Published online Jun 18, 2024. doi: 10.5500/wjt.v14.i2.91081
Revised: March 8, 2024
Accepted: March 25, 2024
Published online: June 18, 2024
Processing time: 175 Days and 22.5 Hours
Endoscopic management is the first-line therapy for post-liver-transplant anas
To compare the safety and efficacy profile of different stenting durations using Kaffes stents.
Adult liver transplant recipients aged 18 years and above who underwent ERCP were retrospectively identified during a 10-year period through a database query. Unplanned admissions post-Kaffes stent insertion were identified manually through electronic and scanned medical records. The main outcome was the incidence of complications when stents were left indwelling for 3 months vs 6 months. Stent efficacy was calculated via rates of stricture recurrence between patients that had stenting courses for ≤ 120 d or > 120 d.
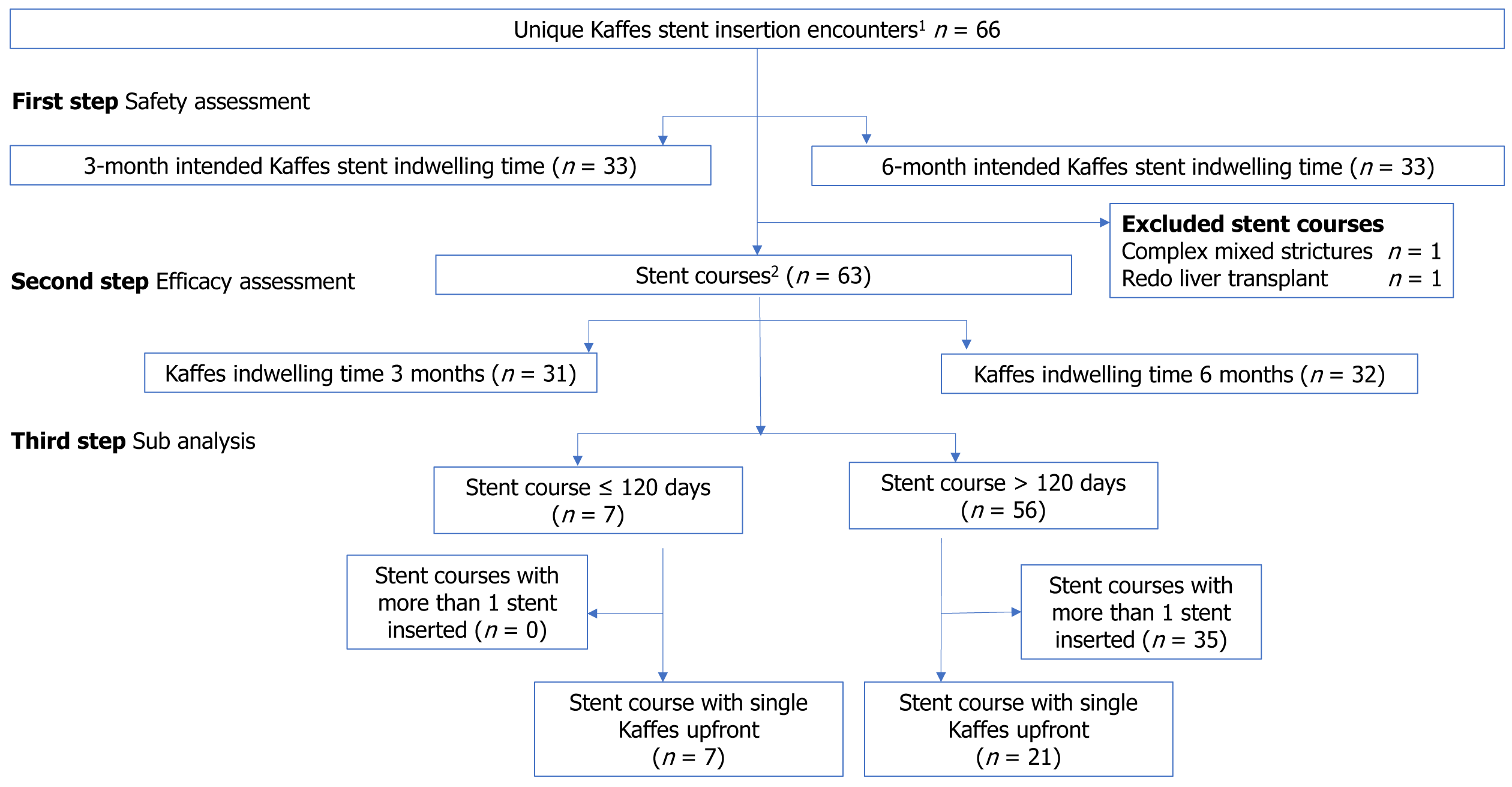
During the study period, a total of 66 ERCPs with Kaffes insertion were performed in 54 patients throughout their stenting course. In 33 ERCPs, the stent was removed or exchanged on a 3-month interval. No pancreatitis, perforations or deaths occurred. Minor post-ERCP complications were similar between the 3-month (abdominal pain and intraductal migration) and 6-month (abdominal pain, septic shower and embedded stent) groups - 6.1% vs 9.1% respectively, P = 0.40. All strictures resolved at the end of the stenting course, but the stenting course was variable from 3 to 22 months. The recurrence rate for stenting courses lasting for up to 120 d was 71.4% and 21.4% for stenting courses of 121 d or over (P = 0.03). There were 28 patients that were treated with a single ERCP with Kaffes, 21 with removal after 120 d and 7 within 120 d. There was a significant improvement in stricture recurrence when the Kaffes was removed after 120 d when a single ERCP was used for the entire stenting course (71.0% vs 10.0%, P = 0.01).
Utilising a single Kaffes intraductal fully-covered metal stent for at least 4 months is safe and efficacious for the management of post-transplant anastomotic strictures.
Core Tip: Biliary strictures are the most common complication post orthotopic liver transplantation. This retrospective study evaluates the safety and efficacy of managing such strictures using intraductal fully-covered metal stent (Kaffes) for different durations. The results show that a single Kaffes stent indwelling for at least 4 months is safe and effective for treating post liver transplant anastomotic strictures.
- Citation: Lim C, Ng J, Sarraf B, Vaughan R, Efthymiou M, Zorron Cheng Tao Pu L, Chandran S. Safety and efficacy of Kaffes intraductal self-expanding metal stents in the management of post-liver transplant anastomotic strictures. World J Transplant 2024; 14(2): 91081
- URL: https://www.wjgnet.com/2220-3230/full/v14/i2/91081.htm
- DOI: https://dx.doi.org/10.5500/wjt.v14.i2.91081
Biliary anastomotic strictures are the most common complication post liver transplant (LT)[1], affecting 5%-13% of cases[2]. Post-transplant anastomotic strictures (PTAS) typically occur within 5-8 months post-operatively, although occasionally these have been reported to appear between 7 d and 11 years following LT[3]. While early onset strictures tend to be due to technical issues during surgery such as duct size mismatch, postoperative bile leak and excessive caute
Regardless of its timing, endoscopic management is the first-line therapy in most cases, due to superior success rates and less invasive nature compared to radiological approaches, as well as lower complication rates compared to surgical interventions[1,3,6-10].
Over time, the initial strategy focused mainly on endoscopic retrograde cholangiopancreatography (ERCP)-guided balloon dilatation has been superseded by a combination of balloon dilatation and serial stenting with improved stricture resolution (30% vs 75%)[11], and reduced recurrence rates (31% vs 62%)[12]. The current standard of care involves 3-monthly treatments of stricture dilation/stepwise upsizing of multiple plastic stents (MPS) until the cholangiogram confirms stricture resolution[13], which typically follows a 12-month stenting course[14]. Following adequate endoscopic management of the stricture, there is enduring patency of up to 90%[11,12,15], and both graft and patient survival rates approximate those without a history of PTAS[16].
Due to challenges arising from the coronavirus disease 2019 (COVID-19) pandemic, in 2020, our cohort of patients were faced with delays in scheduled replacements of their stents. At this point, discussions within our Endoscopy Unit around how to address this challenging scenario had begun. While traditional self-expanding metal stents (SEMS) would potentially offer a longer occlusion-free indwelling time, they were limited by migration risk of up to 75%[17].
However, the unique intraductal fully covered SEMS (FCSEMS) with an antimigration waist named Kaffes stent (Niti-S Kaffes, Taewoong Medical, Gyeonggi-do, South Korea) was already in use at our centre, with good anecdotal outcomes and other groups reporting excellent PTAS resolution and reduced risk of stent migration[18-20]. Routinely, this stent was replaced at 3 monthly intervals at our centre prior to COVID-19, similarly to the plastic stenting protocol.
During the COVID–19 pandemic, our unit recommendation shifted to removing these fully-covered intraductal SEMS in 6 rather than 3 months based on reassuring data from the literature[2,3,21,22]. We hence aim with this retrospective study to evaluate the safety of different stent indwelling periods and efficacy of different stenting course durations using intraductal FCSEMS (Kaffes).
In this single centre retrospective cohort study, we investigated the safety profile of Kaffes FCSEMS via assessing post-ERCP complications, and then evaluated efficacy by appraising the stricture resolution and recurrence rates. Finally, a subanalysis was performed on stent courses where a single Kaffes FCSEMS was utilised upfront.
Adult LT recipients (> 18 years) who underwent ERCPs for PTAS between December, 2012 and May, 2023 at the sole LT hospital in the state of Victoria (located in Melbourne, Australia) were retrospectively identified through an endoscopy reporting software (Provation). All encounters that involved a Kaffes stent were included. All Kaffes encounters were cross matched manually by three of the authors (Sarraf B, Lim C and Ng J), through electronic and scanned medical records, and divided into two groups based on the intended duration of Kaffes indwelling time of 3- vs 6-months. All unplanned readmissions occurring within 30 d of any Kaffes insertion procedures were identified and assessed for post-ERCP complications.
Data on the same cohort of LT recipients who underwent Kaffes insertion were then reorganised based on stenting courses. Each stenting course commenced when a biliary stent was inserted for PTAS where there was no indwelling biliary stent at the start of the procedure; and concluded when the biliary stent was removed after PTAS resolution (defined as lack of resistance on sweeping biliary tree and lack of waist on cholangiogram), and at the end of the procedure the patient was left unstented. These stenting courses commonly included consecutive intervening stent changes, but in some cases consisted of a single stent staying in place for the whole stenting course duration. In the event of stricture recurrence, subsequent endoscopic stenting was considered a fresh stenting course.
When assessing for stent efficacy (stricture resolution and recurrence), atypical stent courses with mixed aetiology strictures and prematurely terminated courses were excluded. Non-PTAS strictures commonly present as multi-level strictures that would not be amenable to Kaffes stenting, and have a different pathophysiology that may pose as a confounding factor. One of the stent courses terminated prematurely due to graft failure from other causes, resulting in stent removal together with the explanted organ, therefore making it impossible to analyse the effect of Kaffes stenting on stricture progress. It was noted that in the remaining stent courses, it appeared that most of the stricture recurrences occurred in cases where Kaffes stent was left indwelling for less than 121 d. Subsequently, these remaining stent courses were divided into separate groups of ≤ 120 d vs > 120 d. To better analyse the effect of Kaffes stenting, a subanalysis was performed on these courses where Kaffes was inserted upfront.
Demographic data including age at the time of the ERCP, gender, indication for transplantation and time between LT and ERCP were collected.
ERCP procedure characteristics were extracted from clinical notes, intra-operative and anaesthetic records, medication charts and ERCP reports generated at the time of the procedure. Specifically, ERCP-related information was retrieved including indication, intervention details and stent characteristics. Readmission data included time between the ERCP and readmission, length of stay, reason for readmission and procedural-related complications leading to readmission.
Post-ERCP pancreatitis was defined as per the Atlanta criteria[23]. Episodes of pancreatitis fulfilling these criteria and occurring within 14 d after ERCP were included. Cholangitis was defined following the 2018 Tokyo Guidelines[21]. Abdominal pain was defined as any non-specific abdominal pain that could not be attributed to any other known cause such as post-ERCP pancreatitis. Bleeding was defined as clinical evidence of bleeding, haematemesis and/or melaena, or a drop in haemoglobin by 2 g/L without other cause. Perforation was defined by evidence of gas or luminal content outside the gastrointestinal tract as determined by imaging. Mortality was tracked over 30 d following ERCP.
Statistics were calculated using SPSS 26.0.0.0 (IBM SPSS Statistics© Copyright IBM Corporation 1989, 2019, Armonk, NY, United States). Normality was assessed with the Shapiro-Wilk test and showed outcomes analysed were not normally distributed. Hence, Mann Whitney tests were used for statistical analyses. Median and interquartile ranges (IQR) were used for continuous variables. Frequencies and percentages were used for categorical variables.
This study was reviewed and granted approval by the Austin Health Office for Research.
54 LT recipients who met our study’s criteria were identified, and 10 of these had more than one ERCP procedures for Kaffes stent insertion, with a total of 66 unique Kaffes stent insertion encounters for biliary PTAS (Figure 1). All LT recipients received deceased donor grafts, and the most common indication was hepatoma (Table 1). Median time between transplant and Kaffes stent insertion was 79 wk in the 3-month group (IQR 33-149 wk) and 80 wk in the 6-month group (IQR 18–240 wk). Median duration of ERCPs for Kaffes insertion was 24 min (IOR 17-30).
| Demographic data of patients | Stent indwelling time | |
| 3 months | 6 months | |
| Median age at the time of Kaffes stent insertion, yr (IQR, yr) | 57 (48-64) | 61 (53-69) |
| Gender, n (%) | ||
| Male | 20 (60.6) | 19 (57.6) |
| Female | 13 (39.4) | 14 (42.4) |
| Transplant indication, n (%)1 | ||
| Hepatoma | 8 (14.3) | 9 (16.1) |
| Non-alcoholic steatohepatitis | 9 (16.1) | 5 (8.9) |
| Alcoholic cirrhosis | 6 (10.7) | 7 (12.5) |
| Hepatitis C | 8 (14.3) | 7 (12.5) |
| Hepatitis B | 4 (7.1) | 1 (1.8) |
| Autoimmune hepatitis | 3 (5.4) | 6 (10.7) |
| Cryptogenic cirrhosis | 6 (10.7) | 3 (5.4) |
| Acute liver failure | 2 (3.6) | 3 (5.4) |
| Primary biliary cirrhosis | 2 (3.6) | 3 (5.4) |
| Primary sclerosing cholangitis | 2 (3.6) | 3 (5.4) |
| Polycystic liver | 2 (3.6) | 3 (5.4) |
| Alpha 1 antitrypsin deficiency | 2 (3.6) | 3 (5.4) |
| Other | 2 (3.6) | 3 (5.4) |
| Graft size, n (%) | ||
| Complete liver graft | 31 (93.9) | 31 (93.9) |
| Partial liver graft | 2 (6.1) | 2 (6.1) |
| Biliary anastomosis anatomy, n (%) | ||
| End to end | 33 (100.0) | 31 (93.9) |
| End to side | 0 (0.0) | 2 (6.1) |
| Transplant complications, n (%) | ||
| History of rejection | 19 (63.3) | 12 (36.4) |
| History of bile leak | 5 (16.7) | 6 (18.2) |
| History of hepatic artery thrombosis | 1 (3.3) | 2 (6.1) |
| History of previous anastomotic stricture | 28 (93.3) | 31 (93.9) |
| Time between transplant and Kaffes insertion, wk (IQR, wk) | 79 (33-149) | 80 (18-240) |
Kaffes-insertion encounters were divided equally into two groups of 33 based on the intended duration of Kaffes stent indwelling time of 3- vs 6-months (see Table 2 for details). 23 cases in the 3-month group, and 6 cases in the 6-month group, were performed prior to the COVID-19 lockdown movement control orders.
| ERCP characteristics | 3 months | 6 months |
| Indication for ERCP, n (%)1 | ||
| Stricture | 31 (93.9) | 31 (93.9) |
| Choledocolithiasis | 3 (9.1) | 5 (15.2) |
| Cholangitis | 2 (6.1) | 8 (24.2) |
| Other2 | 12 (36.4) | 6 (18.2) |
| ERCP setting, n (%) | ||
| Inpatient | 11 (33.3) | 12 (36.4) |
| Outpatient | 22 (66.6) | 21 (63.6) |
| Imaging prior to ERCP, n (%) | ||
| Ultrasound | 13 (39.4) | 16 (48.5) |
| CT abdomen | 15 (45.5) | 18 (54.5) |
| Magnetic resonance cholangiopancreatography | 19 (57.6) | 22 (66.7) |
| At least one of the above modalities | 28 (84.8) | 29 (87.9) |
| Technical details, n (%) | ||
| Sphincterotomy | 2 (6.1) | 2 (6.1) |
| Dilatation | 8 (24.2) | 8 (24.2) |
| Previous stent in situ, n (%) | ||
| Nil | 15 (45.5) | 20 (60.6) |
| Plastic | 18 (54.5) | 13 (39.4) |
| Indication for Kaffes, n (%) | ||
| New stricture | 9 (27.3) | 16 (48.5) |
| Persistent stricture, failed plastic stent program | 20 (60.6) | 15 (45.5) |
| Persistent stricture, failed metal stent program | 0 (0.0) | 1 (3.0) |
| Persistent stricture, failed combination stents | 1 (3.3) | 0 (0.0) |
| Migration of previous stent | 0 (0.0) | 0 (0.0) |
| Stricture recurrence | 3 (9.1) | 1 (3.0) |
Comparing the 3-month-indewelling group vs the 6-month-indewelling group, 7 vs 16 (21.2% vs 48.5%) of these patients had known duct mismatch following transplant, and 66.7% vs 36.4% had a history of rejection. Both groups had similar frequencies of history of bile leak and hepatic artery thrombosis, and more than 90% in both groups had known PTAS prior to ERCP (Table 1).
There were no cases of post-ERCP pancreatitis, perforation, or death within 30 d of Kaffes stent deployment/removal in either arm (Table 3). Patients in both arms only received rectal indomethacin in < 20% of cases, mostly due to a history of prior sphincterotomies. While one patient in each arm had inadvertent pancreatic duct cannulation, only the case from the 3-month group received prophylactic pancreatic stent. More than 85% of patients from both groups were administered prophylactic antibiotics during stent insertion.
| Post-ERCP complications | 3 months | 6 months | P value |
| Any complication, n (%) | 3 (6.1) | 3 (9.1) | 0.40 |
| Pancreatitis, n (%) | |||
| Episodes | 0 (0.0) | 0 (0.0) | 1.00 |
| During Kaffes insertion | |||
| Prophylactic rectal indomethacin | 2 (6.1) | 4 (12.1) | 0.40 |
| Pancreatic duct cannulation | 1 (3.0) | 1 (3.0) | 1.00 |
| Pancreatic duct stent insertion1 | 1 (3.0) | 0 (0.0) | 0.32 |
| Cholangitis, n (%) | |||
| Episodes | 0 (0.0) | 1 (3.0) | 0.32 |
| Prophylactic antibiotics at Kaffes insertion | 30 (90.9) | 29 (87.9) | 0.69 |
| Piperacillin/Tazobactam | 20 (60.6) | 25 (75.8) | |
| Ciprofloxacin | 1 (3.0) | 0 (0.0) | |
| Ceftriaxone | 8 (24.2) | 0 (0.0) | |
| Other | 1 (3.1) | 4 (12.1) | |
| Bleeding2 | 1 (3.3) | 0 (0.0) | 1.00 |
| Admission3 | 2 (6.7) | 3 (9.1) | 0.31 |
| Stent migration | 1 (3.3) | 0 (0.0) | 0.29 |
Comparing the 3-month to the 6-month group, there were similar numbers of minor post-ERCP complications (6.1% vs 9.1%, P = 0.40) as follows. One patient from each group were admitted overnight for observation of self-limiting abdominal pain. One patient from the 3-month group was noted to have downstream migration of Kaffes stent when presenting for planned stent removal, at which point stricture had resolved and patient was asymptomatic, concluding the stent course. In the 6-month group, one patient with a history of septic showers post-ERCP developed transient cholangitis within 24 h of elective observatory admission despite prophylactic antibiotics; another patient experienced difficulty in removal of Kaffes stent which was partly embedded. After sweeping the biliary tree, another Kaffes stent was placed stent-in-stent to induce local pressure necrosis, and a pigtail stent was placed alongside the 2nd Kaffes to permit biliary drainage. 3 wk later all 3 stents were easily retrieved, followed by balloon dilation and a switch to MPS strategy, with the total stenting course duration being 22 months – there has not been recurrence since.
Of the 66 encounters of Kaffes stent insertion above, two encounters were part of the same stenting course (Kaffes replaced by another Kaffes). Two stenting courses were not included in the efficacy analysis (recurrence), one due to presence of complex mixed anastomotic and ischemic strictures and ongoing stenting was determined as being related mostly to the ischaemic component; and the other underwent a redo LT in the middle of the course for graft cirrhosis and failure with ischemic strictures hence hindering assessment of the end of the stenting course.
Of the remaining 63 ERCP encounters, 31 Kaffes indwelling time of 3 months, and 32 involved Kaffes indwelling time of 6 months. Median duration of follow up was 860 d (IQR 531–1533 d). Looking at all episodes of Kaffes insertion, 30 out of 31 in the 3-month-group and 30 out of 32 in 6-month group had stricture resolution on fluoroscopy and were hence left unstented (i.e., concluded the stenting course), regardless of the total stenting course duration (Table 4).
| Stricture outcomes | 3 months | 6 months | P value |
| Stricture resolution on stent extraction, n (%) | 30 (96.8) | 30 (93.8) | 0.72 |
| Stricture recurrence, n (%) | 14 (42.4) | 3 (9.1) | 0.01a |
| Median days to recurrence since stent removal, d (IQR, d) | 273 (73-1192) | 431 (7-617) |
Stenting courses ranged from 3 to 22 months, and were split into two groups: ≤ 120 d or > 120 d. While there was no statistical difference in stricture resolution on Kaffes removal between the two groups (100.0% vs 89.3%, P = 0.66), there was a higher rate of recurrence when stent course lasted less than 121 d (71.4% vs 21.4%, P = 0.03) (Table 5). The time-to-recurrence was close to 8 months in the former group and over one year for the later. When considering only the stent courses where Kaffes was the final stent, results were similar (Table 6).
| Stricture outcomes | ≤ 120 d | > 120 d | P value |
| n | 7 | 56 | |
| Median duration of stent course, d (IQR, d) | 95 (90-116) | 196 (176-296) | |
| Stricture resolution on Kaffes removal, n (%) | 7 (100.0) | 50 (89.3) | 0.66 |
| Recurrence | |||
| n (%) | 5 (71.4) | 12 (21.4) | 0.03a |
| Median days to recurrence (IQR) | 248 (104–812) | 368 (259-1084) | 0.33 |
| Stricture outcomes | ≤ 120 d | > 120 d | P value |
| n | 7 | 50 | |
| Median duration of stent course, d (IQR, d) | 95 (90-116) | 193 (178-281) | |
| Stricture resolution at end of course, n (%) | 7 (100.0) | 50 (100.0) | 1.00 |
| Recurrence | |||
| n (%) | 5 (71.4) | 9 (18.0) | 0.02a |
| Median days to recurrence (IQR) | 248 (104-812) | 431 (264-1522) | 0.24 |
In the subanalysis of 28 patients that were treated with a single ERCP with Kaffes stent, 7 had stent removal within 120 d and 21 over 120 d. Median duration of stent courses were 95 d (90-116 d) vs 183 d (167-193 d). Recurrence rate was statistically higher when the stenting course with a single Kaffes had it removed within 120 d (71.0% vs 10.0%, P = 0.01). Median d to recurrence was numerically higher but did not reach statistical difference (Table 7).
| Stricture outcomes | ≤ 120 d | > 120 d | P value |
| n | 7 | 21 | |
| Median duration of stent course, d (IQR, d) | 95 (90-116) | 183 (167-193) | |
| Stricture resolution on Kaffes removal, n (%) | 7 (100.0) | 20 (95.0) | 1.00 |
| Recurrence | |||
| n (%) | 5 (71.0) | 2 (10.0) | 0.01a |
| Median days to recurrence (IQR) | 248 (27-812) | 1139 (785-1493) | 0.19 |
While current guidelines recommend a treatment protocol of 3-monthly sequential dilatation/stenting of PTAS, there is no data specifically looking into 3 vs 6 months of FCSEMS placement[24]. To our knowledge, this is the first study reporting on the safety of 6-month stenting interval in the LT setting. Despite theoretical concerns about risks of secondary localised ischemic stricture formation, our study demonstrated similar stricture resolution in both the 3- and 6-month stent indwelling groups when compared to previous publications[18,19,25]. Stricture recurrence rates amongst the 6-month indwelling group was less than those reported in the literature (Table 8). Our study also showed reduced stent migration rates compared to previous studies[26-28], and the only case with migration in our study likely occurred due to early stricture resolution.
| Ref. | Sissingh et al[22], 2023 | Warner et al[18], 2020 | Martins et al[27], 2018 | Tal et al[26], 2017 | Cote et al[28], 2016 | Kaffes et al[19], 2014 |
| n | 80 | 62 | 59 | 48 | 73 | 20 |
| FCSEMS removal protocol, months | 6 | 3 | 6 | 4-6 | 6-12 | 3 |
| Median stent indwelling time, months | NA | 10 | 5 | 6 | 6 | 4 |
| Stricture resolution (%) | 93 | 96 | 83 | 100 | 89 | 100 |
| Stricture recurrence (%) | 33 | 25 | 32 | 21 | 15 | 30 |
| Migration rate (%) | 16 | NA | 10 | 21 | 45 | 0 |
| Acute pancreatitis (%) | 7 | 5 | 13 | NA | 6 | NA |
| Cholangitis rate (%) | 7 | 4 | 2 | NA | 3 | 10 |
| Bile duct perforation (%) | 0 | 2 | NA | NA | NA | NA |
The unique design of Kaffes intraductal FCSEMS likely accounts for the low rate of migration and improved recurrence rate. Compared to conventional stents, Kaffes has a mid-stent waist which produces a radial force towards the stent centre, and nestles entirely within the biliary duct, protecting it from being dragged vertically. Also, the Kaffes stents were able to provide continuous dilation to the maximum diameter soon after deployment, compared to plastic stents that were only able to achieve target diameter within the final 3 months of the stenting course.
The most reported complications following endoscopic treatment of PTAS are cholangitis (7%-40%) pancreatitis (5%) and perforation (up to 2%)[18,19]. Despite our study’s retrospective nature and relatively small number of patients treated, our cohort of patients did not demonstrate a significantly different complication rate regardless of prolonged or standard duration for stent indwelling time. Antibiotic coverage and stenting the pancreatic duct following inadvertent duct cannulation likely conferred a protective effect.
When considering the efficacy of Kaffes stenting to manage PTAS, rates of stricture resolution on stent removal were similar regardless of duration of stent indwelling time or sequence of stent type throughout the stent course. However, recurrence rates were statistically less when stents were indwelling for at least 4 months. Even when Kaffes stent was placed upfront and utilised as the sole stent during the stenting course, this finding holds true. This suggests that placing a Kaffes stent for at least 4 months would be the ideal duration for PTAS management, negating the need for multiple repeated courses of ERCPs.
This study suggests that a 6 monthly schedule utilising intraductal FCSEMS to manage PTAS is both safe and efficacious, and offers an exciting alternative approach to the current standard of care. In particular, a single course of Kaffes stenting for at least 4 months (121 d) is sufficient if there is stricture resolution on removal fluoroscopy, and has low risk of stricture recurrence. Larger randomized control trials should be considered to explore the promising results of this retrospective study.
| 1. | Park JS, Kim MH, Lee SK, Seo DW, Lee SS, Han J, Min YI, Hwang S, Park KM, Lee YJ, Lee SG, Sung KB. Efficacy of endoscopic and percutaneous treatments for biliary complications after cadaveric and living donor liver transplantation. Gastrointest Endosc. 2003;57:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Graziadei IW, Schwaighofer H, Koch R, Nachbaur K, Koenigsrainer A, Margreiter R, Vogel W. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl. 2006;12:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (3)] |
| 4. | Fasullo M, Patel M, Khanna L, Shah T. Post-transplant biliary complications: advances in pathophysiology, diagnosis, and treatment. BMJ Open Gastroenterol. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 5. | Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Villa NA, Harrison ME. Management of Biliary Strictures After Liver Transplantation. Gastroenterol Hepatol (N Y). 2015;11:316-328. [PubMed] |
| 7. | Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 455] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Pfau PR, Kochman ML, Lewis JD, Long WB, Lucey MR, Olthoff K, Shaked A, Ginsberg GG. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Judah JR, Draganov PV. Endoscopic therapy of benign biliary strictures. World J Gastroenterol. 2007;13:3531-3539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Williams ED, Draganov PV. Endoscopic management of biliary strictures after liver transplantation. World J Gastroenterol. 2009;15:3725-3733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Schwartz DA, Petersen BT, Poterucha JJ, Gostout CJ. Endoscopic therapy of anastomotic bile duct strictures occurring after liver transplantation. Gastrointest Endosc. 2000;51:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Zoepf T, Maldonado-Lopez EJ, Hilgard P, Malago M, Broelsch CE, Treichel U, Gerken G. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl. 2006;12:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Pasha SF, Harrison ME, Das A, Nguyen CC, Vargas HE, Balan V, Byrne TJ, Douglas DD, Mulligan DC. Endoscopic treatment of anastomotic biliary strictures after deceased donor liver transplantation: outcomes after maximal stent therapy. Gastrointest Endosc. 2007;66:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Franzini T, Sagae VMT, Guedes HG, Sakai P, Waisberg DR, Andraus W, D'Albuquerque LAC, Sethi A, de Moura EGH. Cholangioscopy-guided steroid injection for refractory post liver transplant anastomotic strictures: a rescue case series. Ther Adv Gastrointest Endosc. 2019;12:2631774519867786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Costamagna G, Pandolfi M, Mutignani M, Spada C, Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 272] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Rizk RS, McVicar JP, Emond MJ, Rohrmann CA Jr, Kowdley KV, Perkins J, Carithers RL Jr, Kimmey MB. Endoscopic management of biliary strictures in liver transplant recipients: effect on patient and graft survival. Gastrointest Endosc. 1998;47:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Devière J, Nageshwar Reddy D, Püspök A, Ponchon T, Bruno MJ, Bourke MJ, Neuhaus H, Roy A, González-Huix Lladó F, Barkun AN, Kortan PP, Navarrete C, Peetermans J, Blero D, Lakhtakia S, Dolak W, Lepilliez V, Poley JW, Tringali A, Costamagna G; Benign Biliary Stenoses Working Group. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology. 2014;147:385-95; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 18. | Warner B, Harrison P, Farman M, Devlin J, Reffitt D, El-Sherif Y, Khorsandi SE, Prachalias A, Cerisuelo MC, Menon K, Jassem W, Srinivasan P, Vilca-Melendez H, Heneghan M, Heaton N, Joshi D. A unique type of fully covered metal stent for the management of post liver transplant biliary anastomotic strictures. BMC Gastroenterol. 2020;20:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Kaffes A, Griffin S, Vaughan R, James M, Chua T, Tee H, Dinesen L, Corte C, Gill R. A randomized trial of a fully covered self-expandable metallic stent versus plastic stents in anastomotic biliary strictures after liver transplantation. Therap Adv Gastroenterol. 2014;7:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Aepli P, St John A, Gupta S, Hourigan LF, Vaughan R, Efthymiou M, Kaffes A. Success and complications of an intra-ductal fully covered self-expanding metal stent (ID-FCSEMS) to treat anastomotic biliary strictures (AS) after orthotopic liver transplantation (OLT). Surg Endosc. 2017;31:1558-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 484] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 22. | Sissingh NJ, de Vries BA, Inderson A, van Hoek B, van der Heide F, van Hooft JE. Intraductal fully covered self-expandable metal stent versus multiple plastic stents for treating biliary anastomotic strictures after liver transplantation. Gastrointest Endosc. 2023;97:704-712.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4691] [Article Influence: 360.8] [Reference Citation Analysis (48)] |
| 24. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 534] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 25. | Kao D, Zepeda-Gomez S, Tandon P, Bain VG. Managing the post-liver transplantation anastomotic biliary stricture: multiple plastic versus metal stents: a systematic review. Gastrointest Endosc. 2013;77:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Tal AO, Finkelmeier F, Filmann N, Kylänpää L, Udd M, Parzanese I, Cantù P, Dechêne A, Penndorf V, Schnitzbauer A, Friedrich-Rust M, Zeuzem S, Albert JG. Multiple plastic stents versus covered metal stent for treatment of anastomotic biliary strictures after liver transplantation: a prospective, randomized, multicenter trial. Gastrointest Endosc. 2017;86:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 27. | Martins FP, De Paulo GA, Contini MLC, Ferrari AP. Metal versus plastic stents for anastomotic biliary strictures after liver transplantation: a randomized controlled trial. Gastrointest Endosc. 2018;87:131.e1-131.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Coté GA, Slivka A, Tarnasky P, Mullady DK, Elmunzer BJ, Elta G, Fogel E, Lehman G, McHenry L, Romagnuolo J, Menon S, Siddiqui UD, Watkins J, Lynch S, Denski C, Xu H, Sherman S. Effect of Covered Metallic Stents Compared With Plastic Stents on Benign Biliary Stricture Resolution: A Randomized Clinical Trial. JAMA. 2016;315:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0