Published online Mar 18, 2025. doi: 10.5500/wjt.v15.i1.100413
Revised: October 3, 2024
Accepted: November 1, 2024
Published online: March 18, 2025
Processing time: 103 Days and 21.1 Hours
Acute liver failure (ALF) is a severe condition characterized by rapid deterioration of liver function in individuals without preexisting liver disease. Liver transplantation (LT) is the most impactful treatment. Yellow fever (YF) is an infectious disease that primarily affects the liver and has a high mortality rate. However, LT can be a viable option for treating rare cases with extensive liver involvement. However, the criteria for assessing the severity of ALF and det
To present necessary adjustments to established scoring systems for ALF secondary to YF.
This was an observational, retrospective, single-center study. Fourteen consecutive patients with confirmed ALF due to YF were monitored in the intensive care unit by a specialized liver transplant team during a three-month epidemic outbreak in Brazil. During hospitalization, general supportive therapeutic measures were implemented, and the patients were regularly assessed using the King's College criteria and the Clichy-Villejuif criteria to determine the severity of liver failure. LT is considered a viable measure for patients with signs of end-stage liver failure.
Eight of 14 (57%) patients developed severe neurological alterations within the first 96 hours after hospital admission. Four patients underwent emergency LT, and despite a moderate viral infection of the graft after transplantation, the 5-year survival rate was 50%. Although the King's College criteria and the Clichy-Villejuif criteria are the main scoring systems for ALF, they are insufficient for predicting the risk of mortality in this context, primarily because of low serum bilirubin levels in the final stage of the disease and significant disparities between coagulation abnormalities and patient severity.
To ensure good applicability in cases of YF-induced ALF, the authors suggest adaptations to the King's College and Clichy-Villejuif criteria.
Core Tip: This study describes the application of current liver transplantation (LT) scoring systems, the King's College criteria and the Clichy-Villejuif criteria, for predicting outcomes in patients with acute liver failure (ALF) due to yellow fever (YF). We focused on 14 patients with confirmed ALF due to YF who were monitored by a liver transplant team during an epidemic in Brazil. Four patients underwent emergency LT following adaptations to the aforementioned scoring systems. The 5-year survival rate was 50%. These findings highlight the need to revise the criteria for transplantation for YF-associated ALF and demonstrate that LT can be a viable, life-saving option in specific cases.
- Citation: Athanasio BDS, Andrade AMF, Costa VV, Castro JF, Garcia SLM, Teixeira MM, Souza DDG, Vidigal PVT, Lima CX. King's College criteria and the Clichy-Villejuif criteria require adjustments for assessing acute liver failure due to yellow fever. World J Transplant 2025; 15(1): 100413
- URL: https://www.wjgnet.com/2220-3230/full/v15/i1/100413.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i1.100413
Acute liver failure (ALF) is a serious condition characterized by rapid deterioration of liver function in individuals without preexisting liver disease, and liver transplantation (LT) has been the most impactful treatment in recent decades. Ongoing assessment of key clinical features in prognostic scoring systems is crucial in determining the outcome of ALF and guiding clinical decisions, including the need for LT.
Despite the inclusion of multiple clinical features in various scoring systems, hepatic encephalopathy, coagulopathy, and serum bilirubin levels remain the most critical because of their direct reflection of liver function and their strong correlation with patient outcomes. For example, hepatic encephalopathy provides a window into the extent of neu
The King's College criteria and the Clichy-Villejuif criteria are the gold standard for determining the need for LT in patients with ALF[3] (Table 1). The performance of these criteria can be improved by considering the underlying cause of the disease and utilizing sequential assessments instead of single time-point estimates, particularly in cases of hyperacute etiologies[4].
| King's College criteria | Clichy-Villejuif criteria | |
| Paracetamol induced liver failure (Acetaminophen) | Non-paracetamol induced liver failure | |
| Arterial potential of hydrogen < 7.3 (irrespective of the grade of encephalopathy) | PT > 100 seconds (INR > 6.5) | Age: Patients over the age of 30 years are at higher risk and are more likely to need a transplant |
| All three of the following | Any three of the following | Factor V levels |
| PT > 100 seconds (INR > 6.5) | Age < 10 years or > 40 years | Factor V level < 20% of normal for patients over 30 years old |
| Serum creatinine > 3.4 mg/dL (300 μmol/L) | Etiology: Non-A hepatitis, non-B hepatitis, and idiosyncratic drug reactions | Factor V level < 30% of normal for patients under 30 years old |
| Grade III or IV encephalopathy | Duration of jaundice before encephalopathy > 7 days. Prothrombin time > 50 seconds (INR > 3.5). Serum bilirubin > 17.5 mg/dL (300 μmol/L) | |
| Grade of encephalopathy | ||
| Presence of grade III or IV encephalopathy indicates a severe case | ||
Yellow fever (YF) is a mosquito-borne viral illness caused by an arbovirus of the family Flaviviridae, genus Flavivirus, encompassing positive-sense single-stranded RNA viruses. YF is an infectious disease endemic to forest areas in Africa and America and has a high mortality rate[5].
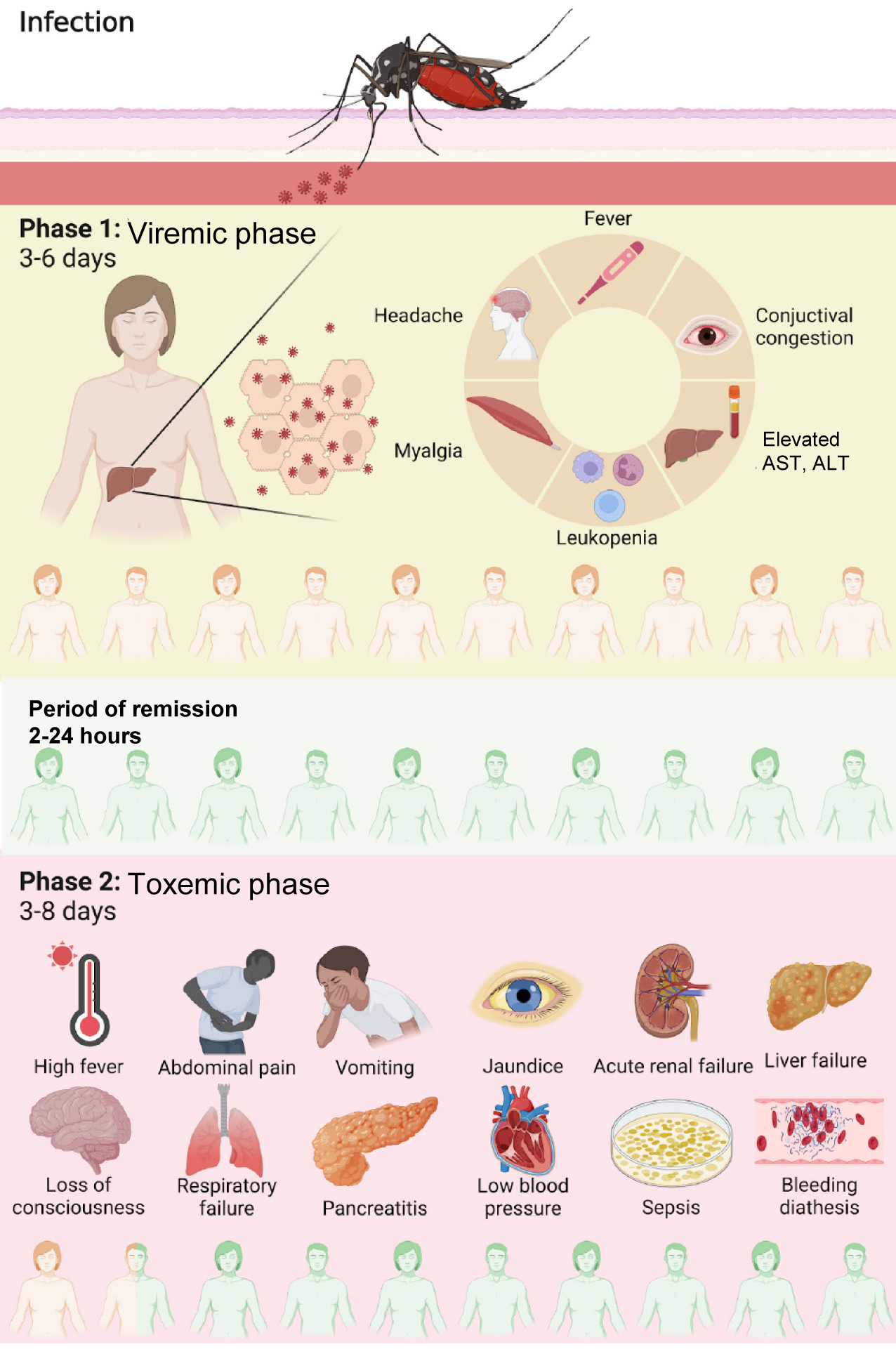
YF is typically described as a biphasic disease (Figure 1). After an incubation period of 3–6 days, the first phase, called the "viremic phase", begins. Symptom onset is typically abrupt, with fever, chills, asthenia, headache, back pain, generalized myalgia, nausea, and dizziness. Symptom onset may be followed by a "period of remission", during which the fever and symptoms disappear for approximately 24 hours. The second phase, called the "toxemic phase", affects 15%–25% of infected individuals and is characterized by the resurgence of high fever associated with vomiting, epigastric pain, jaundice, renal dysfunction, and hemorrhagic diathesis. YF is a disease characterized by multisystem involvement, but the liver is the most affected organ, making ALF a common cause of death[5]. Despite a comprehensive under
YF was epidemic in Brazil between June YF and June 2018[9]. In this context, patients who developed ALF were evaluated for LT. In a pioneering effort worldwide, the first LTs for ALF caused by YF were performed[6].
The objective of this study was to describe and discuss the application of these criteria for assessing the severity of ALF caused by YF in a series of 14 cases from a single center.
This was an observational, retrospective, single-center study conducted at the Liver Transplant Unit of Felicio Rocho Hospital. The study population comprised 14 patients who were admitted to the intensive care unit with confirmed ALF due to YF contracted during an epidemic in Brazil between January and February 2018. All patients were monitored by the LT team and were followed for 6 years by the medical team. Clinical, laboratory, and radiological data were evaluated both prospectively and retrospectively during this period.
All participants signed an informed consent form after being thoroughly informed about the study, and the study protocol was approved by the Ethics Committee of the Felicio Rocho Hospital, No. 2.851.504.
The basic care protocol aligned with the guidelines of the local health authority. All patients with suspected YF were admitted and underwent imaging (abdominal ultrasound) and laboratory tests, including a complete blood count, INR, liver function tests, factor V tests, renal function tests, amylase, lipase, blood glucose, serological tests for hepatitis B virus [hepatitis B surface, hepatitis B surface antibody, hepatitis B core antigen immunoglobulin (Ig) G and IgM], hepatitis C (anti-hepatitis C virus), hepatitis A (anti-hepatitis A virus IgG and IgM), serology for YF and dengue, and RNA detection of the YF virus in serum via PCR. Blood samples were collected every 12 hours after patient admission, immediately before discharge, and at each follow-up visit (weekly during the first month, monthly until the 6th month, and semiannually thereafter).
During the toxemic phase of the disease, patients with laboratory-confirmed liver function impairment underwent regular clinical and laboratory evaluations. The King's College criteria and Clichy-Villejuif criteria were used to assess the severity of ALF and determine potential indications for emergency LT. All patients underwent neurological evaluation and cranial computed tomography when they presented with an altered mental status. In cases of severe acute renal failure, continuous hemodialysis was performed. Antibiotics were not routinely administered; they were used only in cases of suspected bacterial infection or as prophylaxis for patients undergoing LT. Plasmapheresis has been utilized in some of the more severe cases.
The cases of patients who developed ALF with neurological alterations were discussed exhaustively in clinical meetings on a daily basis.
The liver transplant team, in consensus with a national committee of experts, recommended LT for patients showing signs of severe liver involvement and no expected survival even with the best supportive treatment. In these cases, the patients were listed for LT from deceased donors, provided on an emergency basis by the National Transplant System.
In cases where it was feasible, orthotopic LT was performed using the classic piggy-back technique. The immunosuppression protocol included induction with 125 mg of methylprednisolone during the anhepatic phase, followed by an additional 125 mg dose at the end of surgery, which was repeated at 24 hours and 48 hours after surgery. On the third postoperative day (POD), 20 mg of oral prednisone was introduced daily, while tacrolimus at a dose of 0.1 mg/kg was administered twice daily (every 12 hours) starting on the second POD. The explanted organs were subjected to histopathological and microbiological analyses.
All patients had lived in rural areas in the ten days preceding the onset of symptoms. The clinical and demographic characteristics of the patients are shown in Table 2.
| Characteristics | Patients |
| Age (years); median (minimum-maximum) | 53 (23-69) |
| Male sex | 13 (92.8) |
| Vaccination for yellow fever more than 10 days ago | 3 (21.4) |
| Comorbidities | |
| Smoking | 3 (21.4) |
| Hypertension | 2 (21.4) |
| Immunosuppressed (previous renal transplant) | 1 (7.1) |
| Diabetes | 0 |
| Time from symptoms onset to admission (days), mean (minimum-maximum) | 4-7 (1-8) |
| Signs and symptoms at hospital admission | |
| Myalgia | 12 (85.7) |
| Fever | 11 (78.6) |
| Jaundice | 11 (78.6) |
| Vomiting | 7 (50) |
| Headache | 6 (42.9) |
| Abdominal pain | 4 (28.6) |
| Diarrhea | 2 (14.3) |
| Distance between exposure location and hospital (km), mean (minimum-maximum) | 1487 (30-291) |
| Rural workers | 6 (42.6) |
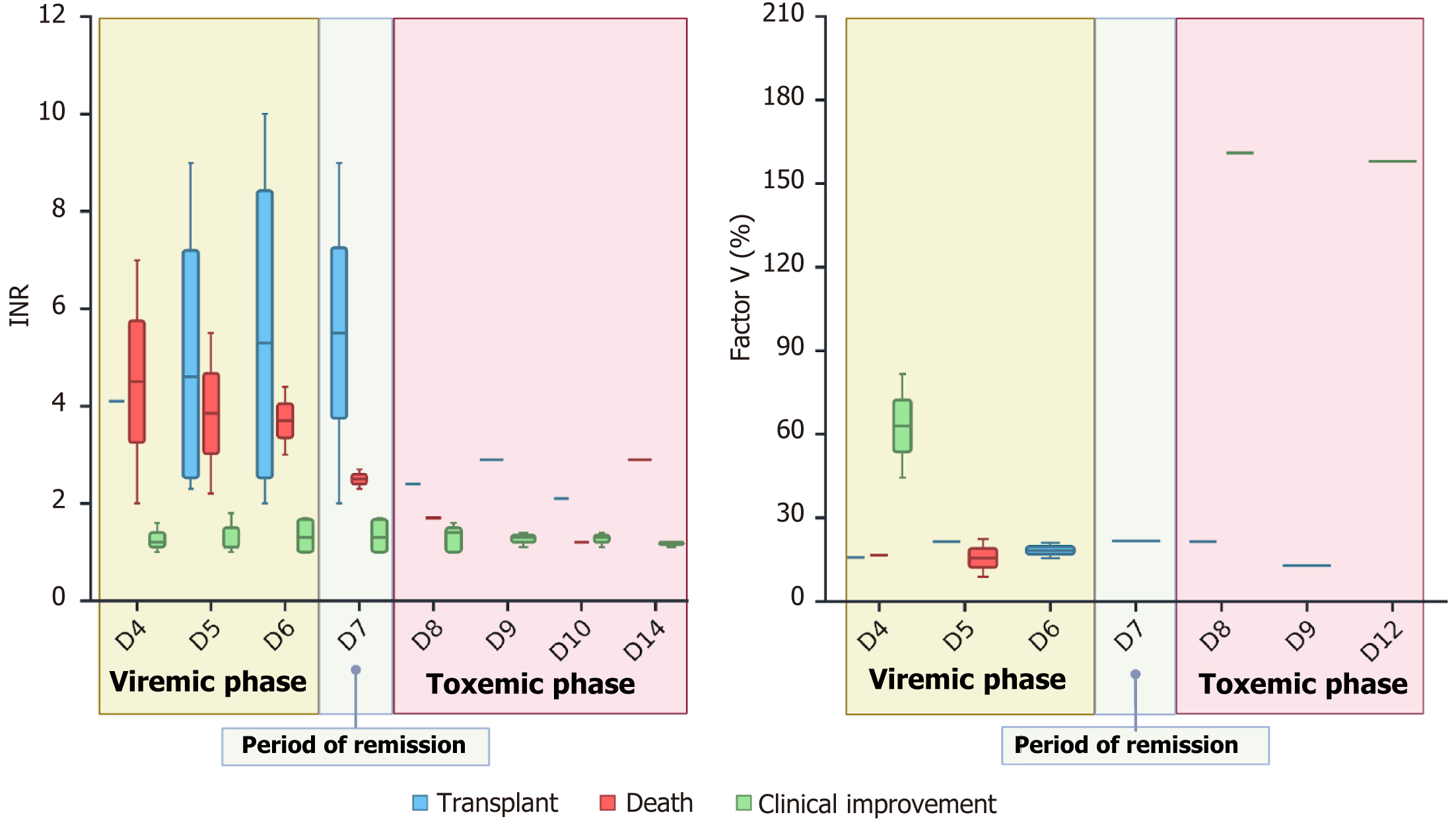
Clinical, laboratory, and histopathological data were obtained through a review of medical records. The laboratory test results are summarized in Table 3, and the evolution of the INR and factor V are detailed in Table 4. In addition to neurological changes, the INR was ≥ 3.5 (a minor criterion) in 4 patients and greater than 6.5 (a major criterion). Thus, an INR greater than 3.5 is indicative of severe disease and therefore correlated with an unfavorable outcome. However, 4 other patients did not have an INR above 3.5 but still had unfavorable outcomes. The King’s College criteria could not be used to predict disease severity in these patients, who therefore could not be listed for transplantation (Figure 2).
| Patients | Age (years) | Gender | Platelets (mL) | Aspartate aminotransferase (IU/L) | Alanine aminotransferase (IU/L) | Bilirubin (mg/dL) | International normalized ratio | Creatinine (mg/dL) | Lactate (IU/L) | Encephalopathy | Transplantation | Outcome |
| 1 | 53 | M | 29000 | 9693 | 2282 | 17.2 | 2.9 | 12.4 | 133 | 4 | No | Dead |
| 2 | 64 | M | 26000 | 7900 | 4648 | 3.54 | 2.3 | 6.48 | 61 | 2 | Yes | Alive |
| 3 | 37 | M | 58000 | 8151 | 7206 | 8.16 | > 10 | 7.1 | 164 | 3 | Yes | Dead |
| 4 | 49 | M | 89000 | 5160 | 2463 | 5.12 | 1.81 | 8.74 | 40 | No | No | Alive |
| 5 | 39 | M | 58000 | 8707 | 4509 | 6.81 | 2.9 | 8.81 | 125 | 4 | No | Dead |
| 6 | 58 | M | 12000 | 12590 | 7446 | 21.9 | 1.65 | 1.17 | 42 | No | No | Alive |
| 7 | 69 | M | 35000 | 8572 | 3987 | 5.9 | > 10 | 8.11 | 135 | 3 | Yes | Dead |
| 8 | 57 | M | 29000 | 8451 | 3854 | 10.1 | 4.4 | 7.49 | 184 | 4 | No | Dead |
| 9 | 57 | F | 46000 | 28266 | 9680 | 4.47 | > 10 | 5.06 | 115 | 4 | No | Dead |
| 10 | 63 | M | 56000 | 3650 | 2215 | 5.41 | 1.12 | 0.77 | 20 | No | No | Alive |
| 11 | 23 | M | 14000 | 9471 | 3926 | 13.47 | 1.7 | 0.92 | 20 | No | No | Alive |
| 12 | 46 | M | 79000 | 2791 | 2126 | 01.02 | 1.1 | 1.2 | 8 | No | No | Alive |
| 13 | 47 | M | 38000 | 12207 | 2992 | 8.15 | 2.9 | 1.46 | 41 | 3 | Yes | Alive |
| 14 | 40 | M | 49000 | 157 | 288 | 0.49 | 1.1 | 0.82 | 15 | No | No | Alive |
| Cases | Concentrated fresh plasma | D4 | D5 | D6 | D7 | D8 | D9 | D10 | D12 | D14 | D4 | D5 | D6 | D7 | D8 | D9 | D10 | D12 | D14 | Day of onset of neurological symptoms | Neurological symptoms | Outcome |
| International normalized ratio | Factor V (%) | |||||||||||||||||||||
| Case 1 | Yes | - | - | - | - | 1.7 | - | 1.2 | - | 2.9 | - | - | - | - | - | - | - | - | - | D10 | Initial slowing with rapid progression to stupor and coma | Death |
| Case 9 | Yes | > 7 | 5.5 | - | - | - | - | - | - | - | - | 8.8 | - | - | - | - | - | - | - | D5 | Drowsiness and flapping with rapid progression to coma | Death |
| Case 12 | Yes | - | - | 4.4 | 2.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | D5 | Drowsiness, disorientation, progression to stupor and coma | Death |
| Case 13 | Yes | 2 | 2.2 | 3 | 2.7 | - | - | - | - | - | 16.6 | 22.4 | - | - | - | - | - | - | - | D5 | Recurrent seizures | Death |
| Case 4 | Yes | 4.1 | 6.6 | 7.9 | > 9 | - | - | - | - | - | - | - | 15.6 | - | - | - | - | - | - | D6 | Agitation was intubated due to respiratory failure | Transplant |
| Case 8 | No | - | 2.3 | 2 | - | - | - | - | - | - | 15.8 | 21.5 | - | - | - | - | - | - | - | D5 | Slowing and mild disorientation | Transplant |
| Case 11 | Yes | - | > 9 | > 10 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | D4 | Drowsiness and flapping with rapid progression to coma | Transplant |
| Case 14 | Yes | - | 2.6 | 2.7 | 2 | 2.4 | 2.9 | 2.1 | - | - | - | - | 21 | 21.7 | 21.5 | 12.8 | - | - | - | D9 | Slowing, flapping, progression to stupor and coma | Transplant |
| Case 2 | No | 1.1 | 1.1 | 1 | 1 | 1 | - | - | - | - | - | - | - | - | 161 | - | - | - | - | - | Clinical improvement | |
| Case 3 | No | 1 | 1 | 1 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Clinical improvement | |
| Case 5 | No | 1.6 | 1.8 | 1.7 | 1.6 | 1.4 | 1.3 | 1.3 | - | 1.2 | - | - | - | - | - | - | - | 158 | - | D10 | Clinical improvement | |
| Case 6 | No | - | - | 1.7 | 1.7 | 1.5 | - | - | - | 1.1 | 81.6 | - | - | - | - | - | - | - | - | - | Clinical improvement | |
| Case 7 | No | 1.4 | 1.5 | 1.6 | 1.7 | 1.6 | 1.4 | 1.4 | - | 1.2 | 44.4 | - | - | - | - | - | - | - | - | D9 | Mental confusion and disorientation, complete recovery. Bacterial sepsis | Clinical improvement |
| Case 10 | No | 1.2 | 1.1 | 1 | 1 | 1 | 1.1 | 1.1 | - | - | - | - | - | - | - | - | - | - | - | - | Clinical improvement | |
The factor V level was particularly useful in some cases, as negative outcomes could not be predicted using the King’s College criteria for some patients because their INR was less than 3.5. All the factor V levels in these patients were below 20%. Two of these patients underwent LT and had favorable post-transplantation outcomes. Transplantation was indicated for these patients according to the modified Clichy criteria.
In all patients, the total bilirubin (TB) levels progressively increased, peaking on the 14th day after symptom onset. The critical period for discussing the indications for LT was between days 4 and 10 after symptom onset, when neurological symptoms suggesting ALF presented. In all cases, TB levels were below 7 mg/dL, which was significantly lower than the cutoff value of 17.5 mg/dL in the King's College criteria. Importantly, in all 8 patients who either died or underwent LT, clinical jaundice was noted between days 3 and 4 of progression. Considering that neurological symptoms appeared between days 5 and 10, we can affirm that all patients in this group had both jaundice and encephalopathy for 7 days or less.
Among the 14 patients, 10 presented with neurological symptoms. Table 4 illustrates the relationship between the development of neurological symptoms and patient outcomes. Changes in mental status were progressive and associated with a poor prognosis, as 8 out of 10 patients progressed to liver failure or death. All patients presented developed neurological symptoms between days 4 and 10 after clinical presentation. This period corresponds chronologically to the toxemic phase, characterized by peak viremia and the most severe clinical manifestations. Patients experienced sensory depression, necessitating orotracheal intubation for airway protection on average 13 hours after the onset of neurological symptoms, indicating very rapid progression to severe disease. Only two patients who developed neurological symptoms did not progress to liver failure or death, and there was subsequent laboratory confirmation of bacterial sepsis.
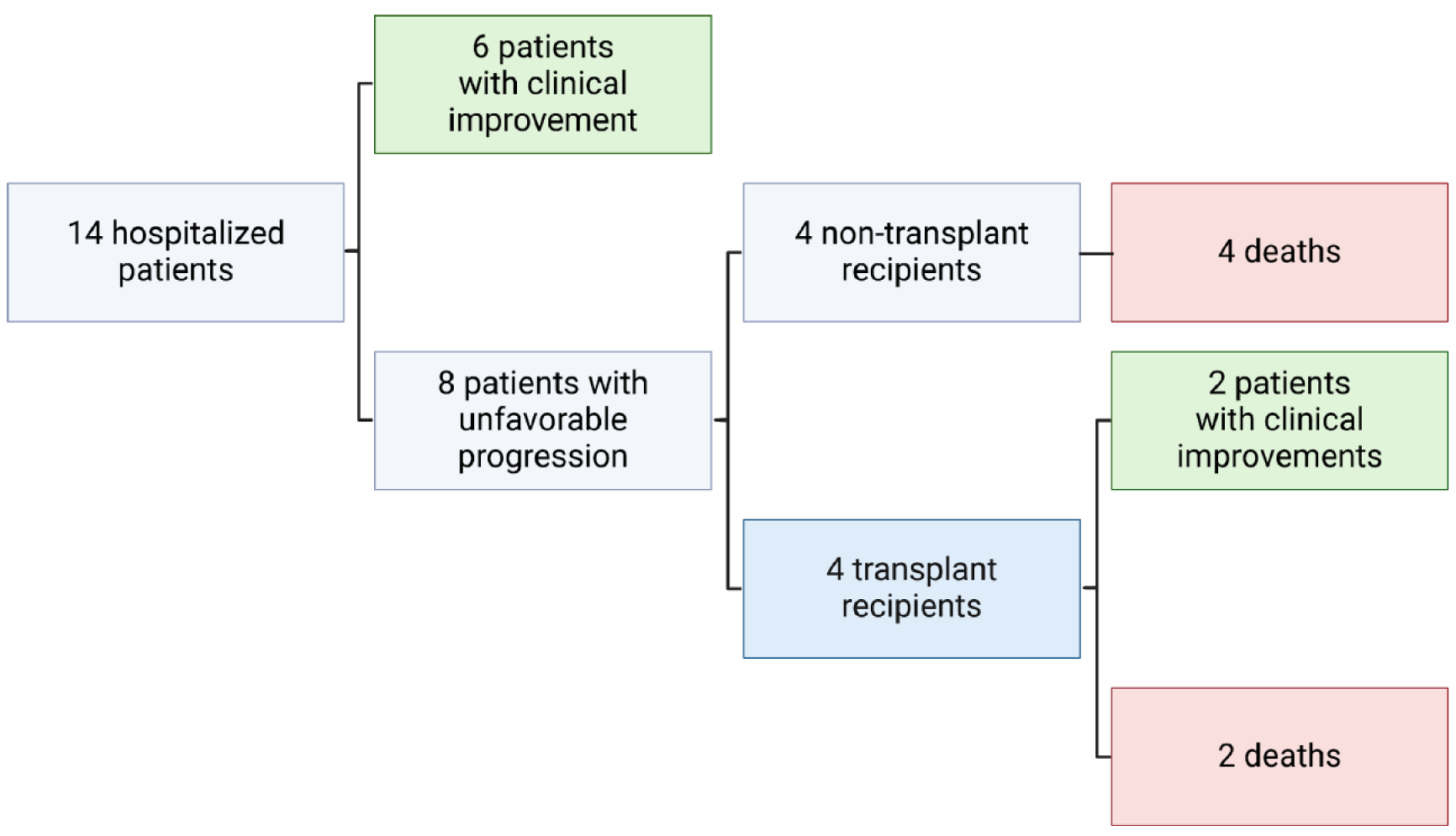
All the final outcomes are listed in Figure 3. Patients were divided into two groups according to their progression. The first group included 6 patients whose prognosis was favorable, as defined by clinical improvement (CI) without any intervention. The second group included 8 patients whose prognosis was unfavorable, as defined by liver loss and the need for LT, or death. All the patients in the second group who did not undergo LT died; among the patients who underwent LT, 2 showed signs of CI after LT, and 2 died.
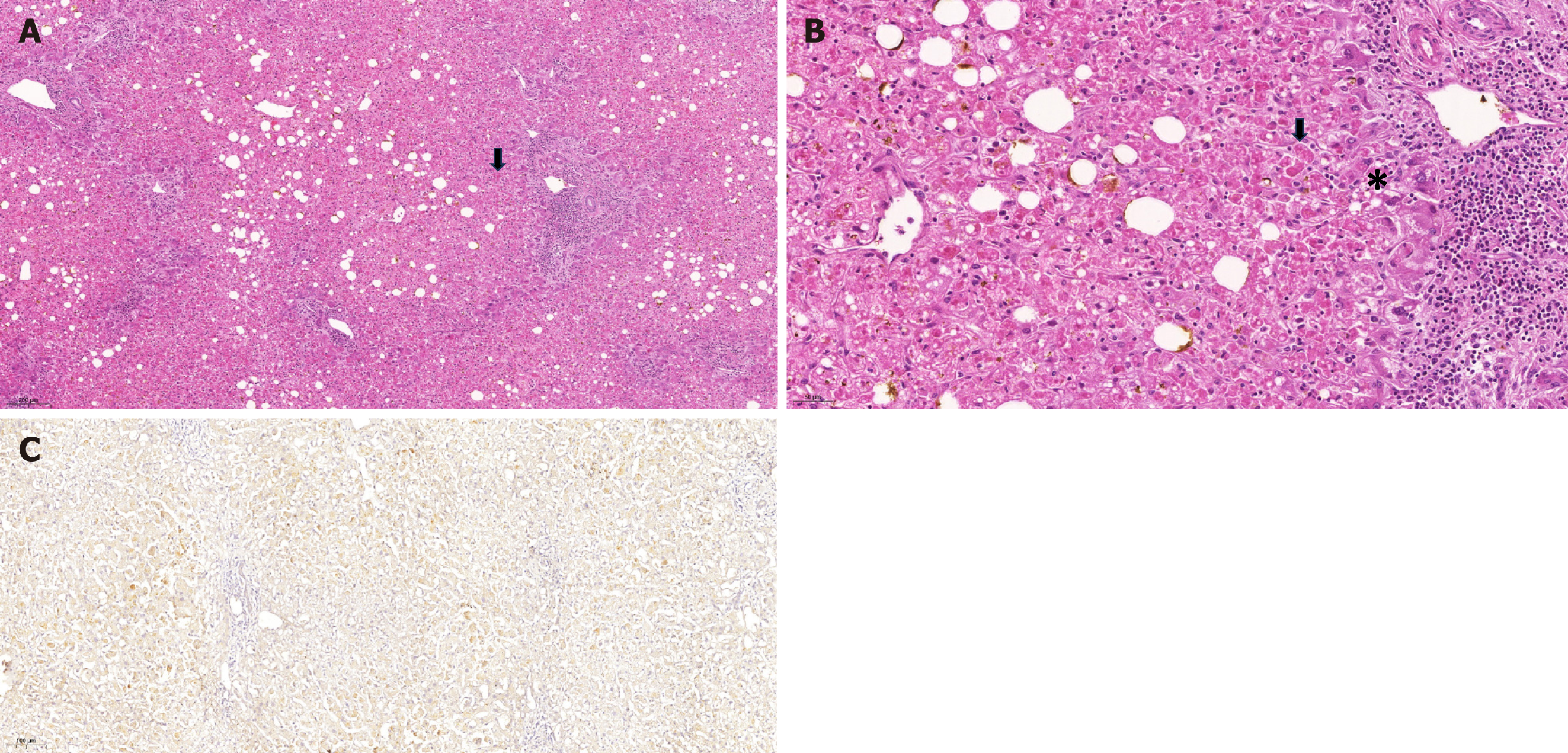
The explants of the four patients who underwent LT were subjected to anatomopathological evaluation. The weight of the explants ranged from 1007 g to 1263 g. There were no signs of arterial or venous thrombus or focal lesions in the explants. Microscopic analysis (Figure 4) revealed hepatocyte necrosis associated with inflammatory infiltration of discrete mononuclear cells (especially in zone 2) as well as frequent acidophilic degeneration of hepatocytes, namely, Councilman–Rocha–Lima bodies; macrovesicular and microvesicular steatosis; and areas of hemorrhage. There was a clear dissociation between the severity of hepatocyte cell injury and the inflammatory infiltrate, which was mild, located mainly in the portal tracts, and composed mainly of lymphocytes, macrophages in some cases, and neutrophils in rare cases. None of the patients had arteritis, bile duct injury, or interstitial collagen deposition.
Among the patients who underwent LT, two required surgical intervention due to hemorrhage at the surgical site on the second POD. At this time, graft samples were obtained. Two aspects stood out in the histological evaluation of these samples: (1) The presence of changes suggestive of YF; and (2) The difference in findings between the two patients, such as a more extensive and severe injury in cases that later resulted in death. The biopsied liver grafts were subjected to immunohistochemical analysis using an antibody against flavivirus envelope protein 4G2, which confirmed the presence of viral particles in the cytoplasm of hepatocytes. There was notable difference between the samples from the two patients. The first patient with histological findings indicating severe disease presented a large quantity of viral particles distributed throughout the section. The second patient presented only a small quantity of viral particles in zone 2. The liver graft from another patient who underwent LT was subjected postmortem biopsy. The findings were consistent with a severe case of YF, as described in other cases, and here, immunohistochemistry confirmed viral reinfection in the graft. However, the liver graft from one patient who underwent transplantation was not biopsied.
All 6 patients who experienced favorable outcomes in this study were followed for at least 6 months after discharge and did not exhibit any complications related to atrial fibrillation. None of them developed late or chronic liver failure.
Both patients who underwent LT and survived (patients 8 and 14) were followed for more than 5 years after LT. These patients had good graft function and a good quality of life without physical or neurological sequelae. One of the patients (patient 8) experienced episodes of acute cellular rejection requiring corticosteroid pulse therapy 3 years after LT; this patient had chronic renal dysfunction related to the use of calcineurin inhibitors, which were subsequently replaced by everolimus. Patient 14 has not presented any late complications to date and is on a low dose of tacrolimus.
Determining which patients with ALF who will likely survive with optimal supportive care and those who will likely die without LT remains challenging. Prognostic scoring systems can be used to identify patients who will not survive without LT and prevent surgeons from performing procedures on patients who are unlikely to benefit from an otherwise life-saving measure. While no model is flawless, the prognosis of ALF is determined on the basis of its etiology, severity according to laboratory and clinical indicators, and rate of progression.
In this study, we evaluated the use of the King's College criteria and the Clichy-Villejuif criteria in assessing the severity of ALF and the need for LT in patients with YF-induced ALF. Our findings revealed that these criteria, which have been proven useful in the assessment of other causes of ALF, cannot be used to accurately predict the risk of mortality in the context of YF-induced ALF. Moreover, the reliance of the King's College criteria on serum bilirubin levels and coagulation abnormalities did not align with the clinical severity observed in our patients.
The primary challenge encountered in this study was the discrepancy between laboratory markers and clinical presentation. Notably, even though our patients had severe clinical manifestations, their serum bilirubin levels were low during the critical period when decisions to perform LT were made. Additionally, coagulation abnormalities, as indicated by the INR, do not consistently correlate with patient outcomes. For example, some patients with INRs below the threshold for transplantation still experienced poor outcomes, whereas others with elevated INRs survived with only supportive care.
The inclusion of factor V levels in our evaluation provided additional insight into the severity of liver failure in these patients. Factor V levels below 20% were associated with worse outcomes, suggesting that this marker may be a valuable addition to existing criteria for assessing YF-induced ALF.
The 50% survival rate after LT in our cohort underscores the potential for LT to be a life-saving intervention for selected patients with YF-induced ALF. The presence of viral particles in liver grafts after transplantation highlights the complexity of managing YF in transplant recipients, emphasizing the need for tailored immunosuppressive protocols and vigilant monitoring.
Global warming has a profound effect on the dissemination of arboviruses, which are diseases transmitted by mosquitoes that were once predominantly confined to tropical regions. With 215 countries/territories potentially suitable for the most well-known vectors of arboviral diseases and an increasing number of cases reported in more than half of these countries/territories, arboviral diseases are indeed a source of concern for public health worldwide[10]. As temperatures continue to increase worldwide, vectors such as Aedes aegypti are expanding into temperate climates. Current research suggests that the incidence of mosquito-borne diseases could increase dramatically in response to climate change[11]. Increased intercontinental travel facilitates the spread of these viruses, as infected individuals can transport pathogens across borders, resulting in outbreaks in new areas. The expansion of mosquito habitats and extensive global travel underscores the urgency of a coordinated international response to mitigate the impact of arboviruses in a warming world. In recent years, cases of YF have been reported in unvaccinated travelers returning from endemic regions to countries such as Belgium[12], China[13] and the Netherlands World Health Organization[14]. The influence of global warming on the transmission of arboviral diseases is further increased by extensive intercontinental travel, a hallmark of globalization. Increased intercontinental travel facilitates the spread of these viruses, as infected individuals can transport pathogens across borders, resulting in outbreaks in new areas. This interconnectedness means that local authorities in temperate countries, which have been historically spared from such diseases, must now be vigilant and proactive in their public health strategies. The expansion of mosquito habitats and increased global travel underscores the urgency of a coordinated international response to mitigate the impact of arboviruses in a warming world.
This study has several limitations that should be considered. First, the sample size was relatively small, comprising only 14 patients, which may limit the generalizability of the results. Selection bias is likely owing to the retrospective, single-center nature of the study, and the applicability of the study findings to other settings is limited. Additionally, because of its uniqueness, the YF epidemic in Brazil may not reflect typical clinical scenarios elsewhere. Prospective, multicenter, large-sample studies are needed to validate our findings and to refine the proposed modifications to the King's College criteria and Clichy-Villejuif criteria for YF-induced ALF.
Our findings indicate that modifications to the King's College criteria and Clichy-Villejuif criteria are essential to enhance their accuracy in predicting ALF caused by YF. First, incorporating factor V levels as an additional marker is recommended, as levels below 20% are linked to negative outcomes and have been shown to be more useful than the INR is in some instances. Moreover, the reliance of the King’s College criteria on serum bilirubin levels should be recon
This study highlights the limitations of current scoring systems for LT in predicting outcomes for patients with ALF due to YF. The King's College criteria and Clichy-Villejuif criteria, while valuable for other etiologies of ALF, require adjustments to improve their applicability in the context of YF. Our findings suggest that incorporating additional markers, such as factor V levels, and considering the unique pathophysiology of YF can enhance prognostic accuracy and guide clinical decision-making.
LT can be a viable, life-saving option for patients with YF-induced ALF, as demonstrated by the survival of patients who undergo this procedure. The need for revised transplantation criteria tailored to the specific challenges of YF is evident. Future research should focus on validating these adjusted criteria in larger cohorts and exploring the underlying mechanisms of YF to develop more effective treatment strategies.
The authors would like to thank all the patients who agreed to participate in the study.
| 1. | Borkakoty A, Kumar P, Taneja S. Hepatic Encephalopathy. N Engl J Med. 2017;376:186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1517] [Cited by in RCA: 1335] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 3. | Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 765] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 4. | Ichai P, Legeai C, Francoz C, Boudjema K, Boillot O, Ducerf C, Mathurin P, Pruvot FR, Suc B, Wolf P, Soubrane O, Le Treut YP, Cherqui D, Hannoun L, Pageaux GP, Gugenheim J, Letoublon C, Saric J, Di Martino V, Abergel A, Chiche L, Antonini TM, Jacquelinet C, Castaing D, Samuel D; French Liver Transplant Teams. Patients with acute liver failure listed for superurgent liver transplantation in France: reevaluation of the Clichy-Villejuif criteria. Liver Transpl. 2015;21:512-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 463] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 6. | Song ATW, Abdala E, de Martino RB, Malbouisson LMS, Tanigawa RY, Andrade GM, Ducatti L, Doi AM, Pinho JRR, Gomes-Gouvêa MS, Malta FM, Arantes RM Jr, Tonacio AC, Figueira Pinto L, Haddad LBP, Santos VR, Pinheiro RSN, Nacif LS, Galvão FHF, Alves VAF, Andraus W, Carneiro D'Albuquerque LA. Liver Transplantation for Fulminant Hepatitis Attributed to Yellow Fever. Hepatology. 2019;69:1349-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Duarte-neto AN, Cunha MDP, Marcilio I, Song ATW, de Martino RB, Ho Y, Pour SZ, Dolhnikoff M, Saldiva PHN, Duarte MIS, Takakura CF, Lima FR, Tanigawa RY, Iglezias SD, Kanamura CT, dos Santos ABG, Perondi B, Zanotto PMDA, D’albuquerque LAC, Alves VAF. Yellow fever and orthotopic liver transplantation: new insights from the autopsy room for an old but re-emerging disease. Histopathology. 2019;75:638-648. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Kallas EG, D'Elia Zanella LGFAB, Moreira CHV, Buccheri R, Diniz GBF, Castiñeiras ACP, Costa PR, Dias JZC, Marmorato MP, Song ATW, Maestri A, Borges IC, Joelsons D, Cerqueira NB, Santiago E Souza NC, Morales Claro I, Sabino EC, Levi JE, Avelino-Silva VI, Ho YL. Predictors of mortality in patients with yellow fever: an observational cohort study. Lancet Infect Dis. 2019;19:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | da Rocha MM, Codeço CT, da Silva CMFP. Spatiotemporal Evolution of the Yellow Fever Epidemic in Southeast Brazil from 2016 to 2019. Vector Borne Zoonotic Dis. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Leta S, Beyene TJ, De Clercq EM, Amenu K, Kraemer MUG, Revie CW. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int J Infect Dis. 2018;67:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 309] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 11. | Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. 2019;13:e0007213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 559] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 12. | Bae HG, Drosten C, Emmerich P, Colebunders R, Hantson P, Pest S, Parent M, Schmitz H, Warnat MA, Niedrig M. Analysis of two imported cases of yellow fever infection from Ivory Coast and The Gambia to Germany and Belgium. J Clin Virol. 2005;33:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Song R, Guan S, Lee SS, Chen Z, Chen C, Han L, Xu Y, Li A, Zeng H, Ye H, Zhang F. Late or Lack of Vaccination Linked to Importation of Yellow Fever from Angola to China. Emerg Infect Dis. 2018;24:1383-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | World Health Organization. Imported case of yellow fever in the Netherlands. 2000. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2000_02_25-en. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/