Published online Aug 18, 2021. doi: 10.5500/wjt.v11.i8.344
Peer-review started: February 22, 2021
First decision: May 5, 2021
Revised: June 16, 2021
Accepted: August 10, 2021
Article in press: August 10, 2021
Published online: August 18, 2021
Processing time: 171 Days and 3.4 Hours
The recently emergent disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), transmitted by droplets and aerosols, was named coronavirus disease 2019 (COVID-19) by World Health Organization. Predominantly, the disease progress is asymptomatic or mild, but one-fifth of the patients advance to severe or critical illness. In severe COVID-19 patients, type-2 T helper cells release numerous cytokines; this excessive immune response is named as cytokine storm. The cytokine storm, which is the hallmark of the COVID-19 induced by the disease and aggravates due to lack of proper immune response, similar to SARS and Middle East respiratory syndrome (MERS), and the disease status may progress forward to acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome, multi-organ dysfunction syndrome, and death. Mesenchymal stromal cell transplantation is up-and-coming in treating many diseases such as HIV, hepatitis B, influenza, coronavirus diseases (SARS, MERS), lung injuries, and ARDS. Upon closer inspection on respiratory diseases, COVID-19, influenza, SARS, and MERS have similarities in patho
Core Tip: Upon closer inspection on respiratory diseases, coronavirus disease 2019 (COVID-19), influenza, severe acute respiratory syndrome, and Middle East respiratory syndrome have similarities in pathogenesis, especially cytokine and immune response profiles. These comparable features in terms of the cytokine storm will provide hints for the treatment of COVID-19. Transplantation of mesenchymal stromal cells provides tissue regeneration and rejuvenation with immunotolerant and immunomodulant properties on damaged tissues by exerting their effects through immune cells.
- Citation: Sütlüoğlu H, Özdemir Ö. May mesenchymal stem cell transplantation be a solution for COVID-19 induced cytokine storm? World J Transplant 2021; 11(8): 344-355
- URL: https://www.wjgnet.com/2220-3230/full/v11/i8/344.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i8.344
The substantial clues of a novel severe acute respiratory syndrome (SARS)-like coronavirus and possible outbreak were predicted with highlighting pandemic preparedness by Ge et al[1] from Wuhan in 2013. Two years after this study, Menachery et al[2] had been likewise drawing attention to a potential risk of SARS coronavirus (SARS-CoV) re-emergence from viruses circulating in bat populations. In late 2019, a few patients related to a seafood market in Wuhan province from China with the symptoms of fever, myalgia, dry cough, dyspnea, headache, sore throat, diarrhea, nausea, vomiting, and SARS-like viral pneumonia were reported[3,4]. Further analyses have shown a novel single-stranded enveloped RNA virus from the Coronaviridae family, and the genome sequence of the virus had 96.2% similarity with other bat betacoronaviruses causing previous diseases [79.6% with SARS, approximately 50% with Middle East respiratory syndrome (MERS)]. The recently emergent disease caused by the SARS-CoV-2 virus, transmitted by droplets and aerosols, was named coronavirus disease 2019 (COVID-19) by World Health Organization (WHO)[5-8]. As a consequence of increasing cases, WHO has announced that the outbreak would be assessed as a pandemic from March 11st, 2020, onward[9]. The disease differs from influenza-related pneumonia in that it is able to progress very seriously, even in young people without comorbidity. To date, mainly being in the first place thousands of bravely health workers like Li Wenliang, more than 4 million deaths and 200 million cases were confirmed, and the outbreak still has destructible impacts on economic and social circumstances[10-12]. The disease currently does not have any curable therapy. Recently discovered vaccines are being commenced to use in many countries with emergency use authorization[13,14]. Several new variants were reported, which are thought to be more infectious from the United Kingdom and numerous countries[15,16]. Due to this manner, the need for new and effective treatments continues.
Predominantly, the disease progress is asymptomatic or mild, but one-fifth of the patients advance to severe or critical illness[17]. In severe COVID-19 patients, type-2 T helper (Th) cells release numerous cytokines; this excessive immune response is named as ‘cytokine storm’, which is the leading cause of lung injury, acute respiratory distress syndrome (ARDS), multi-organ dysfunction syndrome (MODS), and death[18,19]. In this review, we aim to outline the usage of mesenchymal stem cells (MSCs) or, in other words, mesenchymal stromal cells, which have immunosuppressant and immunomodulatory benefits in countless diseases such as graft-vs-host disease, Crohn's disease, and some type of lung injuries, on severe or critically ill COVID-19 patients[20-24].
In the eighty percent of the people who have been exposed to the SARS-CoV-2 via droplets and aerosols from an infected person, the disease remains limited in the upper respiratory tract. However, in the rest of the patients, the virus proceeds to the lower respiratory tract, and with pulmonary involvement, it causes more severe illness. Disease mortality was reported between 0.5 and 2 percent in different studies and changes with obesity, older age, hypertension, and underlying chronic medical conditions[25-27]. The infectious process might occur progressively in a wide range of manifestations with life-threatening cardiovascular, thromboembolic, neurological, and respiratory complications[4,19,28,29]. As compatible with virus-cell invasion pathophysiology, organ involvement is correlated with the expression of host cells' angiotensin-converting enzyme 2 (ACE2) receptor and transmembrane protease, serine 2 (TMPRSS2) enzyme[30,31]. Unfortunately, the ACE2 receptor is widely distributed on the human cell surface, like lung, intestine, liver, kidney, brain, especially the alveolar type II (AT2) cells, capillary endothelium, and the AT2 cells highly express TMPRSS2[32-34]. On the grounds of that, the primary target of the virus is the lung. Moreover, the maladaptive immune response in severely ill patients damages the airways and causes a terrible cytokine storm characterized by elevated blood cytokine levels as a consequence of hyperactivation of the immune cells and impaired feedback mechanism. However, it leads to excessive infiltration of monocytes, macrophages, and T cells in the lungs. Therefore, disease severity in patients is due to not only the viral infection but also the host response. A notable example of this condition might be multisystem inflammatory syndrome in children and multisystem inflammatory syndrome in adults[35,36]. This uncontrolled hyperinflammatory response catalyzes multi-organ damage leading to multi-organ failure, especially of the cardiac, hepatic, and renal systems[18,29]. These organ failures raise the mortality rate, such as most patients with SARS-CoV-2 infection who developed acute kidney injury or have existing chronic kidney disease eventually died[37]. At present, no curative and effective COVID-19 treatment available, and the primary approach to patients is supportive care such as oxygen therapy (such as high flow nasal cannula oxygen therapy, mechanic ventilation), antipyretics, or venous thromboembolism prophylaxis[38,39]. Various drugs and supplementary therapies like antivirals (remdesivir, lopinavir/ritonavir, oseltamivir, favipiravir), antibiotics (azithromycin), immunomodulatory drugs (tocilizumab, hydroxychloroquine, convalescent plasma, anakinra, etc.) are being still investigated, but none of the therapies have reliable evidence[17]. To date, the only drug which is evidenced to decrease the mortality rate in severe and critically ill patients is corticosteroids[40]. Also, a specific agent to alleviate the SARS-CoV-2 induced cytokine storm is not developed as yet, and drugs that are aimed at this phenomenon are non-specific. Suppressing the excessive immune response is the key difficulty of the therapy options[41]. It is thought that people who overcome COVID-19 might have long-term sequels and different organ damages, notably lung and heart[42,43]. Multipotent MSC transplantation could promote lung and other damaged tissue repairs with its differentiation and paracrine secretory properties (exosomes/extracellular vesicles) and may prevent morbidities[44-47].
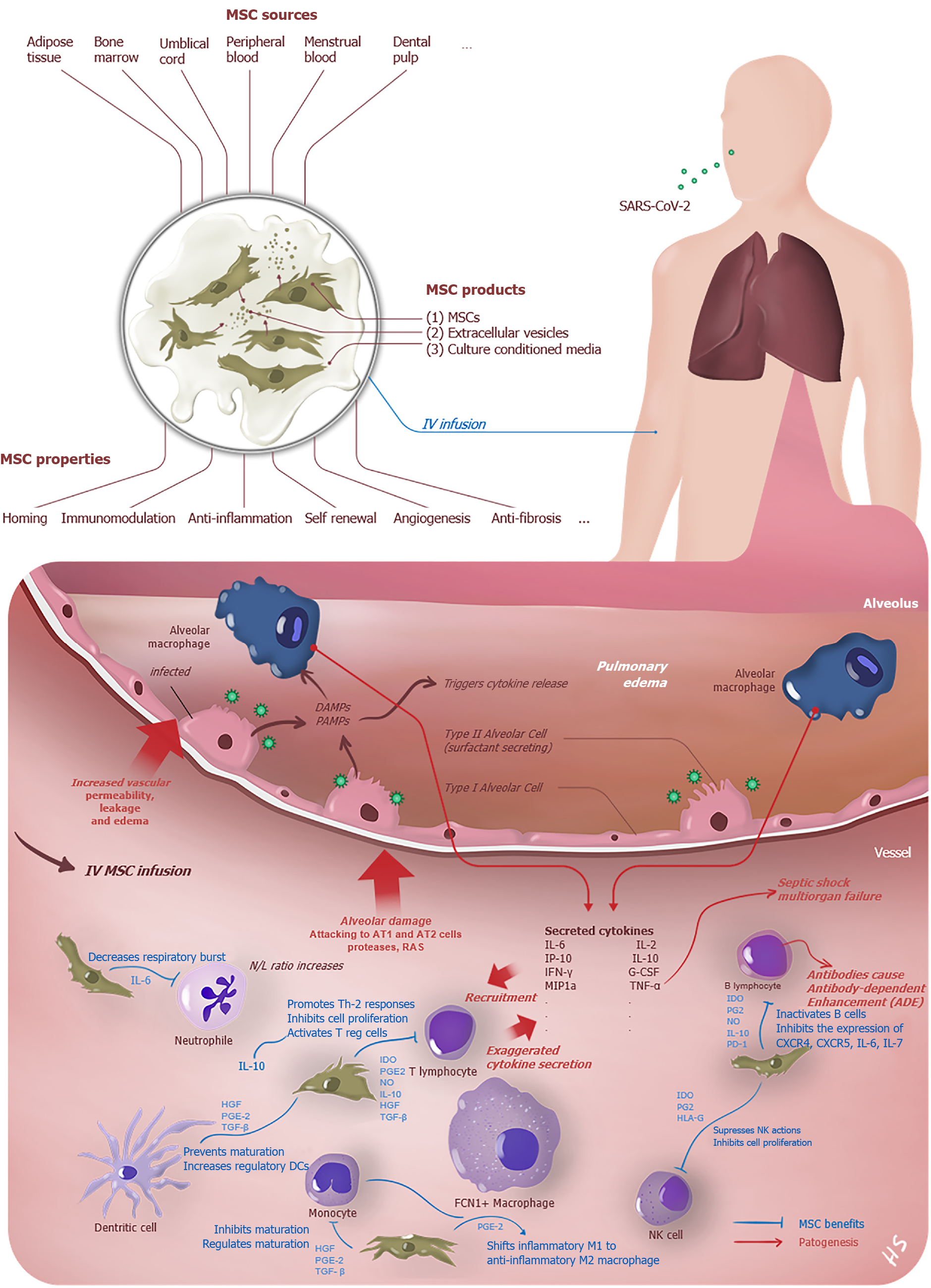
The virus that reaches the lungs from the upper respiratory tract via the ACE2 receptor infects AT2 cells here. After intracellular replication, it spreads to the parenchyma by exocytosis and causes epithelial and endothelial damage. The alveolar macrophages recognize damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) that arise from infected both AT-1 and AT-2 type dead cells, and the initiation of the inflammation is triggered (Figure 1). Thus, numerous chemokines and cytokines are started to secret excessively by lung and peripheral immune cells[18]. The cytokine storm, which is the hallmark of the COVID-19 induced by the disease and aggravates due to lack of proper immune response, similar to SARS and MERS, and the clinical status may progress forward ARDS, systemic inflammatory response syndrome, MODS, and death[48,49].
When the severe patients' laboratory results were analyzed, decreased lymphocyte count, elevated leukocyte count, neutrophils-lymphocytes ratio, a low percentage of monocyte, eosinophils, and basophils have been observed. Besides, Th, T suppressor (Ts), and regulatory T (Treg) cell count were determined as more obviously decreased in severe cases[50]. While studies on the pathophysiology of the disease are continuing, the following substances were found high in patients who suffer from cytokine storm: interleukin (IL)-1β, IL-2, IL-6, IL-7, IL-8, IL-9, IL-10, and IL-17; granulocyte-macrophage colony-stimulating factor (GM-CSF); TNF-α, IFN-γ, and
Together with these results, it is thought immunosuppression might be harmful in the early stages but helpful late stages of the disease. For this reason, the timing of the immunomodulatory therapies is essential[19]. Mortality rate reductive effects of the corticosteroids in patients who are intubated or only taken oxygen support can be explained with their potent anti-inflammatory effects[54]. Immunosuppression is a two-sided sword, and selective application is fundamental. Nevertheless, the ideal candidates for the immunomodulatory therapy in COVID-19 are still unspecified. Even only cytokine-specific therapies like IL-6 inhibitor tocilizumab might cause increasing the risk of sepsis, bacterial pneumonia, gastrointestinal perforation, and hepatotoxicity as a possible consequence of profound immunosuppression[55]. Additionally, indiscriminative and long-lasting immunosuppression has some disadvantages as SARS-CoV-2 progression and secondary infections. Therefore, administration of the short half-live immunosuppressant drugs will be more appropriate management.
There is still no consensus about the biomarkers that can be used for patient selection. However, besides the being need for further studies, it is thought that severe and critically ill patients might benefit from immunomodulatory options including MSC transplantation. Focusing on potential cytokine storm predictors, cytokine level measurement, especially IL-6, is not routine and usually is a "send-out" test. Instead of that, there are more accessible tests such as CRP, D-Dimer, and Ferritin, but their cut-off values vary in different studies. Another disease, hemophagocytic lymphohistiocytosis (HLH), which is induced cytokine storm, has a diagnostic score H score, (it assets temperature, organomegaly, number of cytopenias, triglycerides, fibrinogen, ferritin, aspartate aminotransferase, hemophagocytes on bone marrow aspirate, and known immunosuppression), and modified or a redesigned version of the score will be helpful not only in the management of MSC transplantation but, including other immunomodulatory therapies[56]. Further studies, which take these variables into account, need to be undertaken.
MSCs were firstly described in 1968 by Friedenstein et al[58] with a cluster of cells from bone marrow as colony-forming unit-fibroblasts[57]. Multipotent MSCs were defined by the International Society for Gene & Gene Therapy with three minimal criteria; being plastic adherent, specific surface antigen expression (expressing CD73, CD90, and CD105, lacking the expression of hematopoietic and endothelial markers CD11b, CD14, CD19, CD34, CD45, CD79α, and HLA-DR) and multipotent differentiation potential (capable of in vitro differentiation into adipocyte, chondrocyte and osteoblast lineages)[59]. Recently, The International Society for Cell & Gene Therapy (ISCT) Mesenchymal Stromal Cell (ISCT MSC) committee has advised naming these extraordinary cells as "Mesenchymal Stromal Cells" instead of "Mesenchymal Stem Cells" to clarify nomenclature[60]. In this review, we use the terms as synonyms. These cells derived from limited tissues like adipose, umbilical cord, placenta, synovium, and menstrual blood has such properties as priming, self-renewal, differentiation, immunomodulation & immunoprivilege, angiogenesis & repair, homing mechanism, anti-apoptosis, anti-inflammation & anti-fibrosis, and clinical trials about the benefits on COVID-19 patients continue[61].
MSC transplantation is up-and-coming in treating many diseases such as HIV, Hepatitis B, Influenza, coronaviruses (SARS, MERS), lung injuries, and ARDS. The only treatment of the HLH disease that causes the cytokine storm is stem cell transplantation[62]. Upon closer inspection on respiratory diseases, COVID-19, influenza, SARS, and MERS have similarities in pathogenesis, especially cytokine and immune response profiles. These comparable features in terms of the cytokine storm will provide hints for COVID-19 treatment[56,63]. In influenza A (H5N1) infection-induced lung injury, which acts similar to COVID-19 pro-inflammatory cytokine release, the significant benefits of MSCs in both cytokine profile and alveolar clearance are evidenced[23,64]. Menstrual-blood-derived MSC transplantation has significantly reduced mortality in influenza A (H7N9)-induced ARDS[22]. Mahendiratta et al[65] recently published a systematic review and reported pooled evidence on MSC therapy benefits in SARS-CoV-2, SARS-CoV, MERS-CoV, and ARDS.
While MHC-1 expression of the MSCs provides the escape from Natural killer cells response, minimal MHC-2 expression or absence of this surface protein hampers the CD4+ T cell response. For this reason, they are assumed as hypoimmunogenic[66]. MSCs, provide tissue regeneration and rejuvenation with immunotolerant and immunomodulant properties on damaged tissues by exerting their effects through immune cells[67]. Also, young MSCs might be useful in older adults because aged MSCs contribute to inflammaging and immunosenescence, which may explain the high mortality rate in this population due to COVID-19[68,69]. As well as numerous mechanisms are continuing to be investigated, some of them can be summarized as follows: (1) Inhibition of T cell (significantly cytotoxic CD8+ T cells, Th 1 , Th 17)[61,70-72], B cell proliferation to plasma cell (thus MSCs can reduce the secretion of immunoglobulin), Dendritic cell activation, and apoptosis of T cells; (2) Differentiation of the cytokine profile and cell type of T cells and B cells into anti-inflammatory cytokines such as induces the production of IL-10 and regulatory T cell, regulatory B cell[61,67]; (3) Reduction of production in cytokine storm-related inflammatory factors, such as IL-1α, IL-6, IFN-γ, IL-17, TNF-α (Figure 1)[73]; (4) Promoting the transformation of inflammation-related M1 macrophages to regeneration-related M2 macrophages[74,75]; and (5) MSC products like exosomes and extracellular vesicles that do not contain any cell are thought to have similar effects to MSC transplantation, owing to the soluble mediator profiles they secrete[67,76].
Besides being encouraging and promising, the previous studies had some limitations, and one crucial of them is the small sample size. Also, the outcomes of the studies were not standardized, and most of the outcomes are observatory. Commonly evaluated parameters are CRP, D-dimer, IL-6, IL-10, TNF-α, blood lymphocyte, neutrophil counts, pulmonary involvements in thorax computed tomography, and radiography imaging. Another point is that some studies were assumed as successful, despite having already an ameliorative trend in parameters before transplantation[77,78]. In almost all of the studies, patients had received antibiotics, antivirals, antipyretics, corticosteroids, and supportive treatments (Table 1).
| Ref. | MSC type | Sample size | Dose | Outcome |
| Leng et al[86] | ACE2- MSC | 10 patients (7 MSC + 3 Placebo) | Single infusion 106 cells/kg cells IV, 40 min | A decrease of TNF-α and an increase of anti-inflammatory IL-10 were significant (P < 0.05). Other outcome data consisted of one critically ill patient. Three of the 7 patients who taken MSC discharged in the follow-up period |
| Zhang et al[77] | Human umbilical cord Wharton’s jelly-derived MSCs (hWJCs) | One critically ill patient | Single infusion 106 cells/kg cells IV, 40 min | The patient was discharged 6 d after the administration. They suggested that remarkable amelioration in imaging, laboratory, and clinical test outcomes |
| Sánchez-Guijo et al[87] | Adipose tissue-derived MSC (AT-MSC) | 13 severe ill patients | More than 1 infusion approximately 106 cells/kg cells IV | Two patients died during the follow-up period. They detected a decrease in inflammatory parameters and an increase in total lymphocyte counts 5 d after administration |
| Sengupta et al[88] | Bone marrow MSCs derived exosomes | 24 patients | Single infusion 15 ml ExoFloTM IV | The study's survival rate is 83%, and 71% of the patients were recovered in the study interval. The outcome of the study is a clinical improvement with an average PaO2/FiO2 rate increase of 192% (P < 0.001) |
| Peng et al[89] | UC-MSCs and CP | 1 severe ill patient | Two times infusion plasma volume 400 mL (Total) (1:160 titer SARS-CoV-2 specific IgG); 3 times infusion 106 cells/kg (Total) IV 30-40 min | Lack of response to CP treatment, MSCs were administrated to the patient. After the clinical improvement, the patient was discharged |
| Liang et al[78] | UC-MSCs | 1 critically ill patient | 3 times infusion 5 × 107 cell (each time) with thymosin-a1 IV | Clinical and laboratory improvement had been seen; The patient was discharged 17 d after the first MSC infusion |
| Tang et al[90] | Menstrual blood-derived MSCs | 2 patients | 3 times infusion 106/kg cells | Imaging and laboratory improvement had been seen |
| Shu et al[91] | UC-MSCs | 41 severe ill patients (12 MSC treatment + 29 Placebo) | Single infusion 2 × 106 cells/kg IV 60 min | In treatment arm progression from severe to critical illness and 28-d mortality rate were 0, while 4 patients deteriorated to critical condition and 3 of them died, 28 d mortality rate was 10.34%. The treatment arm’s clinical and laboratory improvements were significantly faster than the placebo group |
| Tao et al[92] | Human umbilical cord blood-derived MSCs | 1 critically ill patient | 5-times infusion 1.5 × 106 cells/kg (each time) IV 60-80 min | After the MSC treatment, related to the clinical condition, the patient had undergone lung transplantation. The patient died 6 d after the transplantation because of the rejection |
| Feng et al[93] | UC-MSCs | 16 severe and critically ill patients | 4 times with one-day intervals 1 × 108 cells once 90 min | The primary outcome was oxygenation index on day 14, and it has improved after UC-MSCs transplantation. On day 28, there is no significant difference between severe and critical types’ mortality rates (6.25%) |
| Guo et al[94] | UC-MSCs | 31 severe and critically ill patients | 106/kg cells in 100 mL saline 200 mL (median volume) for each patient | They reported a significant increase in lymphocyte count, PaO2/FiO2, and decrease CRP, D-Dimer, IL-6, procalcitonin |
Although clinical research is still ongoing, strict ‘Good Manufacturing’ rules are applied in the preparation of MSCs for clinical use[79]. It is seen that these rules are rigorously followed in the studies. The frequently preferred IV MSC dose is 106 cells per kilogram, and the infusion rate is 60 min, but the total dose calculation (e.g., 15 × 107 cells) and multiple injection choices varied in different studies (Table 1). MSCs reach the lungs about venous vascular anatomy through IV administration and have been shown clearance from injured and inflamed lung tissue within 24-48 h[80]. Most of the studies to date have not contained any information about the ACE2 expression of administered MSCs or supposed as lack of ACE2 expression. Nevertheless, Derkeste et al[81] reported that adult bone marrow, adipose tissue, and umbilical-cord derived MSCs highly express ACE2. The same study has shown that placenta-derived MSCs and human-induced pluripotent stem cells are the best sources for COVID-19 treatment because of very low or absence ACE2 expression. Another significant aspect of MSC products is the contained pro-inflammatory cytokine amount. There are concerns regarding the possibility of worsened the cytokine storm by this situation. Moreover, the inflammatory response within the first two hours was reported due to IV MSC infusion[82]. About that, it has been seen a single shot corticosteroid application before the MSC infusion in previous studies. A recent systematic review from Thompson et al[83] has indicated intravascular (IV) MSC transplantation safety. The study has shown an association with fever but not non-fever acute infusional toxicity, infection, thrombotic/embolic events, or malignancy. However, Moll et al[84] have drawn attention to that MSCs highly express the procoagulant tissue factor and could trigger blood clotting in COVID-19 patients already in a hypercoagulable state. Finally, while cell-based strategies have tremendous benefits, it should be kept in mind that treatment costs are still very high, and the developing countries will have difficulties meeting these therapies[85].
Despite to be seen the benefits of MSC and its products in COVID-19, the mechanisms still need to be elucidated. Therefore, the need for the results of ongoing clinical trials and meta-analyses of randomized controlled trials continues. We think that if the costs, ethical, and storage problems of treatments are resolved over time, they might prevent COVID-19 related morbidity and mortality. We foresee that most of these problems will get over with advanced researches on MSC products. However, it should not be overlooked that MSC and MSC-based treatments are still experimental and have pros and cons.
| 1. | Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 1330] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 2. | Menachery VD, Yount BL Jr, Debbink K, Agnihothram S, Gralinski LE, Plante JA, Graham RL, Scobey T, Ge XY, Donaldson EF, Randell SH, Lanzavecchia A, Marasco WA, Shi ZL, Baric RS. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med. 2015;21:1508-1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 573] [Cited by in RCA: 725] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 3. | Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6483] [Cited by in RCA: 5452] [Article Influence: 908.7] [Reference Citation Analysis (0)] |
| 4. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30462] [Article Influence: 5077.0] [Reference Citation Analysis (12)] |
| 5. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14335] [Article Influence: 2389.2] [Reference Citation Analysis (10)] |
| 6. | Tang X, Wu C, Li X, Song Y, Yao X, Wu X, Duan Y, Zhang H, Wang Y, Qian Z, Cui J, Lu J. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev. 2020;7:1012-1023. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1089] [Cited by in RCA: 874] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 7. | Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8473] [Cited by in RCA: 7688] [Article Influence: 1281.3] [Reference Citation Analysis (0)] |
| 8. | van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564-1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5894] [Cited by in RCA: 5712] [Article Influence: 952.0] [Reference Citation Analysis (1)] |
| 9. | World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19. 11 March 2020. [cited 10 February 2021]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. |
| 10. | Tolksdorf K, Buda S, Schuler E, Wieler LH, Haas W. Influenza-associated pneumonia as reference to assess seriousness of coronavirus disease (COVID-19). Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. [cited 10 February 2021]. Available from: https://covid19.who.int. |
| 12. | Green A. Li Wenliang. The Lancet. 2020;395:682. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, Romero JR, Talbot HK, Lee GM, Bell BP, Dooling K. The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Moderna COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 14. | Ledford H. Moderna COVID vaccine becomes second to get US authorization. Nature. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Mahase E. Covid-19: What have we learnt about the new variant in the UK? BMJ. 2020;371:m4944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Parums V. Editorial: Revised World Health Organization (WHO) Terminology for Variants of Concern and Variants of Interest of SARS-CoV-2. Med Sci Monit. 2021;27:e933622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Parasher A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad Med J. 2021;97:312-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 421] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 18. | Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3174] [Cited by in RCA: 2969] [Article Influence: 494.8] [Reference Citation Analysis (0)] |
| 19. | Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383:2255-2273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1324] [Cited by in RCA: 2292] [Article Influence: 382.0] [Reference Citation Analysis (0)] |
| 20. | Sanz-Baro R, García-Arranz M, Guadalajara H, de la Quintana P, Herreros MD, García-Olmo D. First-in-Human Case Study: Pregnancy in Women With Crohn's Perianal Fistula Treated With Adipose-Derived Stem Cells: A Safety Study. Stem Cells Transl Med. 2015;4:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2047] [Cited by in RCA: 2046] [Article Influence: 113.7] [Reference Citation Analysis (5)] |
| 22. | Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, Gao H, Lu X, Yu L, Dai X, Xiang C, Li L. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering (Beijing). 2020;6:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 23. | Loy H, Kuok DIT, Hui KPY, Choi MHL, Yuen W, Nicholls JM, Peiris JSM, Chan MCW. Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus-Associated Acute Lung Injury. J Infect Dis. 2019;219:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 24. | Hu S, Li J, Xu X, Liu A, He H, Xu J, Chen Q, Liu S, Liu L, Qiu H, Yang Y. The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res Ther. 2016;7:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis. 2020;101:138-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 26. | Jehi L, Ji X, Milinovich A, Erzurum S, Merlino A, Gordon S, Young JB, Kattan MW. Development and validation of a model for individualized prediction of hospitalization risk in 4,536 patients with COVID-19. PLoS One. 2020;15:e0237419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 27. | Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1712] [Cited by in RCA: 1716] [Article Influence: 286.0] [Reference Citation Analysis (0)] |
| 28. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11607] [Article Influence: 1934.5] [Reference Citation Analysis (2)] |
| 29. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14867] [Article Influence: 2477.8] [Reference Citation Analysis (1)] |
| 30. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14570] [Article Influence: 2428.3] [Reference Citation Analysis (3)] |
| 31. | Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2713] [Cited by in RCA: 2786] [Article Influence: 464.3] [Reference Citation Analysis (1)] |
| 32. | Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J Virol. 2019;93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 485] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 33. | Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, Tie Y, Fullerton KE. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 986] [Article Influence: 164.3] [Reference Citation Analysis (0)] |
| 34. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4193] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 35. | Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20:453-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 254] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 36. | Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, Lee EH, Paneth-Pollak R, Geevarughese A, Lash MK, Dorsinville MS, Ballen V, Eiras DP, Newton-Cheh C, Smith E, Robinson S, Stogsdill P, Lim S, Fox SE, Richardson G, Hand J, Oliver NT, Kofman A, Bryant B, Ende Z, Datta D, Belay E, Godfred-Cato S. Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection - United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 376] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 37. | Russo E, Esposito P, Taramasso L, Magnasco L, Saio M, Briano F, Russo C, Dettori S, Vena A, Di Biagio A, Garibotto G, Bassetti M, Viazzi F; GECOVID working group. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J Nephrol. 2021;34:173-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect. 2020;26:1259.e5-1259.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 39. | Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1289] [Cited by in RCA: 1326] [Article Influence: 221.0] [Reference Citation Analysis (0)] |
| 40. | Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, Pardo-Hernandez H, Qasim A, Martinez JPD, Rochwerg B, Lamontagne F, Han MA, Liu Q, Agarwal A, Agoritsas T, Chu DK, Couban R, Cusano E, Darzi A, Devji T, Fang B, Fang C, Flottorp SA, Foroutan F, Ghadimi M, Heels-Ansdell D, Honarmand K, Hou L, Hou X, Ibrahim Q, Khamis A, Lam B, Loeb M, Marcucci M, McLeod SL, Motaghi S, Murthy S, Mustafa RA, Neary JD, Rada G, Riaz IB, Sadeghirad B, Sekercioglu N, Sheng L, Sreekanta A, Switzer C, Tendal B, Thabane L, Tomlinson G, Turner T, Vandvik PO, Vernooij RW, Viteri-García A, Wang Y, Yao L, Ye Z, Guyatt GH, Brignardello-Petersen R. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 540] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 41. | Li J, Wang X, Li N, Jiang Y, Huang H, Wang T, Lin Z, Xiong N. Feasibility of Mesenchymal Stem Cell Therapy for COVID-19: A Mini Review. Curr Gene Ther. 2020;20:285-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 43. | Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 44. | Chen S, Cui G, Peng C, Lavin MF, Sun X, Zhang E, Yang Y, Guan Y, Du Z, Shao H. Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Res Ther. 2018;9:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 45. | Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 725] [Article Influence: 103.6] [Reference Citation Analysis (36)] |
| 46. | Liu D, Kong F, Yuan Y, Seth P, Xu W, Wang H, Xiao F, Wang L, Zhang Q, Yang Y. Decorin-Modified Umbilical Cord Mesenchymal Stem Cells (MSCs) Attenuate Radiation-Induced Lung Injuries via Regulating Inflammation, Fibrotic Factors, and Immune Responses. Int J Radiat Oncol Biol Phys. 2018;101:945-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Yang Y, Hu S, Xu X, Li J, Liu A, Han J, Liu S, Liu L, Qiu H. The Vascular Endothelial Growth Factors-Expressing Character of Mesenchymal Stem Cells Plays a Positive Role in Treatment of Acute Lung Injury In Vivo. Mediators Inflamm. 2016;2016:2347938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 871] [Cited by in RCA: 970] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 49. | Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 450] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 50. | Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 3380] [Article Influence: 563.3] [Reference Citation Analysis (1)] |
| 51. | Samaddar A, Grover M, Nag VL. Pathophysiology and Potential Therapeutic Candidates for COVID-19: A Poorly Understood Arena. Front Pharmacol. 2020;11:585888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A; Yale IMPACT Team; Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, Iwasaki A. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1791] [Cited by in RCA: 1638] [Article Influence: 273.0] [Reference Citation Analysis (0)] |
| 53. | Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, Muñoz-Ruiz M, McKenzie DR, Hayday TS, Francos-Quijorna I, Kamdar S, Joseph M, Davies D, Davis R, Jennings A, Zlatareva I, Vantourout P, Wu Y, Sofra V, Cano F, Greco M, Theodoridis E, Freedman JD, Gee S, Chan JNE, Ryan S, Bugallo-Blanco E, Peterson P, Kisand K, Haljasmägi L, Chadli L, Moingeon P, Martinez L, Merrick B, Bisnauthsing K, Brooks K, Ibrahim MAA, Mason J, Lopez Gomez F, Babalola K, Abdul-Jawad S, Cason J, Mant C, Seow J, Graham C, Doores KJ, Di Rosa F, Edgeworth J, Shankar-Hari M, Hayday AC. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26:1623-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 699] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 54. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7575] [Article Influence: 1515.0] [Reference Citation Analysis (7)] |
| 55. | Romani L, Tomino C, Puccetti P, Garaci E. Off-label therapy targeting pathogenic inflammation in COVID-19. Cell Death Discov. 2020;6:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Ryabkova VA, Churilov LP, Shoenfeld Y. Influenza infection, SARS, MERS and COVID-19: Cytokine storm - The common denominator and the lessons to be learned. Clin Immunol. 2021;223:108652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 57. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1248] [Cited by in RCA: 1140] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 58. | Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. [PubMed] |
| 59. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13033] [Article Influence: 685.9] [Reference Citation Analysis (12)] |
| 60. | Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, Nolta J, Phinney DG, Sensebe L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 581] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 61. | Jeyaraman M, John A, Koshy S, Ranjan R, Anudeep TC, Jain R, Swati K, Jha NK, Sharma A, Kesari KK, Prakash A, Nand P, Jha SK, Reddy PH. Fostering mesenchymal stem cell therapy to halt cytokine storm in COVID-19. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Ishii E. Hemophagocytic Lymphohistiocytosis in Children: Pathogenesis and Treatment. Front Pediatr. 2016;4:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 63. | Latreille E, Lee WL. Interactions of Influenza and SARS-CoV-2 with the Lung Endothelium: Similarities, Differences, and Implications for Therapy. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, Lau EH, Nicholls JM, Fang X, Guan Y, Lee JW, Chan RW, Webster RG, Matthay MA, Peiris JS. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci U S A. 2016;113:3621-3626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 65. | Mahendiratta S, Bansal S, Sarma P, Kumar H, Choudhary G, Kumar S, Prakash A, Sehgal R, Medhi B. Stem cell therapy in COVID-19: Pooled evidence from SARS-CoV-2, SARS-CoV, MERS-CoV and ARDS: A systematic review. Biomed Pharmacother. 2021;137:111300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005;2:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 598] [Cited by in RCA: 658] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 67. | Weiss ARR, Dahlke MH. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol. 2019;10:1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 504] [Article Influence: 72.0] [Reference Citation Analysis (15)] |
| 68. | Omarjee L, Perrot F, Meilhac O, Mahe G, Bousquet G, Janin A. Immunometabolism at the cornerstone of inflammaging, immunosenescence, and autoimmunity in COVID-19. Aging (Albany NY). 2020;12:26263-26278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Lee BC, Yu KR. Impact of mesenchymal stem cell senescence on inflammaging. BMB Rep. 2020;53:65-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 70. | Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9:28-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 71. | Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1730] [Cited by in RCA: 1647] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 72. | Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 73. | Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 1104] [Article Influence: 100.4] [Reference Citation Analysis (1)] |
| 74. | Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, Krasnodembskaya AD. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med. 2017;196:1275-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 604] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 75. | Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1595] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 76. | Deffune E, Prudenciatti A, Moroz A. Mesenchymal stem cell (MSc) secretome: A possible therapeutic strategy for intensive-care COVID-19 patients. Med Hypotheses. 2020;142:109769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Zhang Y, Ding J, Ren S, Wang W, Yang Y, Li S, Meng M, Wu T, Liu D, Tian S, Tian H, Chen S, Zhou C. Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther. 2020;11:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 78. | Liang B, Chen J, Li T, Wu H, Yang W, Li Y, Li J, Yu C, Nie F, Ma Z, Yang M, Xiao M, Nie P, Gao Y, Qian C, Hu M. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine (Baltimore). 2020;99:e21429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 79. | Sensebé L, Bourin P, Tarte K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther. 2011;22:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 80. | Armitage J, Tan DBA, Troedson R, Young P, Lam KV, Shaw K, Sturm M, Weiss DJ, Moodley YP. Mesenchymal stromal cell infusion modulates systemic immunological responses in stable COPD patients: a phase I pilot study. Eur Respir J. 2018;51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Desterke C, Griscelli F, Imeri J, Marcoux P, Lemonnier T, Latsis T, Turhan AG, Bennaceur-Griscelli A. Molecular investigation of adequate sources of mesenchymal stem cells for cell therapy of COVID-19-associated organ failure. Stem Cells Transl Med. 2021;10:568-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, Korevaar SS, Mensah FK, Franquesa M, de Bruin RW, Betjes MG, Weimar W, Baan CC. Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev. 2013;22:2825-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 83. | Thompson M, Mei SHJ, Wolfe D, Champagne J, Fergusson D, Stewart DJ, Sullivan KJ, Doxtator E, Lalu M, English SW, Granton J, Hutton B, Marshall J, Maybee A, Walley KR, Santos CD, Winston B, McIntyre L. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. EClinicalMedicine. 2020;19:100249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 84. | Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk HD, Reinke P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front Immunol. 2020;11:1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 85. | Golchin A. Cell-Based Therapy for Severe COVID-19 Patients: Clinical Trials and Cost-Utility. Stem Cell Rev Rep. 2021;17:56-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 86. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 860] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 87. | Sánchez-Guijo F, García-Arranz M, López-Parra M, Monedero P, Mata-Martínez C, Santos A, Sagredo V, Álvarez-Avello JM, Guerrero JE, Pérez-Calvo C, Sánchez-Hernández MV, Del-Pozo JL, Andreu EJ, Fernández-Santos ME, Soria-Juan B, Hernández-Blasco LM, Andreu E, Sempere JM, Zapata AG, Moraleda JM, Soria B, Fernández-Avilés F, García-Olmo D, Prósper F. Adipose tissue-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25:100454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 88. | Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 540] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 89. | Peng H, Gong T, Huang X, Sun X, Luo H, Wang W, Luo J, Luo B, Chen Y, Wang X, Long H, Mei H, Li C, Dai Y, Li H. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Res Ther. 2020;11:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 90. | Tang L, Jiang Y, Zhu M, Chen L, Zhou X, Zhou C, Ye P, Chen X, Wang B, Xu Z, Zhang Q, Xu X, Gao H, Wu X, Li D, Jiang W, Qu J, Xiang C, Li L. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020;14:664-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 91. | Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, Ji N, Zheng Y, Chen X, Shi L, Wu M, Deng K, Wei J, Wang X, Cao Y, Yan J, Feng G. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 92. | Tao J, Nie Y, Wu H, Cheng L, Qiu Y, Fu J, Jiang X. Umbilical cord blood-derived mesenchymal stem cells in treating a critically ill COVID-19 patient. J Infect Dev Ctries. 2020;14:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Feng Y, Huang J, Wu J, Xu Y, Chen B, Jiang L, Xiang H, Peng Z, Wang X. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: A pilot study. Cell Prolif. 2020;53:e12947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 94. | Guo Z, Chen Y, Luo X, He X, Zhang Y, Wang J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit Care. 2020;24:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu LH S-Editor: Yan JP L-Editor: A P-Editor: Li X