Published online May 18, 2022. doi: 10.5500/wjt.v12.i5.88
Peer-review started: December 13, 2021
First decision: March 13, 2022
Revised: March 16, 2022
Accepted: April 20, 2022
Article in press: April 20, 2022
Published online: May 18, 2022
Processing time: 150 Days and 7.5 Hours
Children infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seem to have a better prognosis than adults. Nevertheless, pediatric solid organ transplantation (SOT) has been significantly affected by the unprecedented coronavirus disease 2019 (COVID-19) pandemic during the pre-, peri-, and post-transplant period. Undoubtedly, immunosuppression constitutes a real challenge for transplant clinicians as increased immunosuppression may prolong disease recovery, while its decrease can contribute to more severe symptoms. To date, most pediatric SOT recipients infected by SARS-CoV-2 experience mild disease with only scarce reports of life-threatening complications. As a consequence, after an initial drop during the early phase of the pandemic, pediatric SOTs are now performed with the same frequency as during the pre-pandemic period. This review summarizes the currently available evidence regarding pediatric SOT during the COVID-19 pandemic.
Core Tip: Pediatric patients experience milder symptoms of coronavirus disease 2019 (COVID-19). Pediatric solid organ transplantation during the COVID-19 pandemic represents a real challenge not only for the solid organ transplantation candidates and recipients but also for the transplant clinicians. Immunosuppression increases the risk of COVID-19 but may also provide a benefit against possible infection, as it lowers the risk of a catastrophic hyperinflammatory response from the host. We herein review the currently available evidence regarding pediatric solid organ transplantation during the COVID-19 pandemic.
- Citation: Kakos CD, Ziogas IA, Tsoulfas G. Pediatric transplantation during the COVID-19 pandemic. World J Transplant 2022; 12(5): 88-99
- URL: https://www.wjgnet.com/2220-3230/full/v12/i5/88.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i5.88
Coronavirus disease 2019 (COVID-19) has impacted all people worldwide and particularly people with chronic underlying comorbidities. Specifically, people with weakened immunity either due to an underlying disease or due to immunosuppression are at high risk. Although children represent just 2%-10% of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) diagnostic cases and seem to have less severe disease when compared with adults[1], pediatric solid organ transplantation (SOT) candidates and recipients have been significantly afflicted by the pandemic. The aim of this review is to summarize and discuss the currently available data regarding pediatric SOT during the COVID-19 pandemic.
It is well known now that children experience milder COVID-19 when compared with adults and a lower proportion of children require hospitalization[2,3]. The most frequently reported symptoms are cough and fever, while some pediatric patients may also present with gastrointestinal symptoms[4]. Although fatalities are rare in the pediatric population, 2%-8% of children with COVID-19 will eventually require admission to an intensive care unit[5]. Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 is a post-infectious consequence of pediatric SARS-CoV-2 infection presenting with gastrointestinal, cardiac, renal, or neurologic manifestations[6].
There has been excessive research on why adults experience a more severe form of COVID-19. A key concept is the difference between the pediatric and adult immune systems. Except for the most severe SARS-CoV-2 cases, children appear to preserve CD8+ cytotoxic response[6-8], as they do not face the immunosenescence that normally occurs with aging. Data have also shown that children might have more powerful adaptive immunity[9]. For example, pediatric SARS-CoV-2 patients do not present with either lymphopenia or high neutrophil/lymphocyte ratio[6]. In addition, adults have higher levels of circulating proinflammatory cytokines [interleukin-1β (IL-1β), IL-6, IL-10, IL-12, interferon-γ, tumor necrosis factor-α (TNF-α), C-reactive protein] than pediatric SARS-CoV-2 patients[10-12]. Although in a study from New York City, IL-6 and TNF-α values did not differ from adults[13].
A finding that needs further investigation is the potential role of angiotensin-converting enzyme 2 (ACE2) receptor, which is the main binding protein of SARS-CoV-2 on host cells[14]. ACE2 has been described as an anti-fibrotic and anti-inflammatory agent against pulmonary leak and inflammation, thus higher expression of ACE2 that has been observed in children may contribute to the fact that children are more resistant to SARS-CoV-2[7].
Furthermore, the fact that children typically do not have significant comorbidities, such as arterial hypertension, diabetes mellitus, or congestive heart failure, may contribute to the milder cases of COVID-19 observed. Associated factors that predispose a negative outcome in children with SARS-CoV-2 have not been well defined[15]. Nevertheless, previous studies have identified obesity, hypoxemia at clinical presentation, asthma, congenital heart disease, inherited metabolic syndrome, chromosomal disorders, and ethnicity as risk factors for severe SARS-CoV-2 infection in children[16-19]. Last but not least, another theory suggests that common childhood infections (respiratory syncytial virus, mycoplasma pneumoniae) can carry out cross protection, so children who have recently recovered from these infections may have higher immunoglobin G titers than adults[20,21].
SARS-CoV-2 enters the liver parenchyma through the ACE2 receptor. However, the liver is only rarely affected seriously by the disease, most probably due to its tolerogenic environment[22,23]. The most common hepatic manifestation is an elevation of hepatic transaminases in 6%-27% of pediatric cases and a mild elevation of γ-glutamyl transferase, alkaline phosphatase, and total bilirubin, yet their clinical significance remains unclear[24]. The liver damage may be directly caused by viral infection of the liver cells from medications like remdesivir or lopinavir/ritonavir or from chronic hypoxia[25-27]. High levels of IL-6 and IL-10 are associated with severe SARS-CoV-2 infection but not with SARS-CoV-2-related abnormal liver enzymes[28].
A cohort study from the United States and the United Kingdom demonstrated that adults with chronic liver disease and cirrhosis are prone to increased risk of adverse outcomes following SARS-CoV-2[29]. A study from northern Italy also noted that adults and children with autoimmune liver disease maintained satisfactory health status despite their imbalanced immune system[30]. Another Italian multicenter study that included both cirrhotic and non-cirrhotic liver disease patients demonstrated that 84% of children with chronic liver disease remained healthy during the outbreak[9]. It remains unclear whether children with chronic liver disease experience more severe symptoms.
SARS-CoV-2 can also present with renal manifestations, while several studies suggest that kidney transplantation should be continued during the COVID-19 pandemic under certain precautions[31-34]. Acute kidney injury is mostly associated with immune alterations and direct cytopathic lesions by SARS-CoV-2[35]. Acute tubular injury is also a common yet typically mild manifestation[36]. Comorbidities, such as diabetes mellitus and cardiovascular disease, can delay recovery from acute kidney injury[37]. A multicenter study from Turkey revealed that the incidence of SARS-CoV-2 is higher in pediatric patients on dialysis or after kidney transplantation, yet the authors reported that regional factors, such as the high population, the crowded households, and socioeconomic status in Istanbul, may have contributed to this particular observation in that cohort[38]. They also found that the hospitalization rate was higher in dialysis patients compared with kidney transplantation recipients, potentially due to a higher proportion of asymptomatic disease in kidney transplantation recipients[38].
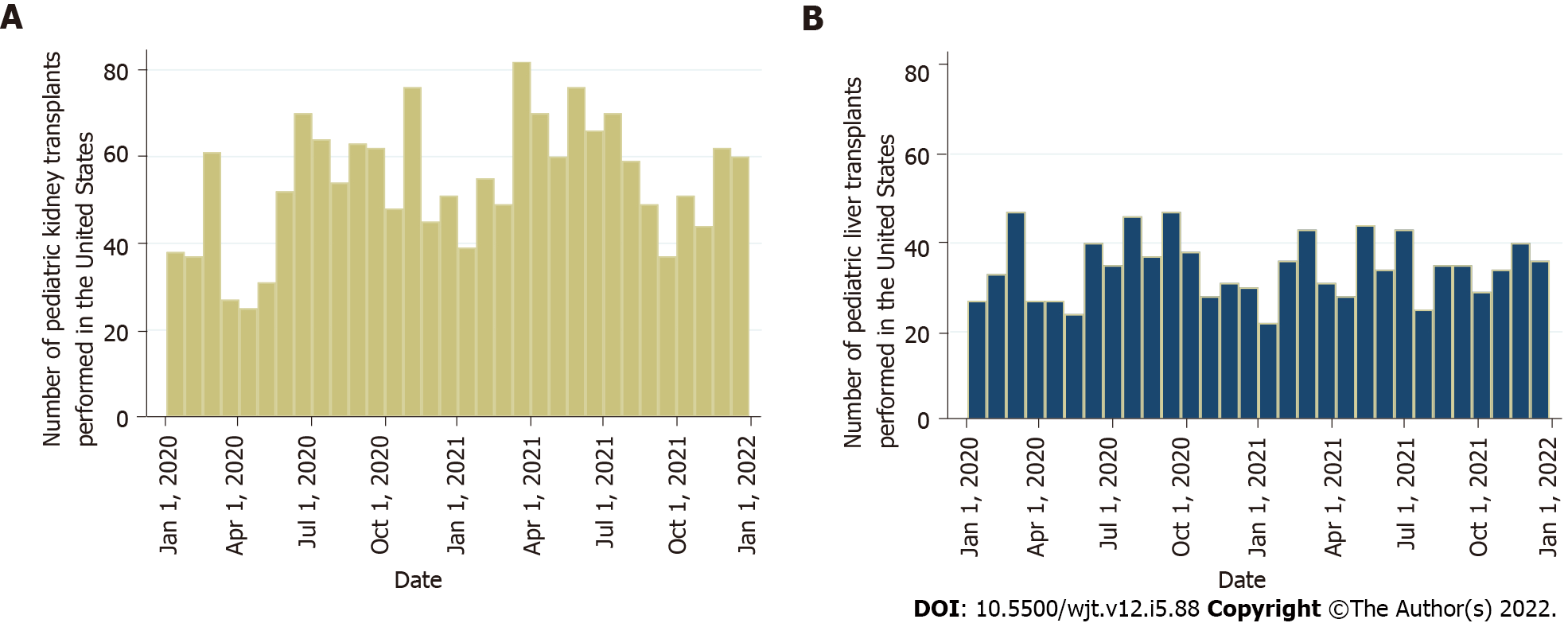
It was inevitable that the COVID-19 pandemic would affect the transplant activity worldwide. A multicenter analysis of the European Reference Network on Pediatric Transplantation showed a substantial reduction of pediatric transplants across Europe[39]. This was related to the precautions and measures to minimize SARS-CoV-2 transmission, the shortage of hospital beds and staff, the restrictions in operation room availability, and a notable decline in the recovery of deceased donor organs, especially during the early phase of the pandemic[40]. Additionally, United States data from the Scientific Registry of Transplant Recipients showed an initial decrease in pediatric kidney transplants from both deceased and living donors by 47% and 82%, respectively[41]. Subsequently, there was a continual increase with numbers reaching the expected pre-pandemic levels by May 2020[41]. The authors also reported a 189% increase in waitlist removal due to mortality or deterioration[41]. Kemme et al[42] used the same registry studying pediatric liver transplantation. They found a decrease in waitlist addition by 25% between March and May of 2020, with Black candidates being affected the most. During the early phase of the pandemic there was a 38% reduction in pediatric liver transplantation, with Black children experiencing an 81% decline in living donor liver transplantation in contrast to White children who faced no change in this category. Overall, White children had a 30% drop in liver transplantation during the pandemic[42]. Figure 1 depicts the number of pediatric kidney and liver transplants performed in the United States between January 1, 2020 and January 1, 2022.
Except for universal recommendations from transplant societies worldwide, there are no mandatory guidelines specific to pediatric SOT during the pandemic. The decision for SOT depends on the urgency of the need for a new organ and the risk-to-benefit ratio. Both pediatric SOT candidates and living donors should follow prevention strategies to reduce potential exposure to SARS-CoV-2 in the pretransplant period. Self-quarantine for 14 d prior to living donation is important, while a negative swab test for both the candidate and the donor upon admission to the hospital should also be required. Particularly in cases of pediatric SOT, the caregiver should also be asymptomatic and have a negative swab test prior to transplant. Further, most transplant societies strongly mandate universal SARS-CoV-2 screening of potential deceased donors before organ procurement[43].
There is no consensus about the optimal time for transplantation when the potential donor had a SARS-CoV-2 infection. In general, it is recommended to avoid grafts from donors with active SARS-CoV-2 infection[44], while there are different acceptance criteria for donors who have recently recovered from the infection[43]. Some transplant societies recommend using a graft from a living donor at least 28 d after symptom resolution irrespective of real-time reverse transcriptase polymerase chain reaction (RT-PCR) positivity. Due to the pulmonary and renal dysfunction associated with SARS-CoV-2 infection, additional considerations may be appropriate when the procedure involves transplantation of lungs or kidneys from a previously infected donor.
There is a scarcity of data regarding the optimal time of SOT if a pediatric candidate is infected by SARS-CoV-2. Ideally, the candidate should be both asymptomatic and have a negative test. Notably, Goss et al[45] reported an uncomplicated liver transplantation in a child positive for SARS-CoV-2 on a nasopharyngeal swab test just 4 wk before transplant. The immunoglobin G specific antibodies persisted for 6 wk after liver transplantation, with unaltered immunosuppression per the center’s standard protocol[45]. Until additional data are available, the risk of the procedure must always be weighed against the risk of deferring SOT.
On another note, technology overall has significantly changed the way people communicate during the COVID-19 pandemic, and thus telemedicine can have a pivotal role on transplant follow-up as it facilitates the general rules for social distancing[46]. However, a German study showed that most young adults who underwent liver transplantation in childhood were afraid to attend medical appointments and 40% reported lower appointment adherence[47]. Additionally, although video consultations might be helpful for follow-up, their acceptance by liver transplantation recipients was lower than expected[47]. It is important that pediatric patients adhere to follow-up appointments after SOT, and their parents should notify the transplant provider of any suspected or proven SARS-CoV-2 exposure and discuss whether additional measures are needed. Careful hand hygiene and avoidance of crowds during the period of high immunosuppression are key strategies for prevention of a possible infection[48].
Finally, several studies have evaluated the SARS-CoV-2 vaccine safety and efficacy in SOT recipients and children, with nearly all of them supporting that the administration of at least two vaccine doses in these patients is safe and efficient[49-55]. There is also an ongoing study approved by Johns Hopkins University examining the levels of SARS-CoV-2 antibodies in children who are organ transplant candidates or recipients before and after they get the SARS-CoV-2 vaccine (IRB00248540).
A confirmed SARS-CoV-2 case requires laboratory evidence of viral detection. The testing strategies vary by geographical location and testing capacity. A nasopharyngeal RT-PCR test is the recommended gold standard. However, a negative RT-PCR test does not definitively exclude SARS-CoV-2 infection, and the reported rates of false negative results vary between 2%-29%[56]. If symptoms persist, a second nasopharyngeal RT-PCR test should be performed after 48-72 h. Depending on the time of the year, an evaluation for other respiratory viruses should be considered. An alternative diagnosis would reduce but not eliminate the possibility of COVID-19, while the detection of another respiratory pathogen may require additional management (e.g., antiviral treatment in case of influenza infection).
Antibody tests should not be used to diagnose acute SARS-CoV-2 infection, while their application to assess the host response after an infection is an area under investigation. It is unknown if pediatric SOT recipients mount a robust serologic response to SARS-CoV-2, and even if they have protective antibodies, the length of this protection is unknown[53-55]. Single center studies from Saudi Arabia and Brazil have shown a relatively high seroprevalence of SARS-CoV-2 in the pediatric kidney transplantation population[57,58]. However, there are concerns for possible false positive antibody results due to cross-reactivity with other coronaviruses[59].
The management of a confirmed case of SARS-CoV-2 in a pediatric SOT recipient is mainly supportive, with supplemental oxygen, nonsteroidal anti-inflammatory drugs, remdesivir, dexamethasone, and SARS-CoV-2 convalescent plasma being the only proven measures that can significantly affect the outcome[26,60,61]. Lopinavir, ritonavir, and hydroxychloroquine have not shown any significant benefit in mortality and morbidity, including the need for mechanical ventilation[60].
A crucial aspect in this group of patients is immunosuppression, which is generally considered a double-edged sword[62]. Increased immunosuppression may increase the viral load and delay recovery, whereas low immunosuppression may contribute to severe COVID-19 forms due to a more robust immune response[63]. In fact, SARS-CoV-2-induced pulmonary injury is mainly driven by excessive activation of the innate immune inflammatory response of the host[64]. Despite that notion, it has been proposed that immunosuppression in immunocompromised children may not actually increase the risk for severe SARS-CoV-2 disease[65]. On the contrary, SOT recipients may benefit from immunosuppressive drugs, as they will dampen the cytokine storm[66,67]. Immunosuppression has not been reported as a stronger risk factor than obesity, chronic comorbidities, or increased age. One possible explanation is that in SARS-CoV-2, unlike other viral agents (e.g., adenovirus, rhinovirus, norovirus, influenza), the host immune response is the main driver of lung tissue damage during infection[65]. Interestingly, a systematic review showed that immunosuppressed patients have a lower incidence of SARS-CoV-2 infection when compared with the general population, and they may exhibit relatively favorable outcomes as compared to other comorbidities[68].
The impact of immunosuppression on COVID-19 severity in pediatric SOT recipients remains unclear. Although complete withdrawal of immunosuppression might not be the optimal approach, individual modifications may be necessary in cases of moderate-to-severe SARS-CoV-2 infection[69]. It seems that some immunosuppression may allow for control of the dysregulated immune response, which is commonly observed in severe SARS-CoV-2 infection[65,69]. Comparative data on immunosuppression management strategies are not yet available. Some authors recommend decreasing or discontinuing cell cycle inhibitors and cautiously reducing calcineurin inhibitors (i.e., cyclosporine, tacrolimus) in moderate-to-severe COVID-19 in adult SOT recipients, while others recommend continuing calcineurin inhibitors and steroids and stopping anti-proliferative medication[70]. It is also thought that calcineurin inhibitors might exert an antiviral effect and inhibit IL-6 and IL-10 pathways, which are involved in the immune dysregulation observed in COVID-19 patients[71]. In addition, certain immunosuppression therapies like mammalian target of rapamycin inhibitors may even have biologic activity against SARS-CoV-2[72].
Transplant centers follow their own strategies based on their institutional experiences. Although the data for pediatric patients are scarce, Colmenero et al[73] observed no adverse outcome with the use of calcineurin inhibitors and mammalian target of rapamycin inhibitors in adult patients. On the other hand, mycophenolate mofetil was associated with severe SARS-CoV-2 infection in a dose-dependent manner[74]. This can be explained by its mechanism of action, as mycophenolate mofetil produces a cytostatic effect on activated lymphocytes[74]. It is well known that SARS-CoV-2 is associated with lymphopenia, so mycophenolate mofetil may exert a synergic and deleterious effect on depleting peripheral lymphocytes[74]. On the contrary, mammalian target of rapamycin inhibitors increase the quality and functionality of memory T cells and reduce the replication of multiple viruses including cytomegalovirus, Epstein-Barr virus and human immunodeficiency virus[75]. Regarding calcineurin inhibitors, some studies have shown in vitro antiviral effects against coronaviruses and that they can ameliorate the cytokine storm[76]. Randomized clinical trials comparing the different immunosuppressive schemas would help us guide management of both adult and pediatric SOT recipients.
If there is strong suspicion for bacterial superinfection, the administration of antibiotics, such as moxifloxacin, levofloxacin, ceftriaxone, vancomycin, or amikacin, can be considered[77-79]. Azithromycin should be used with caution in SOT recipients as it can increase the levels of tacrolimus[80]. These medications have been prescribed mainly in unresponsive cases, which precludes us from deducing meaningful conclusions in the absence of high-quality data.
There are several recent reports of pediatric SOT recipients who have been infected by SARS-CoV-2 (Table 1)[38,57,58,66,77-79,81-98]. For example, Heinz et al[81] reported mild symptoms in a 6-mo-old recipient just 4 d after liver transplantation, while the infection was probably transmitted from the mother-donor. Neither the donor nor the recipient were tested pretransplant due to low availability of rapid testing at the early phase of the pandemic[81]. A multicenter study documented no mortality due to COVID-19 but a high rate of acute liver injury in pediatric liver transplantation recipients[83]. Morand et al[82] reported a coinfection of SARS-CoV-2 and Epstein-Barr virus in a pediatric liver transplantation recipient that was managed with slight reduction of tacrolimus. Nikoupour et al[78] reported a fatal outcome in a 3-year-old liver transplantation recipient after multiorgan failure and cardiorespiratory arrest. Results from the same transplant center reported a 100% death rate in 4 pediatric liver transplantation recipients due to liver failure, implying an increased mortality risk in children[84]. A case report from Texas described a case of multisystem inflammatory syndrome with features of Kawasaki disease in a 3-year-old African American female liver transplantation recipient[86]. The patient did not require transfer to the intensive care unit and was effectively managed with tacrolimus titration[85].
| Ref. | Organ | Number of recipients | Diagnosis method | Center | Outcome | Cause of death |
| Sin et al[83] | Liver | 110 | N/A | International | All alive | N/A |
| Kehar et al[88] | Liver | 47 | RT-PCR test: 39. Serum antibodies: 8 | International | All alive | N/A |
| Fonseca et al[89] | Liver | 12 | RT-PCR test | Hospital Sírio-Libanês, São Paulo, Brazil | All alive | N/A |
| Yuksel et al[90] | Liver | 10 | RT-PCR test | Koç University Hospital, Istanbul, Turkey | All alive | N/A |
| Ali Malekhosseini et al[84] | Liver | 4 | RT-PCR test or chest computed tomography scan | Shiraz Transplant Center, Abu Ali Sina Hospital, Shiraz, Iran | All died | Liver failure |
| Duvant et al[79] | Liver | 1 | Serum antibodies | Hospital Timone Enfants, Marseille, France | Alive | N/A |
| Heinz et al[81] | Liver | 1 | RT-PCR test | Columbia University Vagelos College of Physician and Surgeons, New York, United States | Alive | N/A |
| Morand et al[82] | Liver | 1 | RT-PCR test | La Timone Children Hospital, Marseille, France | Alive | N/A |
| Nikoupour et al[78] | Liver | 1 | RT-PCR test | Shiraz Transplant Center, Abu Ali Sina Hospital, Shiraz, Iran | Dead | Multiorgan failure |
| Soin et al[91] | Liver | 1 | RT-PCR test | Medanta the Medicity, Gurgaon, Delhi, India | Alive | N/A |
| Petters et al[85] | Liver | 1 | RT-PCR test | Baylor College of Medicine, Houston, United States | Alive | N/A |
| Canpolat et al[38] | Kidney | 29 | RT-PCR test | Multicenter, Turkey | All alive | N/A |
| Varnell et al[92] | Kidney | 24 | RT-PCR test | Multicenter (United States) | All alive | N/A |
| Alshami et al[57] | Kidney | 9 | RT-PCR test | King Fahad Specialist Hospital Dammam, Saudi Arabia | All alive | N/A |
| Berteloot et al[86] | Kidney | 5 | RT-PCR test | Hospital Universitaire Necker Enfants Maladies, Paris, France | All alive | N/A |
| Singer et al[93] | Kidney | 5 | RT-PCR test | Cohen Children Medical Center, New York, United States | All alive | N/A |
| Solomon et al[94] | Kidney | 4 | RT-PCR test | Maria Fareri Children’s Hospital, New York, United States | All alive | N/A |
| Levenson et al[87] | Kidney | 1 | RT-PCR test | Louisiana State University Health Sciences Center, New Orleans, Louisiana, United States | Alive | N/A |
| Bush et al[77] | Kidney | 1 | RT-PCR test | University of Florida, Gainesville, United States | Alive | N/A |
| Bock et al[95] | Heart | 20 | RT-PCR test | Loma Linda Children’s Hospital, California, United States | All alive | N/A |
| Lee et al[96] | Heart | 4 | RT-PCR test: 3. Serum antibodies: 1 | Columbia University Irving Medical Center, New York, United States | All alive | N/A |
| Russell et al[97] | Heart | 1 | RT-PCR test | UCLA, California, United States | Alive | N/A |
| Goss et al[66] | Liver, kidney, heart, lung | 26 | RT-PCR test | Multicenter (United States) | All alive | N/A |
| Cleto-Yamane et al[58] | Liver, kidney | 25 | RT-PCR test | Hospital Estadual da Crianca, Rio de Janeiro, Brazil | All alive | N/A |
| Talgam-Horshi et al[98] | Liver, kidney, combined (liver and pancreas) | 25 | RT-PCR test | Schneider Children’s hospital of Israel, Tel Aviv, Israel | All alive | N/A |
There are also some interesting findings in pediatric kidney transplantation recipients. Berteloot et al[86] presented 9 pediatric cases, 7 of whom developed graft arterial stenosis during early follow-up after kidney transplantation. It was reported as immune post viral graft vasculitis triggered by SARS-CoV-2[86]. Levenson et al[87] reported acute kidney injury in an adolescent male kidney transplantation recipient following SARS-CoV-2 infection, with biopsy showing segmental glomerulosclerosis on a background of chronic active antibody-mediated rejection. The case was treated with an overall reduction of immunosuppression, along with anti-inflammatory treatment, which proved to be effective in preserving allograft function while attaining recovery[87]. Finally, a multicenter, multiorgan case series from five transplant centers across the United States demonstrated favorable outcomes in pediatric SOT recipients with COVID-19, which may mirror those of immunocompetent children, with infrequent hospitalizations and minimal additional treatment requirements[66].
Pediatric transplantation is a complex process that requires a combination of resources and specialized professionals and has been significantly impacted by the COVID-19 pandemic. Overall, there was a substantial decrease in pediatric SOT during the early phase of the pandemic, yet recent findings show that pediatric SOT outcomes during the pandemic were favorable. The results on the safety and efficacy on vaccines have been promising, yet further research is required to draw more solid conclusions on the optimal immunosuppressive management of pediatric SOT recipients.
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the United States Government.
| 1. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11607] [Article Influence: 1934.5] [Reference Citation Analysis (2)] |
| 2. | Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53:371-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 362] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 3. | Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, Vidal E, Cogo P. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 346] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 4. | CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1054] [Cited by in RCA: 1073] [Article Influence: 178.8] [Reference Citation Analysis (0)] |
| 5. | Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, Krivec U, Lo Vecchio A, Shingadia D, Soriano-Arandes A, Melendo S, Lanari M, Pierantoni L, Wagner N, L'Huillier AG, Heininger U, Ritz N, Bandi S, Krajcar N, Roglić S, Santos M, Christiaens C, Creuven M, Buonsenso D, Welch SB, Bogyi M, Brinkmann F, Tebruegge M; ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 931] [Cited by in RCA: 863] [Article Influence: 143.8] [Reference Citation Analysis (1)] |
| 6. | Wu H, Zhu H, Yuan C, Yao C, Luo W, Shen X, Wang J, Shao J, Xiang Y. Clinical and Immune Features of Hospitalized Pediatric Patients With Coronavirus Disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3:e2010895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 7. | Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J Pediatr. 2020;87:537-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 8. | Miri SM, Noorbakhsh F, Mohebbi SR, Ghaemi A. Higher prevalence of asymptomatic or mild COVID-19 in children, claims and clues. J Med Virol. 2020;92:2257-2259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Di Giorgio A, Hartleif S, Warner S, Kelly D. COVID-19 in Children With Liver Disease. Front Pediatr. 2021;9:616381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 865] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 11. | Jacques FH, Apedaile E. Immunopathogenesis of COVID-19: Summary and Possible Interventions. Front Immunol. 2020;11:564925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 955] [Cited by in RCA: 956] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 13. | Pierce CA, Preston-Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B, Garforth SJ, Herrera NG, Jangra RK, Morano NC, Orner E, Sy S, Chandran K, Dziura J, Almo SC, Ring A, Keller MJ, Herold KC, Herold BC. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 280] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 14. | Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res. 2020;126:1456-1474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1325] [Cited by in RCA: 1407] [Article Influence: 234.5] [Reference Citation Analysis (1)] |
| 15. | Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, Heidemann SM, Kleinman LC, Sen AI, Hall MW, Priestley MA, McGuire JK, Boukas K, Sharron MP, Burns JP; International COVID-19 PICU Collaborative. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 2020;174:868-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 711] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 16. | Fernandes DM, Oliveira CR, Guerguis S, Eisenberg R, Choi J, Kim M, Abdelhemid A, Agha R, Agarwal S, Aschner JL, Avner JR, Ballance C, Bock J, Bhavsar SM, Campbell M, Clouser KN, Gesner M, Goldman DL, Hammerschlag MR, Hymes S, Howard A, Jung HJ, Kohlhoff S, Kojaoghlanian T, Lewis R, Nachman S, Naganathan S, Paintsil E, Pall H, Sy S, Wadowski S, Zirinsky E, Cabana MD, Herold BC; Tri-State Pediatric COVID-19 Research Consortium. Severe Acute Respiratory Syndrome Coronavirus 2 Clinical Syndromes and Predictors of Disease Severity in Hospitalized Children and Youth. J Pediatr. 2021;230:23-31.e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 17. | Williams N, Radia T, Harman K, Agrawal P, Cook J, Gupta A. COVID-19 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review of critically unwell children and the association with underlying comorbidities. Eur J Pediatr. 2021;180:689-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 18. | Brisca G, Mariani M, Andrea Rotulo G, Pirlo D, Romanengo M, Castagnola E, Piccotti E, Moscatelli A. Clinical course of COVID-19 in children with pre-existing medical conditions. Acta Paediatr. 2021;110:1291-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Harman K, Verma A, Cook J, Radia T, Zuckerman M, Deep A, Dhawan A, Gupta A. Ethnicity and COVID-19 in children with comorbidities. Lancet Child Adolesc Health. 2020;4:e24-e25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Orange JS, Du W, Falsey AR. Therapeutic Immunoglobulin Selected for High Antibody Titer to RSV also Contains High Antibody Titers to Other Respiratory Viruses. Front Immunol. 2015;6:431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Mi B, Chen L, Panayi AC, Xiong Y, Liu G. Serum Mycoplasma pneumoniae IgG in COVID-19: A protective factor. 2020 Preprint. Available from: medRxiv 2020.04.12.20060079. [DOI] [Full Text] |
| 22. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. 2020 Preprint. Available from: bioRxiv 2020.02.03.931766. [DOI] [Full Text] |
| 23. | Li N, Hua J. Immune cells in liver regeneration. Oncotarget. 2017;8:3628-3639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 833] [Cited by in RCA: 792] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 25. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1308] [Article Influence: 218.0] [Reference Citation Analysis (8)] |
| 26. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5829] [Cited by in RCA: 5220] [Article Influence: 870.0] [Reference Citation Analysis (0)] |
| 27. | Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 28. | Zhou YH, Zheng KI, Targher G, Byrne CD, Zheng MH. Abnormal liver enzymes in children and infants with COVID-19: A narrative review of case-series studies. Pediatr Obes. 2020;15:e12723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 389] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 30. | Di Giorgio A, Nicastro E, Speziani C, De Giorgio M, Pasulo L, Magro B, Fagiuoli S, D' Antiga L. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020;73:702-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | López V, Vázquez T, Alonso-Titos J, Cabello M, Alonso A, Beneyto I, Crespo M, Díaz-Corte C, Franco A, González-Roncero F, Gutiérrez E, Guirado L, Jiménez C, Jironda C, Lauzurica R, Llorente S, Mazuecos A, Paul J, Rodríguez-Benot A, Ruiz JC, Sánchez-Fructuoso A, Sola E, Torregrosa V, Zárraga S, Hernández D; Grupo de Estudio GREAT (Grupo Español de Actualizaciones en Trasplante). [Recommendations on management of the SARS-CoV-2 coronavirus pandemic (Covid-19) in kidney transplant patients]. Nefrologia (Engl Ed). 2020;40:265-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, Ross M, Forest S, Goldstein YD, Ajaimy M, Liriano-Ward L, Pynadath C, Loarte-Campos P, Nandigam PB, Graham J, Le M, Rocca J, Kinkhabwala M. Covid-19 and Kidney Transplantation. N Engl J Med. 2020;382:2475-2477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 644] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 33. | Coates PT, Wong G, Drueke T, Rovin B, Ronco P; Associate Editors, for the Entire Editorial Team. Early experience with COVID-19 in kidney transplantation. Kidney Int. 2020;97:1074-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Vistoli F, Furian L, Maggiore U, Caldara R, Cantaluppi V, Ferraresso M, Zaza G, Cardillo M, Biancofiore G, Menichetti F, Russo A, Turillazzi E, Di Paolo M, Grandaliano G, Boggi U; Italian National Kidney Transplantation Network; the Joint Committee of the Italian Society of Organ Transplantation and the Italian Society of Nephrology. COVID-19 and kidney transplantation: an Italian Survey and Consensus. J Nephrol. 2020;33:667-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Bitencourt L, Pedrosa AL, de Brito SBCS, Fróes ACF, de Carvalho ST, Fonseca GG, Ferreira GC, Fradico PF, Simões E Silva AC. COVID-19 and Renal Diseases: An Update. Curr Drug Targets. 2021;22:52-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, Cantaluppi V. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17:751-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 340] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 37. | Yende S, Parikh CR. Long COVID and kidney disease. Nat Rev Nephrol. 2021;17:792-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 38. | Canpolat N, Yıldırım ZY, Yıldız N, Taşdemir M, Göknar N, Evrengül H, Gülmez R, Aksu B, Dursun H, Özçelik G, Yavaşcan Ö, Çiçek RY, Tülpar S, Hacıhamdioğlu DÖ, Nayır A, Alpay H. COVID-19 in pediatric patients undergoing chronic dialysis and kidney transplantation. Eur J Pediatr. 2022;181:117-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Doná D, Torres Canizales J, Benetti E, Cananzi M, De Corti F, Calore E, Hierro L, Ramos Boluda E, Melgosa Hijosa M, Garcia Guereta L, Pérez Martínez A, Barrios M, Costa Reis P, Teixeira A, Lopes MF, Kaliciński P, Branchereau S, Boyer O, Debray D, Sciveres M, Wennberg L, Fischler B, Barany P, Baker A, Baumann U, Schwerk N, Nicastro E, Candusso M, Toporski J, Sokal E, Stephenne X, Lindemans C, Miglinas M, Rascon J, Jara P; ERN TransplantChild. Pediatric transplantation in Europe during the COVID-19 pandemic: Early impact on activity and healthcare. Clin Transplant. 2020;34:e14063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Merola J, Schilsky ML, Mulligan DC. The Impact of COVID-19 on Organ Donation, Procurement and Liver Transplantation in the United States. Hepatol Commun. 2020;5:5-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Charnaya O, Chiang TP, Wang R, Motter J, Boyarsky B, King E, Werbel W, Durand CM, Avery R, Segev D, Massie A, Garonzik-Wang J. Effects of COVID19 Pandemic on Pediatric Kidney Transplant in the United States. Res Sq. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Kemme S, Yoeli D, Sundaram SS, Adams MA, Feldman AG. Decreased access to pediatric liver transplantation during the COVID-19 pandemic. Pediatr Transplant. 2022;26:e14162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | L'Huillier AG, Danziger-Isakov L, Chaudhuri A, Green M, Michaels MG, M Posfay-Barbe K, van der Linden D, Verma A, McCulloch M, Ardura MI. SARS-CoV-2 and pediatric solid organ transplantation: Current knowns and unknowns. Pediatr Transplant. 2021;25:e13986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | American Society of Transplantation. SARS-CoV-2 (Coronavirus, 2019-nCoV): Recommendations and Guidance for Organ Donor Testing. [cited 5 December 2021]. Available from: https://www.myast.org/sites/default/files/Donor%20Testing_100520_revised_ReadyToPostUpdated10-12.pdf. |
| 45. | Goss MB, Munoz FM, Ruan W, Galván NTN, O'Mahony CA, Rana A, Cotton RT, Moreno NF, Heczey AA, Leung DH, Goss JA. Liver transplant in a recently COVID-19 positive child with hepatoblastoma. Pediatr Transplant. 2021;25:e13880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Hussain T, Nassetta K, Badawy SM. Adherence to Immunosuppression Medications among Heart Transplant Recipients: Challenges, Opportunities, and Potential Role of Digital Approaches in the COVID-19 Era. J Cardiovasc Dev Dis. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Kröncke S, Lund LK, Buchholz A, Lang M, Briem-Richter A, Grabhorn EF, Sterneck M. Psychosocial situation, adherence, and utilization of video consultation in young adult long-term pediatric liver transplant recipients during COVID-19 pandemic. Pediatr Transplant. 2021;25:e14121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Gonzalez BE, Michaels MG. Safe living after transplantation or chemotherapy. Pediatr Transpl Oncol Infect Dis. 2021;90-96.e2. [DOI] [Full Text] |
| 49. | Qin CX, Auerbach SR, Charnaya O, Danziger-Isakov LA, Ebel NH, Feldman AG, Hsu EK, McAteer J, Mohammad S, Perito ER, Thomas AM, Chiang TPY, Garonzik-Wang JM, Segev DL, Mogul DB. Antibody response to 2-dose SARS-CoV-2 mRNA vaccination in pediatric solid organ transplant recipients. Am J Transplant. 2022;22:669-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Strauss AT, Hallett AM, Boyarsky BJ, Ou MT, Werbel WA, Avery RK, Tobian AAR, Massie AB, Hamilton JPA, Garonzik-Wang JM, Segev DL. Antibody Response to Severe Acute Respiratory Syndrome-Coronavirus-2 Messenger RNA Vaccines in Liver Transplant Recipients. Liver Transpl. 2021;27:1852-1856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021;325:2204-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 816] [Article Influence: 163.2] [Reference Citation Analysis (0)] |
| 52. | Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM, Segev DL. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann Intern Med. 2021;174:1330-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 53. | Freeman MC, Rapsinski GJ, Zilla ML, Wheeler SE. Immunocompromised Seroprevalence and Course of Illness of SARS-CoV-2 in One Pediatric Quaternary Care Center. J Pediatric Infect Dis Soc. 2021;10:426-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Nailescu C, Khalid M, Wilson AC, Amanat F, Arregui S, Canas J, Hooks J, Krammer F, Schwaderer AL, Hains DS. Assessment of Seroconversion to SARS-CoV-2 in a Cohort of Pediatric Kidney Transplant Recipients. Front Pediatr. 2020;8:601327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Zhang Y, Xu J, Jia R, Yi C, Gu W, Liu P, Dong X, Zhou H, Shang B, Cheng S, Sun X, Ye J, Li X, Zhang J, Ling Z, Ma L, Wu B, Zeng M, Zhou W, Sun B. Protective humoral immunity in SARS-CoV-2 infected pediatric patients. Cell Mol Immunol. 2020;17:768-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Woloshin S, Patel N, Kesselheim AS. False Negative Tests for SARS-CoV-2 Infection - Challenges and Implications. N Engl J Med. 2020;383:e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 567] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 57. | Alshami A, Al Attas R, Azzam A, Mohammed A, Al-Quhaidan N. Detection of SARS-CoV-2 antibodies in pediatric kidney transplant patients. BMC Nephrol. 2021;22:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Cleto-Yamane TL, Rodrigues-Santos G, de Magalhães-Barbosa MC, Moura PG, Vasconcelos RD, Gouveia JLS, de Oliveira AL, Ferreira FC, Shalders AL, de Oliveira MBG, Lima-Setta F, da Cunha AJLA, Prata-Barbosa A. Screening of COVID-19 in outpatient children with cancer or solid organ transplantation: preliminary report. Eur J Pediatr. 2021;180:3237-3241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Lv H, Wu NC, Tsang OT, Yuan M, Perera RAPM, Leung WS, So RTY, Chan JMC, Yip GK, Chik TSH, Wang Y, Choi CYC, Lin Y, Ng WW, Zhao J, Poon LLM, Peiris JSM, Wilson IA, Mok CKP. Cross-reactive Antibody Response between SARS-CoV-2 and SARS-CoV Infections. Cell Rep. 2020;31:107725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 60. | Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, Pardo-Hernandez H, Qasim A, Martinez JPD, Rochwerg B, Lamontagne F, Han MA, Liu Q, Agarwal A, Agoritsas T, Chu DK, Couban R, Cusano E, Darzi A, Devji T, Fang B, Fang C, Flottorp SA, Foroutan F, Ghadimi M, Heels-Ansdell D, Honarmand K, Hou L, Hou X, Ibrahim Q, Khamis A, Lam B, Loeb M, Marcucci M, McLeod SL, Motaghi S, Murthy S, Mustafa RA, Neary JD, Rada G, Riaz IB, Sadeghirad B, Sekercioglu N, Sheng L, Sreekanta A, Switzer C, Tendal B, Thabane L, Tomlinson G, Turner T, Vandvik PO, Vernooij RW, Viteri-García A, Wang Y, Yao L, Ye Z, Guyatt GH, Brignardello-Petersen R. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 540] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 61. | Rahman F, Liu STH, Taimur S, Jacobs S, Sullivan T, Dunn D, Baneman E, Fuller R, Aberg JA, Bouvier N, Rana MM. Treatment with convalescent plasma in solid organ transplant recipients with COVID-19: Experience at large transplant center in New York City. Clin Transplant. 2020;34:e14089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395:1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 63. | Willicombe M, Thomas D, McAdoo S. COVID-19 and Calcineurin Inhibitors: Should They Get Left Out in the Storm? J Am Soc Nephrol. 2020;31:1145-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 64. | Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1858] [Cited by in RCA: 1986] [Article Influence: 331.0] [Reference Citation Analysis (0)] |
| 65. | D'Antiga L. Coronaviruses and Immunosuppressed Patients: The Facts During the Third Epidemic. Liver Transpl. 2020;26:832-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 495] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 66. | Goss MB, Galván NTN, Ruan W, Munoz FM, Brewer ED, O'Mahony CA, Melicoff-Portillo E, Dreyer WJ, Miloh TA, Cigarroa FG, Ranch D, Yoeli D, Adams MA, Koohmaraie S, Harter DM, Rana A, Cotton RT, Carter B, Patel S, Moreno NF, Leung DH, Goss JA. The pediatric solid organ transplant experience with COVID-19: An initial multi-center, multi-organ case series. Pediatr Transplant. 2021;25:e13868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 67. | Seminari E, Colaneri M, Sambo M, Gallazzi I, Di Matteo A, Roda S, Bruno R; COVID19 IRCCS San Matteo Pavia Task Force. SARS Cov-2 infection in a renal-transplanted patient: A case report. Am J Transplant. 2020;20:1882-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 68. | Minotti C, Tirelli F, Barbieri E, Giaquinto C, Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? J Infect. 2020;81:e61-e66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 69. | Romanelli A, Mascolo S. Immunosuppression drug-related and clinical manifestation of Coronavirus disease 2019: A therapeutical hypothesis. Am J Transplant. 2020;20:1947-1948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 70. | Kronbichler A, Gauckler P, Windpessl M, Il Shin J, Jha V, Rovin BH, Oberbauer R. COVID-19: implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol. 2020;16:365-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 71. | Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1169] [Cited by in RCA: 1139] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 72. | Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 600] [Reference Citation Analysis (0)] |
| 73. | Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (1)] |
| 74. | Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1066] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 75. | Bowman LJ, Brueckner AJ, Doligalski CT. The Role of mTOR Inhibitors in the Management of Viral Infections: A Review of Current Literature. Transplantation. 2018;102:S50-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 76. | Tanaka Y, Sato Y, Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5:1250-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 77. | Bush R, Johns F, Acharya R, Upadhyay K. Mild COVID-19 in a pediatric renal transplant recipient. Am J Transplant. 2020;20:2942-2945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 78. | Nikoupour H, Kazemi K, Arasteh P, Ghazimoghadam S, Eghlimi H, Dara N, Gholami S, Nikeghbalian S. Pediatric liver transplantation and COVID-19: a case report. BMC Surg. 2020;20:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Duvant P, Roquelaure B, Morand A, Bosdure E, Garaix F, Zandotti C, Fabre A. A second case of multisystem inflammatory syndrome associated with SARS-CoV-2 in a liver-transplanted child. Pediatr Transplant. 2022;26:e14116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Mori T, Aisa Y, Nakazato T, Yamazaki R, Ikeda Y, Okamoto S. Tacrolimus-azithromycin interaction in a recipient of allogeneic bone marrow transplantation. Transpl Int. 2005;18:757-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 81. | Heinz N, Griesemer A, Kinney J, Vittorio J, Lagana SM, Goldner D, Velasco M, Kato T, Lobritto S, Martinez M. A case of an Infant with SARS-CoV-2 hepatitis early after liver transplantation. Pediatr Transplant. 2020;24:e13778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Morand A, Roquelaure B, Colson P, Amrane S, Bosdure E, Raoult D, Lagier JC, Fabre A. Child with liver transplant recovers from COVID-19 infection. A case report. Arch Pediatr. 2020;27:275-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Sin P, Díaz LA, Martínez M, Vizcaya C, D'Agostino D, Gana JC. Acute Liver Injury Among Pediatric Liver Transplantation Recipients With Coronavirus Disease 2019: An International Collaborative Study. J Pediatr Gastroenterol Nutr. 2021;73:391-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Ali Malekhosseini S, Nikoupour H, Gholami S, Shamsaeefar A, Arasteh P, Kazemi K, Dehghani M, Eghlimi H, Raeisi Shahraki H, Roozbeh J, Rezaianzadeh A, Nikeghbalian S. A Report of 85 Cases of COVID-19 and Abdominal Transplantation From a Single Center: What Are the Associated Factors With Death Among Organ Transplantation Patients. Transplantation. 2021;105:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Petters LM, Vogel TP, Munoz FM, Hernandez JA, Koohmaraie S, Nowicki MJ, Zumbro CE, Mysore KR. Multisystem inflammatory syndrome in children associated with SARS-CoV-2 in a solid organ transplant recipient. Am J Transplant. 2021;21:2596-2599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Berteloot L, Berthaud R, Temmam S, Lozach C, Zanelli E, Blanc T, Heloury Y, Capito C, Chardot C, Sarnacki S, Garcelon N, Lacaille F, Charbit M, Pastural M, Rabant M, Boddaert N, Leruez-Ville M, Eloit M, Sermet-Gaudelus I, Dehoux L, Boyer O. Arterial abnormalities identified in kidneys transplanted into children during the COVID-19 pandemic. Am J Transplant. 2021;21:1937-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Levenson E, Shepherd TN, Aviles D, Craver R, Ehlayel A, Love GL, Simms K, Straatmann C, Ashoor IF. De novo collapsing glomerulopathy in a pediatric kidney transplant recipient with COVID-19 infection. Pediatr Transplant. 2021;25:e14013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Kehar M, Ebel NH, Ng VL, Baquero JER, Leung DH, Slowik V, Ovchinsky N, Shah AA, Arnon R, Miloh T, Gupta N, Mohammad S, Kogan-Liberman D, Squires JE, Sanchez MC, Hildreth A, Book L, Chu C, Alrabadi L, Azzam R, Chepuri B, Elisofon S, Falik R, Gallagher L, Kader H, Mogul D, Mujawar Q, Namjoshi SS, Valentino PL, Vitola B, Waheed N, Zheng MH, Lobritto S, Martinez M. Severe Acute Respiratory Syndrome Coronavirus-2 Infection in Children With Liver Transplant and Native Liver Disease: An International Observational Registry Study. J Pediatr Gastroenterol Nutr. 2021;72:807-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 89. | Fonseca EA, Feier F, Pugliese R, Freitas AF, Porta G, Miura I, Baggio V, Kondo M, Benavides M, Vincenzi R, Roda K, Oliveira CV, Chapchap P, Seda-Neto J. Pediatric liver transplantation activity in a high-volume program during the COVID-19 pandemic in Brazil. Pediatr Transplant. 2021;25:e14112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Yuksel M, Akturk H, Mizikoglu O, Toroslu E, Arikan C. A single-center report of COVID-19 disease course and management in liver transplanted pediatric patients. Pediatr Transplant. 2021;25:e14061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Soin AS, Choudhary NS, Yadav SK, Saigal S, Saraf N, Rastogi A, Bhangui P, Srinivasan T, Mohan N, Saha SK, Gupta A, Chaudhary RJ, Yadav K, Dhampalwar S, Govil D, Gupta N, Vohra V. Restructuring Living-Donor Liver Transplantation at a High-Volume Center During the COVID-19 Pandemic. J Clin Exp Hepatol. 2021;11:418-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Varnell C Jr, Harshman LA, Smith L, Liu C, Chen S, Al-Akash S, Barletta GM, Belsha C, Brakeman P, Chaudhuri A, Fadakar P, Garro R, Gluck C, Goebel J, Kershaw D, Matossian D, Nailescu C, Patel HP, Pruette C, Ranabothu S, Rodig N, Smith J, Sebestyen VanSickle J, Weng P, Danziger-Isakov L, Hooper DK, Seifert M. COVID-19 in pediatric kidney transplantation: The Improving Renal Outcomes Collaborative. Am J Transplant. 2021;21:2740-2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 93. | Singer PS, Sethna C, Molmenti E, Fahmy A, Grodstein E, Castellanos-Reyes L, Fassano J, Teperman L. COVID-19 infection in a pediatric kidney transplant population: A single-center experience. Pediatr Transplant. 2021;25:e14018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Solomon S, Pereira T, Samsonov D. An early experience of COVID-19 disease in pediatric and young adult renal transplant recipients. Pediatr Transplant. 2021;25:e13972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 95. | Bock MJ, Kuhn MA, Chinnock RE. COVID-19 diagnosis and testing in pediatric heart transplant recipients. J Heart Lung Transplant. 2021;40:897-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Lee H, Mantell BS, Richmond ME, Law SP, Zuckerman WA, Addonizio LJ, Lee TM, Lytrivi ID. Varying presentations of COVID-19 in young heart transplant recipients: A case series. Pediatr Transplant. 2020;24:e13780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 97. | Russell MR, Halnon NJ, Alejos JC, Salem MM, Reardon LC. COVID-19 in a pediatric heart transplant recipient: Emergence of donor-specific antibodies. J Heart Lung Transplant. 2020;39:732-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 98. | Talgam-Horshi E, Mozer-Glassberg Y, Waisbourd-Zinman O, Ashkenazi-Hoffnung L, Haskin O, Levi S, Hamdani G, Landau D, Alfandary H. Clinical Outcomes and Antibody Response in COVID-19-Positive Pediatric Solid Organ Transplant Recipients. Pediatr Infect Dis J. 2021;40:e514-e516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Jin X, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ