Published online May 18, 2022. doi: 10.5500/wjt.v12.i5.100
Peer-review started: March 31, 2021
First decision: July 29, 2021
Revised: August 11, 2021
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: May 18, 2022
Processing time: 407 Days and 6.5 Hours
The lack of space, as an indication for a native unilateral nephrectomy for positioning a future kidney graft in the absence of other autosomal dominant polycystic kidney disease-related symptoms, remains controversial.
To evaluate the surgical comorbidity and the impact on graft survival of an associated ipsilateral native nephrectomy during isolated kidney transplantation in patients with autosomal dominant polycystic kidney disease.
One hundred and fifty-four kidney transplantations performed between January 2007 and January 2019 of which 77 without (kidney transplant alone (KTA) group) and 77 with associated ipsilateral nephrectomy (KTIN group), were re
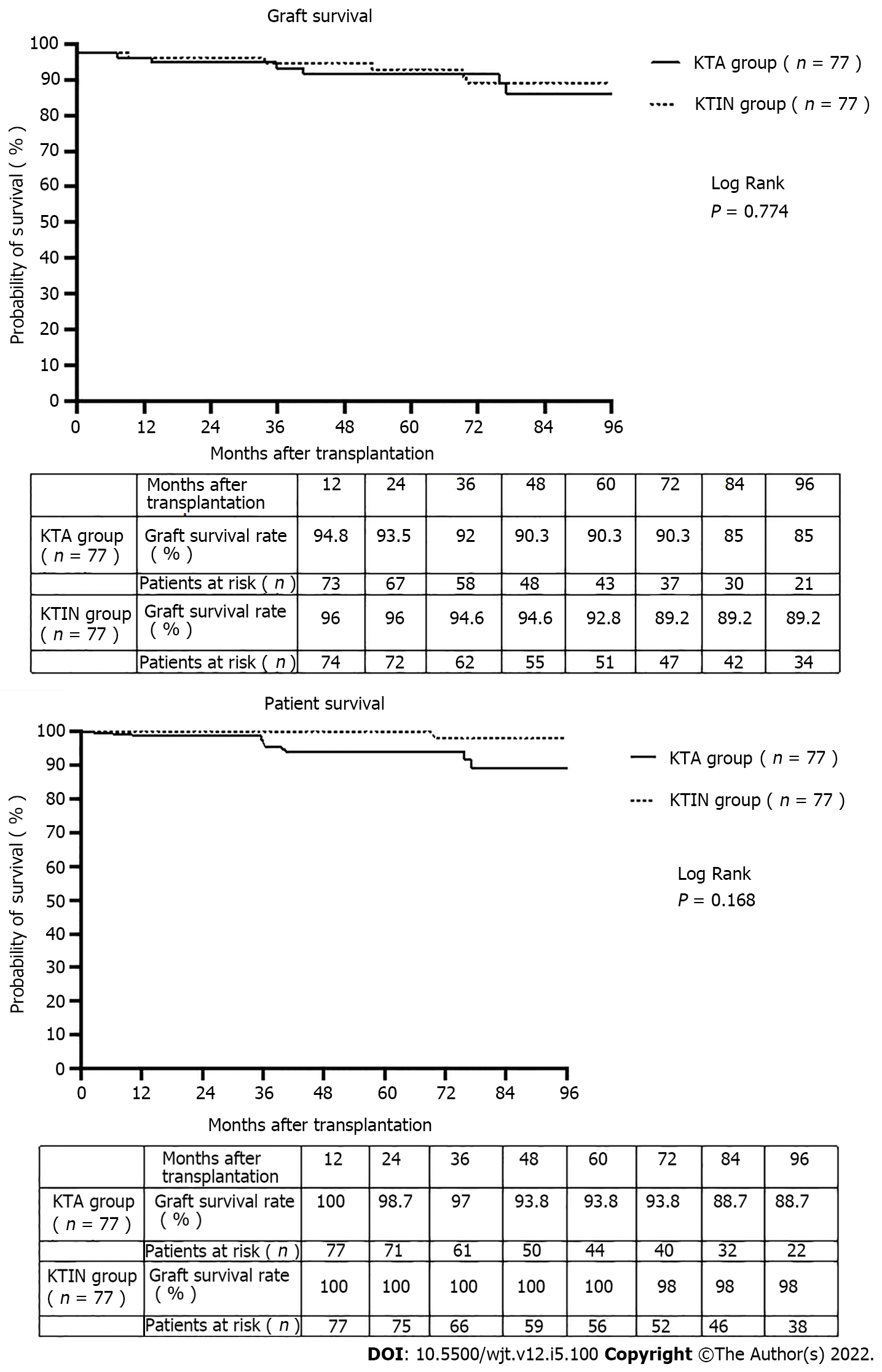
Creation of space for future graft positioning was the main reason (n = 74, 96.1%) for associated ipsilateral nephrectomy. No significant difference in surgical comorbidity (lymphocele, wound infection, incisional hernia, wound hematoma, urinary infection, need for blood transfusion, hospitalization stay, Dindo Clavien classification and readmission rate) was observed between the two study groups. The incidence of primary nonfunction and delayed graft function was comparable in both groups [0% and 2.6% (P = 0.497) and 9.1% and 16.9% (P = 0.230), respectively, in the KTA and KTIN group]. The 1- and 5-year graft survival were 94.8% and 90.3%, and 100% and 93.8%, respectively, in the KTA and KTIN group (P = 0.774). The 1- and 5-year patient survival were 96.1% and 92.9%, and 100% and 100%, respectively, in the KTA and KTIN group (P = 0.168).
Simultaneous ipsilateral native nephrectomy to create space for graft positioning during kidney transplantation in patients with autosomal dominant polycystic kidney disease does not negatively impact surgical comorbidity and short- and long-term graft survival.
Core Tip: The associated surgical comorbidity and graft survival of an ipsilateral nephrectomy during isolated kidney transplantation in patients with autosomal dominant polycystic kidney disease was evaluated. One hundred and fifty-four patients were retrospectively evaluated, of which 77 did and 77 did not undergo associated ipsilateral nephrectomy during the transplantation. In a long-term follow-up, we observed no negative impact on surgical comorbidity and graft survival of a simultaneous ipsilateral native nephrectomy to create space for graft positioning during kidney transplantation in patients with autosomal dominant polycystic kidney disease.
- Citation: Darius T, Bertoni S, De Meyer M, Buemi A, Devresse A, Kanaan N, Goffin E, Mourad M. Simultaneous nephrectomy during kidney transplantation for polycystic kidney disease does not detrimentally impact comorbidity and graft survival. World J Transplant 2022; 12(5): 100-111
- URL: https://www.wjgnet.com/2220-3230/full/v12/i5/100.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i5.100
Autosomal dominant polycystic kidney disease (ADPKD) is one of the most frequent causes of renal failure in Europe and the USA and may affect 2.5% to 10% of dialysis patients. Renal failure is the result of the development and progressive expansion of multiple bilateral cysts in the renal parenchyma, leading to a progressive decline in renal function owing to compression of normal functioning parenchyma by enlarging cysts[1-3].
Clear indications for nephrectomy before transplantation include intractable pain and discomfort, ongoing hematuria, recurrent severe cyst infections, gastrointestinal symptoms such as early satiety, recurrent nephrolithiasis and risk of malignancy[1,2,4]. Unilateral native nephrectomy to create space for graft positioning in an otherwise asymptomatic ADPKD patient is quite often routinely performed in isolated kidney transplant candidates before their activation on the waiting list. This strategy is mainly driven by the fear of increased surgical comorbidity and the possible negative impact of prolonged cold ischemia time and short- and long-term graft survival related to the associated nephrectomy during transplantation. However, many controversies still exist concerning the indication and timing of a unilateral nephrectomy to create space for graft positioning in an asymptomatic kidney transplant candidate suffering from massive enlarged polycystic kidney[3,5,6].
Therefore, this retrospective study aimed to evaluate the surgical comorbidity and the impact on early and late graft survival of an associated ipsilateral native nephrectomy during isolated kidney transplantation in ADPKD patients. Based on these results a symptom-based algorithm is proposed to decide the timing and necessity of a unilateral or bilateral nephrectomy in ADPKD candidates waiting for, or during transplantation.
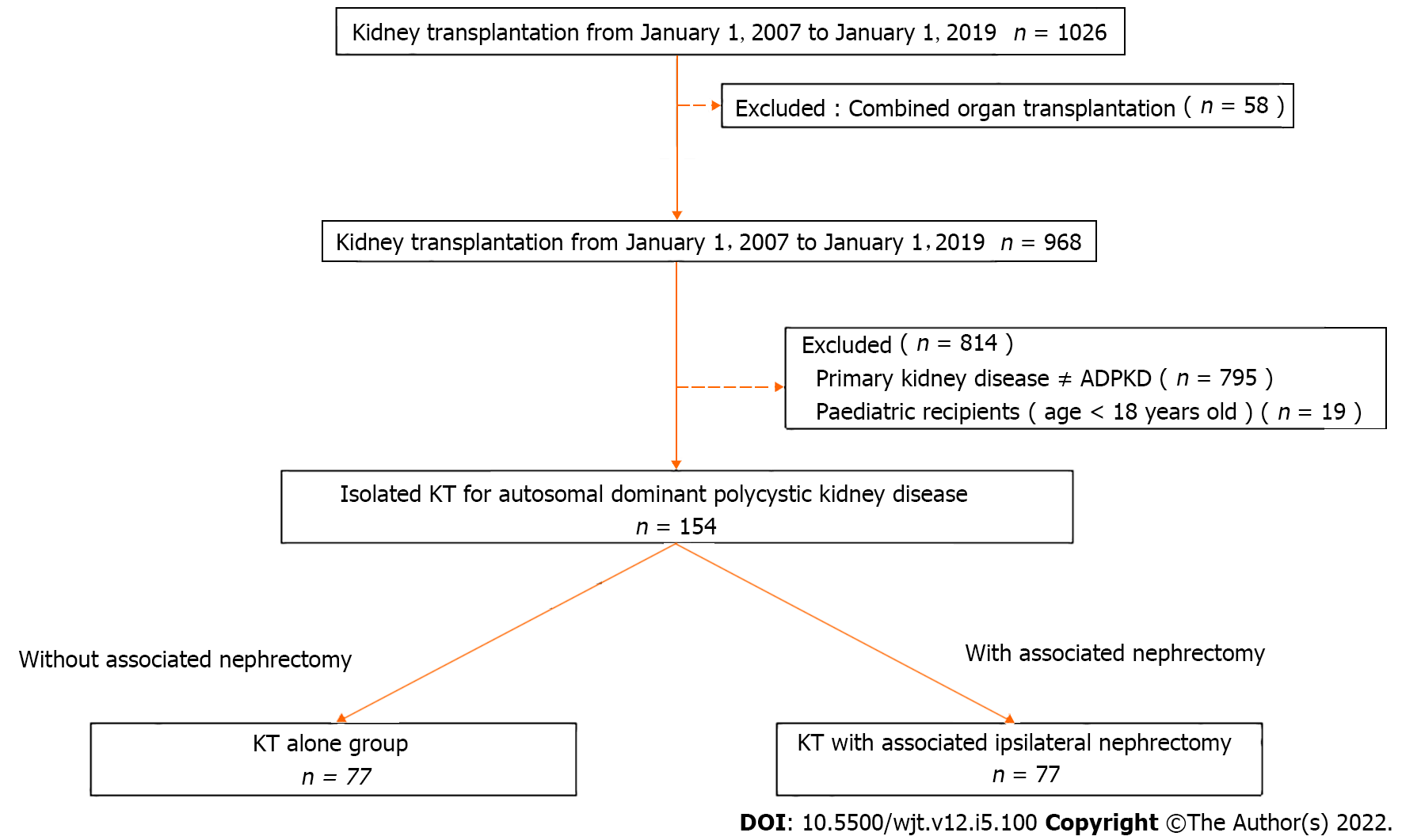
Figure 1 illustrates the selection flowchart of this retrospective study. Between January 1 2007 and January 1 2019, a total of 1026 kidney transplantations were performed at the University Clinics Saint-Luc (Brussels, Belgium) of which 154 patients underwent isolated kidney transplantation for ADPKD. This selection was obtained using the following inclusion criteria: isolated kidney transplant recipient, ADPKD as a primary cause of renal failure, age greater than 18 years old. The exclusion criteria were the following: multi-organ recipients, ADPKD not the primary kidney disease and pediatric recipients. No patients were lost from follow-up. From these 154 ADPKD patients, 77 underwent a kidney transplantation alone (KTA group) and 77 kidney transplantation with associated native ipsilateral nephrectomy (KTIN group) and were retrospectively reviewed. This study was approved by the institutional ethical committee. The following donor characteristics were analyzed: Age, gender, type of donor (living vs deceased) and type of deceased donor (donation after brain death or donation after circulatory death) and cytomegalovirus status. Recipient characteristics included: age, gender, body mass index (BMI), rank of transplant, time on dialysis and residual diuresis before transplantation, Human Leucocyte Antigen (HLA) mismatching, hemoglobin and albumin level before transplantation. Donor and recipient characteristics are presented in Table 1. Combined organ transplantation and ABO incompatible transplantations were excluded.
| KT alone group (n = 77) | KT with associated ipsilateral nephrectomy (n = 77) | P value | |
| Donor characteristics | |||
| Age, yr | 46.23 ± 14.94 | 47.40 ± 14.86 | NS |
| Gender, male/female, n (%) | 42/35 (54.5/45.5) | 37/40 (48.1/51.9) | NS |
| CMV status, negative/positive, n (%) | 32/43 (55.2/47.8) | 26/47 (35.6/64.4) | NS |
| Type of donor, living/deceased donor, n (%) | 6/71 (7.8/92.2) | 21/56 (27.3/72.7) | a |
| Type of deceased donor, DBD/DCD, n (%) | 54/17 (76.1/23.9) | 38/18 (67.9/32.1) | NS |
| Recipient characteristics | |||
| Age, yr | 57.40 ± 9.89 | 53.40 ± 9.12 | NS |
| Gender, male/female, n (%) | 48/29 (62.3/37.7) | 47/30 (61.0/38.9) | NS |
| Body mass index, kg/m² | 25.69 ± 4.00 | 25.33 ± 3.76 | NS |
| Blood group, n (%) | NS | ||
| A | 33 (42.9) | 42 (54.5) | NS |
| B | 5 (6.5) | 4 (5.2) | NS |
| AB | 0 (0) | 3 (3.9) | NS |
| O | 39 (50.6) | 28 (36.4) | NS |
| Pretransplant dialysis versus preemptive kidney transplant, n (%) | 65/12 (84.4/15.6) | 55/22 (71.4/28.6) | NS |
| Residual urine diuresis before transplant, mL | 1057.75 ± 852.84 | 1188.42 ± 818.65 | NS |
| Rank of transplant | NS | ||
| First transplant, n (%) | 73 (94.8) | 76 (98.7) | NS |
| Second transplant, n (%) | 3 (3.9) | 1 (1.3) | NS |
| Third transplant, n (%) | 1 (1.3) | 0 (0) | NS |
| Time on dialysis before transplantation, d | 1105 ± 1198 | 720 ± 757 | NS |
| HLA Mismatching (MM), n (%) | NS | ||
| 0 MM | 11 (14.3) | 6 (7.8) | |
| 1 MM | 8 (10.4) | 7 (9.1) | |
| 2 MM | 30 (39.0) | 16 (30.8) | |
| 3 MM | 23 (29.9) | 30 (39) | |
| 4 MM | 2 (2.6) | 7 (9.1) | |
| 5 MM | 3 (3.9) | 6 (7.8) | |
| 6 MM | 0 (0.0) | 5 (6.5) | |
| Hemoglobin before transplantation, g/dL | 12.47 ± 1.72 | 12.69 ± 1.18 | NS |
| Albumin before transplantation, g/dL | 4.32 ± 0.40 | 4.24 ± 0.41 | NS |
| Peritransplant plasmapheresis treatment, n (%) | 14 (18) | 3 (4) | a |
A standard kidney transplant procedure was performed by a hockey stick incision with a classical vascular reconstruction on the iliac vessels and a ureterovesical anastomosis achieved according to the extravesical approach described by Lich-Gregoir[7-9]. A ureter stent was not routinely used but only according to the surgeon's preference and indication. An associated native ipsilateral nephrectomy was performed, if indicated, before implantation of the kidney graft with cranial extension up to the costal margin of the hockey stick incision by a retroperitoneal approach. Perioperative drainage of multiple renal cysts was frequently performed to facilitate surgical resection. The following surgical characteristics were collected: indications for associated ipsilateral native nephrectomy, total surgical time, anastomosis time (defined as the time from the start of vascular anastomosis to reperfusion of the kidney), cold ischemia time (defined as the time from the start of in situ cold perfusion of the kidney in the deceased donor or ex vivo cold perfusion of the kidney in a living donor to the start of in situ vascular anastomosis in the recipient) and weight of the removed native polycystic kidney.
A triple-drug protocol consisting of tacrolimus (Advagraf, Astellas Pharma BV, Brussels, Belgium), methylprednisolone (Medrol, Pfizer NV, Brussels, Belgium) and mycophenolate mofetil (Cellcept, 2x500 mg/d, NV Roche SA, Brussels, Belgium) was used during the whole study period in all except one patient. Induction therapy with basiliximab (Simulect, Novartis Pharma GmbH, Neurenberg, Germany) on day 0 and 4 and thymoglobuline 1.25 mg/kg (Thymoglobulin, Sanofi Genzyme Europe B.V., Amsterdam, The Netherlands) day 1 until day 4 after transplantation was used in recipients of a living donor graft and a donor after circulatory death, respectively. Plasmapheresis was applied in highly immunized recipients until one month after transplantation. Tacrolimus trough levels (T0) were between 10 and 14 ng/mL, 7 and 10 ng/mL and 5 and 7 ng/mL, during the first month, between the second and third month and from 3 mo after transplantation, respectively. Methylprednisolone was started immediately after transplant at 16 mg/d and tapered (minus 4 mg every 2 wk) to a fixed dose of 4 mg for all recipients at long-term. Co-trimoxazole prophylaxis was given to all patients during the first 6 mo after transplantation. Valganciclovir prophylaxis (900 mg/d for normal kidney function) was given to all patients during the first 6 mo after transplantation with the exception of cytomegalovirus donor seronegative/recipient seronegative patients. Biopsy-proven acute cellular rejection and humoral rejections were treated with methylprednisolone boluses for 3 d and plasmapheresis, respectively.
During the transplant hospitalization, the patients were monitored daily to evaluate comorbidity and kidney function was evaluated by serum creatinine and urine analysis. If primary nonfunction (PNF), delayed graft function (DGF) or vascular problems of the kidney graft were suspected, an urgent ultrasound was performed. Otherwise, a baseline ultrasound was performed at the end of the transplant hospitalization. Ambulatory follow-up of the kidney graft function (measured by serum creatinine and urine analysis) and surgical comorbidity was performed according to local center practice. No protocol, only indication biopsies of the kidney graft were performed after the preceding ultrasound. Every year after transplantation, an ultrasound of the kidney graft and the native kidneys was performed. If malignancy of the native kidneys was suspected, nuclear magnetic resonance was carried out.
The primary endpoint was surgical comorbidity, measured as the incidence of postoperative lymphocele, wound infection, incisional hernia, wound hematoma, urinary infection, need for peritransplant (during and after transplantation) blood transfusion, pulmonary embolism, total hospital stay, readmission rate and surgical complications classified according to the Dindo Clavien classification[10]. Secondary endpoints were the incidence of PNF, DGF (defined as the need for dialysis during the first week after transplantation), venous or arterial kidney graft thrombosis, acute rejection incidence and type of rejection (cellular vs humoral) during the first year after transplantation and the 1- and 5-year patient- and graft survival rate.
Characteristics of the donor, the recipient and the transplant outcome were compared using the chi-square test for categorical variables and the t-test for continuous variables. Continuous variables are provided as means and standard deviations. Log-rank statistics were used with the Kaplan-Meier product-limit method to evaluate the associations of individual covariates with allograft survival. P < 0.05 was considered statistically significant. Statistical analysis and plots were accomplished with SPSS 24.0 statistical software (SPSS, Chicago, IL, USA) and Prism 8.2.0 (Graphpad Software, San Diego, CA, USA).
Donor and recipient characteristics of both study groups were comparable, with the exception of the incidence of living donation, which was significantly higher in the KTIN group compared with the KTA group [21 (27.3%) vs 6 (7.8%), P = 0.003] (Table 1). Peritransplant plasmapheresis was performed in 14 (18%) and 3 (4%) immunized recipients in the KTA and the KTIN group, respectively (P = 0.008).
The main indications for performing an associated ipsilateral native nephrectomy at the same site as the kidney transplantation were lack of space for graft positioning (n = 74; 96.1%), pain (n = 29; 37.7%) and hematuria (n = 30; 39.0%). Pain, as the only reason for ipsilateral nephrectomy, was present in 3 (3.9%) patients. The decision not to perform an associated ipsilateral native nephrectomy was taken by the surgeon during the transplant procedure if enough space for graft positioning in combination with the absence of other ADPKD-related symptoms was estimated at the moment of transplantation. No difference in anastomosis and cold ischemia time was observed between the two study groups (Table 2). The total surgical time was significantly longer in the KTIN group as compared with the KTA group (223.29 ± 71.96 vs 169.07 ± 44.31, respectively; P = 0.005). The mean weight of the removed polycystic kidney was 2073.94 ± 1197.89 g.
| KT alone group (n = 77) | KT with associated ipsilateral nephrectomy (n = 77) | P value | |
| Indications for associated nephrectomy, n (%) | |||
| Creating space for graft positioning, n (%) | 74 (96.1) | ||
| Pain, n (%) | 29 (37.7) | ||
| Recurrent urinary tract infections, n (%) | 11 (14.3) | ||
| Hematuria, n (%) | 30 (39.0) | ||
| Digestive symptoms, n (%) | 3 (3.9) | ||
| Lithiasis, n (%) | 9 (11.7) | ||
| Anastomosis time1, min | 39.61 ± 9.782 | 36.96 ± 10.10 | NS |
| Cold ischemia time, min | 827.56 ± 446.12 | 767.87 ± 436.81 | NS |
| Total surgical time, min | 169.07 ± 44.31 | 223.29 ± 71.96 | a |
| Weight of removed native kidney, g | 2073.94 ± 1197.89 |
No significant difference in surgical comorbidity (lymphocele, wound infection, incisional hernia, wound hematoma, pulmonary embolism, urinary infection, need for peritransplant blood transfusion, hospitalization stay, readmission rate and Dindo Clavien classification) was observed between the two study groups (Table 3).
| KT alone group (n = 77) | KT with associated ipsilateral nephrectomy (n = 77) | P value | |
| Surgical comorbidity | |||
| Lymphocele, n (%) | 5 (6.5) | 7 (9.1) | NS |
| Wound infection, n (%) | 6 (7.8) | 2 (2.6) | NS |
| Incisional hernia, n%) | 0 (0) | 3 (3.9) | NS |
| Wound hematoma, n (%) | 6 (7.8) | 3 (3.9) | NS |
| Pulmonary embolism, n (%) | 1 (1.3) | 0 (0) | NS |
| Urinary infection, n (%) | 14 (18.2) | 8 (10.4) | NS |
| Need for blood transfusion, n (%) | 22 (28.6) | 34 (44.2) | NS |
| Hospital stay after transplantation, d | 15.22 ± 6.662 | 14.81 ± 6.44 | NS |
| Readmission rate during whole follow-up, n (%) | 42 (46.2) | 49 (63.6) | NS |
| Dindo Clavien classification | NS | ||
| Class I | 36 (46.8) | 33 (42.9) | NS |
| Class II | 22 (28.6) | 32 (41.6) | NS |
| Class III | 7 (9.1) | 3 (3.9) | NS |
| Class IV | 12 (15.6) | 9 (11.7) | NS |
| Clinical outcomes | |||
| Primary nonfunction, n (%) | 0 (0) | 2 (2.6) | NS |
| Delayed graft function, n (%) | 7 (9.1) | 13 (16.9) | NS |
| Renal artery thrombosis of kidney graft, n (%) | 2 (2.6) | 0 (0) | NS |
| Renal vein thrombosis of kidney graft, n (%) | 2 (2.6) | 0 (0) | NS |
| Acute rejection episode within 1 year after transplantation, n (%) | 5 (6.5) | 5 (6.5) | NS |
| Cellular, n (%) | 5 (100) | 2 (40) | |
| Humoral, n (%) | 0 (0) | 3 (60) | |
The incidence of PNF and DGF was comparable in both groups [0% vs 2.6% (P = 0.497) and 9.1% vs 16.9% (P = 0.230), respectively, in the KTA and KTIN group] (Table 3). No significant difference in renal artery and vein thrombosis of the kidney graft was observed between the two study groups. In addition, the incidence of acute rejection within one year after transplantation was comparable among the groups.
The 1- and 5-year graft survival were 94.8% and 90.3, and 100% and 93.8%, respectively, in the KTA and KTIN group (P = 0.774) (Figure 2). The 1- and 5-year patient survival were 96.1% and 92.9%, and 100% and 100%, respectively, in the KTA and KTIN group (P = 0.168) (Figure 2).
This retrospective single-center study is one of the largest series to demonstrate the absence of a negative impact on surgical comorbidity and short- and long-term kidney graft function following an associated ipsilateral native nephrectomy to create space for graft positioning during isolated kidney transplantation in ADPKD patients compared with ADPKD kidney transplant recipients without simultaneous nephrectomy.
The lifetime nephrectomy rate of at least one kidney is approximately 20-30% for patients with ADPKD[11,12]. Maintaining native kidneys in ADPKD transplant candidates may help to prevent renal osteodystrophy, anemia, uremia, fluid overload, congestive heart failure, and hyperkalemia[4,13,14]. The advantage of maintaining total native urine output is important for dialysis comfort in patients on the waiting list for transplantation and confers some survival benefits on the waiting list[15]. Even today, the indications and timing for a native unilateral or bilateral nephrectomy in ADPKD candidates for isolated kidney transplantation remain controversial and are quite often center-dependent and based on historical routine and experience. Clear indications for unilateral or bilateral native nephrectomy before transplantation are: (1) Invalidating pain and discomfort; (2) Ongoing hematuria; (3) Recurrent renal cyst infections and gastrointestinal pressure symptoms (e.g., early satiety); (4) recurrent nephrolithiasis (rare); (5) The suspicion of malignancy in those with complex cysts; and (6) Combined liver and kidney transplantation. In the absence of these clear indications, the lack of space for positioning a future kidney graft remains controversial as an indication for performing a unilateral native nephrectomy before transplantation. We agree that a simultaneous ipsilateral nephrectomy to create space during isolated kidney transplantation can be technically challenging, even in the hands of an experienced surgeon. A review of the literature, as illustrated in Table 4, does not demonstrate a significant negative impact of an associated ipsilateral or bilateral nephrectomy during isolated kidney transplantation on surgical comorbidity and early and late allograft and patient survival[16-20]. The advantage of performing the nephrectomy simultaneous with the transplantation is the avoidance of an extra anesthetic/surgical procedure and possible oliguria when performed before transplantation during the time on the waiting list. In line with these previous studies, the risk of losing a kidney graft, in relation to native nephrectomy is extremely low.
| Ref. | Study group (n) | Type of donor | Isolated KT with simultaneous native bilateral or unilateral nephrectomy | KT alone | Study conclusions | |
| Bilateral | Unilateral | |||||
| Nunes P et al[13], 2007 | 1 (143) | LD (6%) + DD (94%) | + | Comparable overall complication rate and graft survival after 5 years if unilateral nephrectomy is performed for creation of space for a renal allograft | ||
| 2 (16) | LD (2%) + DD (98%) | + | ||||
| Kramer A et al[14], 2009 | 1 (20) | LD (100%) | + | Minimal morbidity of an associated bilateral nephrectomy during transplantation and graft and patient survival of 100% during 5-year follow-up | ||
| Skauby MH et al[15], 2012 | 1 (79); 2 (78) | LD (100%) | + | + | Associated bilateral nephrectomy results in a longer hospital stay and more postoperative complications. No difference in 1- and 5-year patient and graft survival | |
| Neeff HP et al[16], 2013 | 1 (100) | LD (38%) + DD (62%) | + | Routine ipsilateral nephrectomy, independent of volume of polycystic kidney, during transplantation is a safe procedure without endangering patient or graft survival. The death of 3 patients in the first year post-transplant is a concern | ||
| Ahmad SB et al[17], 2016 | 1 (66) | LD (100%) | + | In symptomatic patients with ADPKD, the combined procedure is advantageous, especially in terms of patient satisfaction | ||
| 2 (52) | + | |||||
| Current study | 1 (77) | LD (7.8%) + DD (92.2%) | + | Comparable surgical comorbidity and 1- and 5-year patient and graft survival | ||
| 2 (77) | LD (27.3%) + DD (72.7%) | + | ||||
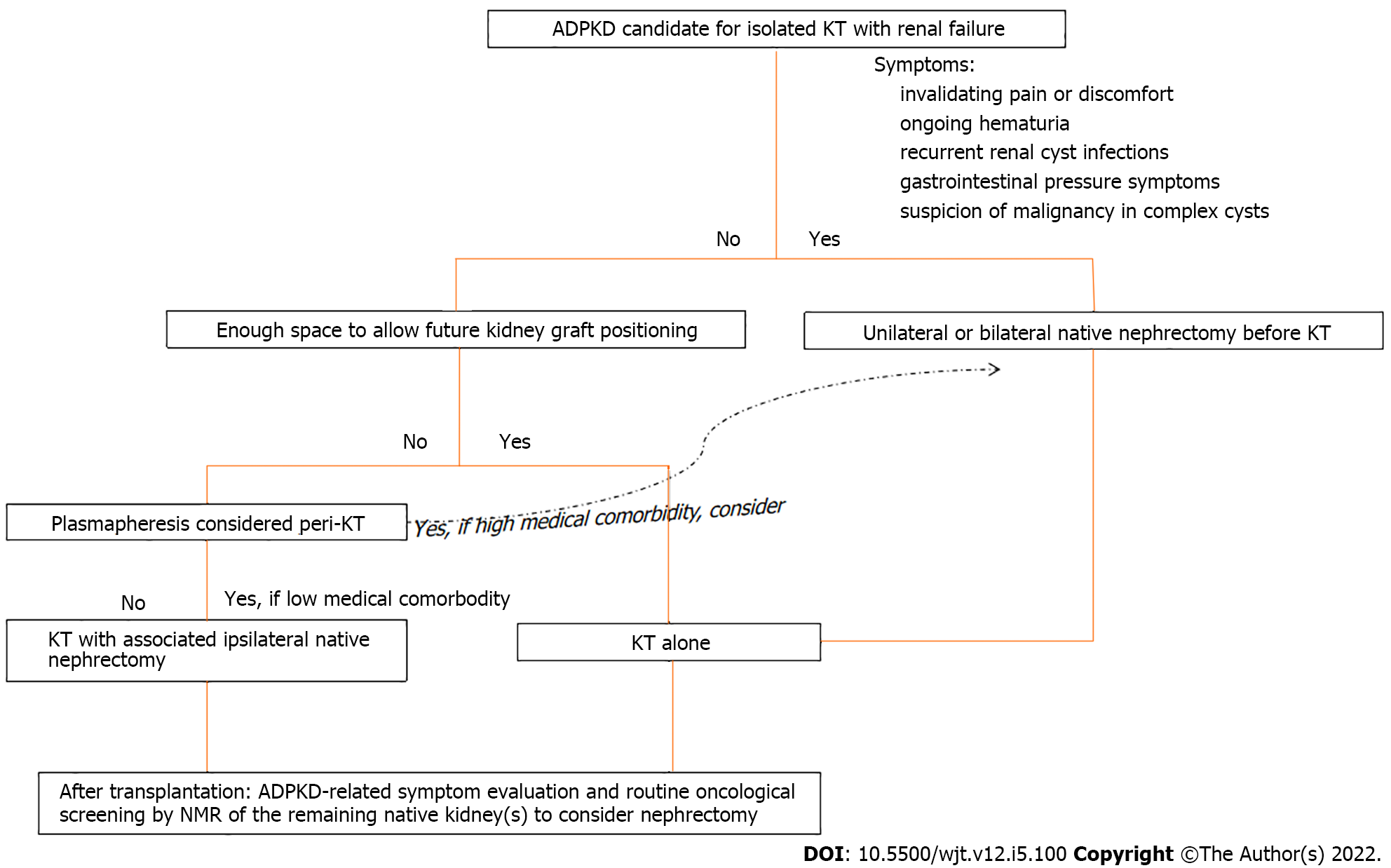
The proposed algorithm to decide the optimal timing of a native nephrectomy in candidates for isolated kidney transplantation is mainly based on ADPKD-related symptoms (Figure 3). In general, we do not perform a native nephrectomy to create space for graft positioning in the absence of ADPKD-related symptoms before transplantation but by preference during the transplantation. Patients with a pretransplant clinical examination showing a polycystic kidney below the level of the umbilicus or a radiological image showing a polycystic kidney extending into the iliac fossa, are very likely to need an ipsilateral native nephrectomy during transplant. Our center policy is to add peritransplant plasmapheresis to the standard immunosuppressive therapy in all high-immunized patients. Therefore, only for high-immunized patients with an expected long waiting time on the transplant list and high associated medical comorbidity, we consider a unilateral nephrectomy to create space for future kidney graft positioning before transplantation with the aim to decrease the risk of plasmapheresis-related surgical complications (bleeding, incisional hernias, blood transfusions, ...) during transplantation. This might explain the difference in the numbers of patients receiving peritransplant plasmapheresis in favor of the KTA group in our study. For high-immunized patients with low associated medical comorbidity, our preference is to perform the associated nephrectomy during the transplantation.
Also, our strategy is to avoid an unnecessary nephrectomy after transplantation. Today, it is unusual for ADPKD patients to require nephrectomies for complications related to their native kidney (< 20%) after transplantation[5]. Nuclear magnetic resonance of the abdomen is routinely performed after transplantation to screen for malignancies in the native polycystic kidney(s). Conflicting data exists regarding the risk of renal cell carcinomas in ADPKD-affected kidneys. While case studies report the occurrence of renal cell carcinomas in ADPKD-affected kidneys[21,22], these tumors may be partly due to acquired renal cystic disease resulting from long-term dialysis[23]. In contrast, data from the Scientific Registry of Transplant Recipients observed a lower cancer risk in polycystic kidney disease recipients. This might be explained by the ADPKD mutations causing clear cyst formation, but it is also possible that these mutations trigger protective cellular mechanisms that prevent cells from undergoing malignant transformation[24]. Ward CJ et al[25] demonstrated that germline mutations in polycystic kidney and hepatic disease 1 were protective against colorectal cancer. However, the observed lower risk of renal cancer in the Scientific Registry of Transplant Recipients can also be explained by the higher incidence of nephrectomies in ADPKD recipients in contrast with their non-ADPKD counterparts[24].
We recognize some limitations in the present study. First, this a retrospective study. Second, in recipients with associated nephrectomy during isolated kidney transplantation, the lower incidence of peritransplant plasmapheresis and higher incidence of living donors could have underestimated the surgical comorbidity in this study group.
In conclusion, simultaneous native ipsilateral nephrectomy to create space for graft positioning during kidney transplantation in ADPKD patients does not detrimentally impact surgical comorbidity and short- and long-term graft survival.
The lack of space, as an indication for a native unilateral nephrectomy for positioning a future kidney graft in the absence of other autosomal dominant polycystic kidney disease (ADPKD)-related symptoms, remains controversial.
Unilateral native nephrectomy to create space for graft positioning in an otherwise asymptomatic ADPKD patient is quite often routinely performed in isolated kidney transplant candidates before their activation on the waiting list. This strategy is mainly driven by the fear of increased surgical comorbidity and the possible negative impact of prolonged cold ischemia time and short- and long-term graft survival related to the associated nephrectomy during transplantation.
To evaluate the surgical comorbidity and the impact on graft survival of an associated ipsilateral native nephrectomy during isolated kidney transplantation in patients with ADPKD.
One hundred and fifty-four kidney transplantations performed between January 2007 and January 2019 of which 77 without (kidney transplant alone (KTA) group) and 77 with associated ipsilateral nephrectomy (KTIN group), were retrospectively reviewed. Demographics and surgical variables were analyzed and their respective impact on surgical comorbidity and graft survival.
No significant difference in surgical comorbidity (lymphocele, wound infection, incisional hernia, wound hematoma, urinary infection, need for blood transfusion, hospitalization stay, Dindo Clavien classification and readmission rate) was observed between the two study groups. The 1- and 5-year graft survival were 94.8% and 90.3%, and 100% and 93.8%, respectively, in the KTA and KTIN group (P = 0.774). The 1- and 5-year patient survival were 96.1% and 92.9%, and 100% and 100%, respectively, in the KTA and KTIN group (P = 0.168).
Simultaneous ipsilateral native nephrectomy to create space for graft positioning during kidney transplantation in patients with ADPKD does not negatively impact surgical comorbidity and short- and long-term graft survival.
More kidney transplant candidates suffering from ADPKD when activated on the waiting list should be proposed for an associated ipsilateral nephrectomy during the transplantation instead of routinely programmed pretransplant nephrectomy.
| 1. | Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1020] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 2. | Kanaan N, Devuyst O, Pirson Y. Renal transplantation in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2014;10:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Akoh JA. Current management of autosomal dominant polycystic kidney disease. World J Nephrol. 2015;4:468-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Kirkman MA, van Dellen D, Mehra S, Campbell BA, Tavakoli A, Pararajasingam R, Parrott NR, Riad HN, McWilliam L, Augustine T. Native nephrectomy for autosomal dominant polycystic kidney disease: before or after kidney transplantation? BJU international. 2011;108:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Gebicki JM. Classic redox. Garrison WM, Jayko ME, Bennett W. Radiation-induced oxidation of protein in aqueous solution. Radiat Res 1962; 16: 483-502. Redox Rep. 2000;5:15-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Argyrou C, Moris D, Vernadakis S. Tailoring the 'Perfect Fit' for Renal Transplant Recipients with End-stage Polycystic Kidney Disease: Indications and Timing of Native Nephrectomy. In Vivo. 2017;31:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Gregoir W. [Congenital vesico-ureteral reflux]. Acta urologica Belgica. 1962;30:286-300. [PubMed] |
| 8. | Lich R, Jr. , Howerton LW, Davis LA. Childhood urosepsis. J Kentucky Medical Association. 1961;59:1177-1179. [RCA] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 190] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Woodruff MF, Nolan B, Robson JS, MacDonald MK. Renal transplantation in man. Experience in 35 cases. Lancet. 1969;1:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vanlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Annals of surgery. 2009;250:187-196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9241] [Article Influence: 543.6] [Reference Citation Analysis (1)] |
| 11. | Sulikowski T, Tejchman K, Zietek Z, Rózański J, Domański L, Kamiński M, Sieńko J, Romanowski M, Nowacki M, Pabisiak K, Kaczmarczyk M, Ciechanowski K, Ciechanowicz A, Ostrowski M. Experience with autosomal dominant polycystic kidney disease in patients before and after renal transplantation: a 7-year observation. Transplant Proc. 2009;41:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Patel P, Horsfield C, Compton F, Taylor J, Koffman G, Olsburgh J. Native nephrectomy in transplant patients with autosomal dominant polycystic kidney disease. Ann R Coll Surg Engl. 2011;93:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Brazda E, Ofner D, Riedmann B, Spechtenhauser B, Margreiter R. The effect of nephrectomy on the outcome of renal transplantation in patients with polycystic kidney disease. Ann Transplant. 1996;1:15-18. [PubMed] |
| 14. |
Pirson Y, Christophe JL, Goffin E. Outcome of renal replacement therapy in autosomal dominant polycystic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association.
European Renal Association. 1996;11 Suppl |
| 15. | Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061-1070. [RCA] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Nunes P, Mota A, Alves R, Figueiredo A, Parada B, Macário F, Rolo F. Simultaneous renal transplantation and native nephrectomy in patients with autosomal-dominant polycystic kidney disease. Transplant Proc. 2007;39:2483-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Kramer A, Sausville J, Haririan A, Bartlett S, Cooper M, Phelan M. Simultaneous bilateral native nephrectomy and living donor renal transplantation are successful for polycystic kidney disease: the University of Maryland experience. J Urol. 2009;181:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Skauby MH, Øyen O, Hartman A, Leivestad T, Wadström J. Kidney transplantation with and without simultaneous bilateral native nephrectomy in patients with polycystic kidney disease: a comparative retrospective study. Transplantation. 2012;94:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Neeff HP, Pisarski P, Tittelbach-Helmrich D, Karajanev K, Neumann HP, Hopt UT and Drognitz O. One hundred consecutive kidney transplantations with simultaneous ipsilateral nephrectomy in patients with autosomal dominant polycystic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association. European Renal Association. 2013;28:466-471. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Ahmad SB, Inouye B, Phelan MS, Kramer AC, Sulek J, Weir MR, Barth RN, LaMattina JC, Schweitzer EJ, Leeser DB, Niederhaus SV, Bartlett ST, Bromberg JS. Live Donor Renal Transplant With Simultaneous Bilateral Nephrectomy for Autosomal Dominant Polycystic Kidney Disease Is Feasible and Satisfactory at Long-term Follow-up. Transplantation. 2016;100:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Hajj P, Ferlicot S, Massoud W, Awad A, Hammoudi Y, Charpentier B, Durrbach A, Droupy S, Benoît G. Prevalence of renal cell carcinoma in patients with autosomal dominant polycystic kidney disease and chronic renal failure. Urology. 2009;74:631-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Jilg CA, Drendel V, Bacher J, Pisarski P, Neeff H, Drognitz O, Schwardt M, Gläsker S, Malinoc A, Erlic Z, Nunez M, Weber A, Azurmendi P, Schultze-Seemann W, Werner M, Neumann HP. Autosomal dominant polycystic kidney disease: prevalence of renal neoplasias in surgical kidney specimens. Nephron Clin Pract. 2013;123:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Ishikawa I, Hayama S, Morita K, Nakazawa T, Yokoyama H, Honda R, Satoh K, Kakuma T. Long-term natural history of acquired cystic disease of the kidney. Ther Apher Dial. 2010;14:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Wetmore JB, Calvet JP, Yu AS, Lynch CF, Wang CJ, Kasiske BL, Engels EA. Polycystic kidney disease and cancer after renal transplantation. J Am Soc Nephrol. 2014;25:2335-2341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Ward CJ, Wu Y, Johnson RA, Woollard JR, Bergstralh EJ, Cicek MS, Bakeberg J, Rossetti S, Heyer CM, Petersen GM, Lindor NM, Thibodeau SN, Harris PC, Torres VE, Hogan MC, Boardman LA. Germline PKHD1 mutations are protective against colorectal cancer. Hum Genet. 2011;129:345-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Belgian Transplant Society; European Society for Organ Transplantation; The Transplantation Society.
Specialty type: Transplantation
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jesus-Silva SG, Brazil S-Editor: Wang LL L-Editor: Webster JR P-Editor: Cai YX