Published online Jun 18, 2023. doi: 10.5500/wjt.v13.i4.147
Peer-review started: January 4, 2023
First decision: January 12, 2023
Revised: February 1, 2023
Accepted: April 20, 2023
Article in press: April 20, 2023
Published online: June 18, 2023
Processing time: 162 Days and 10.9 Hours
Pancreas transplant is the only treatment that establishes normal glucose levels for patients diagnosed with diabetes. However, since 2005, no comprehensive analysis has compared survival outcomes of: (1) Simultaneous pancreas-kidney (SPK) transplant; (2) Pancreas after kidney (PAK) transplant; and (3) Pancreas transplant alone (PTA) to waitlist survival.
To explore the outcomes of pancreas transplants in the United States during the decade 2008-2018.
Our study utilized the United Network for Organ Sharing Standard Transplant Analysis and Research file. Pre- and post-transplant recipient and waitlist characteristics and the most recent recipient transplant and mortality status were used. We included all patients with type I diabetes listed for pancreas or kidney-pancreas transplant between May 31, 2008 and May 31, 2018. Patients were grouped into one of three transplant types: SPK, PAK, or PTA.
The adjusted Cox proportional hazards models comparing survival between transplanted and non-transplanted patients in each transplant type group showed that patients who underwent an SPK transplant exhibited a significantly reduced hazard of mortality [hazard ratio (HR) = 0.21, 95% confidence intervals (CI): 0.19-0.25] compared to those not transplanted. Neither PAK transplanted patients (HR = 1.68, 95%CI: 0.99-2.87) nor PTA patients (HR = 1.01, 95%CI: 0.53-1.95) exper
When assessing each of the three transplant types, only SPK transplant offered a survival advantage compared to patients on the waiting list. PKA and PTA transplanted patients demonstrated no significant differences compared to patients who did not receive a transplant.
Core Tip: The total number of pancreas transplants has been in the decline in United States since 2003/2004. This study aimed to show acceptable survival outcome for diabetic patients receiving pancreas transplant as a cure therapeutic approach.
- Citation: Jarmi T, Brennan E, Clendenon J, Spaulding AC. Mortality assessment for pancreas transplants in the United States over the decade 2008-2018. World J Transplant 2023; 13(4): 147-156
- URL: https://www.wjgnet.com/2220-3230/full/v13/i4/147.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i4.147
The Diabetes Control and Complications Trial demonstrated the advantage of intensive diabetes therapy in delaying the development of macro/microvascular diabetic-related complications and decreasing the overall mortality rate of diabetic patients[1-4]. It is clear, however, from follow-up studies that the risk of developing secondary diabetic complications is not eliminated, and the incidence of hypoglycemic episodes increases over time[5,6]. As a result, pancreas transplant is the only treatment that restores normal glucose metabolism in insulin-dependent diabetic patients[7,8].
Pancreas transplants, in relation to kidney function, fall into three different categories: (1) Simultaneous pancreas-kidney (SPK) transplant in patients with end-stage kidney disease (ESKD); (2) Pancreas after kidney (PAK) transplant; and (3) Pancreas transplant alone (PTA) in patients with no kidney disease[9]. Recipient and graft survival rates and the total number of pancreas transplants had improved in all three categories since the introduction of the procedures. However, around 2003, the number of pancreas transplants started to decline[10]. Multiple events and factors could explain the paradoxical relationship between declining transplants despite improving outcomes[10]. One contributing factor was that during the period, two major studies conducted by Venstrom et al[11] in 2003 and Gruessner et al[12] in 2005 showed inconsistency in reported outcomes of patients and grafts after a pancreas transplant. Subsequently, the overall number of active pancreas transplant centers fell. By 2016, only 11 centers in the United States performed more than 20 pancreas transplants a year, and most centers performed less than 5 transplants annually[10,13]. Consequently, fewer surgeons are adequately trained in pancreas donor recovery and transplant[14,15]. Since the 2003 and 2005 studies, no comprehensive analysis has compared the outcomes of the three categories of pancreas transplant and waitlist survival. To remedy this gap in our understanding, the present study analyzed the mortality of transplanted vs wait-listed patients in all three pancreas recipient categories using United Network for Organ Sharing (UNOS)/IPTR data from May 31, 2008 through May 31, 2018. We hypothesized that since 2005, survival for each type of transplant will have improved. Specifically:
Hypothesis 1: PTA patients will have improved survival compared to those not transplanted.
Hypothesis 2: PAK patients will have improved survival compared to those not transplanted.
Hypothesis 3: SPK patients will have improved survival compared to those not transplanted.
Our study utilized the UNOS Standard Transplant Analysis and Research file[16]. This database contains clinical and follow-up data for all transplants in the United States. Pre- and post-transplant recipient and waitlist characteristics and the most recent recipient transplant and mortality status were used. We included all patients with type I diabetes listed for pancreas or kidney-pancreas transplant between May 31, 2008 and May 31, 2018. Any patients listed for pancreas or pancreas-kidney transplant for the first time before or after those dates were excluded. Patients listed for any organ other than a pancreas, pancreas-kidney simultaneously, or were listed before May 31, 2008, were excluded. Patients under 18 years of age were also removed, as were patients with missing waitlist ID or registration dates.
Patients were grouped into one of three transplant types: SPK, PAK, or PTA. Patients listed for pancreas and kidney transplants at the same time (with overlapping waitlist times) or receiving a pancreas and kidney transplant together were included in the SPK group. Patients listed for their first pancreas transplant on or after May 31, 2008, and with a kidney or kidney-pancreas transplant record before their listing for a pancreas transplant and those receiving a pancreas transplant after having a kidney transplant were included in the PAK group. Finally, patients listed for or who received only a pancreas transplant, having never been listed for or received a kidney transplant, were considered in the PTA group. Patients were considered to have a pancreas transplant if they had a pancreas transplant ID code and date. Patient death was defined as having a death date in the UNOS record, and patients were censored at removal from the waiting list or at the date of the last follow-up unless a death date was present. Waitlist times were calculated as the difference between first registration (INIT_DATE) and waitlist removal date (END_DATE), death date (COMPOSITE_DEATH_DATE), or transplant date (TX_DATE). If a patient was listed at multiple locations or had multiple entries, we determined the unique days between first registration and the removal date, death date, or transplant date. If a candidate was removed for being too sick to undergo their transplant and had a death date after being removed, the time between removal and death was added to the waitlist time. Time from transplant to death or loss to follow-up was calculated as the difference between the transplant date and death or last follow-up date (PX_STAT_DATE).
Descriptive statistics were calculated for transplanted and non-transplanted waitlist patients for each transplant type group. Means, standard deviations, and ranges are used to describe continuous variables. Categorical variables are described by frequency and percentages. Cox regression models comparing transplanted to non-transplanted patients used transplant as a time-dependent covariate, with time on the waitlist as time interval one and time from transplant to death or last follow-up as time interval two for transplanted patients. Adjustment variables included age at waitlist registration, gender, race (white, black, or other), duration of diabetes (years from the date of diabetes onset to date of waitlist registration), body mass index (BMI), Karnofsky functional status score, and presence of peripheral vascular disease (yes or no). BMI and functional status were divided into common clinically relevant groups operationalized into categorical variables. Adjustment variables were not considered as time-varying. Adjusted Cox models comparing survival after transplant between transplant-type groups only included transplanted patients and time from transplant to death or censoring. Additional models for up to 90 d post-transplant, 91 to 365 d post-transplant, and over 1 year post-transplant were also performed to compare survival within each period between transplant-type groups. These models were adjusted for the same variables as the previous set of models, with the addition of years on the waitlist. Hazard ratio (HR) and 95% confidence interval (CI) are reported[17]. All statistical analyses were performed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
There were 9498 patients listed for SPK transplant, 1111 Listed for PAK transplant, and 939 Listed for PTA between May 31, 2008 and May 31, 2018. Of those, 6883 (59.6%) were transplanted, and 926 (8.0%) died on the waitlist. The mean age at listing was 40.6 years (range: 18-73 years), and 6539 (56.6%) patients were male. The majority of patients (7695, 66.6%) were white, 2187 (18.9%) were black, and 1666 (14.4%) were of other races. Almost 12 percent of patients were of Hispanic/Latino ethnicity. Most patients (6931, 62.4%) had a high Karnofsky functional status score, 5347 (46.7%) had a normal BMI, 10217 (90.0%) did not have peripheral vascular disease, and the mean duration of diabetes before registration was 26.5 years (Table 1).
| SPK | PAK | PTA | |||||||
| Transplant (n = 5834) | Waitlist (n = 3664) | P value | Transplant (n = 430) | Waitlist (n = 681) | P value | Transplant (n = 619) | Waitlist (n = 320) | P value | |
| Age at registration (yr) | < 0.001 | 0.04 | 0.68 | ||||||
| mean (SD) | 40.1 (8.7) | 41.0 (9.3) | 40.6 (8.9) | 41.8 (9.5) | 41.7 (10.6) | 41.4 (10.8) | |||
| Range | 18.0 - 69.0 | 18.0 - 73.0 | 22.0 - 67.0 | 18.0 - 66.0 | 18.0 - 68.0 | 20.0 - 70.0 | |||
| Gender | < 0.001 | 0.026 | 0.49 | ||||||
| Female | 2284 (39.1%) | 1663 (45.4%) | 174 (40.5%) | 322 (47.3%) | 378 (61.1%) | 188 (58.8%) | |||
| Male | 3550 (60.9%) | 2001 (54.6%) | 256 (59.5%) | 359 (52.7%) | 241 (38.9%) | 132 (41.2%) | |||
| Race | 0.3 | 0.08 | < 0.001 | ||||||
| White | 3732 (64.0%) | 2295 (62.6%) | 336 (78.1%) | 502 (73.7%) | 567 (91.6%) | 263 (82.2%) | |||
| Black | 1229 (21.1%) | 781 (21.3%) | 37 (8.6%) | 88 (12.9%) | 25 (4.0%) | 27 (8.4%) | |||
| Other | 873 (15.0%) | 588 (16.0%) | 57 (13.3%) | 91 (13.4%) | 27 (4.4%) | 30 (9.4%) | |||
| Ethnicity | 0.22 | 0.7 | 0.012 | ||||||
| Hispanic/Latino | 714 (12.2%) | 480 (13.1%) | 46 (10.7%) | 78 (11.5%) | 23 (3.7%) | 24 (7.5%) | |||
| Non-Hispanic/Non-Latino | 5120 (87.8%) | 3184 (86.9%) | 384 (89.3%) | 603 (88.5%) | 596 (96.3%) | 296 (92.5%) | |||
| Karnofsky score at registration | 0.34 | 0.46 | 0.013 | ||||||
| High | 3441 (60.7%) | 2151 (61.9%) | 291 (70.3%) | 427 (66.6%) | 392 (64.9%) | 229 (74.1%) | |||
| Middle | 2108 (37.2%) | 1259 (36.3%) | 114 (27.5%) | 199 (31.0%) | 195 (32.3%) | 76 (24.6%) | |||
| Low | 121 (2.1%) | 63 (1.8%) | 9 (2.2%) | 15 (2.3%) | 17 (2.8%) | 4 (1.3%) | |||
| BMI at registration | < 0.001 | 0.038 | 0.82 | ||||||
| Normal | 2847 (49.1%) | 1640 (45.2%) | 200 (46.9%) | 287 (42.5%) | 240 (40.1%) | 133 (41.8%) | |||
| Underweight | 95 (1.6%) | 60 (1.7%) | 5 (1.2%) | 8 (1.2%) | 11 (1.8%) | 4 (1.3%) | |||
| Overweight | 2180 (37.6%) | 1311 (36.2%) | 172 (40.4%) | 259 (38.4%) | 231 (38.6%) | 116 (36.5%) | |||
| Obese | 674 (11.6%) | 615 (17.0%) | 49 (11.5%) | 121 (17.9%) | 117 (19.5%) | 65 (20.4%) | |||
| Duration of diabetes (yr) | 0.064 | 0.052 | 0.28 | ||||||
| mean (SD) | 26.3 (9.0) | 26.6 (9.2) | 26.9 (8.6) | 28.0 (9.5) | 26.6 (11.4) | 25.8 (11.7) | |||
| Range | 0.0 - 59.0 | 0.0 - 60.0 | 3.0 - 49.0 | 2.0 - 55.0 | 0.0 - 58.0 | 1.0 - 57.0 | |||
| Peripheral vascular disease at registration | < 0.001 | 0.96 | 0.78 | ||||||
| No | 5253 (91.5%) | 3136 (87.4%) | 384 (91.0%) | 613 (91.1%) | 545 (89.1%) | 286 (89.7%) | |||
| Yes | 488 (8.5%) | 453 (12.6%) | 38 (9.0%) | 60 (8.9%) | 67 (10.9%) | 33 (10.3%) | |||
| Time on waitlist (yr) | < 0.001 | < 0.001 | < 0.001 | ||||||
| mean (SD) | 0.9 (1.0) | 1.8 (1.7) | 1.2 (1.1) | 2.5 (2.0) | 0.6 (0.8) | 1.9 (1.9) | |||
| Range | 0.0 - 7.4 | 0.0 - 10.3 | 0.0 - 5.9 | 0.0 - 9.9 | 0.0 - 6.7 | 0.0 - 10.0 | |||
| Follow-up time after transplant (yr) | |||||||||
| mean (SD) | 3.7 (2.5) | NA | 3.4 (2.6) | NA | 3.3 (2.5) | NA | |||
| Range | 0.0 - 10.1 | NA | 0.0 - 9.1 | NA | 0.0 - 9.1 | NA | |||
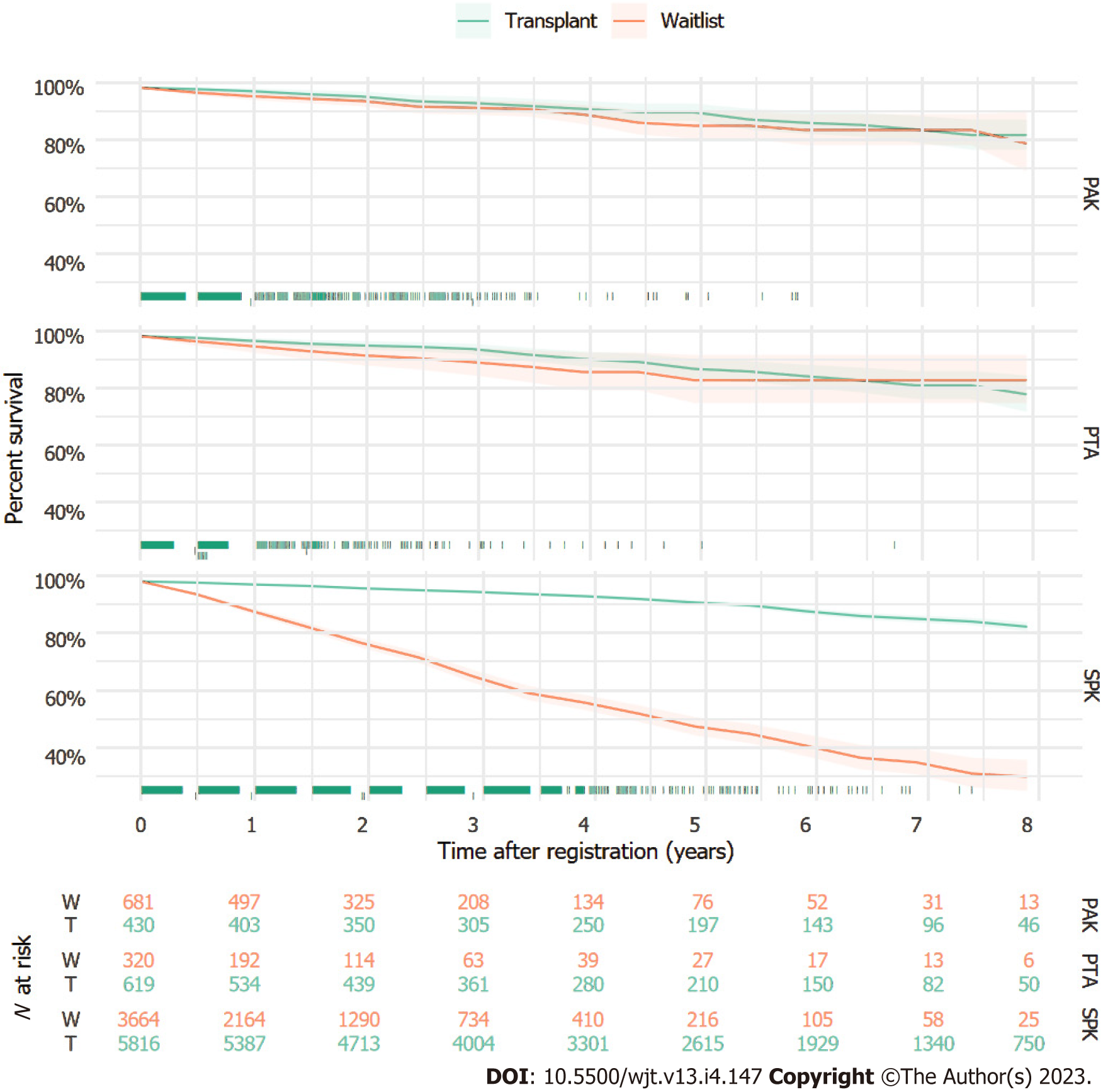
Kaplan-Meier curves for each transplant type vs the wait list over 8 years of follow-up are shown in Figure 1. When considering SPK transplant, there was a significant difference in the survival of the transplanted vs non-transplanted group, starting immediately and growing as time progressed. However, for PAK transplant and PTA, there was no separation between the groups over time, identifying no survival differences.
Results of adjusted Cox proportional hazards models comparing survival between transplanted and non-transplanted patients in each transplant type group are shown in Table 2. SPK transplanted patients exhibited a significantly reduced hazard of mortality (HR = 0.21, 95%CI: 0.19-0.25) compared to those not transplanted. Neither PAK transplanted patients (HR = 1.68, 95%CI: 0.99-2.87) nor PTA patients (HR = 1.01, 95%CI: 0.53-1.95) experienced significantly different hazards of mortality compared to patients who did not receive a transplant. Associations of adjustment variables with mortality varied by transplant type.
| SPK | PAK | PTA | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Transplanted (No) | Reference | Reference | Reference | Reference | Reference | Reference |
| Transplanted (Yes) | 0.21 (0.19, 0.25) | < 0.001 | 1.68 (0.99, 2.87) | 0.06 | 1.01 (0.53, 1.95) | 0.97 |
| Age at registration | 1.01 (1.00, 1.02) | 0.04 | 1.03 (1.00, 1.06) | 0.02 | 1.03 (1.00, 1.06) | 0.03 |
| Gender (F) | Reference | Reference | Reference | Reference | Reference | Reference |
| Gender (M) | 0.97 (0.86, 1.08) | 0.55 | 0.55 (0.35, 0.88) | 0.01 | 1.46 (0.90, 2.37) | 0.13 |
| Race (White) | Reference | Reference | Reference | Reference | Reference | Reference |
| Race (Black) | 1.05 (0.91, 1.22) | 0.47 | 1.82 (0.89, 3.71) | 0.1 | 0.34 (0.05, 2.54) | 0.29 |
| Race (Other) | 0.74 (0.62, 0.89) | 0.001 | 0.77 (0.34, 1.74) | 0.53 | 1.27 (0.45, 3.61) | 0.66 |
| BMI (Normal) | Reference | Reference | Reference | Reference | Reference | Reference |
| BMI (Obese) | 0.76 (0.63, 0.90) | 0 | 0.46 (0.21, 1.04) | 0.06 | 0.80 (0.39, 1.65) | 0.54 |
| BMI (Overweight) | 0.87 (0.77, 0.99) | 0.03 | 0.66 (0.40, 1.10) | 0.11 | 0.85 (0.50, 1.46) | 0.57 |
| BMI (Underweight) | 1.16 (0.77, 1.75) | 0.48 | 3.15 (0.90, 10.96) | 0.07 | 1.76 (0.41, 7.64) | 0.45 |
| Duration of diabetes (yr) | 1.00 (0.99, 1.01) | 0.96 | 0.99 (0.96, 1.02) | 0.62 | 0.98 (0.96, 1.01) | 0.21 |
| Karnofsky score (High) | Reference | Reference | Reference | Reference | Reference | Reference |
| Karnofsky score (Low) | 0.98 (0.60, 1.60) | 0.94 | 2.77 (0.96, 7.95) | 0.06 | 3.07 (0.39, 24.14) | 0.29 |
| Karnofsky score (Middle) | 1.42 (1.26, 1.60) | < 0.001 | 0.89 (0.52, 1.52) | 0.66 | 2.22 (1.35, 3.64) | 0 |
| Peripheral vascular disease (No) | Reference | Reference | Reference | Reference | Reference | Reference |
| Peripheral vascular disease (Yes) | 1.40 (1.19, 1.66) | < 0.001 | 0.98 (0.44, 2.16) | 0.95 | 0.99 (0.47, 2.12) | 0.99 |
Results of adjusted Cox proportional hazards models comparing post-transplant survival between the transplant-type groups are shown in Table 3. In the model that utilized all post-transplant follow-up time, PAK transplant recipients showed a significantly increased mortality hazard compared to SPK transplant recipients (HR = 1.46, 95%CI: 1.07-2.01). In the model using only up to 90 d of follow-up, PTA recipients showed a significantly reduced hazard compared to SPK transplant recipients (HR = 0.21, 95%CI: 0.05-0.88). Patients in the PAK group also showed a reduced hazard in the 90 d after transplant compared to those in the SPK group, although the association was not significant (HR = 0.25, 95%CI: 0.06-1.03). In the model using 91-365 d of follow-up, no significant differences in mortality hazard were observed between the three groups. In the model using over one year of follow-up time, the PAK transplanted group exhibited a significantly increased hazard compared to the SPK group (HR = 1.59, 95%CI: 1.11-0.30), and the PTA group showed a higher hazard than the SPK group, though the association was not statistically significant (HR = 1.36, 95%CI: 0.96-1.92).
| Overall | Up to 90 d post-transplant | 91-365 d post-transplant | Greater than 1 yr post-transplant | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Transplant type (SPK) | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Transplant type (PAK) | 1.46 (1.07, 2.01) | 0.02 | 0.25 (0.06, 1.03) | 0.06 | 1.89 (0.89, 4.02) | 0.1 | 1.59 (1.11, 2.30) | 0.01 |
| Transplant type (PTA) | 1.22 (0.91, 1.65) | 0.19 | 0.21 (0.05, 0.88) | 0.03 | 1.31 (0.64, 2.68) | 0.46 | 1.36 (0.96, 1.92) | 0.08 |
Solid organ pancreas transplant is a complex procedure for which significant progress, in terms of immunosuppressive and surgical advancement, has been made over the past 5 decades. However, despite the advancement in immunomodulatory medications and surgical techniques, the number of pancreas transplants in the United States has declined significantly since 2003/2004[18,19]. The current study found that simultaneous pancreas-kidney transplant offered a survival advantage compared to patients on the waiting list. PAK transplant and PTA patients demonstrated no significant differences compared to patients who did not receive a transplant. As mentioned, two milestone studies demonstrated divergent results regarding pancreas transplant outcomes that are important to consider in light of the current results. The 2005 study conducted by Gruessner et al[12] showed survival results to be improved, while the 2003 study conducted by Venstrom et al[11] showed negative survival benefits.
In the category of PTA, the Gruessner study showed that the overall hazard ratio was 0.66 (95%CI: 0.39–1.12), favoring transplantation, while the Venstrom study showed the overall hazard ratio was 1.57 (95%CI: 0.98-2.53) favoring a no transplantation strategy. In our study, we analyzed data from the decade 2008-2018, and found recipients of PTA to have better survival results compared to the previous analysis conducted by Venstrom et al[11] and offered non-inferior outcomes when compared to patients on the waiting list (HR = 1.01, 95%CI: 0.53-1.95). As a result, there is mixed support for hypothesis 1, as survival has improved compared to the Venstrom study but has not improved compared to the Grussner study. For PAK transplanted patients, Gruessner and colleagues found no overall difference for transplant (HR = 0.92, 95%CI: 0.69-1.12), but Venstrom et al[11] (HR = 1.42, 95%CI: 1.03-1.94) found a worse outcome. Our results, however, showed PAK transplanted patients to have an increased but not significant risk of death after transplant compared to waiting list patients (HR = 1.68, 95%CI: 0.99-2.87). As a result, there is also mixed support for hypothesis 2 as we found worse survival outcomes than Gruessner et al[12], but better survival than the Venstrom study. Finally, previous studies and ours favored transplantation in the SPK transplant category. Specifically, the Gruessner study identified an HR of 0.29 (95%CI: 0.27-0.33), and the Venstrom study identified an HR of 0.43 (95%CI: 0.39-0.48). Compared to patients on the waiting list, the mortality HR for SPK transplant recipients in the current study was 0.21 (95%CI: 0.19-0.25). As a result, there is support for hypothesis 3 as our results indicate improved survival compared to the previous studies.
When we considered the SPK transplant recipients’ category as the analysis reference and broke down the follow-up period to: (1) Up to 90 d post-transplant; (2) 91 to 365 d post-transplant; and (3) Greater than 1 year post-transplant, we found an increased mortality risk among patients with PTA; however, the result was not significant (HR = 1.22, 95%CI: 0.91-1.65) (P = 0.19). The increased mortality risk was significant among patients in the PAK category (HR = 1.46, 95%CI: 1.07- 2.01) (P = 0.02). However, it is unclear why PAK transplant offers less survival benefit when compared to SPK transplant and the waiting list. This is more puzzling, especially if the expected sequence of PAK transplant is to receive a kidney from a living donor first, followed by a pancreas from a deceased donor. This sequence of events should offer a better survival than our results and previously published ones. Therefore, more analysis is needed to dissect all characteristics and conditions associated with the PAK category.
In relation to diseases that could influence poor outcomes, we also reviewed the impact of peripheral vascular disease (PVD) on the survival of the study patients. Patients diagnosed with PVD have a 3-fold increased risk of dying from all causes and a 6-fold increased risk of dying from cardiovascular disease within 10 years compared with patients without PVD[20-22]. Diabetic patients with PVD and those younger than 75 years have a 23% increase in mortality rate vs 7% among the control group[23]. We found patients from the SPK category group to have a lower incidence of PVD (8.5%) when compared to waitlist patients (12.6%) (P = 0.001). In the adjusted Cox proportional hazards models comparing transplanted to non-transplanted patients within each transplant category, SPK transplanted patients with PVD showed a significantly increased mortality risk compared to wait-listed patients (HR = 1.40, 95%CI: 1.19-1.66, P = 0.001). This could add a biased survival advantage when patients with less PVD are selected to proceed with SKP transplants after bypassing patients with more PVD on the waiting list. When reviewing the impact of BMI on the survival of the study patients, we found a paradoxical benefit of obesity among transplanted patients compared to wait-listed patients. This association was significant in the SPK category (HR = 0.76, 95%CI: 0.63-0.90) (P = 0.00) but was not significant in the PAK and PTA categories. The controversial advantage of obesity among patients with ESKD was shown before. Abbott et al[24] performed a retrospective analysis of the United States Renal Data System (USRDS) Dialysis Morbidity and Mortality Wave II Study patients who started dialysis in 1996 and were followed until October 31, 2001. They concluded that BMI ≥ 30 kg/m2 was associated with improved survival in hemodialysis patients.
These results, in total, could be seen as an advancement in the field of transplantation and diabetic care in general. When we consider the consensus of the previous two studies and ours in favoring survival among patients who received SPK transplant, we are likely seeing a result of the remarkably high mortality rate among patients with end-stage kidney disease[25]. As a result, the benefit after an SPK transplant would appear to be more a consequence of resolving the kidney disease[25]. On the other hand, the lack of differences identified in the PAK and PTA groups, despite improved surgical and medical management techniques, likely points to similar progress in diabetic care in terms of medical technology, which improved the survival of diabetic patients with standard insulin therapy[26]. Patients with advanced diabetic disease may most benefit from PAK transplant or PTA. Previous studies have shown improved cost-effectiveness and quality of life for these groups compared to diabetic management through insulin alone[27].
Our study showed the survival advantage of SKP transplants compared to patients on the waiting list over the last decade. However, PAK transplant and PTA demonstrated no significant differences compared to patients who did not receive a transplant.
Pancreas transplant is the only treatment that establishes normal glucose levels for patients diagnosed with diabetes. A significant advancement in management of diabetes associated with significant improvement in diabetic patients outcome has been achieved within the last decade. During the same period of time, there has been a noticeable decline in pancreas transplant procedures in the United States. In order to outline the importance of pancreas transplant as the only incurable treatment available for diabetes that could lead to normal glycemic status of these patients, we analyzed the outcome of pancreas transplant vs diabetic standard of care in the United States from 2008 to 2018.
A noticeable and significant decline of pancreas transplantation in the United States since 2004 has led to a decrease in the number of transplant centers that perform such procedure. This decline has led to a significant limitation among transplant surgeons and transplant physicians that are caring for patients receiving pancreas transplant. This study was to highlight the benefit of pancreas transplant in curing diabetes and to emphasize the potential benefit of pancreas transplantation in order to increase the number of diabetic patients that could receive this curative therapy.
The objective of this study was to bring pancreas transplant as a curative treatment, that could achieve glycemic control among diabetic patients, to the attention of transplant and endocrinology stakeholders. With the current technological advancement in treatment of diabetes, still a significant number of patients suffer from acute hyper and hypoglycemic events in addition to the chronic complications of diabetes. We hope that our research will at the current body of knowledge that supports pancreas transplant as a definitive treatment for diabetes and will encourage more clinical trials to compare standard of care for diabetes vs organ transplantation.
Our study utilized the United Network for Organ Sharing Standard Transplant Analysis and Research file. This database contains clinical and follow-up data for all transplants in the United States since 1988. We included all patients with type I diabetes listed for pancreas or kidney-pancreas transplant between May 31, 2008 and May 31, 2018 and compared their outcome with the patients that had type 1 diabetes and were being listed and waiting for an organ transplant.
The adjusted Cox proportional hazards models comparing survival between transplanted and non-transplanted patients in each transplant type group showed simultaneous pancreas and kidney transplant patients to exhibit a significantly reduced hazard of mortality [hazard ratio (HR) = 0.21, 95% confidence interval (CI): 0.19-0.25] compared to those not transplanted. Neither transplanted patients (HR = 8, 95%CI: 0.99-2.87) nor pancreas transplant alone patients (HR = 1.01, 95%CI: 0.53-1.95) experienced significantly different hazards of mortality compared to patients who did not receive a transplant.
Our study showed the survival advantage of simultaneous kidney and pancreas transplants compared to patients on the waiting list over the last decade. Patients who underwent pancreas transplant alone demonstrated no significant differences compared to patients who did not receive a transplant, which could highlight the importance of pancreas transplant alone despite the advancement in the technology of insulin delivery and diabetic management over the last decade.
We hope that our study will encourage future clinical trials to randomize patients between diabetic standard of care vs transplantation. Meanwhile, we are conducting further studies to address disparities among patients who are receiving pancreas transplant vs remaining on the waiting list. We are aiming to identify any barriers among minorities that could prevent their access to transplant evaluation and to receive an organ transplantation.
| 1. | Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney Int. 1995;47:1703-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 513] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 818] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 3. | Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16450] [Article Influence: 498.5] [Reference Citation Analysis (4)] |
| 4. | Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116:874-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 366] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 666] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 6. | The effect of intensive diabetes therapy on the development and progression of neuropathy. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1995;122:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 399] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | Aref A, Zayan T, Pararajasingam R, Sharma A, Halawa A. Pancreatic transplantation: Brief review of the current evidence. World J Transplant. 2019;9:81-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (2)] |
| 8. | Dean PG, Kukla A, Stegall MD, Kudva YC. Pancreas transplantation. BMJ. 2017;357:j1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | White SA, Shaw JA, Sutherland DE. Pancreas transplantation. Lancet. 2009;373:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Stratta RJ, Fridell JA, Gruessner AC, Odorico JS, Gruessner RW. Pancreas transplantation: a decade of decline. Curr Opin Organ Transplant. 2016;21:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA. 2003;290:2817-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant. 2004;4:2018-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 208] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Kandaswamy R, Skeans MA, Gustafson SK, Carrico RJ, Tyler KH, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2013 Annual Data Report: pancreas. Am J Transplant. 2015;15 Suppl 2:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Fridell JA, Rogers J, Stratta RJ. The pancreas allograft donor: current status, controversies, and challenges for the future. Clin Transplant. 2010;24:433-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Marang-van de Mheen PJ, Hilling DE, Dirkes MC, Baranski AG. Surgical injuries of pancreatic allografts during procurement. Clin Transplant. 2011;25:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Organ Procurement and Transplantation Network. Sharing UNfO. Data request instructions: STAR FILES. Available from: https://optn.transplant.hrsa.gov/data/request-data/data-request-instructions/. |
| 17. | Therneau TM. Lumley T, Elizabeth A, Cynthia C. Survival Analysis. 2013. Available from: https://cran.r-project.org/web/packages/survival/survival.pdf. |
| 18. | Benjamens S, Leemkuil M, Margreiter C, Huurman VA, Leuvenink HG, Pol RA. A steady decline in pancreas transplantation rates. Pancreatology. 2019;19:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Boggi U, Vistoli F, Andres A, Arbogast HP, Badet L, Baronti W, Bartlett ST, Benedetti E, Branchereau J, Burke GW 3rd, Buron F, Caldara R, Cardillo M, Casanova D, Cipriani F, Cooper M, Cupisti A, Davide J, Drachenberg C, de Koning EJP, Ettorre GM, Fernandez Cruz L, Fridell JA, Friend PJ, Furian L, Gaber OA, Gruessner AC, Gruessner RWG, Gunton JE, Han DJ, Iacopi S, Kauffmann EF, Kaufman D, Kenmochi T, Khambalia HA, Lai Q, Langer RM, Maffi P, Marselli L, Menichetti F, Miccoli M, Mittal S, Morelon E, Napoli N, Neri F, Oberholzer J, Odorico JS, Öllinger R, Oniscu G, Orlando G, Ortenzi M, Perosa M, Perrone VG, Pleass H, Redfield RR, Ricci C, Rigotti P, Paul Robertson R, Ross LF, Rossi M, Saudek F, Scalea JR, Schenker P, Secchi A, Socci C, Sousa Silva D, Squifflet JP, Stock PG, Stratta RJ, Terrenzio C, Uva P, Watson CJE, White SA, Marchetti P, Kandaswamy R, Berney T. First World Consensus Conference on pancreas transplantation: Part II - recommendations. Am J Transplant. 2021;21 Suppl 3:17-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1889] [Cited by in RCA: 1826] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 21. | Mohler ER 3rd. Peripheral arterial disease: identification and implications. Arch Intern Med. 2003;163:2306-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, Easton JD, Gavin JR 3rd, Greenland P, Hankey G, Hanrath P, Hirsch AT, Meyer J, Smith SC, Sullivan F, Weber MA; Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003;163:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 406] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Mueller T, Hinterreiter F, Luft C, Poelz W, Haltmayer M, Dieplinger B. Mortality rates and mortality predictors in patients with symptomatic peripheral artery disease stratified according to age and diabetes. J Vasc Surg. 2014;59:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, Agodoa LY, Cruess DF, Kimmel PL. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Foster BJ, Mitsnefes MM, Dahhou M, Zhang X, Laskin BL. Changes in Excess Mortality from End Stage Renal Disease in the United States from 1995 to 2013. Clin J Am Soc Nephrol. 2018;13:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Zimmerman C, Albanese-O'Neill A, Haller MJ. Advances in Type 1 Diabetes Technology Over the Last Decade. Eur Endocrinol. 2019;15:70-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Jarmi T, Thao V, Borah BJ, Brennan E, Moriarty JP, Spaulding AC. Comparing Outcomes and Cost Between Pancreas Transplant and Standard of Care in Patients With Type 1 Diabetes. Pancreas. 2022;51:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atapattu N; Emran TB, Bangladesh S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Xu ZH