Published online Aug 18, 2022. doi: 10.5500/wjt.v12.i8.268
Peer-review started: January 7, 2022
First decision: March 9, 2022
Revised: March 24, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: August 18, 2022
Processing time: 223 Days and 0.9 Hours
Patients with a history of solid organ transplantation (SOT) or hematopoietic stem cell transplantation (HSCT) are at an increased risk of developing post-transplant lymphoproliferative disorder (PTLD). The gastrointestinal (GI) tract is commonly affected as it has an abundance of B and T cells.
To determine typical GI-manifestations, risk factors for developing PTLD, and management.
Major databases were searched until November 2021.
Non-case report studies that described GI manifestations of PTLD, risk factors for developing PTLD, and management of PTLD were included. Nine articles written within the last 20 years were included in the review. All articles found that patients with a history of SOT, regardless of transplanted organ, have a prope
GI tract manifestations may be nonspecific; therefore, consideration of risk factors is crucial for identifying GI-PTLD. Like other lymphoma variants, PTLD is very aggressive making early diagnosis key to prognosis. Initial treatment is reduction of immunosuppression which is effective in more than 50% of cases; however, additional therapy including rituximab, chemotherapy, and surgery may also be required.
Core Tip: Patients with a history of solid-organ or hematopoietic stem cell transplantation are at an increased risk of developing post-transplant lymphoproliferative disorder (PTLD). The gastrointestinal (GI) tract is commonly affected as it has an abundance of B and T-cells. GI tract manifestations may be nonspecific; therefore, consideration of risk factors is crucial for identifying GI-PTLD. Like other lymphoma variants, PTLD is very aggressive making early diagnosis key to prognosis. Initial treatment is reduction of immunosuppression which is effective in more than 50% of cases; however, additional therapy including surgery and chemotherapy may also be required. We performed a systematic review of GI-PTLD to better describe GI manifestations, risk factors for disease, and management of GI-PTLD.
- Citation: Reiche W, Tauseef A, Sabri A, Mirza M, Cantu D, Silberstein P, Chandan S. Gastrointestinal manifestations, risk factors, and management in patients with post-transplant lymphoproliferative disorder: A systematic review. World J Transplant 2022; 12(8): 268-280
- URL: https://www.wjgnet.com/2220-3230/full/v12/i8/268.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i8.268
While primary and secondary lymphoid neoplasms only constitute 1%-4% of all gastrointestinal (GI) malignancies[1,2]; post-transplant lymphoproliferative disorder (PTLD) is one of the most common post-transplant malignancies within the GI tract. PTLD is a lymphoma variant which can manifest in patients having solid organ transplantation (SOT) or hematopoietic stem cell transplantation (HSCT). Patients with a history of SOT are at increased risk of developing PTLD which may be more prone to develop in the GI tract. Review of the typical GI symptoms and timing of symptom development will be invaluable to the clinician caring for patients with a transplantation history, especially as this patient population continues to grow. Risk factors for developing PTLD are important to identify as PTLD can present in a myriad of ways and clinical suspicion greatly aids in timely evaluation and treatment for PTLD. We performed a review of the GI manifestations of PTLD. We described risk factors associated with the development of PTLD. Additionally, we reviewed the management of patients diagnosed with PTLD and associated complications.
This review has been in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA)[5].
An expert librarian conducted a systematic literature search using a priori protocol to identify studies reporting on GI-PTLD manifestations, risk factors for the development of GI-PTLD, and management of GI-PTLD. The search strategies included “gastrointestinal manifestations”, “risk factors”, “manage
This systematic review included studies that evaluated GI manifestations of PTLD. Studies were included irrespective of primary organ transplantation. Information was gathered from nine of the most relevant articles pertaining to GI-PTLD. Additional studies were incorporated to provide background on PTLD manifestations, risk factors, imaging, treatment, and outcomes. Studies reporting performance in pediatric age groups (< 18 years), conference abstracts, and non-English studies were excluded. Studies were restricted to full text. Two authors decided on the final selection (Reiche W, Tauseef A). Details presented in PRISMA flow diagram (Supplementary Figure 1).
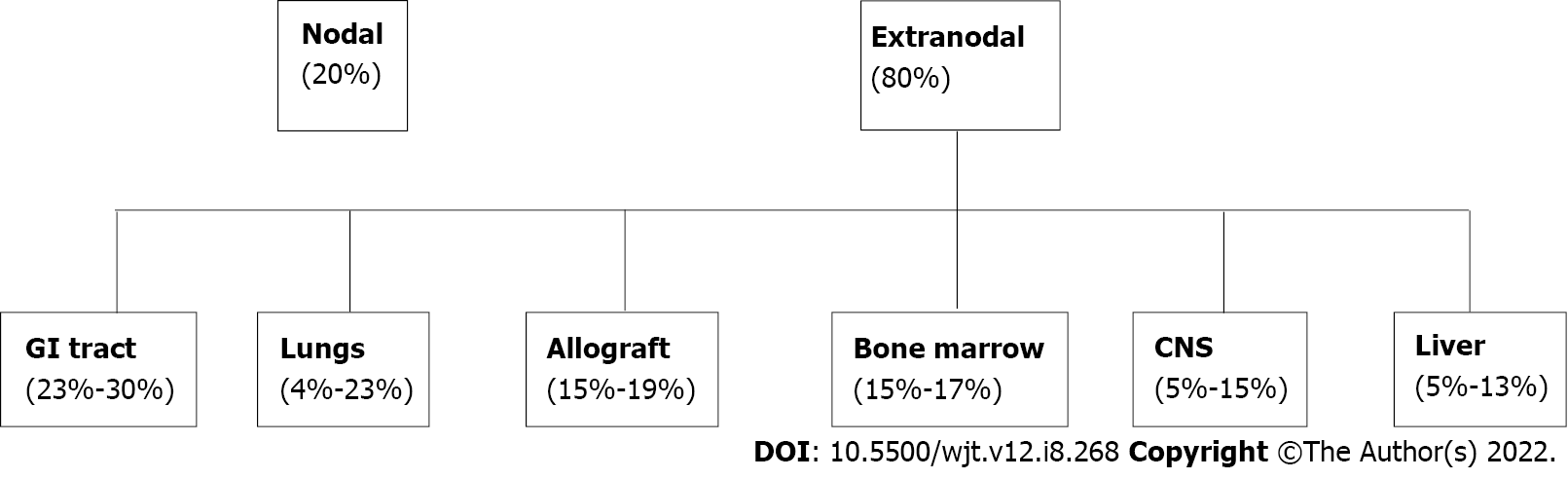
PTLD can manifest as nodal or more commonly as extranodal disease occurring in solid organ tissue outside of lymph nodes[6]. The most involved extranodal sites are the GI tract (23%-30%), lungs (4%-23%), bone marrow (15%-17%), central nervous system (5%-15%), liver (5%-13%), and the allograft itself (15%-19%)[7-9] in Figure 1. The GI tract is one of the most affected organs due to the preponderance of B and T lymphocytes which are prone to develop malignant change[10]. Patients with GI-PTLD usually present with nonspecific constitutional symptoms including fatigue, fever, night sweats, lymphadenopathy, and weight loss[11,12]. Not uncommonly, patients may also have nausea, vomiting, abdominal pain, abdominal fullness, diarrhea or increased ostomy output, and occult or evident bleeding. PTLD may present as a small bowel obstruction, GI bleeding, gastric or intestinal perforation, or obstruction[13].
One study evaluating the location of PTLD found the stomach was one of the most common sites of involvement. Out of a total of 472 patients, 56 patients (11.9%) had gastric PTLD while 415 patients (88.1%) had PTLD in other locations[13] (Table 1). The small bowel is another common area of involvement, PTLD of the small bowel was diagnosed in 50% of patients having PTLD after small bowel transplantation (SBT)[14]. In patients requiring surgery for GI-PTLD complications, organ involvement varied: Small bowel (50%), proximal right colon (31.2%), and stomach, duodenum, and transverse colon (6.2%)[15].
| Ref. | Localization of PTLD | Time from transplant to PTLD (yr) | Classification | |
| Monomorphic | Polymorphic | |||
| Wozniak et al[17] | Small bowel: 9/19 | 7.4 | 9/19 | 10/19 |
| Colorectal: 3/19 | ||||
| Liver: 2/19 | ||||
| Koo et al[14] | Small bowel: 11/12 | 2.7 | 1/12 | 8/12 |
| Colorectal: 1/12 | ||||
| Khedmat et al[13] | Stomach + small bowel: 13/45 | 4.1 | 23/39 | 13/39 |
| Stomach + pancreas: 3/45 | ||||
| Stomach + liver: 7/45 | ||||
| Stomach: 56/472 | ||||
| Khedmat et al[16] | Colorectal + liver: 10/73 | 4.1 | 36/57 | 18/57 |
| Colorectal + small bowel: 22/73 | ||||
| Colorectal + stomach: 2/73 | ||||
| Colorectal: 81/563 | ||||
| Cruz Jr et al[15] | Colorectal: 6/17 | 7.2 | 13/17 | 3/17 |
| Small bowel: 11/17 | ||||
| Ganne et al[18] | Small bowel: 1/8 | 4.8 | 0/2 | 2/2 |
| Stomach: 1/8 | ||||
SOT or HSCT are known risk factors for developing lymphoma or other lymphoproliferative disorders[11]. While studies have shown GI-PTLD can develop after most types of transplant, the incidence of PTLD after intestinal transplantation was determined to be higher than other types of SOT[16]. The mean time to PTLD varies and is dependent on host factors and transplant type. One study found the mean time for development of PTLD is 1 year for patients having HSCT, while the time to PTLD presentation may be up to 7 years after SOT[11,16-18]. The mean interval from transplantation to PTLD diagnosis after SBT was 2.7 years[14]. After liver transplantation, the average time from transplantation to diagnosis of PTLD was 7.2 years[15].
Induction and maintenance regimens are selected based on the risk of acute organ rejection associated with the transplant. T-cell depleting therapy (recombinant anti-thymocyte globulin), interleukin-2 receptor subunit alpha (IL2RA), or no immunosuppression may be used for induction therapy. For instance, for adult heart transplants, T-cell depleting therapy is most commonly used for induction; however half of transplant patients do not receive induction[19]. In lung transplants, induction therapy is used nearly 80% of the time and most commonly IL2RA are used[20,21]. For kidney transplants, induction therapy is provided 90% of the time and is usually T-cell depleting therapy[22,23]. Most commonly, induction is not used after liver transplant[24,25]. For pancreas transplant induction, T-cell depleting therapy is most commonly used (90%)[26]. Lastly, intestinal transplant induction is usually comprised of T-cell depleting agents (63.9%) or no induction (27.8%)[27]. Current trends in maintenance immunosuppression therapy for pancreas, heart, lung, kidney, liver, intestinal transplants are as follows: Pancreas transplants most often use tacrolimus, mycophenolate mofetil (MMF) and nearly 70% of patients are on corticosteroids[26]. Heart transplant maintenance therapy most often includes tacrolimus and MMF and corticosteroids are used nearly 50% of the time[19]. Lung transplants typically are treated with tacrolimus, MMF, and corticosteroids (80%)[21]. Kidney transplants are either treated with tacrolimus, MMF, and corticosteroids (54.1%) or tacrolimus and MMF (36.8%)[22,23]. Liver transplants are typically treated with tacrolimus, MMF, and steroids in 65% of patients[24,25]. Intestinal transplants are treated with tacrolimus (73%) and corticosteroids may be used (37.4%)[28].
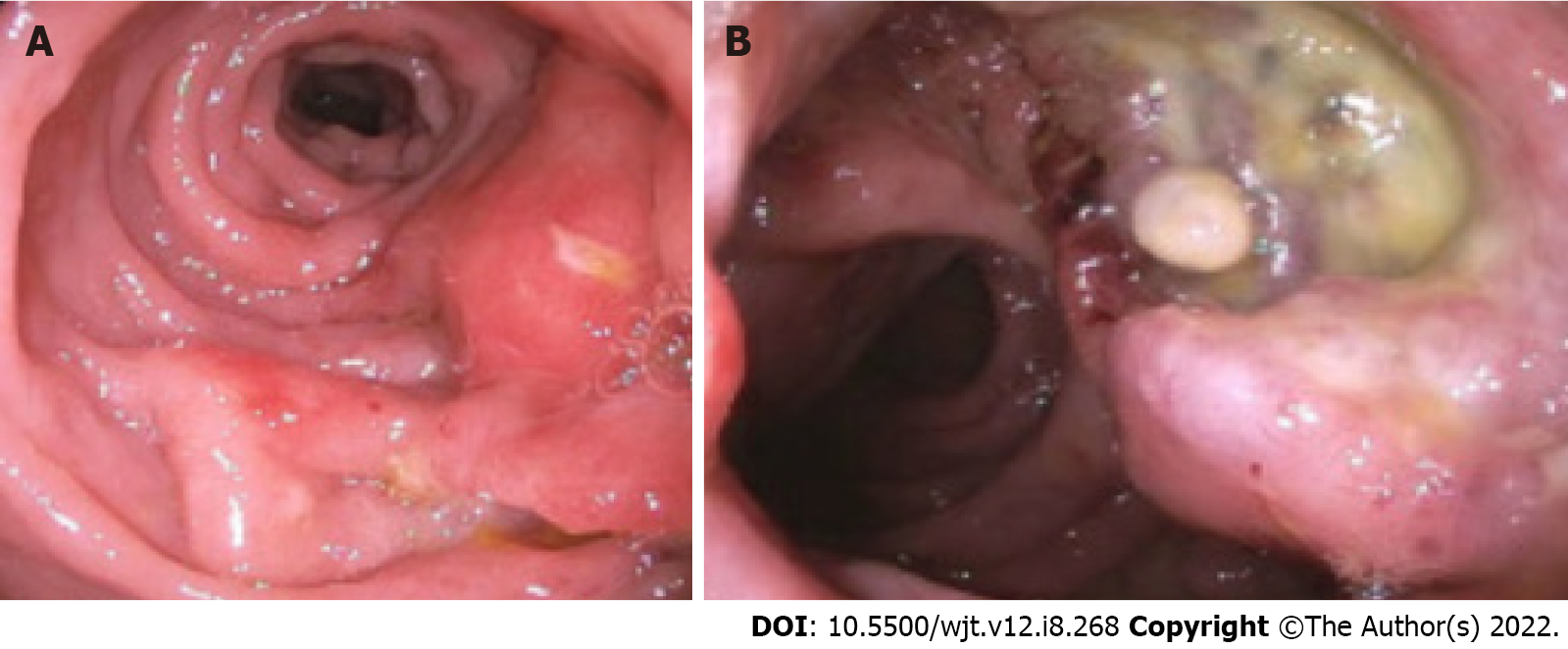
Imaging findings of GI-PTLD are variable and can appear as wall thickening, dilatation, an eccentric or exophytic mass, luminal ulceration, short segment intussusception, and soft tissue nodules in the peritoneum (Figure 2)[12]. Solid organ involvement is usually in the form of infiltrating lesions appearing as a solitary or a multi-nodular mass[17]. Additional risk factors for developing GI-PTLD include induction immunosuppression, prolonged duration of immunosuppression, younger age, fewer human leukocyte antigen matches, use of anti-lymphocyte antibodies, prior splenectomy, cytomegalovirus (CMV), Epstein-Barr virus (EBV), hepatitis C, and human herpesvirus 8 (HHV-8)[10,12,15,17,29,30] (Table 2). EBV is the most common risk factor for developing PTLD, risk is higher in recipients who are initially seronegative but develop positivity after transplantation[15,31,32]. EBV can be transmitted via the graft; however, non-leukoreduced blood products also have the potential to transmit EBV[31]. EBV is present in 60%-70% of patients diagnosed with PTLD[30]. CMV can increase the likelihood of developing PTLD by seven times[33,34]. Hepatitis C and HHV-8 are also risk factors for developing PTLD especially when patients have EBV seropositivity[33]. If more than one risk factor is present, there appears to be cumulative risk[35].
| Ref. | Noted findings regarding GI-PTLD |
| Plummer et al[11] | PTLD presentation is non-specific. Prognosis is variable dependent on burden of disease, age at the time of diagnosis, and morphological subtype |
| Small et al[7] | EBV infection is crucial in the pathophysiology of PTLD. EBV+ patients are more likely to respond to RIS. Chemotherapy can be utilized after RIS if RIS appears unsuccessful |
| Dako et al[12] | Imaging of PTLD involving GI tract is variable. Imaging of PTLD may appear as a large mass, luminal ulceration, intussusception, or soft tissue nodules |
| Wozniak et al[17] | Risk of acute cellular rejection increased when treatment for PTLD occurred. Notable risk factors for PTLD include chronic immunosuppression, viral infection, and increased time from transplantation |
| Koo et al[14] | Incidence rate of PTLD after small bowel transplantation was up to 50% |
| Khedmat et al[13] | Clinical presentation of PTLD is nonspecific. Early treatment with RIS, rituximab, chemotherapy, or surgical therapy, if indicated, can decrease mortality rates |
| Khedmat et al[16] | Patients with PTLD and colorectal symptoms were noted to have a higher risk of metastatic disease. Colorectal PTLD may occur more frequently and may be more aggressive in men compared to women. Multi-organ failure may be more common in men compared to women if there is colorectal PTLD |
| Cruz Jr et al[15] | Surgical intervention uncommonly required for PTLD. Most common surgical need is for intestinal obstruction |
| Ganne et al[18] | PTLD was found to respond to rituximab irrespective of EBV status. Patients with higher EBV titers usually benefited from combination RIS, rituximab, and CHOP therapy. EBV-specific donor cytotoxic lymphocyte infusions may be effective but may lead to graft rejection. GI bleeding may be a presenting feature of disease |
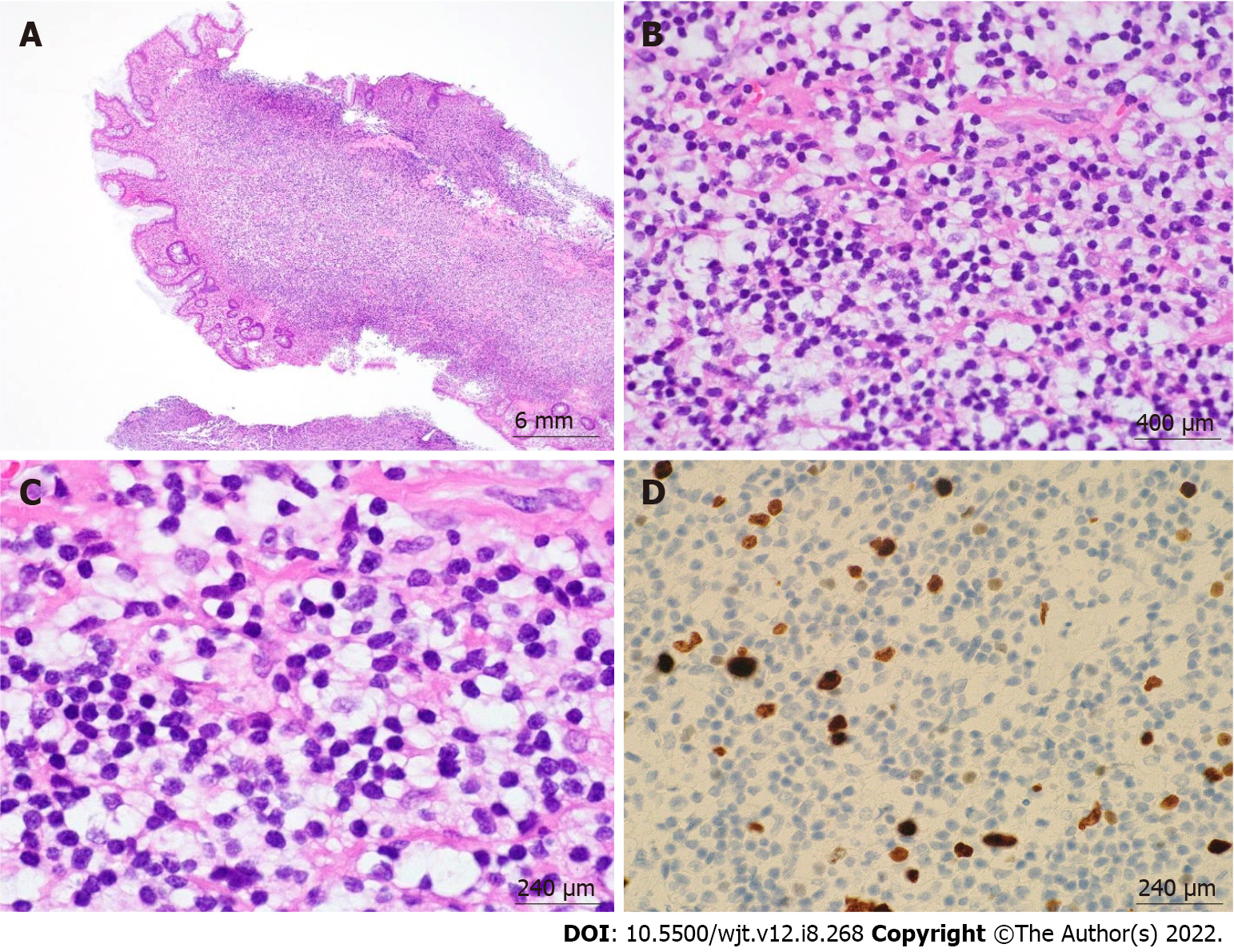
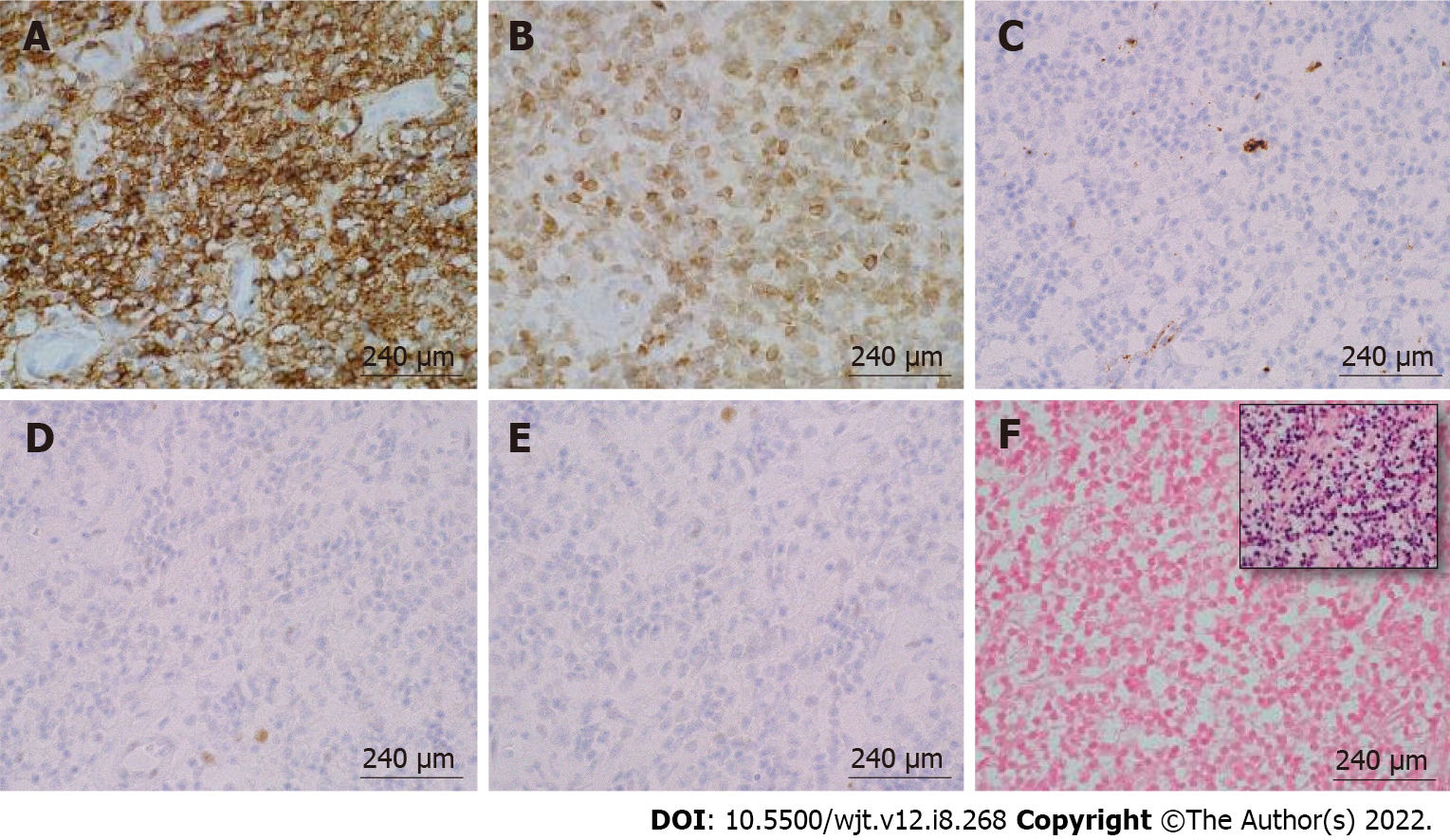
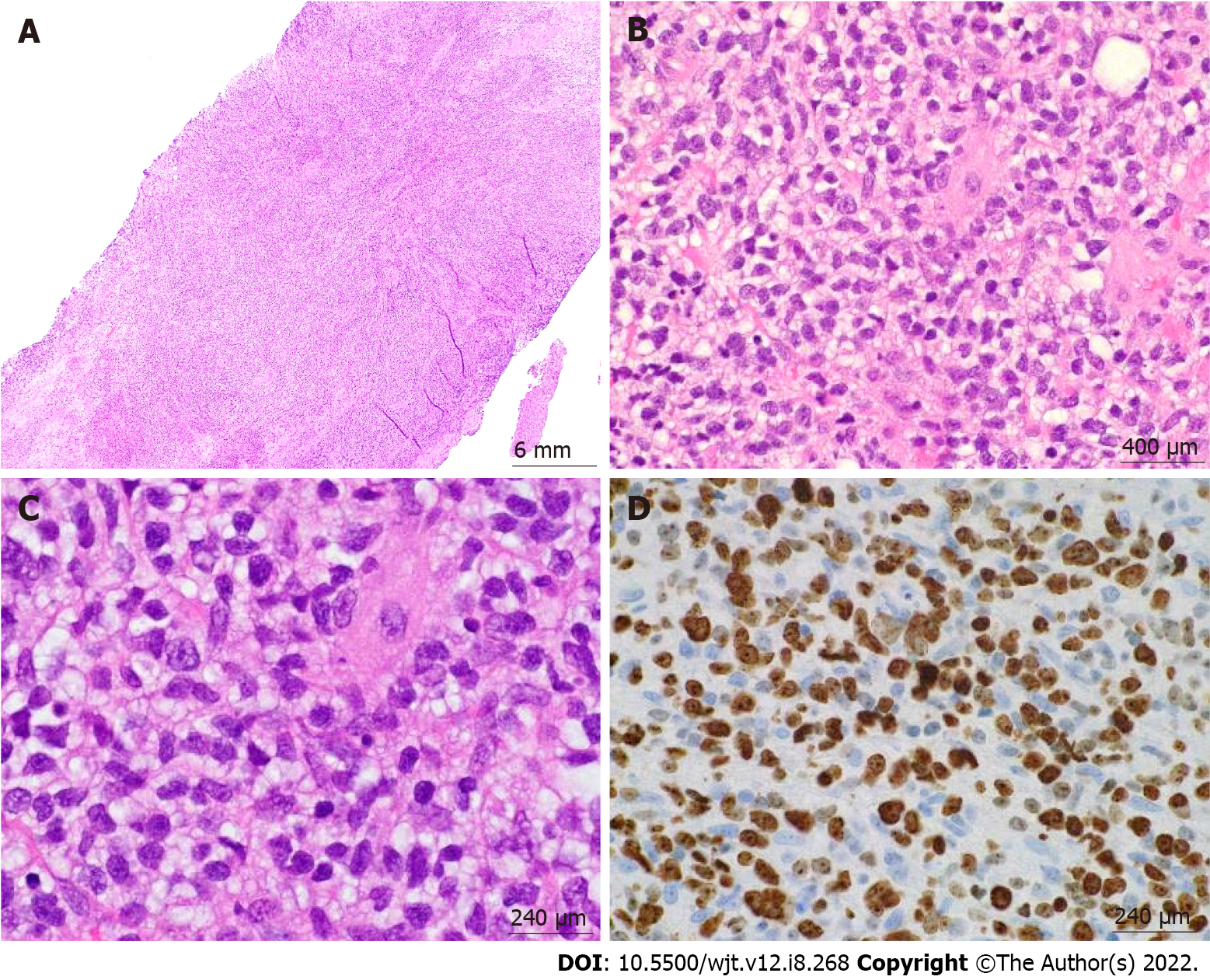
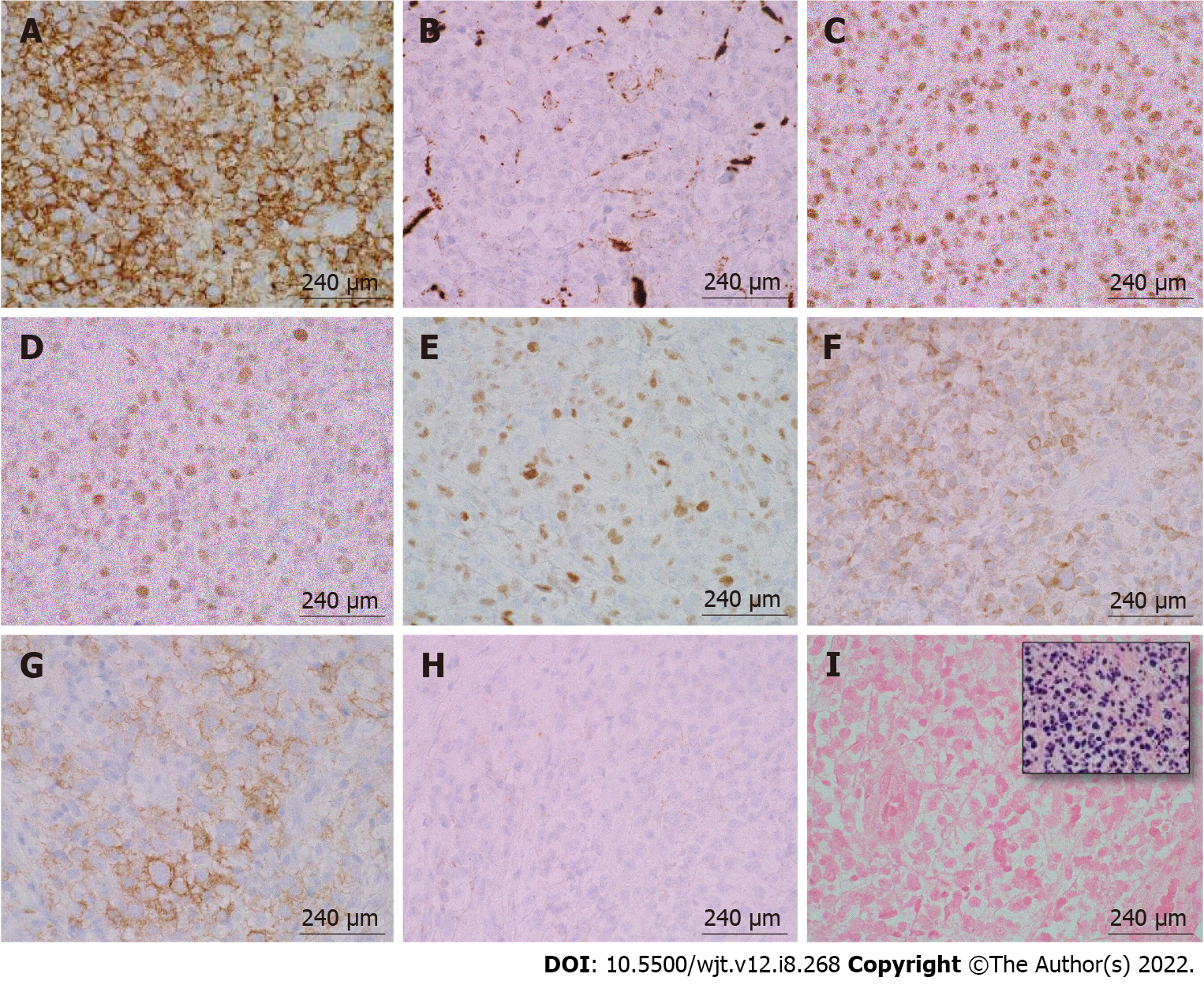
According to the revised 2017 World Health Organization classification of tumors of hematopoietic and lymphoid tissues, PTLD is categorized into four major groups based on morphologic pattern: Non-destructive PTLD, monomorphic PTLD, polymorphic PTLD, and classic Hodgkin lymphoma PTLD. Apart from the polymorphic group, all other groups are further sub-categorized. Non-destructive PTLD are usually EBV-positive and are characterized by architectural preservation of the involved tissue without features suggestive of malignant lymphoma. The subcategories for non-destructive PTLD include plasmacytic hyperplasia, florid follicular hyperplasia, and infectious mononucleosis PTLD. Monomorphic or polymorphic PTLD may follow non-destructive PTLD lesions; however, most non-destructive PTLD have polyclonal B-cells. Polymorphic-PTLD are characterized by a heterogenous population that includes immunoblasts, plasma cells, and small to moderate sized lymphoid cells that efface the architecture of lymph nodes or may form destructive lesions but do not fulfill the criteria for lymphoma. Most cases of polymorphic-PTLD are EBV-positive. Monomorphic PTLD comprise 60%-80% of all PTLD and fulfill criteria for B-cell or T/natural killer-cell neoplasms (Figures 3-6). The least common form of PTLD are the classic Hodgkin lymphoma PTLD which are almost always EBV-positive[36].
Distinction is often made between early PTLD and late PTLD. The former more often associated with EBV positivity and graft involvement while less commonly associated with monomorphic morphology and less often presenting as extranodal disease[8]. Treatment has not been found to differ based on this categorization[29,37,38]. Studies comparing early vs late PTLD have not shown a significant difference in survival[39,40]. Determination of EBV status is a crucial first step after the diagnosis of GI-PTLD has been made. EBV-specific cytotoxic T-cell immunity or donor lymphocyte infusions have been used as second line therapies if reduction of immunosuppression (RIS) or rituximab is not working, patients with EBV may be more responsive to RIS than patients without EBV[7,18]. However, there is no approved treatment in the United States or Europe. Several studies have failed to show improvement with antivirals alone in instances when patients have EBV and PTLD[29,31,,40,,41,].
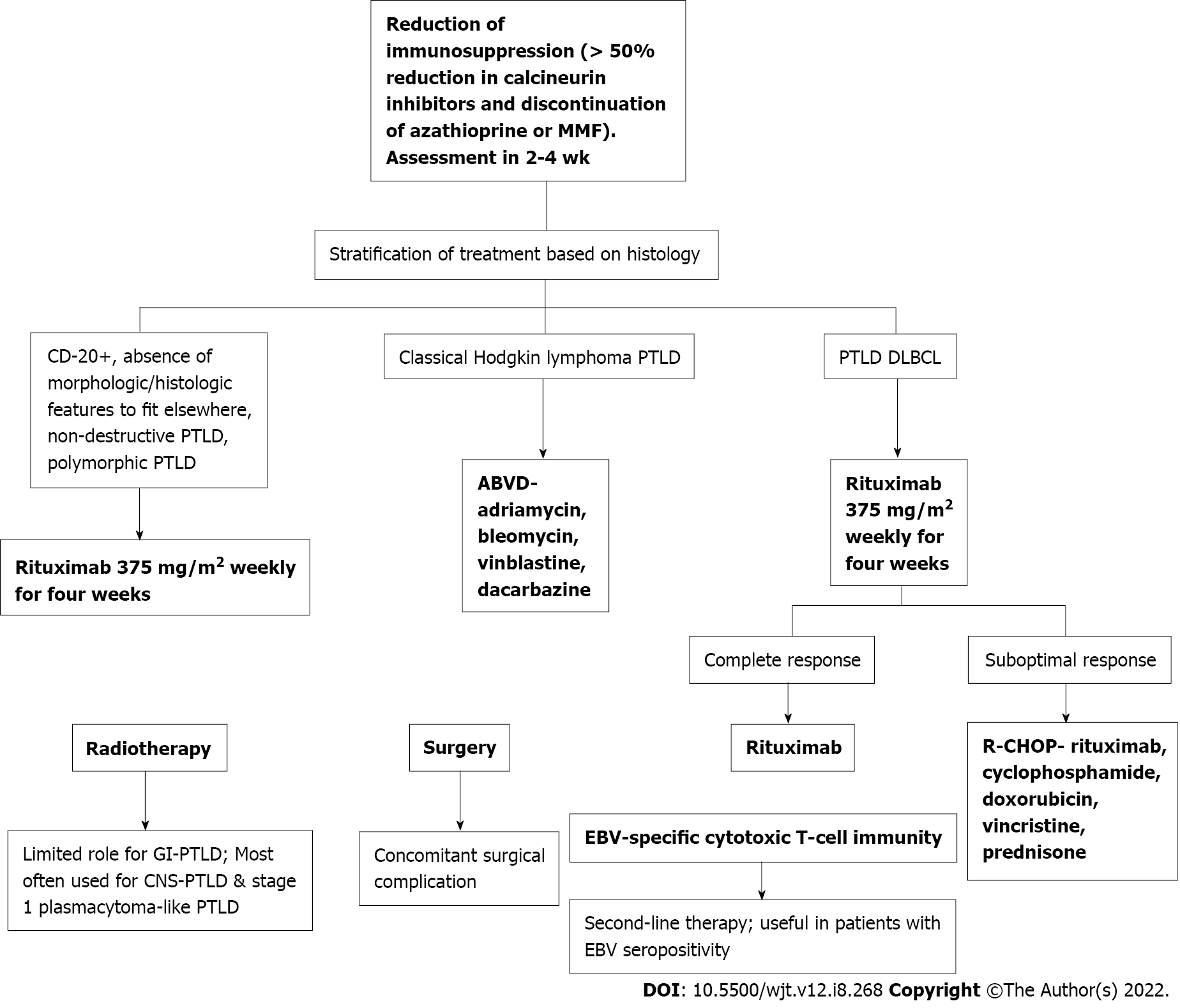
Once GI-PTLD diagnosis has been confirmed with endoscopic biopsy, patients can be managed with RIS, chemotherapy, and surgical intervention for complications[11]. The most important first step in treatment is RIS[11,15]. Immunosuppressant therapy is usually decreased to 50% for calcineurin-inhibitors (cyclosporine, tacrolimus) and MMF or azathioprine, if also prescribed, are discontinued[42]. In the largest study to date evaluating the efficacy of standard RIS, response was nearly 45%. Rates of up to 80% have been reported[31]. More than 70% of the time, RIS will be efficacious regardless of PTLD subtype, EBV status, and early vs late disease. RIS may not be sufficient in monomorphic PTLD[43]. RIS may not work if the disease is bulky, the cancer stage is severe, if multi-organ dysfunction is present, if quick treatment is needed, or for older adults[33,44]. Although beneficial as the first step in manage
If RIS is not sufficient, patients should be considered for antiviral therapy, rituximab, and chemotherapy[7]. Treatment is dependent on the PTLD subtype. Classical Hodgkin lymphoma PTLD is treated with standard adriamycin, bleomycin, vinblastine, and dacarbazine. Patients with PTLD diffuse large B-cell lymphoma type are treated according to the PTLD-1 trial with rituximab induction (weekly rituximab for four weeks) followed by stratification based on response. Patients in clinical remission may be treated with maintenance rituximab weekly for 4 wk. Patients with a suboptimal response may be treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (Figure 7)[45].
Rituximab has been found to be an effective therapy for PTLD. In one study including 8 patients with PTLD after SOT, complete resolution of PTLD was observed in 7 cases. Rituximab was administered at a dose of 375 mg/m2 once a week for four consecutive weeks. Additionally, this study found patients with PTLD usually respond to anti-CD20 monoclonal antibodies irrespective of EBV status[18]. Radiotherapy has been found to have a favorable effect in stage 1 plasmacytoma-like PTLD; however, it is infrequently used for solitary PTLD. Radiotherapy is most often utilized in treatment for central nervous system PTLD[45].
Surgery should be considered in patients who develop GI complications including perforation, hemorrhage, and most commonly intestinal obstruction. Surgical resection is rarely considered in patients as PTLD tends to be multi-focal. A retrospective review of 5677 patients after isolated liver transplantation found only 16 patients developed post-transplantation GI complications associated with PTLD requiring surgical intervention. Overall mortality in this cohort was 69% and most patients died within the first year of explorative laparotomy. This same study found initial mortality higher in patients receiving surgery; however, long-term outcomes do not appear to be affected[15]. Prognosis is dependent on burden of disease, location of PTLD, morphological subtype, and other patient-related factors[11]. Once present, PTLD progression is aggressive; however, early appropriate treatment can decrease mortality rates. In one study comparing gastric PTLD and non-gastric PTLD, patients developing GI-PTLD had survival rates of 71% and 54% at one and five years, respectively[13].
Mortality rates in patients requiring surgery compared to rituximab and chemotherapy found no significant difference between treatment type. Mortality associated with surgical treatment was 16%, like that observed in patients who received rituximab. While mortality rates in patients treated with chemotherapy and radiotherapy with interferon alfa were 42.6% and 33%, respectively[13]. A favorable response to treatment has been noted in EBV-positive patients as they were more responsive to RIS compared to EBV-negative patients[29]. Similarly, a favorable outcome was also noted in patients who had localization to the stomach[13].
Conversely, colorectal involvement has been associated with a more severe disease presentation than PTLD involving non-colorectal sites. In one study, 75% of patients who developed colorectal symptoms had multi-organ involvement, significantly higher than the control group[16]. This same study found colorectal involvement was more likely in men. Male transplant patients developed colorectal PTLD more often than women 19.3% to 8.5%, respectively. Similarly, male transplant patients had a significantly shorter time from transplantation to diagnosis of the disease.
PTLD should be considered in patients with a history of SOT or HSCT as the large resident lymphocyte population in the GI tract has increased potential to develop malignancy. PTLD should be suspected to occur sooner after HSCT, within 1 year, and on average 4 to 5 years after SOT. However, there are multiple factors which appear to have a role in the time to development such as level of immunosuppression and presence of concomitant disease. Transplant type also appears to impact time to development as induction, maintenance, and the extent of inherent lymphoid tissue in the graft all contribute to the relative risk of developing PTLD. For instance, PTLD occurred sooner on average after small bowel transplant and later for liver transplant; in the studies reviewed, there was an approximate 4.5-year difference in time to onset of PTLD. Induction therapy, associated with increased risk of PTLD, is less frequently used after liver transplant while it is commonly used after intestinal transplants. The small bowel also has a greater supply of lymphoid tissue compared to the liver.
Diagnosis of PTLD can be problematic as the clinical spectrum and diagnostic testing are nonspecific. The illness script of PTLD is highly variable ranging from nonspecific abdominal symptoms to overt hemorrhage, perforation, or obstruction. The stomach and small intestine are the most frequently involved organs in the GI tract making clinical questioning and inquiry of symptoms which may represent pathology in these organs important. Imaging findings of PTLD in the GI tract may range from focal intraluminal disease to perforation or metastatic disease. The various clinical presentations and wide-ranging imaging findings make it difficult to specifically identify PTLD by clinical presentation or imaging alone.
Consideration of PTLD should increase in patients with risk factors. Most importantly determination of EBV-status and risk factors for EBV infection need to be determined. EBV infection has been noted to increase the risk of PTLD by 6-76 times[46]. As mentioned previously, elucidation of details surrounding the transplant including transplant type, determination of RIS regimen including whether induction therapy was utilized are important. As transplantation continues to increase, so will the number of patients at risk for development of PTLD[14,19,22,23,25].
Like other lymphomas, PTLD is aggressive and mortality rates improve with early treatment. Prognosis and treatment are dependent on time of disease presentation, morphological subtype of PTLD, and concomitant systemic disease. The most important step in management is RIS; which is usually efficacious. Subsequently, rituximab and chemotherapy based on morphologic subtype have been found to be effective[18]. Differences in outcomes between surgery and treatment with rituximab are not well elucidated, nor is the role of endoscopy in management of PTLD. Broadly, treatment must consider both the risk of acute graft rejection and worsening lymphoproliferative disorder.
This study is a systematic review elucidating GI manifestations, associations, and management of GI-PTLD. Key points after review of the included studies are the presentation, imaging, and direct appearance of GI-PTLD is highly variable making clinical suspicion essential for timely diagnosis. Patients with nonspecific GI symptoms, and history of organ transplantation, should be evaluated for GI-PTLD. Early detection is key for prognosis. Lastly, treatment is dependent on several factors and may include RIS, rituximab, chemotherapy, surgery, or a combination of these interventions. Initial treatment is intuitive and technically easy; however, RIS can be associated with acute graft rejections.
Post-transplant lymphoproliferative disorder (PTLD) is one of the most common post-transplant malignancies within the gastrointestinal (GI) tract. PTLD is a lymphoma variant which can manifest in patients having solid organ transplantation (SOT) or hematopoietic stem cell transplantation (HSCT).
The current understanding of GI manifestations of PTLD including timing to development, risk factors for development, and treatment is limited by small sample size. Previous studies have noted a propensity for the GI tract to develop PTLD; therefore, more information regarding when it may develop, how it manifests, and treatments are needed especially as transplantation becomes more prevalent.
To identify the timing and clinical presentation of GI-PTLD, risk factors for its development, and treatment.
We performed a systematic review after an extensive literature search.
The timing of GI-PTLD is variable but on average develops 4-5 years following SOT and may occur within 1 year after HSCT. Presentation may be insidious including nonspecific abdominal discomfort to fulminant hemorrhage, perforation, or obstruction. GI-PTLD is most likely to develop in the small intestine and stomach. Transplant type, level of induction and maintenance immunosuppression, Epstein-Barr virus-status among other risk factors increase the likelihood one may develop PTLD. PTLD is aggressive and mortality improves with early treatment which is dependent on extent of disease, and morphological subtype. The most important step of therapy is reduction of immunosuppression (RIS) which usually is effective.
The presentation, imaging, and direct appearance of GI-PTLD is highly variable making clinical suspicion key for diagnosis. Early detection is key for prognosis; therefore, consideration of risk factors is essential. Treatment is dependent on several factors and may include RIS, rituximab, chemotherapy, surgery, or a combination of these interventions. Initial treatment is intuitive and technically easy; however, RIS can be associated with acute graft rejections.
This study suggests ascertainment of risk factors is crucial for increasing clinical suspicion when assessing patients who may have GI-PTLD. The clinical and radiological presentation of GI-PTLD is highly variable; therefore, a high index of suspicion for GI-PTLD must be maintained so that early endoscopic diagnosis may allow for targeted treatment. Future prospective studies are needed to better elucidate incidence rates of GI-PTLD and the role of endoscopy in treatment.
| 1. | Loehr WJ, Mujahed Z, Zahn FD, Gray GF, Thorbjarnarson B. Primary lymphoma of the gastrointestinal tract: a review of 100 cases. Ann Surg. 1969;170:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 161] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | McSwain B, Beal JM. Lymphosarcoma of the Gastro-Intestinal Tract: Report of Twenty Cases. Ann Surg. 1944;119:108-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 3. | Lahon B, Mordant P, Thabut G, Georger JF, Dauriat G, Mal H, Lesèche G, Castier Y. Early severe digestive complications after lung transplantation. Eur J Cardiothorac Surg. 2011;40:1419-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Timrott K, Vondran FW, Jaeger MD, Gottlieb J, Klempnauer J, Becker T. Incidence and outcome of abdominal surgical interventions following lung transplantation--a single center experience. Langenbecks Arch Surg. 2011;396:1231-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 18002] [Article Influence: 1058.9] [Reference Citation Analysis (1)] |
| 6. | Kinch A, Baecklund E, Backlin C, Ekman T, Molin D, Tufveson G, Fernberg P, Sundström C, Pauksens K, Enblad G. A population-based study of 135 Lymphomas after solid organ transplantation: The role of Epstein-Barr virus, hepatitis C and diffuse large B-cell lymphoma subtype in clinical presentation and survival. Acta Oncol. 2014;53:669-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Small S, Barnea Slonim L, Williams C, Karmali R. B Cell Lymphomas of the GI Tract. Curr Gastroenterol Rep. 2021;23:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Gupta D, Mendonca S, Chakraborty S, Chatterjee T. Post Transplant Lymphoproliferative Disorder. Indian J Hematol Blood Transfus. 2020;36:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Dierickx D, Tousseyn T, Sagaert X, Fieuws S, Wlodarska I, Morscio J, Brepoels L, Kuypers D, Vanhaecke J, Nevens F, Verleden G, Van Damme-Lombaerts R, Renard M, Pirenne J, De Wolf-Peeters C, Verhoef G. Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. 2013;54:2433-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Neuringer IP. Posttransplant lymphoproliferative disease after lung transplantation. Clin Dev Immunol. 2013;2013:430209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Plummer RM, Linden MA, Beckman AK. Update on B-cell lymphoproliferative disorders of the gastrointestinal tract. Semin Diagn Pathol. 2021;38:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Dako F, Hota P, Kahn M, Kumaran M, Agosto O. Post-lung transplantation abdominopelvic complications: the role of multimodal imaging. Abdom Radiol (NY). 2020;45:1202-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Khedmat H, Ghamar-Chehreh ME, Amini M, Agah S, Taheri S. Localization of post-transplant lymphoproliferative disorders to the stomach might be associated with favorable outcome: a systematic review. Saudi J Kidney Dis Transpl. 2014;25:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Koo J, Dawson DW, Dry S, French SW, Naini BV, Wang HL. Allograft biopsy findings in patients with small bowel transplantation. Clin Transplant. 2016;30:1433-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Cruz RJ Jr, Ramachandra S, Sasatomi E, DiMartini A, de Vera M, Fontes P, Hughes C, Humar A. Surgical management of gastrointestinal posttransplant lymphoproliferative disorders in liver transplant recipients. Transplantation. 2012;94:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Khedmat H, Amini M, Ghamar-Chehreh ME. Colorectal involvement by post-transplant lymphoproliferative disorders: a review of 81 cases. Saudi J Kidney Dis Transpl. 2014;25:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Wozniak LJ, Mauer TL, Venick RS, Said JW, Kao RL, Kempert P, Marcus EA, Hwang V, Cheng EY, Busuttil RW, McDiarmid SV, Farmer DG. Clinical characteristics and outcomes of PTLD following intestinal transplantation. Clin Transplant. 2018;32:e13313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Ganne V, Siddiqi N, Kamaplath B, Chang CC, Cohen EP, Bresnahan BA, Hariharan S. Humanized anti-CD20 monoclonal antibody (Rituximab) treatment for post-transplant lymphoproliferative disorder. Clin Transplant. 2003;17:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Colvin M, Smith JM, Ahn Y, Skeans MA, Messick E, Bradbrook K, Gauntt K, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2020 Annual Data Report: Heart. Am J Transplant. 2022;22 Suppl 2:350-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 20. | Valapour M, Lehr CJ, Skeans MA, Smith JM, Uccellini K, Goff R, Foutz J, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Lung. Am J Transplant. 2020;20 Suppl s1:427-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 21. | Valapour M, Lehr CJ, Skeans MA, Smith JM, Miller E, Goff R, Mupfudze T, Gauntt K, Snyder JJ. OPTN/SRTR 2020 Annual Data Report: Lung. Am J Transplant. 2022;22 Suppl 2:438-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 22. | Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, Foutz J, Wainright JL, Snyder JJ, Kasiske BL, Israni AK. OPTN/SRTR 2018 Annual Data Report: Kidney. Am J Transplant. 2020;20 Suppl s1:20-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 323] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 23. | Lentine KL, Smith JM, Hart A, Miller J, Skeans MA, Larkin L, Robinson A, Gauntt K, Israni AK, Hirose R, Snyder JJ. OPTN/SRTR 2020 Annual Data Report: Kidney. Am J Transplant. 2022;22 Suppl 2:21-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 24. | Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20 Suppl s1:193-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 321] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 25. | Kwong AJ, Ebel NH, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Foutz J, Gauntt K, Cafarella M, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2020 Annual Data Report: Liver. Am J Transplant. 2022;22 Suppl 2:204-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 284] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 26. | Kandaswamy R, Stock PG, Miller J, White J, Booker SE, Israni AK, Snyder JJ. OPTN/SRTR 2020 Annual Data Report: Pancreas. Am J Transplant. 2022;22 Suppl 2:137-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Smith JM, Weaver T, Skeans MA, Horslen SP, Miller E, Noreen SM, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Intestine. Am J Transplant. 2020;20 Suppl s1:300-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Horslen SP, Smith JM, Weaver T, Cafarella M, Foutz J. OPTN/SRTR 2020 Annual Data Report: Intestine. Am J Transplant. 2022;22 Suppl 2:310-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Dierickx D, Habermann TM. Post-Transplantation Lymphoproliferative Disorders in Adults. N Engl J Med. 2018;378:549-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 319] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 30. | Evens AM, Roy R, Sterrenberg D, Moll MZ, Chadburn A, Gordon LI. Post-transplantation lymphoproliferative disorders: diagnosis, prognosis, and current approaches to therapy. Curr Oncol Rep. 2010;12:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Allen UD, Preiksaitis JK; AST Infectious Diseases Community of Practice. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 32. | Peters AC, Akinwumi MS, Cervera C, Mabilangan C, Ghosh S, Lai R, Iafolla M, Doucette K, Preiksaitis JK. The Changing Epidemiology of Posttransplant Lymphoproliferative Disorder in Adult Solid Organ Transplant Recipients Over 30 Years: A Single-center Experience. Transplantation. 2018;102:1553-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Al-Mansour Z, Nelson BP, Evens AM. Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep. 2013;8:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 34. | Ru Y, Chen J, Wu D. Epstein-Barr virus post-transplant lymphoproliferative disease (PTLD) after hematopoietic stem cell transplantation. Eur J Haematol. 2018;101:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, Horowitz MM, Jaffe ES, Kingma DW, Travis LB, Flowers ME, Martin PJ, Deeg HJ, Curtis RE. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992-5001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 36. | Swinnen LJ, LeBlanc M, Grogan TM, Gordon LI, Stiff PJ, Miller AM, Kasamon Y, Miller TP, Fisher RI. Prospective study of sequential reduction in immunosuppression, interferon alpha-2B, and chemotherapy for posttransplantation lymphoproliferative disorder. Transplantation. 2008;86:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Quinlan SC, Pfeiffer RM, Morton LM, Engels EA. Risk factors for early-onset and late-onset post-transplant lymphoproliferative disorder in kidney recipients in the United States. Am J Hematol. 2011;86:206-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Luskin MR, Heil DS, Tan KS, Choi S, Stadtmauer EA, Schuster SJ, Porter DL, Vonderheide RH, Bagg A, Heitjan DF, Tsai DE, Reshef R. The Impact of EBV Status on Characteristics and Outcomes of Posttransplantation Lymphoproliferative Disorder. Am J Transplant. 2015;15:2665-2673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 39. | Paranjothi S, Yusen RD, Kraus MD, Lynch JP, Patterson GA, Trulock EP. Lymphoproliferative disease after lung transplantation: comparison of presentation and outcome of early and late cases. J Heart Lung Transplant. 2001;20:1054-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Ghobrial IM, Habermann TM, Macon WR, Ristow KM, Larson TS, Walker RC, Ansell SM, Gores GJ, Stegall MD, McGregor CG. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation. 2005;79:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC, Olthoff KM, Schuster SJ, Nasta SD, Stadtmauer EA, Tsai DE. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant. 2006;6:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, Marcus R, Parameshwar J, Ramsay A, Newstead C; Haemato-oncology Task Force of the British Committee for Standards in Haematology and British Transplantation Society. Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients - BCSH and BTS Guidelines. Br J Haematol. 2010;149:693-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 43. | Dotti G, Fiocchi R, Motta T, Gamba A, Gotti E, Gridelli B, Borleri G, Manzoni C, Viero P, Remuzzi G, Barbui T, Rambaldi A. Epstein-Barr virus-negative lymphoproliferate disorders in long-term survivors after heart, kidney, and liver transplant. Transplantation. 2000;69:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Reshef R, Vardhanabhuti S, Luskin MR, Heitjan DF, Hadjiliadis D, Goral S, Krok KL, Goldberg LR, Porter DL, Stadtmauer EA, Tsai DE. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder(★). Am J Transplant. 2011;11:336-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 45. | Trappe RU, Dierickx D, Zimmermann H, Morschhauser F, Mollee P, Zaucha JM, Dreyling MH, Dührsen U, Reinke P, Verhoef G, Subklewe M, Hüttmann A, Tousseyn T, Salles G, Kliem V, Hauser IA, Tarella C, Van Den Neste E, Gheysens O, Anagnostopoulos I, Leblond V, Riess H, Choquet S. Response to Rituximab Induction Is a Predictive Marker in B-Cell Post-Transplant Lymphoproliferative Disorder and Allows Successful Stratification Into Rituximab or R-CHOP Consolidation in an International, Prospective, Multicenter Phase II Trial. J Clin Oncol. 2017;35:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 46. | Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJ. Epstein-Barr Virus-Positive Posttransplant Lymphoproliferative Disease After Solid Organ Transplantation: Pathogenesis, Clinical Manifestations, Diagnosis, and Management. Transplant Direct. 2016;2:e48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American College of Gastoenterology, 58606; American Association for the Study of Liver Diseases, 259789.
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Bellini MI, Italy; Bos S, United Kingdom; Ferreira GSA, Brazil; Naserian S, France; Sharma D, India S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ