©The Author(s) 2026.
World J Transplant. Mar 18, 2026; 16(1): 114044
Published online Mar 18, 2026. doi: 10.5500/wjt.v16.i1.114044
Published online Mar 18, 2026. doi: 10.5500/wjt.v16.i1.114044
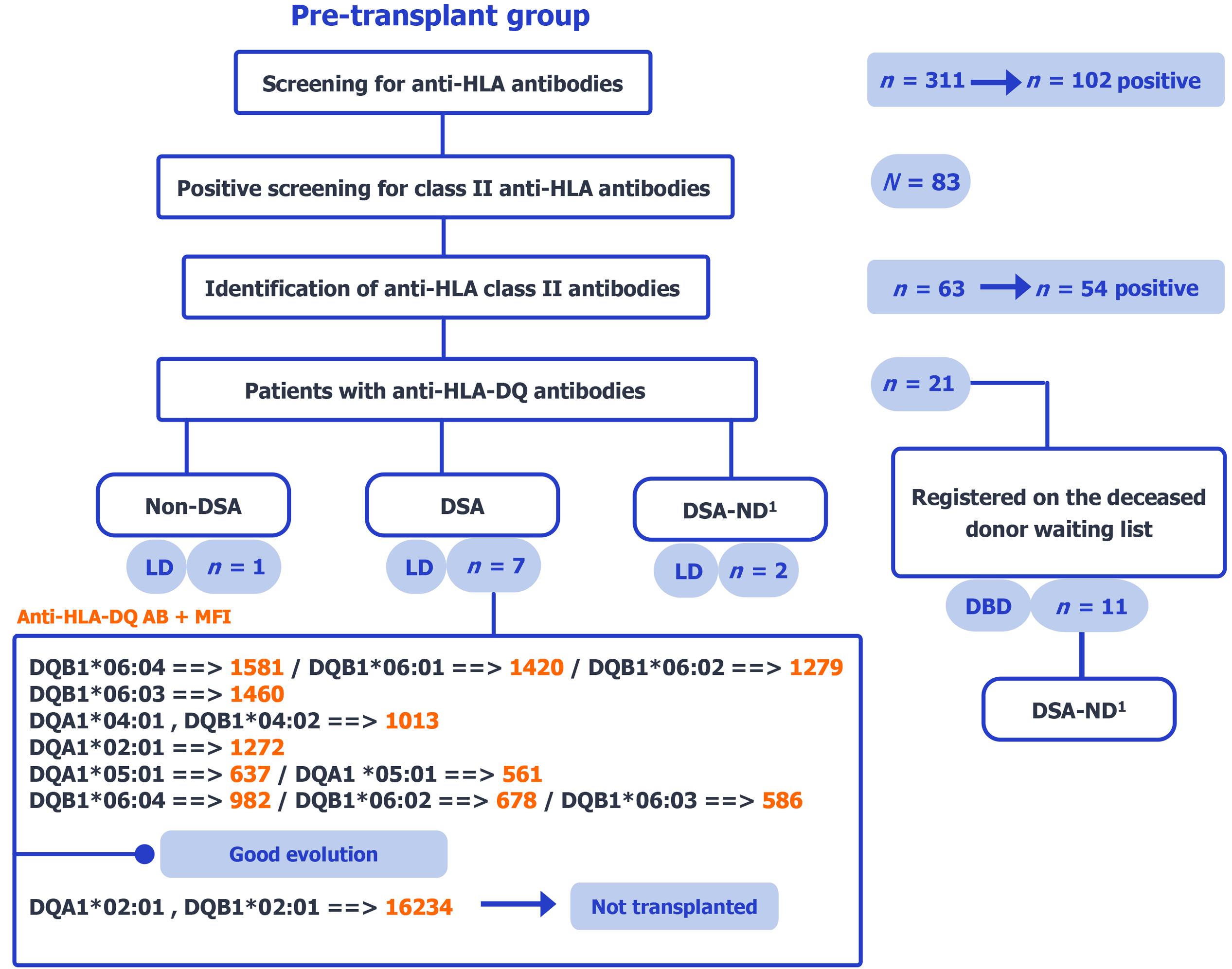
Figure 1 Pre-transplant group flow chart.
The flowchart illustrates the step-by-step selection process, from initial anti-human leukocyte antigen (HLA) antibody screening to the identification of patients with preformed anti-HLA-DQ donor-specific antibodies, and their classification according to donor type, donor typing availability, median fluorescence intensity levels, and transplant eligibility. Primary analysis: Confirmed donor HLA-DQ (LD n = 7); undetermined reported separately (living donor n = 2; brain-dead donor n = 11). 1DSA-ND indicates cases where donor human leukocyte antigen-DQ typing was not performed, preventing confirmation of donor specificity. HLA: Human leukocyte antigen; DSA: Donor-specific antibodies; LD: Living donor; ND: Not determined; AB: Antibodies; MFI: Median fluorescence intensity.
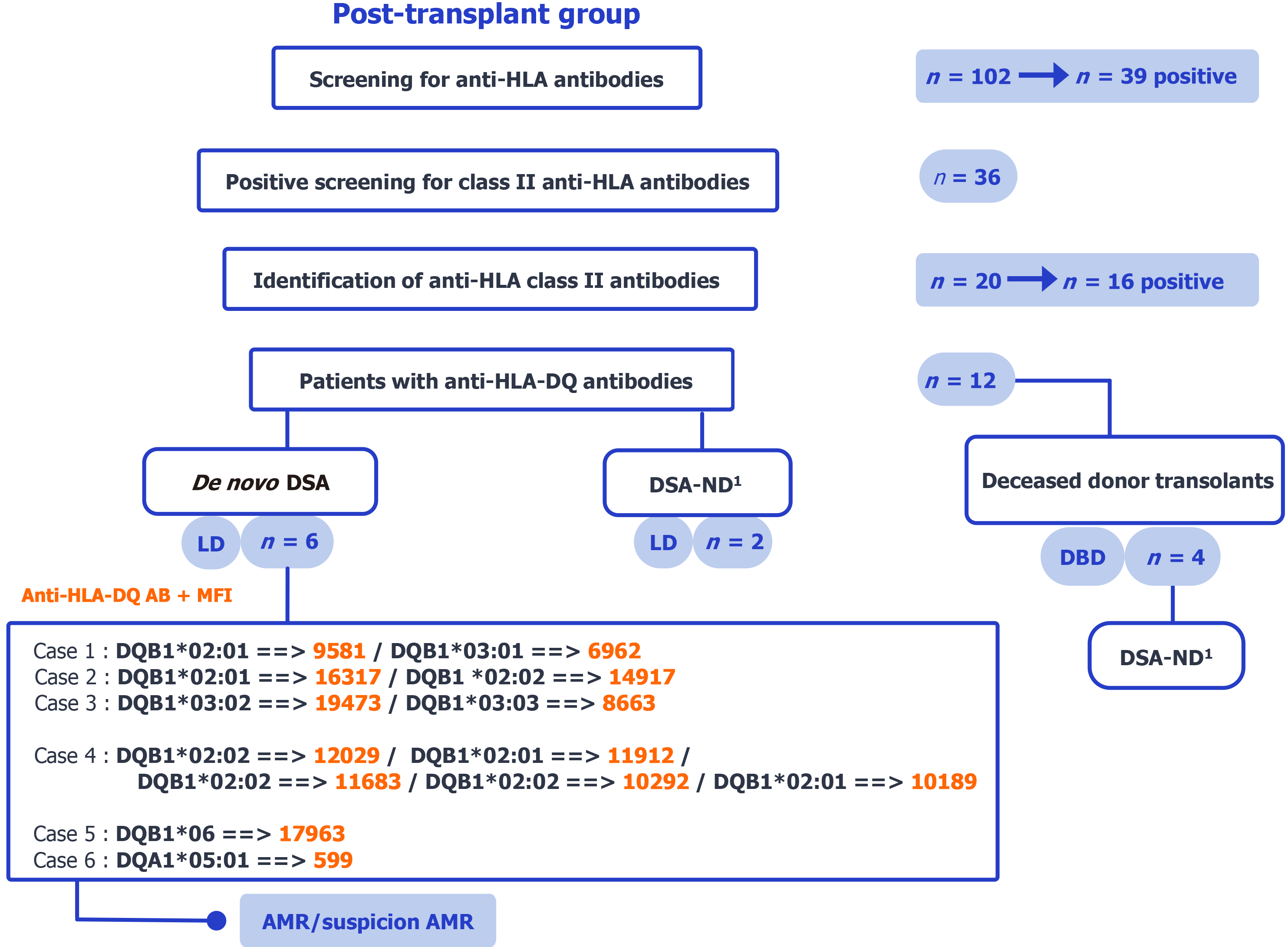
Figure 2 Post-transplant group flow chart.
The flowchart illustrates the selection process for post-transplant patients who developed de novo anti-human leukocyte antigen (HLA)-DQ donor-specific antibodies. It shows the classification of these patients according to donor type (living or deceased), the availability of donor HLA-DQ typing, and the immunological profile based on detected donor-specific antibodies specificities and median fluorescence intensity levels. Primary analysis: Confirmed donor HLA-DQ (living donor, n = 6); undetermined reported separately (living donor, n = 2; brain-dead donor, n = 4). 1DSA-ND indicates cases where donor human leukocyte antigen-DQ typing was not performed, preventing confirmation of donor specificity. HLA: Human leukocyte antigen; DSA: Donor-specific antibodies; LD: Living donor; DBD: Brain-dead donor; AB: Antibodies; MFI: Median fluorescence intensity.
- Citation: Guissouss O, Achiaou K, El Turk J, Mourachid A, Cheggali A, Medkouri G, Ramdani B, Benghanem Gharbi M, Taoudi Benchekroun M, Bennani S. Preformed vs de novo anti-human leukocyte antigens-DQ antibodies in kidney transplantation: A retrospective study. World J Transplant 2026; 16(1): 114044
- URL: https://www.wjgnet.com/2220-3230/full/v16/i1/114044.htm
- DOI: https://dx.doi.org/10.5500/wjt.v16.i1.114044