Published online Mar 18, 2025. doi: 10.5500/wjt.v15.i1.98003
Revised: September 26, 2024
Accepted: October 28, 2024
Published online: March 18, 2025
Processing time: 165 Days and 17 Hours
In the absence of effective antimicrobials, transplant surgery is not viable, and antirejection immunosuppressants cannot be administered, as resistant infections compromise the life-saving goal of organ transplantation.
To evaluate the efficacy of antimicrobials in preventing resistance in solid organ transplant recipients.
A systematic review was conducted using a search methodology consistent with the preferred reporting items for systematic reviews and meta-analyses. This review included randomized clinical trials that evaluated the efficacy of antimicrobial agents (prophylactic or therapeutic) aimed at preventing antimicrobial resistance. The search strategy involved analyzing multiple databases, including PubMed/MEDLINE, Web of Science, Embase, Scopus, and SciELO, as well as examining gray literature sources on Google Scholar. A comprehensive electronic database search was conducted from the databases’ inception until May 2024, with no language restrictions.
After the final phase of the eligibility assessment, this systematic review ultimate
All clinical trials reported significant proportions of antimicrobial-resistant microorganisms following inter
Core Tip: This systematic review evaluates the efficacy of antimicrobial agents in preventing resistance among solid organ transplant recipients. Despite varying results across studies, maribavir and valganciclovir showed promise in reducing resistance rates compared to controls. However, overall, significant proportions of antimicrobial-resistant microorganisms were observed post-intervention, highlighting the urgent need for further high-quality randomized clinical trials to validate these findings and improve outcomes in organ transplantation.
- Citation: Ardila CM, Yadalam PK, Ramírez-Arbelaez J. Efficacy of antimicrobials in preventing resistance in solid organ transplant recipients: A systematic review of clinical trials. World J Transplant 2025; 15(1): 98003
- URL: https://www.wjgnet.com/2220-3230/full/v15/i1/98003.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i1.98003
Antimicrobial resistance is a worldwide health issue that requires urgent, collaborative efforts across multiple sectors, countries, and disciplines[1,2]. Antimicrobial resistance disproportionately affects the most at-risk groups, including immunocompromised individuals and solid organ transplant recipients (SOTR)[2]. Even with improvements in surgical procedures, infection control, and preventive measures, microbial infections persist as the primary cause of infections following solid organ transplantation[3]. In the absence of effective antimicrobials, transplant surgery is not viable, and antirejection immunosuppressants cannot be administered, as resistant infections compromise the life-saving goal of organ transplantation[1-3]. Additionally, the incidence of bacterial infections caused by vancomycin-resistant enterococci, as well as multidrug-resistant and extensively drug-resistant Enterobacteriaceae and non-fermenting gram-negative bacteria, has steadily risen over the past several decades[3,4]. Antibiotic abuse is also responsible for the rising incidence of Clostridioides difficile (C. difficile) infection in SOTR, contributing to more frequent recurrences, graft loss, and mortality[3,5]. The increasing occurrence of Candida species intolerant to fluconazole and Aspergillus fumigatus resistant to azoles is worrisome[6]. Additionally, the emergence of ganciclovir-resistant cytomegalovirus (CMV) following solid organ trans
To mitigate infection risks following solid organ transplantation, appropriate prophylactic medications should be administered both immediately before and after the surgery[3]. Moreover, measures such as strict hand hygiene, contact isolation, and selective bowel decontamination are essential to limit the transmission of multidrug-resistant and ex
Evaluating clinical trials dedicated to the management of antimicrobials in preventing resistance among SOTR is of paramount importance. These trials provide critical insights into the efficacy of various antimicrobial regimens and strategies in real-world settings, helping to identify the most effective practices for reducing the incidence of resistant infections. By rigorously assessing different antimicrobial protocols, clinical trials contribute to the optimization of treatment guidelines, ensuring that SOTR receive the best possible care. Furthermore, these trials can uncover potential adverse effects and resistance patterns associated with specific antimicrobials, guiding clinicians in making informed decisions that balance efficacy and safety.
The relevance of a systematic review in this context cannot be overstated. This systematic review, being the first of its kind in this area, will synthesize and critically appraise the existing evidence from clinical trials, offering a comprehensive overview of the current knowledge on antimicrobial efficacy in preventing resistance among SOTR. Such a review will not only highlight gaps in the current research but also provide a robust evidence base for future studies and policymaking. By collating and analyzing data from multiple sources, this systematic review aims to establish a clear under
A systematic review was conducted using a search methodology consistent with the preferred reporting items for systematic reviews and meta-analyses criteria[10]. The review methodology was officially registered with PROSPERO.
The systematic review was structured around a question developed using the population, intervention, comparison, and outcomes framework:
In SOTR (population), how effective are antimicrobial agents (intervention) compared to placebo, no intervention, or alternative regimens (comparison) in preventing antimicrobial resistance (outcomes)?
The primary outcome assessed was the incidence of antimicrobial resistance. Secondary outcomes included rates of infection, graft survival, patient mortality, and adverse effects associated with antimicrobial usage.
This review included randomized clinical trials that evaluated the efficacy of antimicrobial agents (prophylactic or therapeutic) aimed at preventing antimicrobial resistance. The review focused on studies involving SOTR and those comparing antimicrobial agents with placebo, no intervention, or other antimicrobial regimens. Exclusion criteria included observational studies, case reports, case series, editorials, reviews, commentaries, non-human studies, and clinical trials involving transplant types other than solid organ transplants.
The search strategy involved analyzing multiple databases, including: PubMed/MEDLINE, Web of Science, Embase, Scopus, and SciELO, as well as examining gray literature sources on Google Scholar. A comprehensive electronic database search was conducted from the databases’ inception until May 2024, with no language restrictions. Additionally, further records were identified by reviewing the reference lists and citations of all selected full-text articles for potential inclusion in the study.
The search contained the following terms: “Solid Organ Transplant” OR “Kidney Transplant” OR “Liver Transplant” OR “Heart Transplant” OR “Lung Transplant” OR “Pancreas Transplant” AND “Antimicrobial Agents” OR “Antibiotics” OR “Antifungal Agents” OR “Antiviral Agents” OR “Antimicrobial Prophylaxis” AND “Antimicrobial Resistance” OR “Bacterial Resistance” OR “Multidrug Resistance” OR “Fungal Resistance” OR “Viral Resistance” AND “Infections” OR “Bacterial Infections” OR “Fungal Infections” OR “Viral Infections”.
These search techniques utilize syntax and operators specific to each database to retrieve articles pertinent to defined queries. Modifications may be necessary to tailor the exploration functionality and syntax rules of each individual database.
Table 1 presents the search strategies employed for each respective database utilizing the designated terms.
| Database | Search strategy |
| PubMed/MEDLINE | [“Organ Transplantation” (MeSH) OR “Kidney Transplantation” (MeSH) OR “Liver Transplantation” (MeSH) OR “Heart Transplantation” (MeSH) OR “Lung Transplantation” (MeSH) OR “Pancreas Transplantation” (MeSH)] AND [“Anti-Bacterial Agents” (MeSH) OR “Antibiotics” (MeSH) OR “Antifungal Agents” (MeSH) OR “Antiviral Agents” (MeSH) OR “Antimicrobial Prophylaxis” (MeSH)] AND [“Drug Resistance, Microbial” (MeSH) OR “Drug Resistance, Bacterial” (MeSH) OR “Drug Resistance, Multiple, Bacterial” (MeSH) OR “Drug Resistance, Fungal” (MeSH) OR “Drug Resistance, Viral” (MeSH)] AND [“Infections” (MeSH) OR “Bacterial Infections” (MeSH) OR “Fungal Infections” (MeSH) OR “Viral Infections” (MeSH)] |
| Scopus | TITLE-ABS-KEY (“Organ Transplantation” OR “Kidney Transplantation” OR “Liver Transplantation” OR “Heart Transplantation” OR “Lung Transplantation” OR “Pancreas Transplantation”) AND (“Anti-Bacterial Agents” OR “Antibiotics” OR “Antifungal Agents” OR “Antiviral Agents” OR “Antimicrobial Prophylaxis”) AND (“Drug Resistance, Microbial” OR “Drug Resistance, Bacterial” OR “Drug Resistance, Multiple, Bacterial” OR “Drug Resistance, Fungal” OR “Drug Resistance, Viral”) AND (“Infections” OR “Bacterial Infections” OR “Fungal Infections” OR “Viral Infections”) |
| SciELO | [(“Organ Transplantation” OR “Kidney Transplantation” OR “Liver Transplantation” OR “Heart Transplantation” OR “Lung Transplantation” OR “Pancreas Transplantation”) AND (“Anti-Bacterial Agents” OR “Antibiotics” OR “Antifungal Agents” OR “Antiviral Agents” OR “Antimicrobial Prophylaxis”) AND (“Drug Resistance, Microbial” OR “Drug Resistance, Bacterial” OR “Drug Resistance, Multiple, Bacterial” OR “Drug Resistance, Fungal” OR “Drug Resistance, Viral”) AND (“Infections” OR “Bacterial Infections” OR “Fungal Infections” OR “Viral Infections”)] |
| Embase | (‘Organ Transplantation’/exp OR ‘Kidney Transplantation’/exp OR ‘Liver Transplantation’/exp OR ‘Heart Transplantation’/exp OR ‘Lung Transplantation’/exp OR ‘Pancreas Transplantation’/exp) AND (‘Anti-Bacterial Agents’/exp OR ‘Antibiotics’/exp OR ‘Antifungal Agents’/exp OR ‘Antiviral Agents’/exp OR ‘Antimicrobial Prophylaxis’/exp) AND (‘Drug Resistance, Microbial’/exp OR ‘Drug Resistance, Bacterial’/exp OR ‘Drug Resistance, Multiple, Bacterial’/exp OR ‘Drug Resistance, Fungal’/exp OR ‘Drug Resistance, Viral’/exp) AND (‘Infections’/exp OR ‘Bacterial Infections’/exp OR ‘Fungal Infections’/exp OR ‘Viral Infections’/exp) |
| Web of Science | TS = (“Organ Transplantation” OR “Kidney Transplantation” OR “Liver Transplantation” OR “Heart Transplantation” OR “Lung Transplantation” OR “Pancreas Transplantation”) AND TS = (“Anti-Bacterial Agents” OR “Antibiotics” OR “Antifungal Agents” OR “Antiviral Agents” OR “Antimicrobial Prophylaxis”) AND TS = (“Drug Resistance, Microbial” OR “Drug Resistance, Bacterial” OR “Drug Resistance, Multiple, Bacterial” OR “Drug Resistance, Fungal” OR “Drug Resistance, Viral”) AND TS = (“Infections” OR “Bacterial Infections” OR “Fungal Infections” OR “Viral Infections”) |
| Google Scholar | “Organ Transplantation” OR “Kidney Transplantation” OR “Liver Transplantation” OR “Heart Transplantation” OR “Lung Transplantation” OR “Pancreas Transplantation” AND “Anti-Bacterial Agents” OR “Antibiotics” OR “Antifungal Agents” OR “Antiviral Agents” OR “Antimicrobial Prophylaxis” AND “Drug Resistance, Microbial” OR “Drug Resistance, Bacterial” OR “Drug Resistance, Multiple, Bacterial” OR “Drug Resistance, Fungal” OR “Drug Resistance, Viral” AND “Infections” OR “Bacterial Infections” OR “Fungal Infections” OR “Viral Infections” |
Two researchers independently assessed the relevance of titles and abstracts, followed by a comprehensive evaluation of full-text articles. An unbiased assessment of full texts was conducted to ascertain eligibility. Discrepancies were resolved through discussion; in cases of persistent disagreement, a third expert was consulted. The Kappa test was employed to measure interobserver agreement, with a score exceeding 80 indicating high consistency.
Two researchers independently collected data using customized extraction methods. A comparative analysis was conducted to ensure uniformity in the gathered data. Information regarding patients, interventions, controls, microorganisms studied, incidence of new infections, and adverse events was compiled from the reviewed articles. Additionally, systematic documentation of details such as authorship, publication year, and country of origin was conducted.
Two researchers assessed the risk of bias and the quality of the included clinical trials using the Jadad scale[11]. The Jadad scale is a five-point system that evaluates three key criteria: Randomization, blinding, and withdrawals/dropouts. Scores range from 0 to 5, with higher scores indicating better quality; a score of 0-2 points indicates low quality, while a score of 3-5 points indicates high quality. For randomization, the trial receives 1 point if it is described as randomized, an addi
Information from the trials was collected utilizing descriptive statistics, encompassing mean variances, standard deviation values, and ranges, particularly focusing on continuous outcomes. If the studies exhibited notable homo
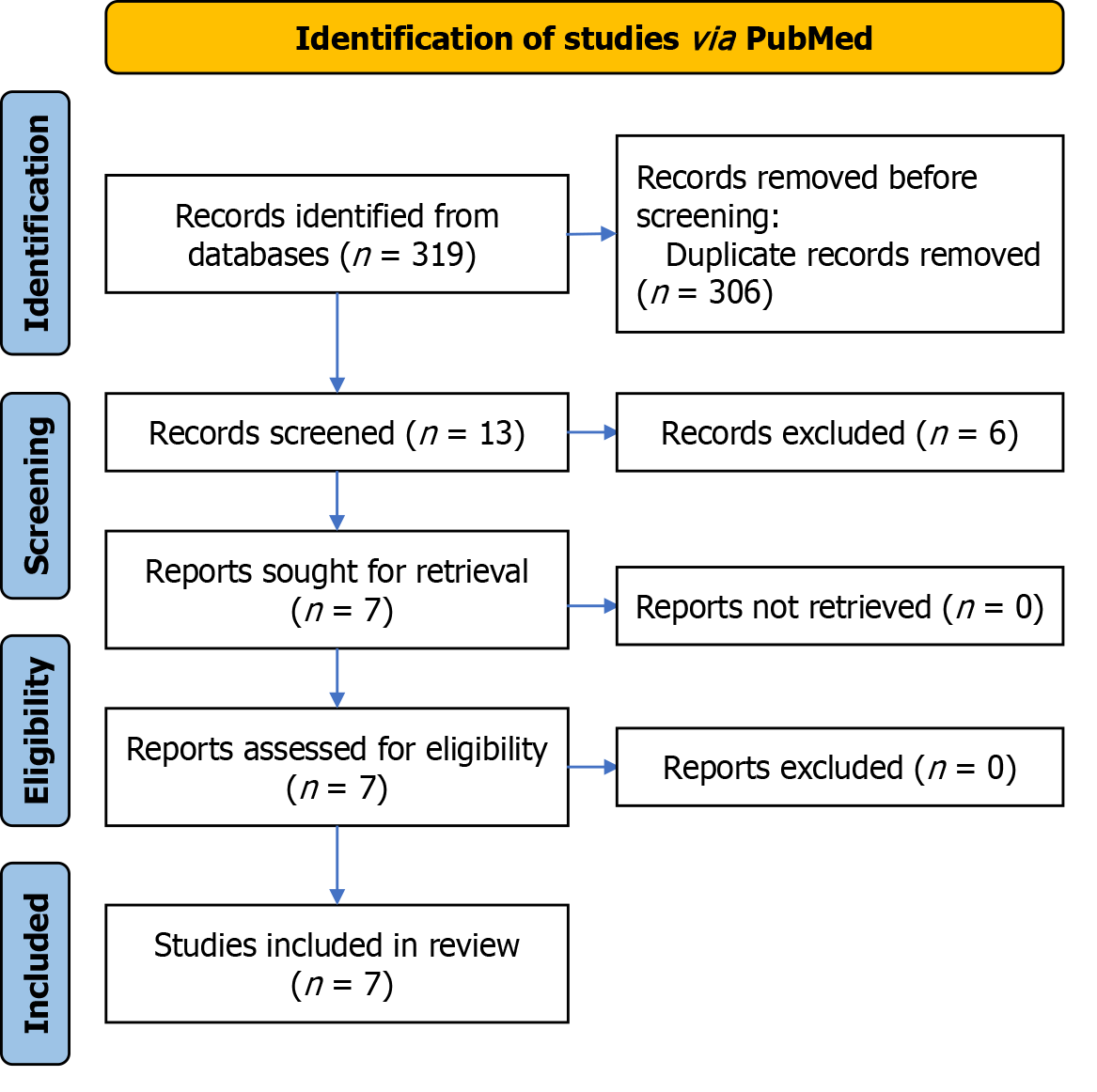
Following the search protocol, 319 studies were identified in electronic databases. After removing duplicates and applying eligibility criteria, 13 documents underwent a comprehensive full-text review. The studies were excluded primarily because they were not randomized clinical trials or because they did not investigate microorganisms with previously reported resistance. After the final phase of the eligibility assessment, this study ultimately included 7 articles. Figure 1 provides a detailed depiction of the examination flowchart.
Table 2 summarizes the main characteristics of the seven randomized clinical trials included in this review[12-18]. The review encompasses trials published between 2004[18] and 2022[12,13] examining data from a total of 2318 patients. Most of these studies were conducted in North America. This table also indicates the microorganisms studied, with a particular focus on CMV. Consequently, the most frequently used medications were maribavir, valganciclovir, and ganciclovir.
| Ref. | Patients | Intervention/control | Microorganisms studied | Incidence of antimicrobial resistance | Adverse events |
| Rock et al[12] | 1085 | Routine and discharge patient room cleaning using ultraviolet-C light vs routine and discharge patient room cleaning | Vancomycin-resistant enterococci and Clostridioides difficile | 6.52 vs 6.68 per 1000 patient-days in vancomycin-resistant enterococci | Not reported |
| Avery et al[13] | 352 | Maribavir 400 mg twice daily versus valganciclovir/ganciclovir, foscarnet, or cidofovir | Cytomegalovirus | 44.4% vs 76.1% (P < 0.05) | 40 deaths, dysgeusia, nausea, diarrhea |
| Fariñas et al[14] | 105 | Colistin-neomycin vs no treatment | Multidrug-resistant | 9.4% vs 13.5% (P > 0.05) | Diarrhea |
| Papanicolaou et al[15] | 120 | Maribavir 400, 800, or 1200 mg at a ratio of 1:1:1 | Cytomegalovirus | 30%, 37.5%, 27.5% (P > 0.05) | 32 deaths, dysgeusia, nausea |
| Boivin et al[16] | 275 | Ganciclovir vs valganciclovir | Cytomegalovirus | 2.3% vs 3.6% (P > 0.05) | 2 deaths, gastrointestinal problem |
| Boivin et al[17] | 80 | Valganciclovir vs ganciclovir | Cytomegalovirus | 2.5% vs 2.5% (P > 0.05) | 1 death, 23 grafts rejected, abdominal pain, diarrhea |
| Boivin et al[18] | 301 | Valganciclovir vs ganciclovir | Cytomegalovirus | 0% vs 6.1% (P < 0.05) | 1 graft rejected |
Of concern, all clinical trials showed significant proportions of resistant microorganisms after the interventions, with no statistically significant differences between the groups (mean resistance: 13.47% vs 14.39% in the control group), except for two studies that demonstrated greater efficacy of maribavir[13] and valganciclovir[18] (mean resistance: 22.2% vs 41.1% in the control group; P < 0.05). On the other hand, Ultraviolet-C light cleaning[12] and the combination of colistin-neomycin did not show significant differences in the incidence of resistance in the studied microorganisms[14].
The total reported deaths in three clinical trials were 75[13,15-17] and there were 24 graft rejections in two studies[17,18]. Moreover, dysgeusia, nausea, and diarrhea were also among the most reported adverse events.
Most of the included studies demonstrate a high risk of bias due to poor blinding and lack of proper randomization methods, except for Papanicolaou et al[15], which meets all the Jadad criteria for high methodological quality. Studies by Avery et al[13] and Fariñas et al[14] both scored 3, showing good methodological quality with some risks of bias. These results suggest that the overall quality of evidence from these trials might be compromised, affecting the reliability of the conclusions drawn from the systematic review (Table 3).
This systematic review underscores the ongoing challenge of antimicrobial resistance in SOTR, despite the use of prophylactic and therapeutic antimicrobial agents. While some interventions showed promise, a significant proportion of resistant microorganisms persisted across studies, highlighting a critical gap in current antimicrobial strategies. The lack of statistically significant differences between most intervention groups suggests that current protocols may be inadequate in addressing the complexity of resistance in this vulnerable population. These findings call for high-quality randomized clinical trials to explore innovative therapies and optimize existing regimens for better long-term outcomes.
Addressing resistance to anti-CMV medications is particularly challenging. Current drugs, like valganciclovir and ganciclovir, target the CMV DNA polymerase (UL54) and rely on activation by the viral UL97 protein kinase. Mutations in CMV genes coding for UL54 or UL97 can lead to resistance[19]. Maribavir, however, has a distinct mechanism of action that does not require activation by UL97[20] and binds to a different site on UL97 compared to valganciclovir and ganciclovir[13], making it effective against strains resistant to these drugs due to UL54 or UL97 mutations[21,22]. This positions maribavir as a promising option for treating resistant CMV infections, though it cannot be used alongside valganciclovir or ganciclovir, as it inhibits the UL97 protein kinase[13].
Two clinical trials in SOTR with resistant CMV demonstrated the efficacy of maribavir. Avery et al[13] found that maribavir was more effective than ganciclovir, valganciclovir, foscarnet, or cidofovir in clearing CMV viremia by week 8, with fewer cases of neutropenia and acute renal injury. Maribavir also cleared viremia more rapidly, an important factor as persistent viremia can signal clinical deterioration. The CMV consensus forum confirmed that treating viremia prevents disease, with viral load serving as a surrogate endpoint in CMV clinical studies[23]. These results highlight maribavir’s potential to transform CMV treatment by effectively targeting drug-resistant strains while maintaining an improved safety profile[13]. Similarly, Papanicolaou et al[15] found that maribavir at doses of 400-1200 mg BID cleared CMV viremia in two-thirds of transplant recipients with resistant infections within six weeks, with no additional adverse effects, confirming its safety and viability as a long-term treatment option for this high-risk group.
Two clinical trials demonstrated that resistance rates to ganciclovir were comparable whether treated with oral valganciclovir or intravenous ganciclovir[16,17]. Although drug resistance mutations significantly impact viral load reduction and early kinetics, they do not consistently correlate with adverse clinical outcomes[16]. The incidence of ganciclovir resistance in SOTR varies based on the specific group studied, immunosuppressive drugs used, antiviral approach, and resistance detection techniques[24]. Reported rates of ganciclovir-resistant CMV mutations range from 0% in seropositive kidney transplant patients to 27% in lung transplant recipients[24,25], consistent with the findings of this review.
In another trial, the effectiveness of oral colistin-neomycin in preventing multidrug-resistant Enterobacterales infections in SOTR was examined. Resistance to colistin was detected in 5.6% of the experimental group and 1.9% of the control group, suggesting that this intervention did not reduce infection rates and may contribute to antimicrobial resistance[14,26-28]. Additionally, Rock et al[12] found that supplementing routine cleaning with Ultraviolet (UV)-C disinfection did not significantly reduce new infections of vancomycin-resistant enterococci or C. difficile in SOTR. While UV-C light can eliminate pathogens from surfaces[29-31], its clinical impact on reducing multidrug-resistant organisms and C. difficile infections remains inconclusive, likely due to the evolving nature of UV-C disinfection practices[28,29].
Overall, this review underscores the persistent challenge of antimicrobial resistance in SOTR. The substantial rates of resistance, graft rejection, and mortality reported across trials emphasize the need for optimizing treatment strategies. Adverse events like dysgeusia, nausea, and diarrhea further highlight the delicate balance between therapeutic efficacy and tolerability. While some interventions showed promise, the widespread prevalence of resistant microorganisms indicates an urgent need for more rigorous studies to assess long-term outcomes and safety profiles of these treatments.
Future research should prioritize longitudinal, multicenter trials with larger sample sizes to assess the long-term efficacy of antimicrobial interventions in preventing resistance in SOTR. These studies should explore combination therapies with different mechanisms of action and include robust surveillance programs to monitor resistance patterns. Additionally, focusing on patient-centered outcomes, such as quality of life and functional status, alongside comprehensive profiling of adverse events, is crucial. Cost-effectiveness analyses should also be integrated to evaluate the economic impact of these interventions. Addressing these aspects will offer a deeper understanding of optimal antimicrobial strategies and their implications for both patient care and healthcare resource management.
This review identified significant risks of bias in most included studies, primarily due to inadequate blinding and randomization. Notably, Papanicolaou et al[15] adhered to the Jadad et al[11] criteria, demonstrating high methodological quality. Avery et al[13] and Fariñas et al[14] showed moderate quality with some biases. These limitations in study quality may affect the reliability of conclusions regarding the efficacy of antimicrobial interventions in preventing resistance among transplant recipients. Similar issues were observed in other systematic reviews assessing clinical trials[32,33]. To improve future research, rigorous study design should be prioritized, including strict blinding, appropriate allocation concealment, and robust randomization methods.
Several limitations of this systematic review warrant consideration. Significant heterogeneity in study designs, interventions, and outcome measures across trials complicates direct comparison and synthesis of results. The focus on specific antimicrobial agents or pathogens also limits the generalizability of findings to broader transplant populations and different healthcare settings. Additionally, publication bias may skew results, with positive outcomes more likely to be published. Short follow-up periods in some trials may not capture long-term outcomes, such as late-onset infections or resistance development. Incomplete reporting of adverse events and variations in microbiological testing methods further complicate interpretation of the evidence. Future research should address these limitations to enhance the robustness and applicability of findings for clinical practice and policy in transplant medicine.
This systematic review highlights the significant challenges in managing infections among SOTR, particularly due to high rates of antimicrobial resistance observed across a variety of pathogens post-intervention. While certain agents, such as maribavir and valganciclovir, demonstrate efficacy against CMV infections, antimicrobial effectiveness varies broadly depending on the pathogen and clinical context. These findings underscore the need for individualized treatment strategies that account for pathogen profiles and patient-specific factors. Methodological limitations, including biases from inadequate blinding and randomization, may affect the reliability of findings across the reviewed studies. The clinical impact of antimicrobial resistance is considerable, with high incidences of mortality and graft rejection reported in transplant populations. To advance the field, there is an urgent need for well-designed, multicenter trials to validate results, standardize treatment protocols, and explore novel therapeutic options for a broader spectrum of infections, ultimately aiming to optimize antimicrobial strategies and improve transplant outcomes.
| 1. | Guardabassi L, Butaye P, Dockrell DH, Fitzgerald JR, Kuijper EJ; ESCMID Study Group for Veterinary Microbiology (ESGVM). One Health: a multifaceted concept combining diverse approaches to prevent and control antimicrobial resistance. Clin Microbiol Infect. 2020;26:1604-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | So M, Walti L. Challenges of Antimicrobial Resistance and Stewardship in Solid Organ Transplant Patients. Curr Infect Dis Rep. 2022;24:63-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Silva JT, Aguado JM. Current state of antimicrobial stewardship and organ transplantation in Spain. Transpl Infect Dis. 2022;24:e13851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Oriol I, Sabé N, Simonetti AF, Lladó L, Manonelles A, González J, Tubau F, Carratalà J. Changing trends in the aetiology, treatment and outcomes of bloodstream infection occurring in the first year after solid organ transplantation: a single-centre prospective cohort study. Transpl Int. 2017;30:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Hosseini-Moghaddam SM, Luo B, Bota SE, Husain S, Silverman MS, Daneman N, Brown KA, Paterson JM. Incidence and Outcomes Associated With Clostridioides difficile Infection in Solid Organ Transplant Recipients. JAMA Netw Open. 2021;4:e2141089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Fernández-Ruiz M, Cardozo C, Salavert M, Aguilar-Guisado M, Escolà-Vergé L, Muñoz P, Gioia F, Montejo M, Merino P, Cuervo G, García-Vidal C, Aguado JM; CANDIPOP Project, the CANDI-Bundle Group; GEIRAS-GEMICOMED (SEIMC)REIPI. Candidemia in solid organ transplant recipients in Spain: Epidemiological trends and determinants of outcome. Transpl Infect Dis. 2019;21:e13195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Escribano P, Rodríguez-Sánchez B, Díaz-García J, Martín-Gómez MT, Ibáñez-Martínez E, Rodríguez-Mayo M, Peláez T, García-Gómez de la Pedrosa E, Tejero-García R, Marimón JM, Reigadas E, Rezusta A, Labayru-Echeverría C, Pérez-Ayala A, Ayats J, Cobo F, Pazos C, López-Soria L, Alastruey-Izquierdo A, Muñoz P, Guinea J; ASPEIN study group. Azole resistance survey on clinical Aspergillus fumigatus isolates in Spain. Clin Microbiol Infect. 2021;27:1170.e1-1170.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Ramírez-Arbeláez JA, Arroyave-Zuluaga RL, Barrera-Lozano LM, Hurtado V, González-Arroyave D, Ardila CM. Relationship between Intraoperative Bile Culture Outcomes and Subsequent Postoperative Infectious Complications: A Retrospective Cohort Study. Biomed Res Int. 2024;2024:3930130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Ardila CM, Bedoya-García JA. Antimicrobial resistance in dentistry. Oral Dis. 2024;30:805-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 5225] [Article Influence: 1045.0] [Reference Citation Analysis (1)] |
| 11. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 13048] [Article Influence: 434.9] [Reference Citation Analysis (3)] |
| 12. | Rock C, Hsu YJ, Curless MS, Carroll KC, Ross Howard T, Carson KA, Cummings S, Anderson M, Milstone AM, Maragakis LL. Ultraviolet-C Light Evaluation as Adjunct Disinfection to Remove Multidrug-Resistant Organisms. Clin Infect Dis. 2022;75:35-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Avery RK, Alain S, Alexander BD, Blumberg EA, Chemaly RF, Cordonnier C, Duarte RF, Florescu DF, Kamar N, Kumar D, Maertens J, Marty FM, Papanicolaou GA, Silveira FP, Witzke O, Wu J, Sundberg AK, Fournier M; SOLSTICE Trial Investigators. Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results From a Phase 3 Randomized Clinical Trial. Clin Infect Dis. 2022;75:690-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 14. | Fariñas MC, González-Rico C, Fernández-Martínez M, Fortún J, Escudero-Sanchez R, Moreno A, Bodro M, Muñoz P, Valerio M, Montejo M, Nieto J, Ruiz-San Millan JC, Casafont-Morencos F, Martinez-Martínez L, Fariñas-Álvarez C; ENTHERE Study Group, the Group for Study of Infection in Transplantation of the Spanish Society of Infectious Diseases and Clinical Microbiology (GESITRA-SEIMC) and the Spanish Network for Research in Infectious Diseases (REIPI). Oral decontamination with colistin plus neomycin in solid organ transplant recipients colonized by multidrug-resistant Enterobacterales: a multicentre, randomized, controlled, open-label, parallel-group clinical trial. Clin Microbiol Infect. 2021;27:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Papanicolaou GA, Silveira FP, Langston AA, Pereira MR, Avery RK, Uknis M, Wijatyk A, Wu J, Boeckh M, Marty FM, Villano S. Maribavir for Refractory or Resistant Cytomegalovirus Infections in Hematopoietic-cell or Solid-organ Transplant Recipients: A Randomized, Dose-ranging, Double-blind, Phase 2 Study. Clin Infect Dis. 2019;68:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 16. | Boivin G, Goyette N, Rollag H, Jardine AG, Pescovitz MD, Asberg A, Ives J, Hartmann A, Humar A. Cytomegalovirus resistance in solid organ transplant recipients treated with intravenous ganciclovir or oral valganciclovir. Antivir Ther. 2009;14:697-704. [PubMed] |
| 17. | Boivin G, Goyette N, Gilbert C, Humar A, Covington E. Clinical impact of ganciclovir-resistant cytomegalovirus infections in solid organ transplant patients. Transpl Infect Dis. 2005;7:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Boivin G, Goyette N, Gilbert C, Roberts N, Macey K, Paya C, Pescovitz MD, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Covington E. Absence of cytomegalovirus-resistance mutations after valganciclovir prophylaxis, in a prospective multicenter study of solid-organ transplant recipients. J Infect Dis. 2004;189:1615-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | El Chaer F, Shah DP, Chemaly RF. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood. 2016;128:2624-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 20. | Chou S, Marousek GI. Accelerated evolution of maribavir resistance in a cytomegalovirus exonuclease domain II mutant. J Virol. 2008;82:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Biron KK, Harvey RJ, Chamberlain SC, Good SS, Smith AA 3rd, Davis MG, Talarico CL, Miller WH, Ferris R, Dornsife RE, Stanat SC, Drach JC, Townsend LB, Koszalka GW. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002;46:2365-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 294] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Chou S, Wu J, Song K, Bo T. Novel UL97 drug resistance mutations identified at baseline in a clinical trial of maribavir for resistant or refractory cytomegalovirus infection. Antiviral Res. 2019;172:104616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Natori Y, Alghamdi A, Tazari M, Miller V, Husain S, Komatsu T, Griffiths P, Ljungman P, Orchanian-Cheff A, Kumar D, Humar A; CMV Consensus Forum. Use of Viral Load as a Surrogate Marker in Clinical Studies of Cytomegalovirus in Solid Organ Transplantation: A Systematic Review and Meta-analysis. Clin Infect Dis. 2018;66:617-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Gilbert C, Boivin G. Human cytomegalovirus resistance to antiviral drugs. Antimicrob Agents Chemother. 2005;49:873-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Limaye AP. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin Infect Dis. 2002;35:866-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Errico G, Gagliotti C, Monaco M, Masiero L, Gaibani P, Ambretti S, Landini MP, D'Arezzo S, Di Caro A, Parisi SG, Palù G, Vespasiano F, Morsillo F, Moro ML, Procaccio F, Ricci A, Grossi PA, Pantosti A, Nanni Costa A; SInT Collaborative Study Group. Colonization and infection due to carbapenemase-producing Enterobacteriaceae in liver and lung transplant recipients and donor-derived transmission: a prospective cohort study conducted in Italy. Clin Microbiol Infect. 2019;25:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Tacconelli E, Mazzaferri F, de Smet AM, Bragantini D, Eggimann P, Huttner BD, Kuijper EJ, Lucet JC, Mutters NT, Sanguinetti M, Schwaber MJ, Souli M, Torre-Cisneros J, Price JR, Rodríguez-Baño J. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin Microbiol Infect. 2019;25:807-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 28. | Carling PC. The need for clinically relevant studies of non-touch disinfecting systems. J Hosp Infect. 2013;84:340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Health Quality Ontario. Portable Ultraviolet Light Surface-Disinfecting Devices for Prevention of Hospital-Acquired Infections: A Health Technology Assessment. Ont Health Technol Assess Ser. 2018;18:1-73. [PubMed] |
| 30. | Otter JA, Yezli S, Salkeld JA, French GL. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control. 2013;41:S6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 343] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 31. | Rock C, Curless MS, Nowakowski E, Ross T, Carson KA, Trexler P, Carroll K, Maragakis LL. UV-C Light Disinfection of Carbapenem-Resistant Enterobacteriaceae from High-Touch Surfaces in a Patient Room and Bathroom. Infect Control Hosp Epidemiol. 2016;37:996-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Ardila CM, Bedoya-García JA, Arrubla-Escobar DE. Antibiotic resistance in periodontitis patients: A systematic scoping review of randomized clinical trials. Oral Dis. 2023;29:2501-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Ardila CM, Bedoya-García JA. Microbial resistance to oral antiseptics used in hospitalized patients: A systematic scoping review of randomized clinical trials. Spec Care Dentist. 2023;43:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/