©The Author(s) 2025.
World J Transplant. Dec 18, 2025; 15(4): 101926
Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.101926
Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.101926
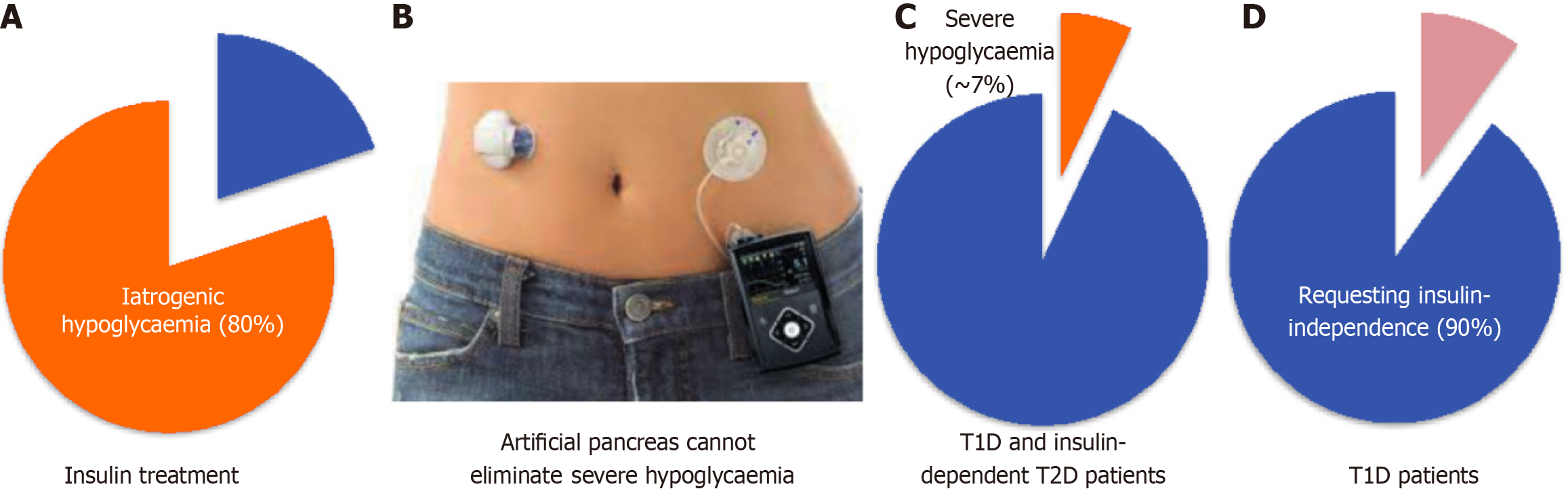
Figure 1 Problems of current insulin replacement therapy.
A: Insulin therapies are associated with iatrogenic hypoglycaemia (blood glucose concentration < 70 mg/dL, 80%); B: Sensor-enhanced insulin pumps (known as artificial pancreas). Insulin pumps and continuous glucose monitoring only reduce but do not eliminate severe hypoglycaemic events/episodes (blood glucose concentration < 54 mg/dL); C: The incidence of severe hypoglycaemia associated with insulin therapies including artificial pancreas is approximate 7% in type 1 diabetes and insulin-requiring type 2 diabetes patients; D: Over 90% type 1 diabetes patients wish for insulin-independence. T1D: Type 1 diabetes; T2D: Type 2 diabetes.
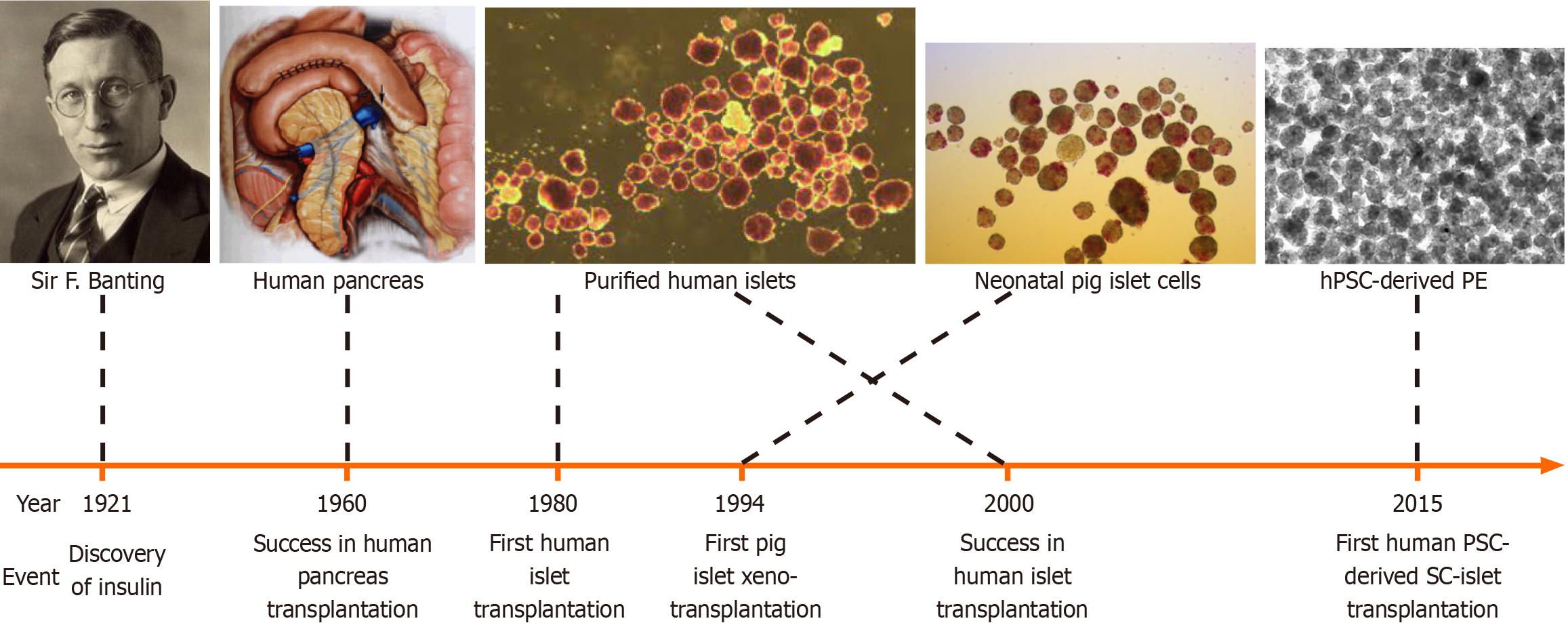
Figure 2 Graphic view of the journey towards the cure of type 1 diabetes.
The scientific and clinical sectors have begun the endeavour to develop a curative therapy for type 1 diabetes after the discovery of insulin in 1921. The first human pancreas transplantation was successfully performed in 1960, the first alloislet transplantation was carried out in 1980 and the Edmonton protocol was first reported in 2000. The first pig xenoislet clinical trial was performed in 1994 and first human pluripotent stem cell-derived insulin-secreting cells underwent clinical trials in 2015. For more details, please read the relevant texts.
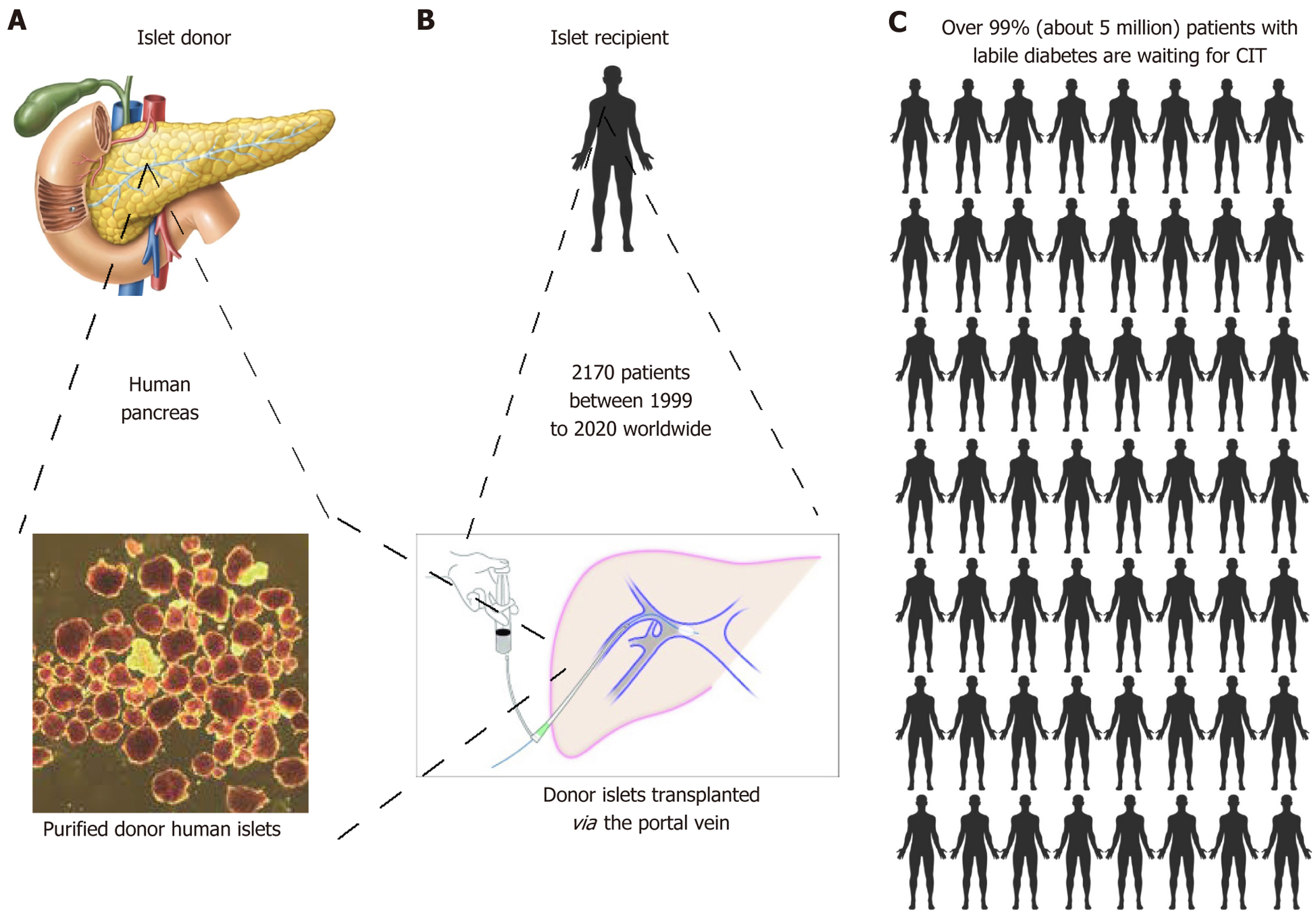
Figure 3 Graphic presentation of clinical islet transplantation.
A-C: Donated islets are purified from donor pancreas (A) and transplanted via the portal vein of the recipient patient (B). A total of 2170 patients received clinical islet transplantation worldwide over 20 years. The donated islets reside in the liver to perform the regulatory roles of glucose homeostasis. Nevertheless, due to the profound shortage of donated pancreases, over 99% of the 5 million patients with labile diabetes were unfortunately not able to receive the life-saving clinical islet transplantation therapy (C).
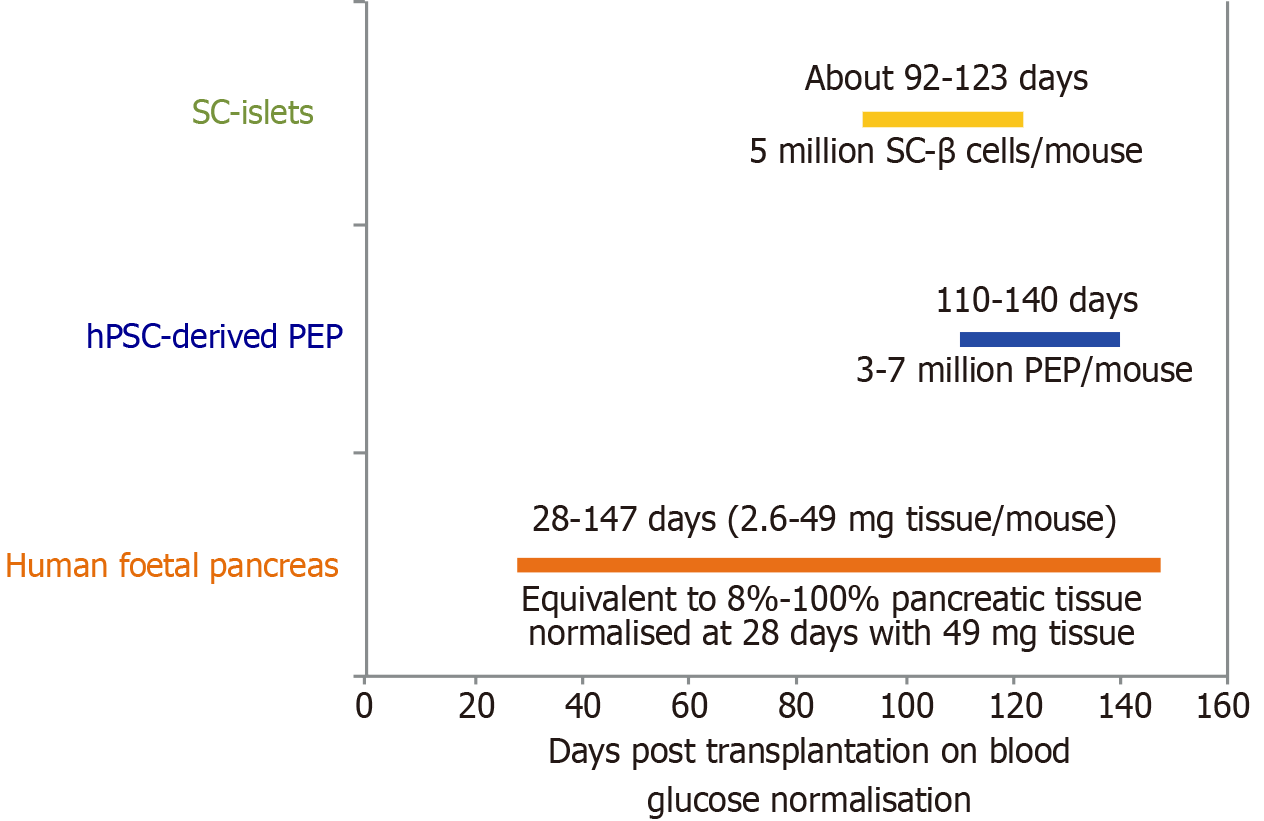
Figure 4 Preclinical proof-of-concept of human pluripotent stem cell-derived insulin secreting cells for blood glucose normalisation.
Using transplantation of human foetal pancreas tissues in mice as a positive control[95], human pluripotent stem cell-derived pancreatic endoderm precursors required 110-140 days for blood glucose normalisation in diabetic mice[96], whereas human pluripotent stem cell-derived insulin secreting cells (known as SC-β cells or islets) required approximately 92-123 days for blood glucose normalisation[97]. hPSC: Human pluripotent stem cell; PEP: Pancreatic endoderm precursor.
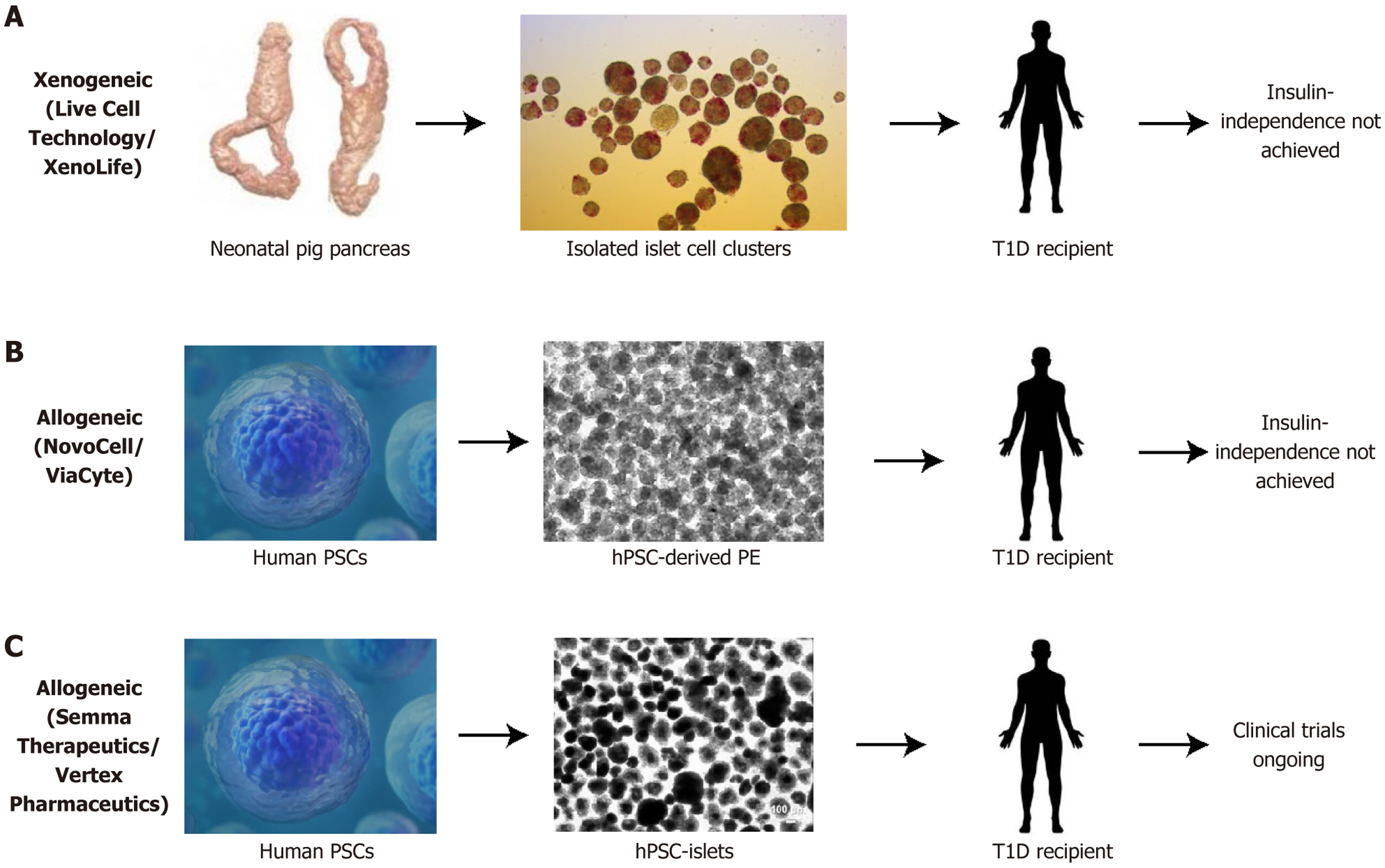
Figure 5 World challenge in manufacturing mature, fine-tuned cells for transplantation.
A: Clinical trials in xenotransplantation of immature pig islet cells to recipient patients have never achieved insulin independence; B: Clinical trials in allotransplantation of human pluripotent stem cell-derived pancreatic precursor cells to recipient patients never achieved insulin independence; C: Clinical trials in allotransplantation of human pluripotent stem cell-derived insulin-secreting cells (known as SC-islets) are currently ongoing. PSC: Pluripotent stem cell; hPSC: Human pluripotent stem cell; T1D: Type 1 diabetes.
- Citation: Jiang H, Henley D, Jiang FX. Towards curing type 1 diabetes: Prospects and challenges of allogeneic or xenogeneic donor islet cell transplantation. World J Transplant 2025; 15(4): 101926
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/101926.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.101926