Published online Jun 18, 2023. doi: 10.5500/wjt.v13.i4.157
Peer-review started: March 17, 2023
First decision: April 13, 2023
Revised: April 18, 2023
Accepted: May 4, 2023
Article in press: May 4, 2023
Published online: June 18, 2023
Processing time: 90 Days and 15.5 Hours
Blood transfusion is common during the peri-transplantation period. The incidence of immunological reactions to blood transfusion after kidney tran
To examine the risk of graft rejection and loss in patients who received blood transfusion in the immediate peri-transplantation period.
We conducted a single-center retrospective cohort study of 105 kidney recipients, among them 54 patients received leukodepleted blood transfusion at our center between January 2017 and March 2020.
This study included 105 kidney recipients, of which 80% kidneys were from living-related donors, 14% from living-unrelated donors, and 6% from deceased donors. Living-related donors were mostly first-degree relatives (74.5%), while the rest were second-degree relatives. The patients were divided into transfusion (n = 54) and non-transfusion (n = 51) groups. The average hemoglobin level at which blood transfusion was commenced was 7.4 ± 0.9 mg/dL. There were no differences between the groups in terms of rejection rates, graft loss, or death. During the study period, there was no significant difference in creatinine level progression between the two groups. Delayed graft function was higher in the transfusion group; however, this finding was not statistically significant. A high number of transfused packed red blood cells was significantly associated with increased creatinine levels at the end of the study.
Leukodepleted blood transfusion was not associated with a higher risk of rejection, graft loss, or death in kidney transplant recipients.
Core Tip: Blood transpfusion in patient undergoing kidney transplantation has long been avoided for the fear for the potential risk of reciepient's immunization and potential rejection. This study addresses the risks of peri-transplantation outcomes of blood transfusion.
- Citation: Bukhari MA, Alhomayani FK, Al Eid HS, Al-Malki NK, Alotaibi ME, Hussein MA, Habibullah ZN. Is peri-transplant blood transfusion associated with worse transplant outcomes? A retrospective study. World J Transplant 2023; 13(4): 157-168
- URL: https://www.wjgnet.com/2220-3230/full/v13/i4/157.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i4.157
Anemia is common in the early post-kidney transplant period[1-3]. The causes of this anemia are multiple and may include blood loss during the surgical operation, erythropoietin deficiency, iron deficiency as a result of previous end-stage renal disease along with delayed graft function (DGF), and adverse reactions to immunosuppressive agents. In some cases, blood transfusion is an essential life-saving practice. Blood transfusion is widely used in the early post-transplantation period following surgery[2].
However, blood transfusion is not without risks. Exposure to non-self human leukocyte antigens (HLAs) can lead to the formation of anti-HLA antibodies or allosensitization[2-4]. Donor-specific antibodies (DSAs) can develop in kidney recipients after receiving a blood transfusion[2]. HLA sensitization may have negative clinical impacts, including, an increased risk of rejection, and graft loss. Despite these risks, clinical guidelines do not provide specific recommendations for blood transfusion during the perioperative period[4]. This uncertainty could be due to the assumption that post-kidney transplant patients receive immunosuppressive agents, which could reduce the possibility of allosensitization[4,5].
The incidence of immunological reactions to blood transfusion after kidney transplantation and their consequences on graft outcomes have not been extensively studied. Our study aimed to examine the risk of graft rejection and graft loss in patients who receive a blood transfusion in the immediate post-transplantation period and those who were on immunosuppressive therapy.
This was a single-center retrospective cohort study of kidney transplantation recipients who received either deceased or living-donor kidneys at Al-Hada Armed Forces Hospital, Taif, Saudi Arabia between January 2017 and March 2020. No other solid-organ transplantation or spontaneous kidney-pancreas transplantation was performed at our center during the study period.
We surveyed kidney transplant recipients who received a blood transfusion in the peri-transplant period (one week prior to transplantation and one month after the surgery). The control group included patients who underwent kidney transplantation during the same period but did not require blood transfusion. At our institution, only leukodepleted blood products are administered to kidney transplant candidates and kidney transplant recipients; this applied to the kidney recipients enrolled in this study. Data were obtained from the patients’ electronic files in the hospital. However, blood product type (leuko-depleted vs non-leuko-depleted) was confirmed from the blood bank records. We excluded recipients who were < 18 years of age, those with previous organ transplantation, those who required desensitization prior to transplantation, those on a calcineurin inhibitors (CNIs) avoidance protocol, and those who required permanent withdrawal of one or more of their immunosuppressive therapies.
During the study period, Immunosuppression protocol in our hospital consisted of induction therapy with either antithymocyte globulin (cumulative dose of 4-6 mg/kg) or basiliximab (two intravenous doses of 20 mg on post-op days 0 and 4) and maintenance immunosuppression with tacrolimus (targeting a tacrolimus level of 8-10 ng/mL in the first three months then 4-6 ng/mL), an antimetabolite (mycophenolate mofetil 1 gm twice daily) and prednisone (tapered to a maintenance dose of 5 mg daily).
Primary outcomes were biopsy-proven rejection, DGF, graft loss within the first 18 mo post-transplantation and death of any cause during the same time period. Post-transplant kidney biopsy and DSA identification were not performed routinely in this cohort but rather on a for-cause basis. Secondary outcomes were changes in creatinine levels during the study period, infections, and urological complications. Both cellular and antibody-mediated rejections were accounted for. Graft loss was defined as the need for another renal replacement therapy. Identification of DSAs was performed using a Luminex single-bead antigen solid-phase assay with a cutoff of 1000 mean fluorescence intensity.
All analyses were performed using SPSS version 26 (IBM, Armonk, NY, United States). Continuous variables are denoted as mean ± SD for normally distributed variables or median (interquartile range) for non-normally distributed variables. The Shapiro-Wilk test was used to assess the normality of continuous variables to guide the selection of a parametric or nonparametric test for the comparison of variables. The variables were compared using the Welch’s t-test, Student’s t-test, and Mann-Whitney-U test. Categorical variables were presented as frequencies and percentages and were compared using the χ2 or Fisher's exact tests as appropriate. All independent variables from the univariate linear regression analysis with P < 0.05 were entered into a multivariate linear regression model to examine their association with creatinine changes. All reported P values were two-sided and P values < 0.05 were considered to indicate a statistical significance.
A total of 124 kidney transplant surgeries were performed at Al-Hada Armed Forces Hospital during the study period (between January 2017 and March 2020). Nineteen patients were excluded they were < 18 years (three recipients), had a previous kidney transplant (five recipients), required desensitization prior to surgery (nine recipients), had ABO incompatibility (1 recipient), and lacked sufficient information (one recipient). The final analysis included data from 105 recipients. The patients were divided into two groups: Blood transfusion (54 recipients) and non-transfusion (51 recipients) groups (Table 1). The transplant recipients in our cohort had a higher prevalence of male sex (77 recipients: 73%), and most kidney transplantations were from living-related (84 recipients, 80%) than living-unrelated (15 recipients, 14%) donors or from deceased-donor kidney transplantation (six recipients; 6%). The median number of HLA mismatches was three in both groups. Basiliximab was the most commonly used agent for induction (62 recipients; 59%). All the recipients in our cohort received a tacrolimus-based regimen with an average tacrolimus level during the study time of 7 ng/mL (6-8 ng/mL).
| Total cohort, 105 | Non-transfusion, 51 (48.6%) | Transfusion, 54 (51.4%) | P value | |
| Age (mean ± SD) | 39.7 ± 14.5 | 40.5 ± 13.9 | 38.9 ± 15.1 | 0.583 |
| Gender | ||||
| Female | 28 (26.7) | 10 (19.6) | 18 (33.3) | 0.127 |
| Male | 77 (73.3) | 41 (80.4) | 36 (66.7) | |
| Type of transplantation | ||||
| LRKTx | 84 (80) | 43 (84.3) | 41 (75.9) | 0.566 |
| LURKTx | 15 (14.3) | 6 (11.8) | 9 (16.7) | |
| DDKTx | 6 (5.7) | 2 (3.9) | 4 (7.4) | |
| HLA mismatch | 3 (1-4) | 3 (0-4) | 3 (2-4) | 0.152 |
| Cause of ESRD | ||||
| Diabetes | 18 (17.1) | 7 (13.7) | 11 (20.4) | 0.331 |
| GN | 26 (24.8) | 11 (21.6) | 15 (27.8) | |
| Hypertension | 18 (17.1) | 12 (23.5) | 6 (11.1) | |
| PCKD | 1 (1) | 1 (2) | 0 (0) | |
| Urological | 7 (6.7) | 2 (3.9) | 5 (9.3) | |
| Other | 35 (33.3) | 18 (35.3) | 17 (31.5) | |
| Donor's age | 33 ± 8.6 | 32.4 ± 8.4 | 33.5 ± 8.8 | 0.562 |
| Induction therapy | ||||
| ATG | 42 (40) | 21 (41.2) | 21 (38.9) | 1 |
| Basiliximab | 62 (59) | 30 (58.8) | 32 (59.3) | |
| No induction | 1 (1) | 0 (0) | 1 (1.9) | |
| Maintenance immunosuppression | ||||
| CNI used tacrolimus | 105 (100) | 51 (100) | 54 (100) | |
| Average CNI level | 7 (6-8) | 7 (6-8) | 7 (6-8) | 0.743 |
| Antimetabolite used (MMF) | 105 (100) | 51 (100) | 54 (100) |
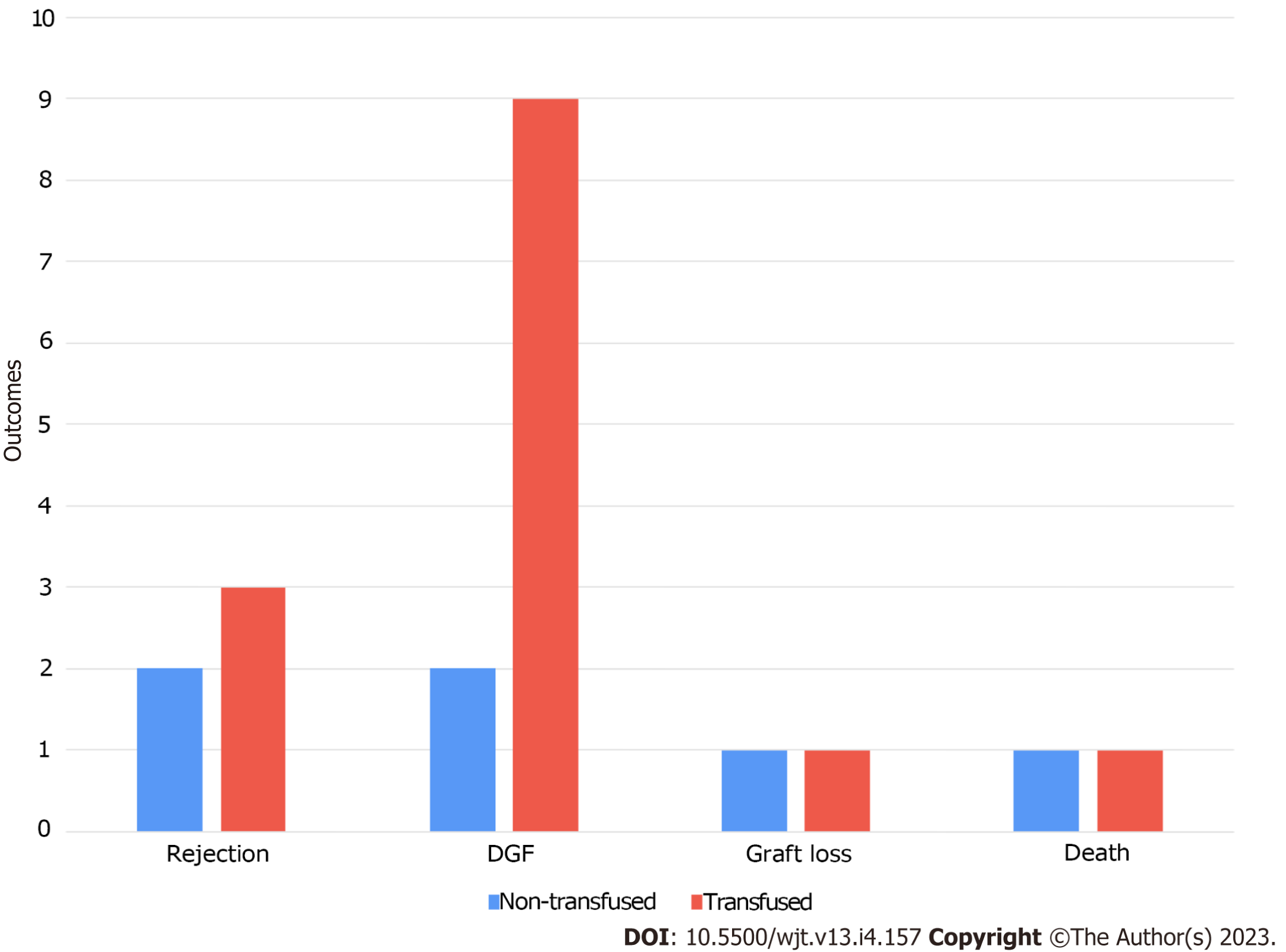
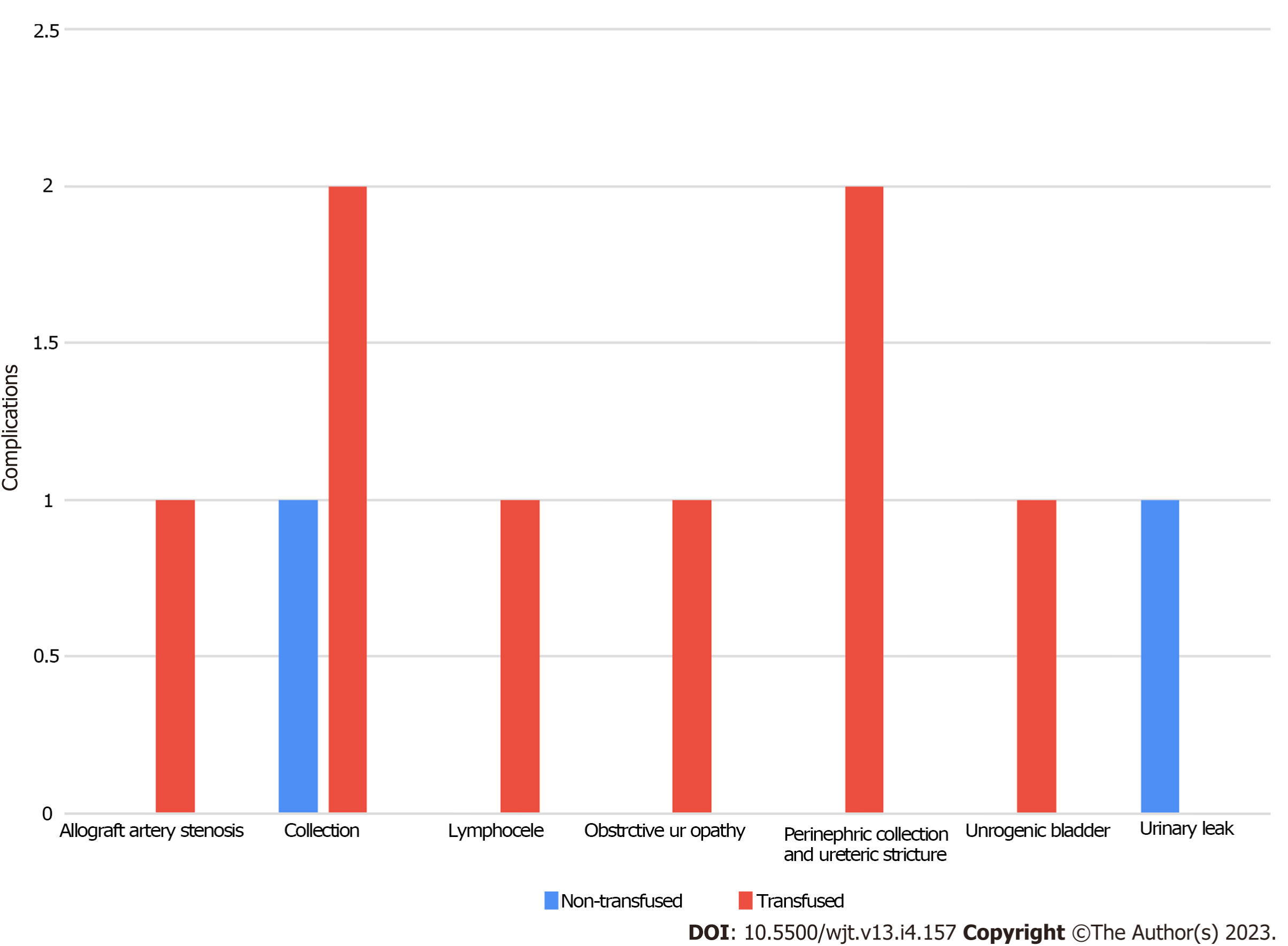
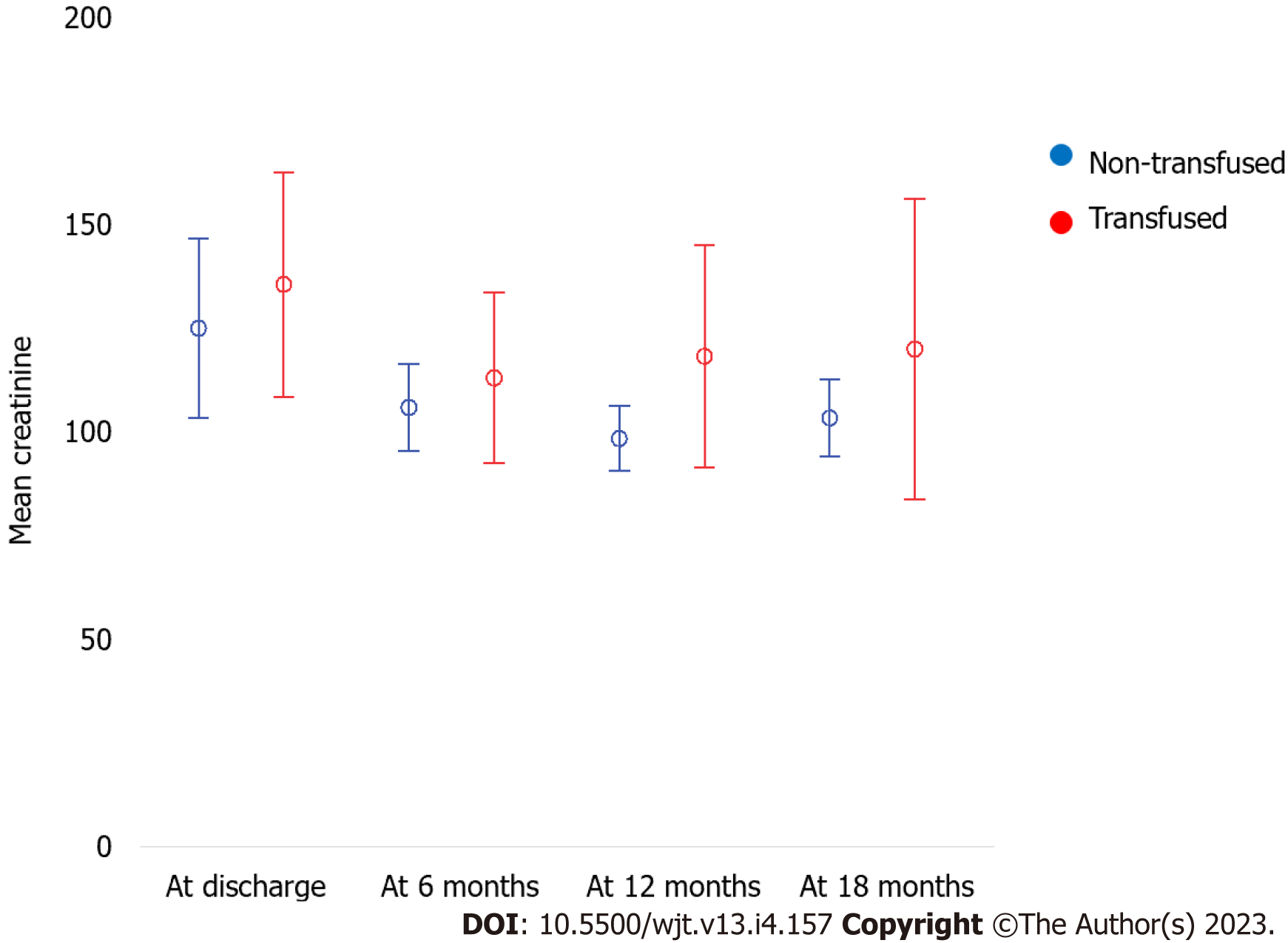
Approximately 85 (69%) recipients in our cohort had anemia [hemoglobin (Hb) of < 12 g/dL]; however, only 57 (54%) recipients received blood transfusions. Among the 57 recipients who received blood transfusion 31 recipients (54%) received only 1 unit, 15 (26%) received two units, 7 (12%) received three units, 3 (5%) received four units, and only 1 (2%) received nine units of blood. The average Hb at the time of transplantation was significantly higher in the non-transfusion group (11.2 mg/dL vs 9.8 mg/dL, P < 0.001) (Table 2). In the transfusion group, the average Hb level at which blood transfusion was initiated was 7.4 ± 0.9 mg/dL. There were no significant differences in infectious and non-infectious complications between the two groups (Table 3). Additionally, there was no significant difference in graft loss or all-cause death between the two groups (Table 4, Figures 1 and 2). There was no significant difference in creatinine level progression between the two groups during the study period (Figure 3).
| Total, 105 | Non-transfused, 51 (48.6%) | Transfused, 54 (51.4%) | P value | |
| Hemoglobin at transplantation | 10.5 ± 1.7 | 11.2 ± 1.6 | 9.8 ± 1.6 | < 0.001 |
| Hemoglobin at blood transfusion | 7.4 ± 0.9 | |||
| Hemoglobin after transfusion | 9.2 ± 1.1 | |||
| Number of blood transfusion units given | 1 (0-1.5) | 1 (0-1.5) |
| Total, 105 | Non-transfusion, 51 (48.6%) | Transfusion, 54 (51.4%) | P value | |
| No. of infections | 0 (0-1) | 1 (0-1) | 0 (0-1.25) | 0.554 |
| Types of infections | ||||
| none | 55 (52.4) | 25 (49) | 30 (55.6) | 0.745 |
| UTI | 30 (28.6) | 14 (27.5) | 16 (29.6) | 0.832 |
| Pnemonia | 1 (1) | 1 (2) | 0 (0) | 0.486 |
| TB | 1 (1) | 0 (0) | 1 (1.9) | 1 |
| BK | 7 (6.7) | 5 (9.8) | 2 (3.7) | 0.261 |
| Bactremia | 4 (3.8) | 1 (2) | 3 (5.6) | 0.618 |
| Epidediymo-orchitis | 2 (1.9) | 1 (2) | 1 (1.9) | 1 |
| Gastroenteritis | 2 (1.9) | 2 (3.9) | 0 (0) | 0.234 |
| Herpes zoster | 1 (1) | 0 (0) | 1 (1.9) | 1 |
| Infected AVF | 1 (1) | 1 (2) | 0 (0) | 0.486 |
| Perianal abcess | 1 (1) | 1 (2) | 0 (0) | 0.486 |
| COVID-19 | 9 (8.6) | 6 (11.8) | 3 (5.6) | 0.311 |
| URTI | 2 (1.9) | 1 (2) | 1 (1.9) | 1 |
| CMV | 12 (11.4) | 6 (11.8) | 6 (11.1) | 1 |
| Urological complications | ||||
| None | 95 (90.5) | 49 (96.1) | 46 (85.2) | 0.484 |
| Allograft artery stenosis | 1 (1) | 0 (0) | 1 (1.9) | |
| Collection | 3 (2.9) | 1 (2) | 2 (3.7) | |
| Lymphocele | 1 (1) | 0 (0) | 1 (1.9) | |
| Obstrctive uropathy | 1 (1) | 0 (0) | 1 (1.9) | |
| Perinephric collection and ureteric stricture | 2 (1.9) | 0 (0) | 2 (3.7) | |
| Unrogenic bladder | 1 (1) | 0 (0) | 1 (1.9) | |
| Urinary leak | 1 (1) | 1 (2) | 0 (0) | |
| Urological complications | ||||
| No | 95 (90.5) | 49 (96.1) | 46 (85.2) | 0.094 |
| Yes | 10 (9.5) | 2 (3.9) | 8 (14.8) | |
| CNI withdrawal | 1 (1) | 0 (0) | 1 (1.9) | 1 |
| Duration from Tx | 3 d | |||
| MMF withdrawal | 1 (1) | 0 (0) | 1 (1.9) | 1 |
| Duration from Tx | 3 d | |||
| Steroids withdrawal | 0 (0) | 0 (0) | 0 (0) |
| Total, 105 | Non-transfusion, 51 (48.6%) | Transfusion, 54 (51.4%) | P value | |
| Rejection | 5 (4.8) | 2 (3.9) | 3 (5.6) | 1 |
| Rejection type | ||||
| ABMR | 2 (40) | 1 (50) | 1 (33.3) | 1 |
| Cellular | 3 (60) | 1 (50) | 2 (66.7) | |
| Graft loss | 4 (3.8) | 1 (2) | 3 (5.6) | 0.618 |
| Death | 2 (1.9) | 1 (2) | 1 (1.9) | 1 |
| DGF | 11 (10.5) | 2 (3.9) | 9 (16.7) | 0.053 |
| Serum creatinine | ||||
| At discharge | 141 ± 124.1 | 123 ± 56.7 | 158 ± 163 | 0.770 |
| 6 mo | 108 ± 40.7 | 107.1 ± 28 | 108.9 ± 50.4 | 0.825 |
| 12 mo | 109.1 ± 51.3 | 101.9 ± 22.9 | 117 ± 69.8 | 0.182 |
| 18 mo | 126 ± 168.7 | 106.2 ± 26.5 | 147.4 ± 241.5 | 0.735 |
| Creatinine difference: At 18 mo-at discharge | -19.4 ± 61.5 | 12.3 ± 253.8 | 0.439 |
Rejection occurred in five recipients in our cohort; three had cellular rejection and two had antibody-mediated rejections (Table 5). All rejection episodes were biopsy-proven, and three occurred in the transfusion group; nevertheless, there was no significant difference in the rate of rejection between the two groups. Additionally, during the study period, there was an improvement in serum creatinine levels in all the patients with rejection in both groups. None of the recipients with allograft rejection lost their grafts during the study period. However, one of the recipients died due to coronavirus disease 2019 pneumonia with a functioning graft. There were no statistically significant differences in age, sex, type of transplantation, HLA mismatch, induction therapy, or CNI levels between patients who developed rejection and those who did not.
| Non-rejection, 100 | Rejection, 5 | P value | |
| Age (mean ± SD) | 36.5 (28.25-51.75) | 36 (21-46.5) | 0.383 |
| Gender | |||
| Female | 28 (28) | 0 (0) | 0.321 |
| Male | 72 (72) | 5 (100) | |
| Type of transplantation | |||
| LRKTx | 81 (81) | 3 (60) | 0.172 |
| LURKTx | 13 (13) | 2 (40) | |
| DDKTx | 6 (6) | 0 (0) | |
| HLA mismatch | 3 (1-4) | 3 (1-4) | 0.729 |
| Cause of ESRD | |||
| Diabetes | 18 (18) | 0 (0) | 0.199 |
| GN | 23 (23) | 3 (60) | |
| Hypertension | 18 (18) | 0 (0) | |
| PCKD | 1 (1) | 0 (0) | |
| Urological | 6 (6) | 1 (20) | |
| Other | 34 (34) | 1 (20) | |
| Donor's age | 32 (26-39) | 28 (25.5-43.5) | 0.981 |
| Induction therapy | |||
| ATG | 41 (41) | 1 (20) | 0.438 |
| Basiliximab | 58 (58) | 4 (80) | |
| No induction | 1 (1) | 0 (0) | |
| Average CNI level | 7 (6-8) | 8 (6.5-9) | 0.311 |
| Hb at transplantation | 10.65 (9.025-11.3) | 10.7 (9.05-12.45) | 0.792 |
| Hb at blood transfusion | 7.4 (6.8-8) | 7.81 | 0.138 |
| Hb after transfusion | 8.9 (8.4-10) | 101 | 0.382 |
| No of blood transfusion unites given | 1 (0-1) | 1 (0-3) | 0.491 |
| Serum creatinine | |||
| At discharge | 111 (83.25-142.75) | 151 (113.5-222.5) | 0.096 |
| 6 mo | 102.5 (80.5-123) | 120 (80-156) | 0.420 |
| 12 mo | 99.5 (81.75-119.25) | 124.5 (92.5-140.75) | 0.201 |
| 18 mo | 105 (84.25-119) | 107 (72-) | 0.894 |
| Death | 1 (1) | 1 (20) | 0.093 |
| DGF | 9 (9) | 2 (40) | 0.084 |
| No. of infections | 0 (0-1) | 1 (0-2) | 0.651 |
| Types of infections | |||
| None | 53 (53) | 2 (40) | 0.188 |
| UTI | 30 (28.6) | 29 (29) | 1 |
| Pnemonia | 1 (1) | 1 (1) | 1 |
| TB | 1 (1) | 1 (1) | 1 |
| BK | 7 (6.7) | 7 (7) | 1 |
| Bactremia | 4 (3.8) | 4 (4) | 1 |
| Epidediymo-orchitis | 2 (1.9) | 2 (2) | 1 |
| Gastroenteritis | 2 (1.9) | 1 (1) | 0.093 |
| Herpes zoster | 1 (1) | 1 (1) | 1 |
| Infected AVF | 1 (1) | 1 (1) | 1 |
| Perianal abcess | 1 (1) | 1 (1) | 1 |
| COVID-19 | 9 (8.6) | 8 (8) | 0.367 |
| URTI | 2 (1.9) | 1 (1) | 0.093 |
| CMV | 11 (11) | 1 (20) | 0.462 |
| Urological complications | |||
| None | 90 (90) | 5 (100) | 1 |
| Allograft artery stenosis | 1 (1) | 0 (0) | |
| Collection | 3 (3) | 0 (0) | |
| Lymphocele | 1 (1) | 0 (0) | |
| Obstrctive uropathy | 1 (1) | 0 (0) | |
| Perinephric collection and ureteric stricture | 2 (2) | 0 (0) | |
| Unrogenic bladder | 1 (1) | 0 (0) | |
| Urinary leak | 1 (1) | 0 (0) | |
| Urological complications | |||
| No | 90 (90) | 5 (100) | 1 |
| Yes | 10 (10) | 0 (0) |
The incidence of DGF was higher in the transfusion group; however, this difference was not statistically significant. In contrast, analysis of the predictors of DGF using multivariate logistic regression showed that age [adjusted odds ratio, 1.06, 95% confidence interval (CI): 1.012-1.111; P = 0.014] and blood transfusion (adjusted odds ratio 5.649, 95%CI: 1.106-28.848; P = 0.037) were significant independent risk factors for DGF. There were no significant differences in graft loss or all-cause death mortality between the two groups.
We conducted a multiple linear regression analysis to examine the association between creatinine change (the difference between creatinine at the end of the study and baseline creatinine) as a dependent variable and eligible study variables as independent variables. We found that a higher number of transfused packed red blood cells was significantly associated with increased creatinine levels at the end of the study (B = 20.14; SE = 6.99; P = 0.004), whereas a higher creatinine level at discharge was associated with milder creatinine increase over the study period (B = -0.79; SE = 0.12; P < 0.001) (Table 6).
| B | SE | 95%CI | P value | |
| (Intercept) | 70.23 | 18.72 | 33.54 to 106.92 | < 0.001 |
| Male vs female | -1.74 | 18.59 | -38.17 to 34.69 | 0.925 |
| No. of PRBCs | 20.14 | 6.99 | 6.45 to 33.84 | 0.004 |
| Creatinine at discharge | -0.79 | 0.12 | -1.03 to -0.54 | < 0.001 |
Anemia is a common condition during the peri-transplantation period. The rate of anemia during this period varies significantly. In a retrospective cohort study, Vanrenterghem et al[1] reported an anemia rate of 38% in a transplant population[1]. However, in a recent prospective study, 64% of the study cohort had anemia that requiring blood transfusion in the first month after transplantation[2]. The transfusion rate post-transplantation has been repeatedly reported to be between 37%-75%[4,6-8]. This high prevalence of transfusion has also been observed in pediatric populations. For instance, Richards et al[7] reported that the prevalence of transfusion was approximately 50% with a higher prevalence in younger children[7]. In our study, the anemia rate was toward the higher end of the above mentioned range at 69% however, only 54% of our cohort required blood transfusion.
Anemia carries significant risk in kidney transplant recipients. A drop of Hb level > 30% of its pre-transplant level was reported to be associated with higher all-cause graft failure and longer length of hospital stay, with a greater risk in those who required blood transfusion of > 3 units and those with longer cold ischemia time[9]. However, the effect of peri-transplantation blood transfusion on graft outcome have not been well established. For instance, in a study by Daloul et al[4], blood transfusion was not associated with a greater risk of worse graft outcomes[4]; however, Massicotte-Azarniouch et al[6] revealed that blood transfusion is associated with a greater risk of graft loss[6]. This is also supported by the findings form a recent study that included more than 1000 recipients, which showed that early blood transfusion post-transplantation didn’t lead to de novo DSAs formation[10]. Our study did not find any association between blood transfusion and graft loss or mortality. The link between blood transfusion and graft or patient loss might be a cofounding factor because patients with advanced allograft dysfunction are commonly anemic. Similarly, sick patients with multiple comorbidities are usually anemic and may require blood transfusions.
HLA molecules in the blood products are known to cause HLA allosensitization for blood transfusion recipients[11-13]. Various strategies have been attempted to avoid HLA allosensitization after blood transfusion, including leuko-reduced (leuko-depleted) blood products[11,14], HLA-selected blood products[15], and autologous blood transfusion[16]. However, the protective effects of these strategies are not well established[11,14-19]. In our study, we decided to account only for leuko-depleted blood products because this is a widely used technique in our blood bank.
The effect of blood transfusion on DSA formation and antibody-mediated rejection are not well understood. Few studies have examined the development of de-novo HLA antibodies after blood transfusions in transplant populations. While some studies found that de-novo HLA antibodies and DSAs have a negative impact on the transplant[2,20], other studies have doubted the significance of HLA antibody development in the setting of immunosuppression therapy[3,8,11]. For instance, In Ferrandiz et al[2] reported that antibody-mediated rejection occurred in 6% of kidney transplant recipients who required blood transfusion post-surgery compared with 1.4% in a non-transfusion group (P = 0.04)[2]. In contrast, in a study by Jalalonmuhali et al[3] involving 699 patients, there was no differences in the development of HLA antibodies or de-novo HLA-DSA and rejection between the transfusion and none transfusion groups[3]. Similarly in our study, the rejection rate in the transfusion group was approximately 5%, with no difference between the two groups.
Multiple factors are associated with an increased risk of poor transplant outcomes after blood transfusion. In a previous prospective observational study, worse transplantation outcomes were linked to the number of transfusion episodes (pre and post-transplantation), regardless of the total number of transfusion units[20]. In another study, poor transplantation outcomes were linked to the number of transfusion units (> 3 units)[9]. In our study, creatinine levels tended to increase toward the end of the follow-up period in the transfusion group, but this finding was not statistically significant.
It is noteworthy that maintenance immunosuppression therapy in studies that found a significant increase in rejection risk after transfusion was cyclosporine-based[2,20] while studies in which the maintenance immunosuppression regimen was tacrolimus-based showed no significant increase in rejection rate between transfusion and non-transfusion groups[3,8]. Our study is consistent with this observation as rejection rate was not significantly different between the two groups in our tacrolimus-based study cohort.
Although blood transfusion after kidney transplantation did not have an impact on patient survival, a cross-sectional study of 1198 liver transplant recipients showed a significant increase in mortality rate in patients who received a large number of blood transfusion units. Average blood transfusion units in expired patients was 5.92 ± 5.91 compared to 3.74 ± 4.23 in alive patients (95%CI: 1.47–2.88)[21].
In this study, there was a tendency toward higher DGF rates in the transfusion group. Although this finding was not statistically significant, it was in line with that of MacIsaac et al[9], in which the rate of DGF in transplant patients was up to 26%[9]. Similarly, in a retrospective cohort study on 1258 kidney transplant recipients who were followed for a median of 1405 d, DGF was as high as 41% in a transfusion group vs 15% in a non-transfusion group (P < 0.0001)[6]. In a study by Fidler et al[20], DGF was associated with a higher risk of combined patient and graft loss at a hazard ratio of 2.5 (1.5-4.5) on univariate analysis; However, this difference disappeared on multivariate analysis. It’s difficult to determine whether DGF is a cause, or a result of blood transfusion based on the available literature.
This study has limitations. This was a single-center retrospective cohort study. The lack of routine DSA and allograft biopsy restricted the inclusion of clinically insignificant DSAs and non-apparent rejections. Moreover, our cohort was predominantly males, which limits the generalizability of our findings.
Leukodepleted blood transfusion in the peri-transplantation period was not associated with a higher risk of rejection, graft loss, or patient loss. Further investigations are needed to address the link between peri-transplantation blood transfusions, DGF and DSA formation.
Blood transfusion is common during the peri-transplantation period. The incidence of immunological reactions to blood transfusion after kidney transplantation and their consequences on graft outcomes have not been extensively studied.
Blood transfusion during the peri-transplantation period is very common and its safety need to be studied.
To examine the risk of graft rejection and loss in patients who received blood transfusion in the immediate peri-transplantation period.
A retrospective cohort study of 105 kidney recipients who received leukodepleted blood transfusions at our center between January 2017 and March 2020.
Of 105 kidney recipients were divided into transfusion (n = 54) and non-transfusion (n = 51) groups. There were no differences between the two groups in terms of rejection rates, graft loss, or death. There was no significant difference in creatinine level progression between the two groups. A high number of transfused packed red blood cells was significantly associated with increased creatinine levels at the end of the study.
Leukodepleted blood transfusion was not associated with a higher risk of rejection, graft loss, or death in kidney transplant recipients.
Leukodepleted blood transfusion in the peri-transplantation period is likely safe.
| 1. | Vanrenterghem Y, Ponticelli C, Morales JM, Abramowicz D, Baboolal K, Eklund B, Kliem V, Legendre C, Morais Sarmento AL, Vincenti F. Prevalence and management of anemia in renal transplant recipients: a European survey. Am J Transplant. 2003;3:835-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Ferrandiz I, Congy-Jolivet N, Del Bello A, Debiol B, Trébern-Launay K, Esposito L, Milongo D, Dörr G, Rostaing L, Kamar N. Impact of Early Blood Transfusion After Kidney Transplantation on the Incidence of Donor-Specific Anti-HLA Antibodies. Am J Transplant. 2016;16:2661-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Jalalonmuhali M, Carroll RP, Tsiopelas E, Clayton P, Coates PT. Development of de novo HLA donor specific antibodies (HLA-DSA), HLA antibodies (HLA-Ab) and allograft rejection post blood transfusion in kidney transplant recipients. Hum Immunol. 2020;81:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Daloul R, Braga JR, Diez A, Logan A, Pesavento T. Early Posttransplant Blood Transfusion and Risk for Worse Graft Outcomes. Kidney Int Rep. 2021;6:986-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Scornik JC, Meier-Kriesche HU. Blood transfusions in organ transplant patients: mechanisms of sensitization and implications for prevention. Am J Transplant. 2011;11:1785-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Massicotte-Azarniouch D, Sood MM, Fergusson DA, Chassé M, Tinmouth A, Knoll GA. Blood Transfusion and Adverse Graft-related Events in Kidney Transplant Patients. Kidney Int Rep. 2021;6:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Richards KM, Spicer RA, Craig E, Kennedy SE. Prevalence and predictors of blood transfusion after pediatric kidney transplantation. Pediatr Nephrol. 2018;33:2177-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Bynum JP, Zachary A, Ness PM, Luo X, Bagnasco S, King KE, Segev DL, Orandi BJ, Warren DS, Fuller A, Ciappi A, Montgomery R, Tobian AAR. Transfusion of leukoreduced blood products and risk of antibody-mediated rejection of renal allografts. Transfusion. 2018;58:1951-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | MacIsaac S, Ramanakumar AV, Saw C, Naessens V, Saberi N, Cantarovich M, Baran D, Paraskevas S, Tchervenkov J, Chaudhury P, Sandal S. Relative decrease in hemoglobin and outcomes in patients undergoing kidney transplantation surgery: A retrospective cohort study. Am J Surg. 2021;222:825-831. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Jouve T, Noble J, Naciri-Bennani H, Dard C, Masson D, Fiard G, Malvezzi P, Rostaing L. Early Blood Transfusion After Kidney Transplantation Does Not Lead to dnDSA Development: The BloodIm Study. Front Immunol. 2022;13:852079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 11. | Scornik JC, Ireland JE, Howard RJ, Fennell RS 3rd, Pfaff WW. Role of regular and leukocyte-free blood transfusions in the generation of broad sensitization. Transplantation. 1984;38:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Terasaki PI, Cai J. Humoral theory of transplantation: further evidence. Curr Opin Immunol. 2005;17:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Vaidya S. Synthesis of new and memory HLA antibodies from acute and chronic rejections versus pregnancies and blood transfusions. Transplant Proc. 2005;37:648-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Karpinski M, Pochinco D, Dembinski I, Laidlaw W, Zacharias J, Nickerson P. Leukocyte reduction of red blood cell transfusions does not decrease allosensitization rates in potential kidney transplant candidates. J Am Soc Nephrol. 2004;15:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Magee BA, Martin J, Cole MP, Morris KG, Courtney AE. Effects of HLA-matched blood transfusion for patients awaiting renal transplantation. Transplantation. 2012;94:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Sasaki H, Chikaraishi T, Furuhata S, Tsutsumi H, Miyano S, Nakazawa R, Nakano T, Kudo H, Kitajima K, Takahashi T, Satoh Y, Kimura K. Autologous blood transfusion for kidney transplant recipients. Transplant Proc. 2008;40:1371-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | van de Watering L, Hermans J, Witvliet M, Versteegh M, Brand A. HLA and RBC immunization after filtered and buffy coat-depleted blood transfusion in cardiac surgery: a randomized controlled trial. Transfusion. 2003;43:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Ohto H, Nomizu T, Kuroda F, Hoshi T, Rokkaku Y. HLA alloimmunization of surgical patients by transfusion with bedside leukoreduced blood components. Fukushima J Med Sci. 2003;49:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Sanfilippo FP, Bollinger RR, MacQueen JM, Brooks BJ, Koepke JA. A randomized study comparing leukocyte-depleted versus packed red cell transfusions in prospective cadaver renal allograft recipients. Transfusion. 1985;25:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Fidler S, Swaminathan R, Lim W, Ferrari P, Witt C, Christiansen FT, D'Orsogna LJ, Irish AB. Peri-operative third party red blood cell transfusion in renal transplantation and the risk of antibody-mediated rejection and graft loss. Transpl Immunol. 2013;29:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Kasraian L, Nikeghbalian S, Karimi MH. Blood Product Transfusion in Liver Transplantation and its Impact on Short-term Survival. Int J Organ Transplant Med. 2018;9:105-111. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sunder T, India; Zhang ZX, Canada S-Editor: Fan JR L-Editor: A P-Editor: Ji MX