, Wang T, Liu Y, Li C, Tang C, Shen Z, Peng X, Deng H. Human pluripotent stem-cell-derived islets ameliorate diabetes in non-human primates. Nat Med. 2022;28:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 101. | Shapiro AMJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J, Wilensky J, Daniels M, Wang RM, Brandon EP, Jaiman MS, Kroon EJ, D'Amour KA, Foyt HL. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. 2021;2:100466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 102. | Vertex. Vertex Announces Positive Results From Ongoing Phase 1/2 Study of VX-880 for the Treatment of Type 1 Diabetes Presented at the American Diabetes Association 84th Scientific Sessions. [cited 1 September 2024]. Available from: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-positive-results-ongoing-phase-12-study-vx-880. |
| 103. | Vertex. Vertex Presents Positive, Updated VX-880 Results From Ongoing Phase 1/2 Study in Type 1 Diabetes at the European Association for the Study of Diabetes 59th Annual Meeting. [cited 15 September 2024]. Available from: https://news.vrtx.com/news-releases/news-release-details/vertex-presents-positive-updated-vx-880-results-ongoing-phase-12. |
| 104. | Vertex. Vertex Reports Second Quarter 2024 Financial Results. [cited 15 September 2024]. Available from: https://investors.vrtx.com/news-releases/news-release-details/vertex-reports-second-quarter-2024-financial-results. |
| 105. | Vertex. Vertex and TreeFrog Therapeutics Announce Licensing Agreement and Collaboration to Optimize Production of Vertex’s Cell Therapies for Type 1 Diabetes. [cited 16 September 2024]. Available from: https://investors.vrtx.com/news-releases/news-release-details/vertex-and-treefrog-therapeutics-announce-licensing-agreement. |
| 106. | Zhu HT, Wang WL, Yu L, Wang B. Pig-islet xenotransplantation: recent progress and current perspectives. Front Surg. 2014;1:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 107. | Groth CG. The potential advantages of transplanting organs from pig to man: A transplant Surgeon's view. Indian J Urol. 2007;23:305-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 108. | Shin JS, Kim JM, Kim JS, Min BH, Kim YH, Kim HJ, Jang JY, Yoon IH, Kang HJ, Kim J, Hwang ES, Lim DG, Lee WW, Ha J, Jung KC, Park SH, Kim SJ, Park CG. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015;15:2837-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 109. | Nagaraju S, Bottino R, Wijkstrom M, Trucco M, Cooper DK. Islet xenotransplantation: what is the optimal age of the islet-source pig? Xenotransplantation. 2015;22:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Kwak K, Park JK, Shim J, Ko N, Kim HJ, Lee Y, Kim JH, Alexander M, Lakey JRT, Kim H, Choi K. Comparison of islet isolation result and clinical applicability according to GMP-grade collagenase enzyme blend in adult porcine islet isolation and culture. Xenotransplantation. 2021;28:e12703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 111. | Mou L, Shi G, Cooper DKC, Lu Y, Chen J, Zhu S, Deng J, Huang Y, Ni Y, Zhan Y, Cai Z, Pu Z. Current Topics of Relevance to the Xenotransplantation of Free Pig Islets. Front Immunol. 2022;13:854883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Rayat GR, Rajotte RV, Hering BJ, Binette TM, Korbutt GS. In vitro and in vivo expression of Galalpha-(1,3)Gal on porcine islet cells is age dependent. J Endocrinol. 2003;177:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 113. | Vanderschelden R, Sathialingam M, Alexander M, Lakey JRT. Cost and Scalability Analysis of Porcine Islet Isolation for Islet Transplantation: Comparison of Juvenile, Neonatal and Adult Pigs. Cell Transplant. 2019;28:967-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 114. | Carvalho Oliveira M, Valdivia E, Verboom M, Yuzefovych Y, Sake HJ, Pogozhykh O, Niemann H, Schwinzer R, Petersen B, Seissler J, Blasczyk R, Figueiredo C. Generating low immunogenic pig pancreatic islet cell clusters for xenotransplantation. J Cell Mol Med. 2020;24:5070-5081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Lu T, Yang B, Wang R, Qin C. Xenotransplantation: Current Status in Preclinical Research. Front Immunol. 2019;10:3060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 116. | Zhou Q, Li T, Wang K, Zhang Q, Geng Z, Deng S, Cheng C, Wang Y. Current status of xenotransplantation research and the strategies for preventing xenograft rejection. Front Immunol. 2022;13:928173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 117. | Bottino R, Knoll MF, Knoll CA, Bertera S, Trucco MM. The Future of Islet Transplantation Is Now. Front Med (Lausanne). 2018;5:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 118. | Fang M, Yang YG, Hu Z. Current status and challenges of pig-to-human organ xenotransplantation. Sci China Life Sci. 2024;67:829-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 119. | Cooper DKC, Pierson RN 3rd. Milestones on the path to clinical pig organ xenotransplantation. Am J Transplant. 2023;23:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 120. | Wolf E, Kemter E, Klymiuk N, Reichart B. Genetically modified pigs as donors of cells, tissues, and organs for xenotransplantation. Anim Front. 2019;9:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 121. | Cooper DKC, Ezzelarab M, Iwase H, Hara H. Perspectives on the Optimal Genetically Engineered Pig in 2018 for Initial Clinical Trials of Kidney or Heart Xenotransplantation. Transplantation. 2018;102:1974-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 122. | Cooper DK, Bottino R. Recent advances in understanding xenotransplantation: implications for the clinic. Expert Rev Clin Immunol. 2015;11:1379-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 123. | Yue Y, Xu W, Kan Y, Zhao HY, Zhou Y, Song X, Wu J, Xiong J, Goswami D, Yang M, Lamriben L, Xu M, Zhang Q, Luo Y, Guo J, Mao S, Jiao D, Nguyen TD, Li Z, Layer JV, Li M, Paragas V, Youd ME, Sun Z, Ding Y, Wang W, Dou H, Song L, Wang X, Le L, Fang X, George H, Anand R, Wang SY, Westlin WF, Güell M, Markmann J, Qin W, Gao Y, Wei HJ, Church GM, Yang L. Extensive germline genome engineering in pigs. Nat Biomed Eng. 2021;5:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 124. | Lin XF. Engineering Immune-Compatible Organs: Genetic Modifications in Pigs for Reduced Rejection in Human Recipients. Anim Mol Breed. 2024;14:106-118. [DOI] [Full Text] |
| 125. | van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, Murase N, Hara H, Ball S, Loveland BE, Ayares D, Lakkis FG, Cooper DK, Trucco M. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9:2716-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 126. | Bottino R, Wijkstrom M, van der Windt DJ, Hara H, Ezzelarab M, Murase N, Bertera S, He J, Phelps C, Ayares D, Cooper DK, Trucco M. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014;14:2275-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 127. | Hawthorne WJ, Salvaris EJ, Phillips P, Hawkes J, Liuwantara D, Burns H, Barlow H, Stewart AB, Peirce SB, Hu M, Lew AM, Robson SC, Nottle MB, D'Apice AJ, O'Connell PJ, Cowan PJ. Control of IBMIR in neonatal porcine islet xenotransplantation in baboons. Am J Transplant. 2014;14:1300-1309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 128. | Hawthorne WJ, Salvaris EJ, Chew YV, Burns H, Hawkes J, Barlow H, Hu M, Lew AM, Nottle MB, O'Connell PJ, Cowan PJ. Xenotransplantation of Genetically Modified Neonatal Pig Islets Cures Diabetes in Baboons. Front Immunol. 2022;13:898948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 129. | Groth CG, Korsgren O, Tibell A, Tollemar J, Möller E, Bolinder J, Ostman J, Reinholt FP, Hellerström C, Andersson A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 384] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 130. | Elliott RB, Escobar L, Tan PL, Muzina M, Zwain S, Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 279] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 131. | Matsumoto S, Tan P, Baker J, Durbin K, Tomiya M, Azuma K, Doi M, Elliott RB. Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant Proc. 2014;46:1992-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 132. | Matsumoto S, Abalovich A, Wechsler C, Wynyard S, Elliott RB. Clinical Benefit of Islet Xenotransplantation for the Treatment of Type 1 Diabetes. EBioMedicine. 2016;12:255-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 133. | Valdés-González RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, Dávila-Pérez R, Elliott RB, Terán L, White DJ. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur J Endocrinol. 2005;153:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 134. | Valdes-Gonzalez R, Rodriguez-Ventura AL, White DJ, Bracho-Blanchet E, Castillo A, Ramírez-González B, López-Santos MG, León-Mancilla BH, Dorantes LM. Long-term follow-up of patients with type 1 diabetes transplanted with neonatal pig islets. Clin Exp Immunol. 2010;162:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 135. | Matsumoto S, Matsumoto K. Clinical Islet Xenotransplantation: Development of Isolation Protocol, Anti-Rejection Strategies, and Clinical Outcomes. Cells. 2024;13:828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 136. | Eisenson DL, Iwase H, Chen W, Hisadome Y, Cui W, Santillan MR, Schulick AC, Gu D, Maxwell A, Koenig K, Sun Z, Warren D, Yamada K. Combined islet and kidney xenotransplantation for diabetic nephropathy: an update in ongoing research for a clinically relevant application of porcine islet transplantation. Front Immunol. 2024;15:1351717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 137. | Markmann JF, Rickels MR, Eggerman TL, Bridges ND, Lafontant DE, Qidwai J, Foster E, Clarke WR, Kamoun M, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hering BJ, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Naji A, Oberholzer J, Posselt AM, Ricordi C, Senior PA, Shapiro AMJ, Stock PG, Turgeon NA. Phase 3 trial of human islet-after-kidney transplantation in type 1 diabetes. Am J Transplant. 2021;21:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 138. | Dieckhoff B, Kessler B, Jobst D, Kues W, Petersen B, Pfeifer A, Kurth R, Niemann H, Wolf E, Denner J. Distribution and expression of porcine endogenous retroviruses in multi-transgenic pigs generated for xenotransplantation. Xenotransplantation. 2009;16:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 139. | Morozov VA, Wynyard S, Matsumoto S, Abalovich A, Denner J, Elliott R. No PERV transmission during a clinical trial of pig islet cell transplantation. Virus Res. 2017;227:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 140. | Cooper DKC, Mou L, Bottino R. A brief review of the current status of pig islet xenotransplantation. Front Immunol. 2024;15:1366530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 141. | Denner J. Xenotransplantation of pig islet cells: Potential adverse impact of virus infections on their functionality and insulin production. Xenotransplantation. 2023;30:e12789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 142. | Ludwig B, Ludwig S, Steffen A, Knauf Y, Zimerman B, Heinke S, Lehmann S, Schubert U, Schmid J, Bleyer M, Schönmann U, Colton CK, Bonifacio E, Solimena M, Reichel A, Schally AV, Rotem A, Barkai U, Grinberg-Rashi H, Kaup FJ, Avni Y, Jones P, Bornstein SR. Favorable outcome of experimental islet xenotransplantation without immunosuppression in a nonhuman primate model of diabetes. Proc Natl Acad Sci U S A. 2017;114:11745-11750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 143. | Ludwig B, Reichel A, Steffen A, Zimerman B, Schally AV, Block NL, Colton CK, Ludwig S, Kersting S, Bonifacio E, Solimena M, Gendler Z, Rotem A, Barkai U, Bornstein SR. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci U S A. 2013;110:19054-19058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 144. | Desai T, Shea LD. Advances in islet encapsulation technologies. Nat Rev Drug Discov. 2017;16:338-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 283] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 145. | Llacua LA, Faas MM, de Vos P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia. 2018;61:1261-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 146. | Kuwabara R, Hu S, Smink AM, Orive G, Lakey JRT, de Vos P. Applying Immunomodulation to Promote Longevity of Immunoisolated Pancreatic Islet Grafts. Tissue Eng Part B Rev. 2022;28:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 147. | Zhi ZL, Khan F, Pickup JC. Multilayer nanoencapsulation: a nanomedicine technology for diabetes research and management. Diabetes Res Clin Pract. 2013;100:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 148. | Kizilel S, Scavone A, Liu X, Nothias JM, Ostrega D, Witkowski P, Millis M. Encapsulation of pancreatic islets within nano-thin functional polyethylene glycol coatings for enhanced insulin secretion. Tissue Eng Part A. 2010;16:2217-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 149. | Wilson JT, Cui W, Chaikof EL. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008;8:1940-1948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 150. | Wiggins SC, Abuid NJ, Gattás-Asfura KM, Kar S, Stabler CL. Nanotechnology Approaches to Modulate Immune Responses to Cell-based Therapies for Type 1 Diabetes. J Diabetes Sci Technol. 2020;14:212-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 151. | Vaithilingam V, Bal S, Tuch BE. Encapsulated Islet Transplantation: Where Do We Stand? Rev Diabet Stud. 2017;14:51-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 152. | Szot GL, Koudria P, Bluestone JA. Transplantation of pancreatic islets into the kidney capsule of diabetic mice. J Vis Exp. 2007;404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 153. | al-Abdullah IH, Anil Kumar MS, Kelly-Sullivan D, Abouna GM. Site for unpurified islet transplantation is an important parameter for determination of the outcome of graft survival and function. Cell Transplant. 1995;4:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 154. | Weber CJ, Hardy MA, Pi-Sunyer F, Zimmerman E, Reemtsma K. Tissue culture preservation and intramuscular transplantation of pancreatic islets. Surgery. 1978;84:166-174. [PubMed] |
| 155. | Ferguson J, Scothorne RJ. Extended survival of pancreatic islet allografts in the testis of guinea-pigs. J Anat. 1977;124:1-8. [PubMed] |
| 156. | Adeghate E, Donáth T. Morphological findings in long-term pancreatic tissue transplants in the anterior eye chamber of rats. Pancreas. 1990;5:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 157. | Pepper AR, Pawlick R, Bruni A, Gala-Lopez B, Wink J, Rafiei Y, Bral M, Abualhassan N, Shapiro AM. Harnessing the Foreign Body Reaction in Marginal Mass Device-less Subcutaneous Islet Transplantation in Mice. Transplantation. 2016;100:1474-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 158. | Sakata N, Tan A, Chan N, Obenaus A, Mace J, Peverini R, Sowers L, Chinnock R, Hathout E. Efficacy comparison between intraportal and subcapsular islet transplants in a murine diabetic model. Transplant Proc. 2009;41:346-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 159. | de Vos P, Hamel AF, Tatarkiewicz K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia. 2002;45:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 160. | Pellegrini S. Alternative transplantation sites for islet transplantation. In: Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas. United States: Academic press, 2020: 833-847. [DOI] [Full Text] |
| 161. | Geng Z, Zhang Q, Li T, Huang T, Wang H, Zhou Q, Deng S, Zhao Y, Li Y, Cheng C, Gonelle-Gispert C, Buhler LH, Wang Y. Advantages of the retroperitoneal retrocolic space as the transplant site for encapsulated xenogeneic islets. Xenotransplantation. 2023;30:e12787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 162. | Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003;3:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 163. | Baidal DA, Ricordi C, Berman DM, Alvarez A, Padilla N, Ciancio G, Linetsky E, Pileggi A, Alejandro R. Bioengineering of an Intraabdominal Endocrine Pancreas. N Engl J Med. 2017;376:1887-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 164. | El Khattabi I, Sharma A. Proper activation of MafA is required for optimal differentiation and maturation of pancreatic β-cells. Best Pract Res Clin Endocrinol Metab. 2015;29:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 165. | Aguayo-Mazzucato C, DiIenno A, Hollister-Lock J, Cahill C, Sharma A, Weir G, Colton C, Bonner-Weir S. MAFA and T3 Drive Maturation of Both Fetal Human Islets and Insulin-Producing Cells Differentiated From hESC. J Clin Endocrinol Metab. 2015;100:3651-3659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 166. | Lezmi E, Jung J, Benvenisty N. High prevalence of acquired cancer-related mutations in 146 human pluripotent stem cell lines and their differentiated derivatives. Nat Biotechnol. 2024;42:1667-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 167. | Augsornworawat P, Hogrebe NJ, Ishahak M, Schmidt MD, Marquez E, Maestas MM, Veronese-Paniagua DA, Gale SE, Miller JR, Velazco-Cruz L, Millman JR. Single-nucleus multi-omics of human stem cell-derived islets identifies deficiencies in lineage specification. Nat Cell Biol. 2023;25:904-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 168. | Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 848] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 169. | Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1-3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J. 1996;13:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 170. | Byrne GW, Stalboerger PG, Du Z, Davis TR, McGregor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011;91:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 171. | Byrne G, Ahmad-Villiers S, Du Z, McGregor C. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation. 2018;25:e12394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 172. | Martens GR, Reyes LM, Li P, Butler JR, Ladowski JM, Estrada JL, Sidner RA, Eckhoff DE, Tector M, Tector AJ. Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs. Transplantation. 2017;101:e86-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 173. | Casu A, Echeverri GJ, Bottino R, van der Windt DJ, He J, Ekser B, Ball S, Ayares D, Cooper DK. Insulin secretion and glucose metabolism in alpha 1,3-galactosyltransferase knock-out pigs compared to wild-type pigs. Xenotransplantation. 2010;17:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 174. | Anand RP, Layer JV, Heja D, Hirose T, Lassiter G, Firl DJ, Paragas VB, Akkad A, Chhangawala S, Colvin RB, Ernst RJ, Esch N, Getchell K, Griffin AK, Guo X, Hall KC, Hamilton P, Kalekar LA, Kan Y, Karadagi A, Li F, Low SC, Matheson R, Nehring C, Otsuka R, Pandelakis M, Policastro RA, Pols R, Queiroz L, Rosales IA, Serkin WT, Stiede K, Tomosugi T, Xue Y, Zentner GE, Angeles-Albores D, Chris Chao J, Crabtree JN, Harken S, Hinkle N, Lemos T, Li M, Pantano L, Stevens D, Subedar OD, Tan X, Yin S, Anwar IJ, Aufhauser D, Capuano S, Kaufman DB, Knechtle SJ, Kwun J, Shanmuganayagam D, Markmann JF, Church GM, Curtis M, Kawai T, Youd ME, Qin W. Design and testing of a humanized porcine donor for xenotransplantation. Nature. 2023;622:393-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 183] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 175. | Han L, He H, Yang Y, Meng Q, Ye F, Chen G, Zhang J. Distinctive Clinical and Pathologic Features of Immature Teratomas Arising from Induced Pluripotent Stem Cell-Derived Beta Cell Injection in a Diabetes Patient. Stem Cells Dev. 2022;31:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 176. | Veres A, Faust AL, Bushnell HL, Engquist EN, Kenty JH, Harb G, Poh YC, Sintov E, Gürtler M, Pagliuca FW, Peterson QP, Melton DA. Charting cellular identity during human in vitro β-cell differentiation. Nature. 2019;569:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 401] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 177. | Egozi A, Llivichuzhca-Loja D, McCourt BT, Bahar Halpern K, Farack L, An X, Wang F, Chen K, Konnikova L, Itzkovitz S. Insulin is expressed by enteroendocrine cells during human fetal development. Nat Med. 2021;27:2104-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/