©The Author(s) 2026.
World J Transplant. Mar 18, 2026; 16(1): 111959
Published online Mar 18, 2026. doi: 10.5500/wjt.v16.i1.111959
Published online Mar 18, 2026. doi: 10.5500/wjt.v16.i1.111959
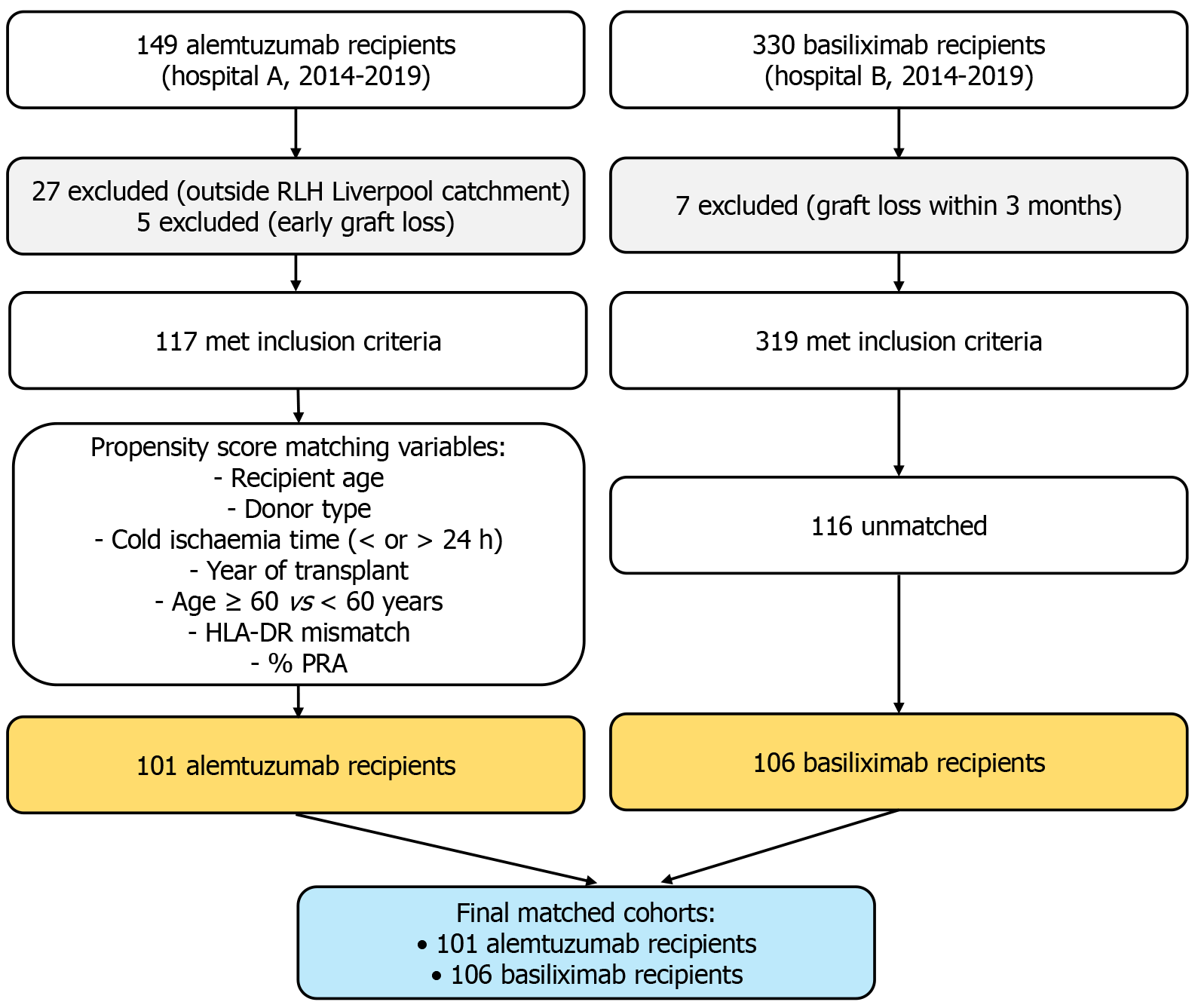
Figure 1 Flow chart showing the selection of kidney transplant recipients who received alemtuzumab or basiliximab induction between 2014 and 2019 at two transplant centers.
Exclusions were based on predefined criteria. Propensity score matching was performed using recipient age, donor type, cold ischemia time (< 24 hours or > 24 hours), year of transplantation, age group (≥ 60 years vs < 60 years), human leucocyte antigen-DR mismatch, and percentage of panel reactive antibody: (1) Cold ischemia time; (2) Human leucocyte antigen; and (3) Royal Liverpool Hospital. CIT: Cold ischemia time; HLA: Human leucocyte antigen; PRA: Panel reactive antibody; RLH: Royal Liverpool Hospital.
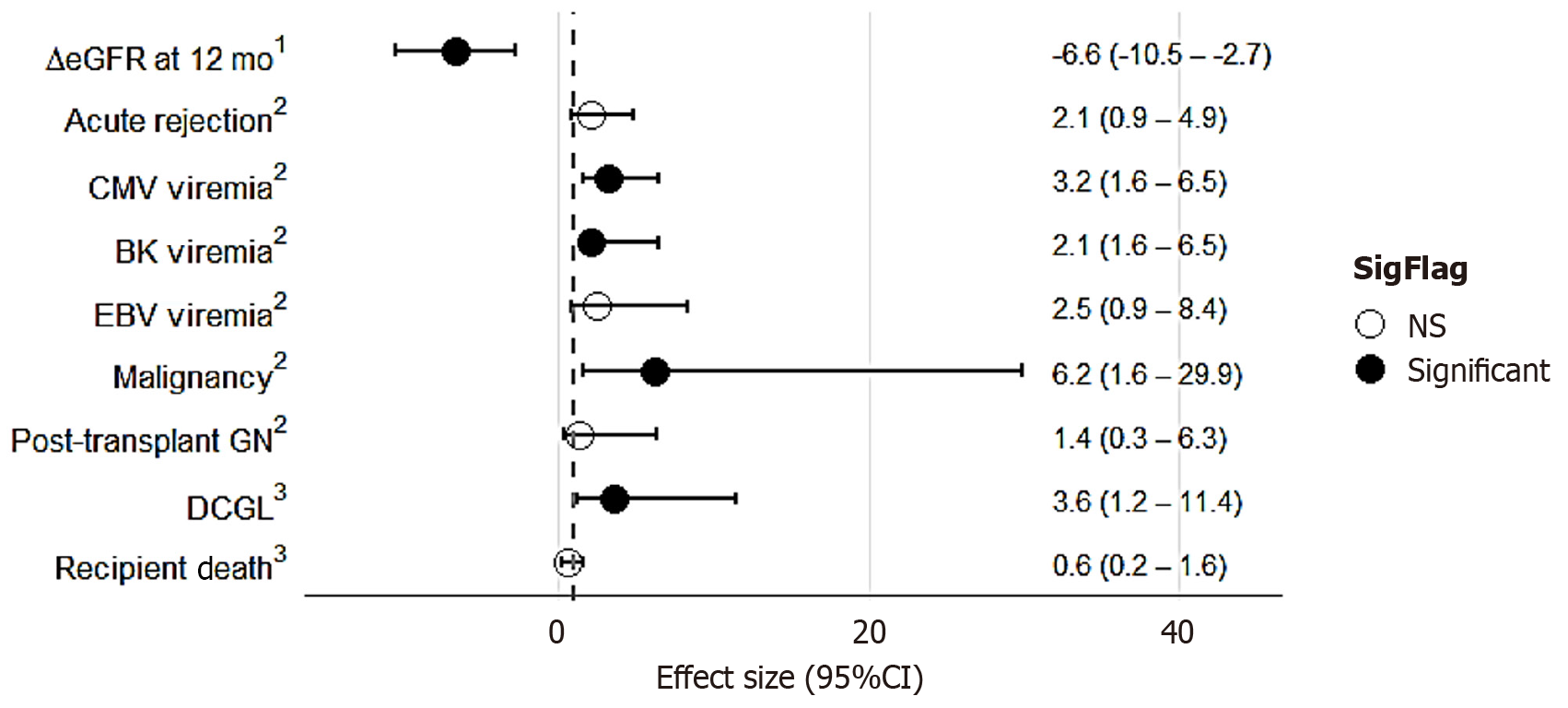
Figure 2 The treatment effects of alemtuzumab induction compared to basiliximab induction (adjusted for confounding factors including propensity scores, primary kidney disease and pre-emptive transplant rate) along with the corresponding 95%CI.
Estimates were calculated using various statistical methods based on the nature of the outcome variable. CMV: Cytomegalovirus; DCGL: Death-censored graft loss; EBV: Epstein-Barr virus; eGFR: Estimated glomerular filtration rate; GN: Glomerulonephritis; NS: Not significant. 1Estimated using linear regression. 2Odd ratio by binary logistic regression. 3Hazard ratio by Cox proportional hazard model.
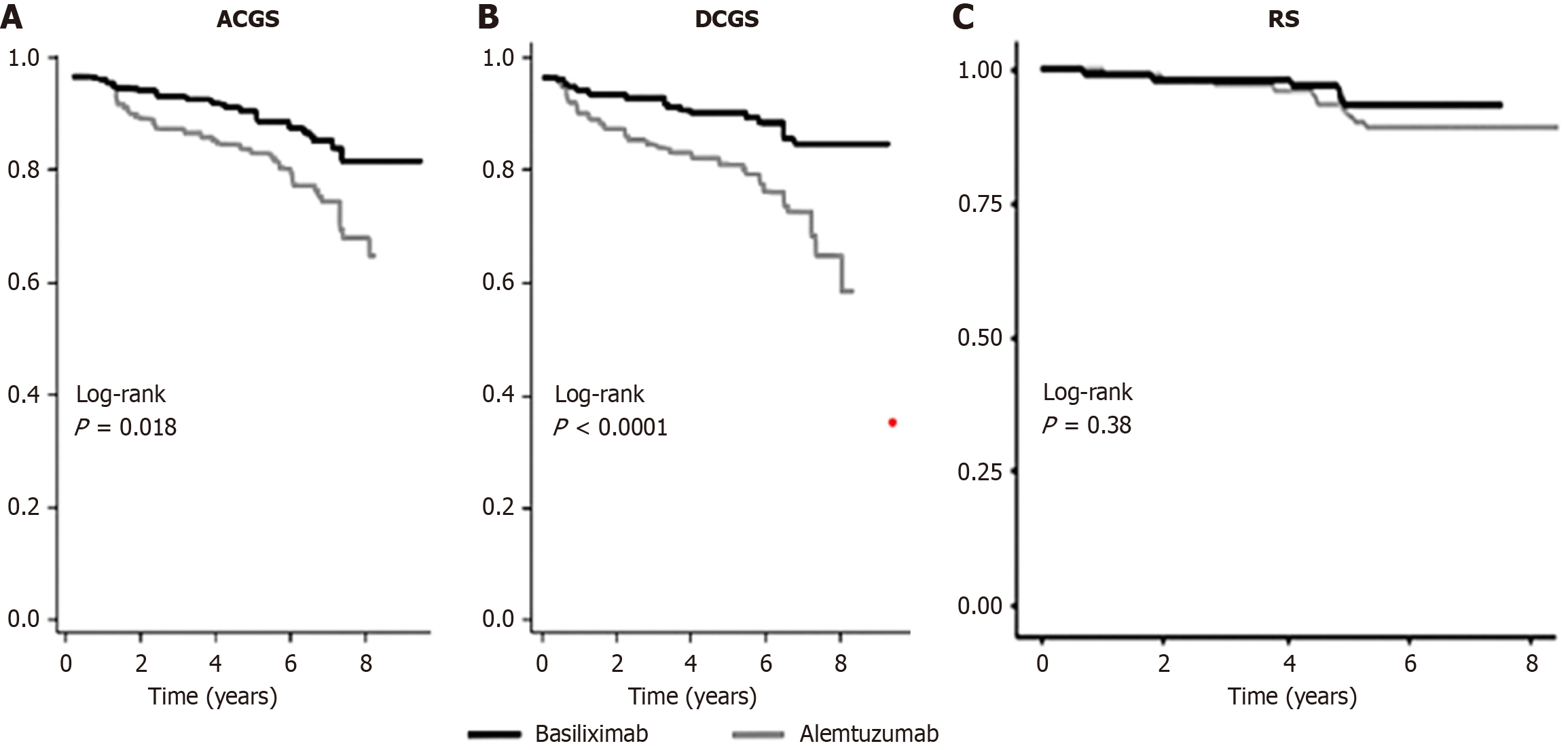
Figure 3 Kaplan-Meier survival curves of all-cause graft survival, death-censored graft survival and recipient survival in patients who received alemtuzumab vs recipients of basiliximab induction.
A: All-cause graft survival; B: Death-censored graft survival; C: Recipient survival. Data derived from a propensity-matched cohort (events = allograft loss and death with functioning graft; allograft loss censored for death and recipient death with functioning graft, respectively). ACGS: All-cause graft survival; DCGS: Death-censored graft survival; RS: Recipient survival.
- Citation: Chukwu CA, Kalra PA, Lowe M, Poulton K, Augustine T, Rao A. Outcomes of basiliximab vs alemtuzumab induction in kidney allograft recipients with matched immunological Profiles: A retrospective cohort study. World J Transplant 2026; 16(1): 111959
- URL: https://www.wjgnet.com/2220-3230/full/v16/i1/111959.htm
- DOI: https://dx.doi.org/10.5500/wjt.v16.i1.111959