Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.100041
Revised: February 23, 2025
Accepted: April 16, 2025
Published online: December 18, 2025
Processing time: 470 Days and 12.1 Hours
Kidney transplant recipients (KTRs) are most vulnerable to infection in the first year after transplantation. Tixagevimab and cilgavimab are neutralizing mono
To describe outcomes of KTRs who received tixagevimab/cilgavimab early posttransplant to prevent coronavirus disease 2019 (COVID-19).
This is a single-center retrospective cohort study of adult KTRs who underwent kidney transplantation from January 1, 2022 to September 30, 2022 and received tixagevimab/cilgavimab 300 mg/300 mg for prevention of COVID-19. Outcomes of interest were adverse events associated with tixagevimab/cilgavimab, COVID-19 breakthrough infection and COVID-19-associated hospitalization and complications. We also conducted a systematic review of the literature for the use of tixagevimab/cilgavimab as pre-exposure prophylaxis for COVID-19 in solid organ transplant recipients (SOTRs) from inception to December 31, 2023.
There were 104 patients included with median age of 50 years (range 21-72 years). Omicron strain of the COVID-19 virus was the predominantly circulating variant at the time of current study. Patients testing positive for COVID-19 were given tixagevimab/cilgavimab for prophylaxis of complications during the median of 3 days (range 0-201 days) after kidney transplant, of whom 97 (93.3%) received the antibodies prior to discharge. No discernable adverse effects attributable to the medication were observed during the time they received prophylaxis. The efficacy of the drug assessed through the absence of breakthrough infections were observed in 91 patients. 13 (12.5%) patients developed COVID-19 breakthrough infections during an overall median follow-up period of 125 days (range 10-257 days) after tixagevimab/cilgavimab. These infections were observed at median 105 days (range 6-211 days) after receiving the prophylactic medication. 5 (4.8%) of overall patients required hospitalization and there were no reported deaths in the cohort. Findings of the systematic review were consistent with our findings wherein tixagevimab/cilgavimab was well tolerated by SOTRs.
Tixagevimab/cilgavimab has a favorable safety profile when administered in newly transplanted kidney recipients. Although breakthrough infections were not uncommon, there was a low rate of hospitalization and no deaths. This study highlights the need to examine the efficacy of novel monoclonal antibodies administered for COVID-19 prophylaxis in newly transplanted recipients.
Core Tip: This study reports on the use of tixagevimab/cilgavimab in newly transplanted kidney recipients. There were no adverse events related to the drug, and a 12.5% breakthrough infection rate, 4.5% hospitalization rate, and no deaths. The results suggest reassuring outcomes in newly transplanted kidney recipients who received tixagevimab/cilgavimab 300 mg/300 mg as prevention for coronavirus disease 2019 during the omicron wave. A systematic literature review of studies reporting outcomes of solid organ transplant recipients who received tixagevimab/cilgavimab found that the drug was well tolerated and a higher dose (300 mg/300 mg) was more protective against breakthrough infections and hospitalization than the lower dose (150 mg/150 mg).
- Citation: El Chediak A, Ahuja D, Bruns C, Simard R, Spence K, Gul A, Forbes RC, Concepcion BP. Prophylactic role of tixagevimab/cilgavimab for COVID-19 in newly transplanted kidney recipients: Single-center experience and review of literature. World J Transplant 2025; 15(4): 100041
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/100041.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.100041
Kidney transplant recipients (KTRs) have worse outcomes and higher mortality when they contract severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1]. This is partly attributed by the comorbidities in transplant recipients patients making them more susceptible to the poor prognosis due to coronavirus disease 2019 (COVID-19) infection. The robust immunosuppression provided to these patients during the induction period makes adds to the enhanced risk[2,3]. In November 2021, the omicron (B.1.1.529) variant was identified as the predominant strain of SARS-CoV-2 worldwide. While the delta variant resulted in higher mortality rates in KTRs, the omicron variant resulted in less severe disease but was more infectious[4]. The lack of mountable anti-spike antibody responses in solid organ transplant recipients (SOTRs) even after 2 doses of the COVID-19 mRNA vaccine suggest that KTRs continue to remain at a higher risk for COVID-19 and breakthrough infections[5]. Similarly, with the omicron variant, data indicates diminished responses after 3 doses of the COVID-19 mRNA vaccine[6,7]. Accordingly, an additional layer of protection for KTRs from SARS-CoV-2 is needed. Monoclonal antibodies were developed to provide enhanced immunity in immunocompromised individuals and their use against SARS-CoV-2 was proposed in those individuals that are at a higher risk due to their low response to the COVID-19 mRNA vaccines[8].
Tixagevimab-cilgavimab (Evusheld®) is a monoclonal antibody combination consisting of two neutralizing antibodies which neutralizes the viral growth by binding to the SARS-CoV-2 spike-protein receptor[9]. These antibodies have an extended half-life and were found to have prophylactic and therapeutic effects in preventing COVID-19[9]. Levin and colleagues studied the role of tixagevimab-cilgavimab in 5197 adults who were identified having an inadequate response to vaccination against COVID-19, an increased risk of exposure to SARS-CoV-2, or both[9]. They observed symptomatic infection in 0.2% of tixagevimab-cilgavimab group as compared to 1.0% patients who received placebo group (relative risk, 0.767 (0.46; 0.90). It provided insights to the protective role of tixagevimab-cilgavimab as a single dose and it was observed to be well tolerated.
The protective effect, however, seems to be dose-dependent. A study by Benotmane and colleagues showed that 150 mg of each antibody does not adequately protect KTRs against the Omicron variant of SARS-CoV-2[10]. Conversely, Al Jurdi et al[11] showed that 300 mg of tixagevimab and 300 mg of cilgavimab is associated with a low risk of breakthrough infections as compared to the low dose (150 mg of each antibody) group. Therefore, the minimum dose of tixagevimab-cilgavimab required to offer protection was established to be 300 mg/300 mg.
Prior studies reporting on outcomes of KTRs who received tixagevimab/cilgavimab have included patients at various timepoints from transplantation. The primary objective of the current study was to observe any adverse outcomes to assess safety profile in the suspectable KTRs population. The efficacy of the medication was assessed through the prevention of breakthrough infections. In addition, we provide a summary of the literature of tixagevimab-cilgavimab and its use in SOTRs. Findings of this study provides insights beyond the prevention of COVID-19 infection to prevent complications in KTRs by highlighting similarity in prophylactic mechanisms.
We conducted a retrospective single-center cohort study of adult patients at Vanderbilt University Medical Center who received a kidney transplant from January 1, 2022, to September 13, 2022. Patients were identified by generating a list of KTRs in the specified time period and performing a manual chart review. The included patients required them to have received tixagevimab/cilgavimab 300 mg/300 mg for prevention of COVID-19. The Vanderbilt University Institutional Review Board (IRB No. 210132) approved the study. The consent was obtained from patients before enrolment into the study and Helsinki declaration was followed.
Before inclusion, all adult patients who received a kidney transplant were offered tixagevimab/cilgavimab 300 mg/300 mg for prevention of COVID-19 prior to discharge from the index hospitalization for transplant. Patients who gave consent received tixagevimab/cilgavimab during inpatient hospitalization and were included. Those who did not give consent during inpatient stay, received an offer again for the medication during subsequent follow-up clinic visits. Patients receiving medication during clinic visit were included as well.
The kidney transplant patients who received medication during the recruitment period comprised the exposure group and the comparator was comprised of kidney transplant patients during the same recruitment period who did not receive the prophylaxis and served as reference.
Clinical characteristics were collected from the electronic medical record and tabulated in a Research Electronic Data Capture tool[12,13]. These included demographic and baseline characteristics (age, sex, race, panel reactive antibody, co-morbidities, kidney function), transplant characteristics (date and type of transplant, Australian Breast Device Registry mismatch, kidney donor profile index, delayed graft function, induction and maintenance immunosuppression), and date of tixagevimab/cilgavimab administration.
Outcomes of interest were adverse events associated with tixagevimab/cilgavimab, COVID-19 breakthrough infection, and COVID-19-associated hospitalization and complications. Breakthrough infection was defined by a positive COVID-19 antigen or polymerase chain reaction test after receiving tixagevimab/cilgavimab. Hospitalizations were attributed to COVID-19 if patients received COVID-19 directed therapies including steroids, immunosuppression adjustment, remdesivir, or tocilizumab. Administration of fluids or treatment of symptoms were not considered COVID-19 directed therapies. For hospitalizations that were ambiguous, two authors (El Chediak A and Concepcion BP) independently adjudicated and came to a consensus as to whether the hospitalization was for COVID-19 directed treatment.
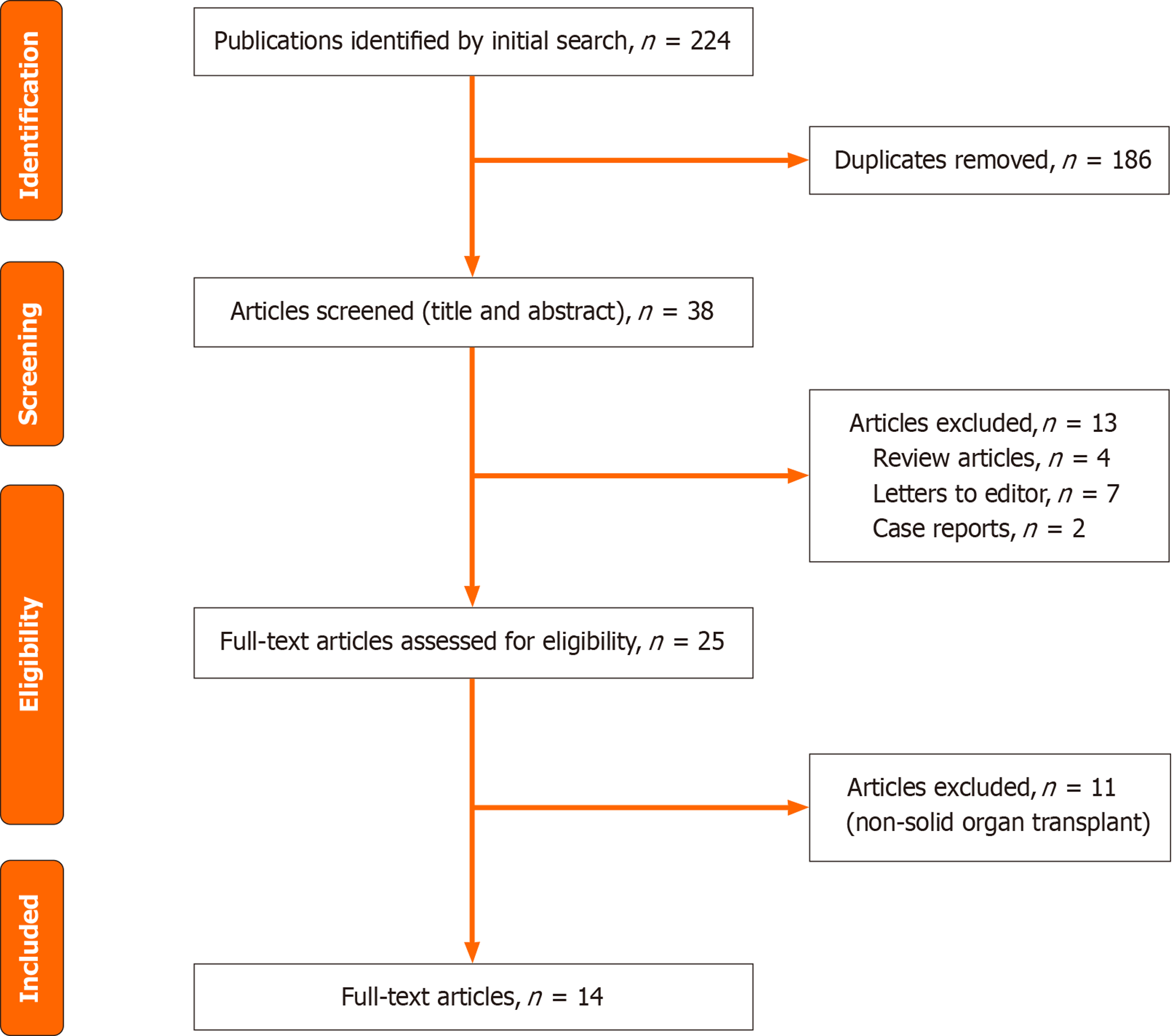
We conducted a systematic review of the literature for the use of tixagevimab/cilgavimab as pre-exposure prophylaxis for COVID-19 in SOTRs from inception to December 31, 2023. The PubMed search engines utilized for literature search include: "Cilgavimab and tixagevimab drug combination" [MeSH terms] OR "cilgavimab/tixagevimab"[tw] OR "tixagevimab/cilgavimab"[tw] OR "Evusheld"[tw] for finding relevant literature for Evusheld. We further combined our search with “AND” Boolean operator for reviewing studies pertaining to transplant. The search engine utilized for this included: “Transplantation” [MeSH terms] OR “Transplant” [tw]. After excluding duplicates, reviewing for relevance, and including those that met our eligibility criteria, 14 studies were selected which are summarized in Table 1. A flowchart illustrating our search criteria is illustrated in Figure 1.
| Ref. | Country | Study design | Intervention | Number of patients | Types of SOTRs | Duration of SOTRs | Age (year) | Female (%) | Outcomes | Conclusions |
| Al Jurdi et al[11] | United States | Retrospective multicenter | 150-150 mg: 90; 300-300 mg: 131 | 444 | Kidney, lung, liver, multi-organ | 3.8 years | 65 (55-72) | 39 | Breakthrough: 5%; hospitalization: 1; death: 0 | Lower breakthrough infection in higher dose group; well tolerated in kidney and lung recipients |
| Sanayei et al[17] | United States | Prospective single center | Dose not specified | 323 | Liver, kidney, pancreas | 7 years | 61.5 (10.5) | 35.5 | Breakthrough: 10.5%; hospitalization: 2; death: NA | Pre-exposure prophylaxis reduced infection; no significant mortality difference |
| Bravo González-Blas et al[18] | Spain | Prospective cohort | 150-150 mg | 55 | Kidney only | 9 years | 67.8 (10) | 51 | Breakthrough: 12.7%; hospitalization: 1; death: 1 | Safe and efficacious in immunocompromised kidney recipients |
| Ordaya et al[19] | United States | Retrospective multicenter | 300-300 mg (92.6%), 150-150 mg (7.4%) | 163 | Heart, heart/lung, heart + abdomen | 2.5 years | 61 (48-69) | 34.4 | Breakthrough: 14.7%; hospitalization: 1; death: 0 | Well tolerated; tacrolimus users more prone to infection |
| Angelico et al[20] | Italy | Single-center observational | All received Tix-cil | 306 | Kidney: 197; liver: 109 | 5.2 years | Kidney: 57.5 | Kidney: 41.1 | Breakthrough: 22.9%; hospitalization: 8; death: 0 | Safe and effective in kidney recipients; comparable to protective antibody titres |
| Morado et al[21] | United States | Retrospective single-center | 300-300 mg (93.3%), 150-150 mg (6.7%) | 90 | Kidney, lung, liver, heart | 94 days | 50.2 (14.5) | 48.9 | Breakthrough: 6.7%; hospitalization: 0; death: 0 | Lower infection rate in Tix-cil group; well tolerated during Omicron |
| Alejo et al[22] | United States | Prospective observational study | 150-150 mg (48%), 300-300 mg (52%) | 392 | Kidney, liver, lung, heart and multi-organ | 150-150 mg: 4.7 years, 300-300 mg: 6.75 years | 64 | 58.2 | Breakthrough: 9.2%; hospitalization: 0.5% | Well-tolerated and improvement in patient reported outcomes |
| Sindu et al[14] | United States | Retrospective single center | 300-300 mg (37.1%) | 546 | Lung | 2.1 years | 67.5 (59.6-73.9) | 39 | Breakthrough: 16.3%; hospitalization: 6.04%; death: 2.2% | Moderate effectiveness in lung transplant recipients |
| Jordan et al[23] | United States | Prospective single center | Tix-cil (41.8%) | 911 | Kidney, heart, lung, lover, multi-organ | Not reported | 60.53 (13.5) | 41.9 | Breakthrough: 35.6%; hospitalization: 2.9%; death: 0 | Reduction in breakthrough infections |
| Benotmane et al[10] | France, Europe | Retrospective single center | 124 (100%) | 124 | Kidney | 5.5 years | 55 | 34.7 | Breakthrough: 91.1%; hospitalization: 12.9%; death: 2.42% | Early administration of prophylaxis in high-risk kidney transplant patients associated with favorable outcomes |
| Kaminski et al[8] | France, Europe | Retrospective single center | 77.42% | 430 | Kidney | 8.2 years | 59.2 | 37.9 | Breakthrough: 19.3%; hospitalization: 3.5%; death: 0.7% | Significant reduction in infections and hospitalizations with a dose dependent benefit |
| Abraham et al[24] | United States | Retrospective single center | 100% | 481 | Kidney, liver, heart, lung, multi-organ | 19 months | 58.8 | 35.1 | Hospitalization: 5.4%; death: 1.6% | Reduced hospitalizations and severity of infections |
| Al Jurdi et al[25] | United States | Retrospective multicenter | 50% | 1266 | Kidney, lung, heart, liver, multi-organ | 4.6 years | 63.5 | 38.3 | Breakthrough: 11.2%; hospitalization: 1.2%; death: 0 | Tix-cil not associated with significant reduction of outcomes in BA.5 period |
| Sindu et al[26] | United States | Retrospective single center | 12.3% | 195 | Lung | 3.3 years | 66.6 | 41.5 | Hospitalization: 57.9%; death: 24.1% | Less virulent omicron strain associated with poorer outcomes in elderly; No significant reduction of adverse outcomes with Tix-cil |
Categorical variables were presented as frequencies (percentages) and continuous variables were presented as medians (range). Wilcoxon Mann Whitney U test and χ2 test were utilized as appropriate to compare groups. A statistical significance was achieved when the P value was < 0.05. Analysis was performed using STATA SE version 15.0 (StataCorp, College Station, TX).
There were 104 patients included with characteristics summarized in Table 2. The median age was 50 years (range 21-72 years). Forty-nine percent of patients were Black while 47% were White. Thirty-nine percent of patients received 2 doses of the COVID-19 mRNA vaccine while 2.9% were unvaccinated. Eighty-three percent of patients received a deceased donor kidney with 17% having delayed graft function. Majority of patients were induced with alemtuzumab (98%) and were maintained on a three-drug immunosuppressive regimen consisting of tacrolimus, mycophenolate and prednisone (96%).
| Characteristics | All patients (n = 104) | No COVID-19 infection | COVID-19 breakthrough | P value |
| Median age (IQR), year | 50 (41-59) | 50 (41-59) | 50 (44-59) | 0.48 |
| Gender, n (%) | 0.33 | |||
| Female | 45 (43.3) | 41 (45.1) | 4 (30.8) | |
| Male | 59 (56.7) | 50 (54.9) | 9 (69.2) | |
| Race, n (%) | 0.65 | |||
| Black | 51 (49.0) | 44 (48.4) | 7 (53.8) | |
| White | 49 (47.1) | 44 (48.4) | 5 (38.5) | |
| Hispanic | 3 (2.9) | 2 (2.2) | 1 (7.7) | |
| Asian/Pacific Islander | 1 (1.0) | 1 (1.1) | 0 | |
| Comorbidities, n (%) | ||||
| Hypertension | 100 (96.1) | 87 (85.6) | 13 (100) | 0.44 |
| Diabetes | 49 (47.1) | 39 (42.9) | 10 (76.9) | 0.21 |
| COPD | 4 (3.8) | 3 (3.3) | 1 (7.7) | 0.44 |
| Asthma | 4 (3.8) | 4 (4.4) | 0 | 0.44 |
| Current smoker | 5 (4.8) | 5 (5.5) | 0 | 0.39 |
| Coronary artery disease | 10 (9.6) | 10 (11.0) | 0 | 0.21 |
| Prior kidney transplant, n (%) | 11 (10.6) | 11 (12.1) | 0 | 0.18 |
| COVID-19 vaccine dose, n (%) | 0.6 | |||
| 0 | 3 (2.9) | 3 (3.3) | 0 | |
| 1 | 6 (5.8) | 5 (5.5) | 1 (7.7) | |
| 2 | 41 (39.4) | 38 (41.8) | 3 (23.1) | |
| 3 | 38 (36.5) | 31 (34.1) | 7 (53.8) | |
| 4 | 16 (15.4) | 14 (15.4) | 2 (15.4) | |
| Donor type, n (%) | 0.45 | |||
| Deceased | 83 (79.8) | 71 (78.0) | 12 (92.3) | |
| Living | 21 (20.2) | 20 (22.0) | 1 (7.7) | |
| KDPI (%), n (%) | 0.27 | |||
| 0-20 | 24 (29.3) | 22 (31.4) | 2 (16.7) | |
| 21-34 | 14 (17.1) | 13 (18.6) | 1 (8.3) | |
| 35-85 | 44 (53.7) | 35 (50.0) | 9 (75.0) | |
| cPRA (%), n (%) | 0.02 | |||
| 0 | 36 (34.6) | 32 (35.2) | 4 (30.8) | |
| 1-20 | 19 (18.3) | 18 (19.8) | 1 (7.7) | |
| 21-79 | 26 (25.0) | 18 (19.8) | 8 (61.5) | |
| 80-97 | 13 (12.5) | 13 (14.3) | 0 | |
| 98-100 | 10 (9.6) | 10 (11.0) | 0 | |
| ABDR Ag mismatch, n (%) | 0.15 | |||
| 0 | 7 (6.7) | 4 (4.4) | 3 (23.1) | |
| 1 | 3 (2.9) | 3 (3.3) | 0 | |
| 2 | 6 (5.8) | 6 (6.6) | 0 | |
| 3 | 17 (16.3) | 16 (17.6) | 1 (7.7) | |
| 4 | 28 (26.9) | 25 (27.5) | 3 (23.1) | |
| 5 | 27 (26.0) | 22 (24.2) | 5 (38.5) | |
| 6 | 16 (15.4) | 15 (16.5) | 1 (7.7) | |
| Delayed graft function, n (%) | 17 (16.3) | 15 (16.5) | 2 (15.4) | 0.92 |
| Induction immunosuppression, n (%) | 102 (98.1) | 89 (97.8) | 13 (100) | 0.59 |
| Alemtuzumab | 2 (1.9) | 2 (2.2) | 0 | |
| Basiliximab | ||||
| Maintenance immunosuppression, n (%) | 1 | |||
| Tacrolimus | 104 (100.0) | 91 (100.0) | 13 (100.0) | |
| Mycophenolate | 104 (100.0) | 91 (100.0) | 13 (100.0) | |
| Prednisone | 96 (92.3) | 84 (92.3) | 12 (92.31) |
Prophylactic medication was administered at a median of 3 days (IQR: 0-201 days) after transplant, of whom 97 (93.3%) received the antibodies prior to discharge from the index transplant hospitalization. The remaining 7 patients received tixagevimab/cilgavimab at 13 days, 23 days, 24 days, 25 days, 28 days, 147 days, 201 days posttransplant.
The enrolled patients did not report any significant adverse events attributing to safety of the medication during the follow up period of 125 days (IQR: 10-257 days). The efficacy was assessed through the prevention of breakthrough infections. During the follow-up period, 13 (12.5%) patients developed breakthrough infection at 105 days (range 6-211 days) after prophylaxis. Two of these patients developed a breakthrough infection after 180 days (diagnosed at 182 days and 211 days posttransplant).
There were 5 (4.8%) patients who required hospitalization due to COVID-19. Two of those 5 patients developed acute kidney injury, and none required intubation or admission to the intensive care unit. Three of the patients received remdesivir and steroids. There were no deaths.
There were 36 patients who were offered tixagevimab/cilgavimab but declined. COVID-19 breakthrough infection in this group was lower at 8.3% (3 of 36 patients) compared to 12.5% in patients who received tixagevimab/cilgavimab. Due to the small number of comparators, there was insufficient power to determine if this difference was statistically significant.
In this study, we found that tixagevimab/cilgavimab 300 mg/300 mg given to newly transplanted kidney recipients has a favorable safety profile. In addition, we found that although breakthrough infections were not uncommon, there was a low rate of hospitalization and no deaths. This data provides reassurance that patients who receive tixagevimab/cilgavimab in the early posttransplant period do rather well even with breakthrough COVID-19 infections. To the best of our knowledge, this report is unique in that this cohort was composed of patients who received tixagevimab/cilgavimab early posttransplant. While several studies have reported outcomes of tixagevimab/cilgavimab in SOTRs which we summarized in our literature review, our report bridges the gap in knowledge of outcomes of KTRs in the early posttransplant period, a patient population that to the best of our knowledge has not been examined exclusively in prior studies.
Our literature search yielded 14 studies which are summarized in Table 1. Our findings were consistent with prior studies wherein tixagevimab/cilgavimab was well tolerated by patients who received solid organ transplants. In addition, the safety profile was particularly better in kidney and lung transplants than other SOTRs. A higher dose (300 mg/300 mg) was more protective against breakthrough COVID-19 infections and hospitalization compared to the lower dose regimen (150 mg/150 mg). Kaminski et al[8] concluded that 150 mg/150 mg dose was insufficient to produce a humoral response compared to higher dose regimen and emphasized preference for a higher dose of 300 mg/300 mg. Benotmane et al[10] studied the usage of tixagevimab/cilgavimab in high and low risk individuals based on comor
Taken together, these prior studies suggest that tixagevimab/cilgavimab dosed at 300/300 mg seems to provide adequate protection to KTRs. In our study, breakthrough infections were not uncommon and occurred at a higher rate compared to prior studies, although most generally remained mild. This finding, however, should not be construed as lack of efficacy in preventing breakthrough infections. Notably, these breakthrough infections occurred at a median of 125 days after tixagevimab/cilgavimab administration, with two patients developing the breakthrough infection greater than 180 days after tixagevimab/cilgavimab administration. We hypothesize that the efficacy of tixagevimab/cilgavimab may wane over time even within the 180-day period in which it is thought to be effective, noting that pharmacokinetic data indicates that tixagevimab/cilgavimab has an extended half-life of approximately 90 days[9]. In addition, breakthrough infections in our cohort also occurred at a higher rate compared to a small comparator group of KTRs who did not receive tixagevimab/cilgavimab. Whether tixagevimab/cilgavimab is less effective in newly transplanted KTRs warrants further study.
This study’s strength is that it focused on a relatively large number of patients who received early administration of tixagevimab/cilgavimab (median of 3 days after kidney transplant). This allows examination of outcomes in a patient population that is at greater risk for infection due to a higher level of immunosuppression early posttransplant. Our findings should be taken in the context of its limitations. This study was a single-center, retrospective observational study design, and limited by the absence of an adequate control group that could provide sufficient statistical power to make meaningful comparisons. The determination of the outcome of COVID-associated hospitalization could be prone to bias although when the outcome was ambiguous, two authors (El Chediak A and Concepcion BP) independently adjudicated and came to a consensus on the outcome.
In summary, our brief report suggests reassuring outcomes in newly transplanted kidney recipients who received tixagevimab/cilgavimab 300 mg/300 mg as prevention for COVID-19 during the omicron wave. Although tixagevimab/cilgavimab is currently no longer approved for use due to viral resistance patterns[16], this study highlights the need to examine the efficacy of novel monoclonal antibodies that are under development in newly transplanted KTRs including appropriate dosing and timing.
| 1. | Ao G, Wang Y, Qi X, Nasr B, Bao M, Gao M, Sun Y, Xie D. The association between severe or death COVID-19 and solid organ transplantation: A systematic review and meta-analysis. Transplant Rev (Orlando). 2021;35:100628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Fishman JA. Infection in renal transplant recipients. Semin Nephrol. 2007;27:445-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 567] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 4. | Cochran W, Shah P, Barker L, Langlee J, Freed K, Boyer L, Scott Anderson R, Belden M, Bannon J, Kates OS, Permpalung N, Mostafa H, Segev DL, Brennan DC, Avery RK. COVID-19 Clinical Outcomes in Solid Organ Transplant Recipients During the Omicron Surge. Transplantation. 2022;106:e346-e347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA. 2021;325:1784-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 6. | Al Jurdi A, Gassen RB, Borges TJ, Lape IT, Morena L, Hullekes F, Efe O, Kotton CN, Riella LV. Antibody Responses Against Emerging SARS-CoV-2 Omicron Lineages After the Fourth Dose of mRNA Vaccine in Kidney Transplant Recipients. Transplantation. 2023;107:e178-e181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Al Jurdi A, Gassen RB, Borges TJ, Lape IT, Morena L, Efe O, Solhjou Z, El Fekih R, Deban C, Bohan B, Pattanayak V, Kotton CN, Azzi JR, Riella LV. Suboptimal antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int. 2022;101:1282-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Kaminski H, Gigan M, Vermorel A, Charrier M, Guirle L, Jambon F, Lacapère A, Ménard C, Moreau K, Neau-Cransac M, Novion M, Pribat F, Taton B, Borde S, Burguet L, Martinez C, Jasiek M, D'Halluin P, Lafon ME, Merville P, Couzi L. COVID-19 morbidity decreases with tixagevimab-cilgavimab preexposure prophylaxis in kidney transplant recipient nonresponders or low-vaccine responders. Kidney Int. 2022;102:936-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, Yuan Y, Seegobin S, Ellery A, Levinson DJ, Ambery P, Arends RH, Beavon R, Dey K, Garbes P, Kelly EJ, Koh GCKW, Near KA, Padilla KW, Psachoulia K, Sharbaugh A, Streicher K, Pangalos MN, Esser MT; PROVENT Study Group. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. N Engl J Med. 2022;386:2188-2200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 542] [Article Influence: 135.5] [Reference Citation Analysis (14)] |
| 10. | Benotmane I, Velay A, Gautier-Vargas G, Olagne J, Obrecht A, Cognard N, Heibel F, Braun-Parvez L, Keller N, Martzloff J, Perrin P, Pszczolinski R, Moulin B, Fafi-Kremer S, Thaunat O, Caillard S. Breakthrough COVID-19 cases despite prophylaxis with 150 mg of tixagevimab and 150 mg of cilgavimab in kidney transplant recipients. Am J Transplant. 2022;22:2675-2681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Al Jurdi A, Morena L, Cote M, Bethea E, Azzi J, Riella LV. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am J Transplant. 2022;22:3130-3136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 12. | Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 17058] [Article Influence: 2436.9] [Reference Citation Analysis (0)] |
| 13. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 40146] [Article Influence: 2361.5] [Reference Citation Analysis (0)] |
| 14. | Sindu D, Razia D, Grief K, Cherrier L, Omar A, Walia R, Tokman S. Pre-exposure Prophylaxis with Tixagevimab-cilgavimab did not Reduce Severity of COVID-19 in Lung Transplant Recipients with Breakthrough Infection. Transplant Direct. 2023;9:e1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 15. | Cochran W, Salto-Alejandre S, Barker L, Langlee J, Freed K, Carter D, Bannon J, Goddard D, Mostafa H, Werbel W, Shah P, Segev D, Brennan D, Avery R. COVID-19 Outcomes in Solid Organ Transplant Recipients Who Received Tixagevimab-cilgavimab Prophylaxis and/or Bebtelovimab Treatment in a Nurse-driven Monoclonal Antibody Program During the Omicron Surge. Transplantation. 2023;107:e60-e61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | U S. Food and Drug Administration. FDA announces Evusheld is not currently authorized for emergency use in the U.S. Jan 26, 2023. [cited 8 December 2023] Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us. |
| 17. | Sanayei AM, Montalvan A, Faria I, Ochalla J, Pavlakis M, Blair BM, Alonso CD, Curry M, Saberi B. Tixagevimab-Cilgavimab Decreases the Rate of SARS-CoV-2 Infection Among Solid Organ Transplant Recipients. Transplant Proc. 2023;55:1784-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Bravo González-Blas L, Menéndez García N, Fernández Prada M, Gago Fraile M, Suárez Fernández ML, Ridao Cano N. [Tixagevimab-cilgavimab as pre-exposure prophylactic treatment against SARS-CoV-2 in kidney transplantation patients]. Nefrologia. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ordaya EE, Higgins EM, Vergidis P, Razonable RR, Beam E. Real-world experience of tixagevimab-cilgavimab pre-exposure prophylaxis in orthotopic heart transplant recipients. Transpl Infect Dis. 2023;25:e14040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Angelico R, Romano F, Coppola L, Materazzo M, Pedini D, Santicchia MS, Cacciola R, Toti L, Sarmati L, Tisone G. Effects of Anti-COVID-19 Vaccination and Pre-Exposure Prophylaxis with Tixagevimab-Cilgavimab in Kidney and Liver Transplant Recipients. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Morado F, Davoudi R, Kawewat-Ho P, Nanda N, Cartus R, Shaikh SA. A single-center review of pre-exposure prophylaxis with tixagevimab-cilgavimab in solid organ transplant recipients. Transpl Infect Dis. 2023;25:e14086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Alejo JL, Kim JD, Chiang TPY, Avery RK, Karaba AH, Jefferis A, Warren DS, Massie AB, Tobian AAR, Segev DL, Werbel WA. Patient-reported outcomes after Tixagevimab and Cilgavimab pre-exposure prophylaxis among solid organ transplant recipients: Safety, effectiveness, and perceptions of risk. Clin Transplant. 2023;37:e14913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Jordan SC, Joung SY, Wang M, Tran TA, Bravo M, Masoom H, Chang C, Mendez M, Sun N, Patel J, Kittleson M, Frias E, Prostko JC, Ebinger JE, Cheng S, Sobhani K. Assessing the post hoc effectiveness of tixagevimab-cilgavimab for prevention of SARS-CoV-2 infections in solid organ transplant recipients. Transpl Infect Dis. 2024;26:e14182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Abraham DL, Lichvar AB, Brubaker AL, Haste N, Chen V, Abeles S, Aslam S, Yam N, Horton L, Chen B, Binkin N, Law N. Risk factors for breakthrough COVID-19 infections in solid organ transplant recipients receiving tixagevimab/cilgavimab for pre-exposure prophylaxis. Transpl Infect Dis. 2023;25:e14125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Al Jurdi A, Morena L, Verhoeff R, Alzahrani N, Kotton CN, Riella LV. Tixagevimab-cilgavimab Preexposure Prophylaxis in Solid Organ Transplant Recipients Is Associated With Fewer Breakthrough SARS-CoV-2 Infections, Except During the BA.5 Period. Transplantation. 2023;107:e238-e240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Sindu D, Razia D, Bay C, Padiyar J, Grief K, Buddhdev B, Arjuna A, Abdelrazek H, Mohamed H, McAnally K, Omar A, Walia R, Schaheen L, Tokman S. Evolving impact of the COVID-19 pandemic on lung transplant recipients: A single-center experience. J Heart Lung Transplant. 2024;43:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/