Published online Jun 22, 2017. doi: 10.5498/wjp.v7.i2.121
Peer-review started: February 12, 2017
First decision: March 28, 2017
Revised: April 10, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: June 22, 2017
Processing time: 142 Days and 15.4 Hours
To evaluate usefulness of single photon emission computed tomography (SPECT) with three-dimensional stereotactic surface projection (3D-SSP) in distinguishing between Alzheimer’s disease (AD) and depression.
We studied 43 patients who presented with both depressive symptoms and memory disturbance. Each subject was evaluated using the following: (1) the Minimal Mental State Examination; (2) the Hamilton Rating Scale for Depression; (3) Clinical Global Impression-Severity scale (CGI-S); and (4) SPECT imaging with 3D-SSP.
The MMSE scores correlated significantly with the maximum Z-scores of AD-associated regions. CGI-S scores correlated significantly with the maximum Z-scores of depression-associated regions. Factor analysis identified three significant factors. Of these, Factor 1 could be interpreted as favouring a tendency for AD, Factor 2 as favouring a tendency for pseudo-dementia, and Factor 3 as favouring a depressive tendency.
We investigated whether these patients could be categorized as types: Type A (true AD), Type B (pseudo-dementia), Type C (occult AD), and Type D (true depression). The factor scores in factor analysis supported the validity of this classification. Our results suggest that SPECT with 3D-SSP is highly useful for distinguishing between depression and depressed mood in the early stage of AD.
Core tip: The present study aimed to evaluate whether statistical analysis of single photon emission computed tomography images by three-dimensional stereotactic surface projection (3D-SSP) is useful for distinguishing between Alzheimer’s disease (AD) and depression. The Minimal Mental State Examination, the Hamilton Rating Scale for Depression, and Clinical Global Impression-Severity scale findings correlated significantly with the Z-scores of AD-associated and depression-associated regions as determined using 3D-SSP analysis. Furthermore, factor analysis identified three significant factors: Factor 1, a tendency for AD; Factor 2, a tendency for pseudo-dementia; and Factor 3, a depressive tendency.
- Citation: Kirino E. Three-dimensional stereotactic surface projection in the statistical analysis of single photon emission computed tomography data for distinguishing between Alzheimer’s disease and depression. World J Psychiatr 2017; 7(2): 121-127
- URL: https://www.wjgnet.com/2220-3206/full/v7/i2/121.htm
- DOI: https://dx.doi.org/10.5498/wjp.v7.i2.121
It is important to distinguish between depression and the depressed mood characteristic of early stage Alzheimer’s disease (AD), but it can be difficult to make this distinction based solely on clinical symptoms. Brain images obtained by magnetic resonance imaging (MRI) and single photon emission computed tomography (SPECT) may be useful for distinguishing between these two conditions even at relatively early stages. Among the different imaging modalities, SPECT has been introduced clinically for making a differential diagnosis of early stage AD because it can detect brain function abnormalities before the appearance of organic changes in the brain better than other techniques. The accuracy of SPECT for diagnosing AD is reportedly 88%[1]. A meta-analysis found that SPECT has a superior specificity to clinical criteria (sensitivity 74% vs 81%, specificity 91% vs 70%) in discriminating AD from vascular dementia, fronto-temporal dementia and non-dementia subjects[2].
Three-dimensional stereotactic surface projection (3D-SSP) is a technique used for the statistical analysis of SPECT images. This technique converts images of the brains of patients with inter-individual variances in morphological features using standard brain coordinates to extract functional information (projected onto the brain surface), followed by statistical processing of the data for visual representation of the extent and severity of the reduction in brain metabolism or brain perfusion[3]. This technique was initially developed to analyze positron emission tomography (PET) images, and Minoshima et al[4] used 3D-SSP to show that patients with AD had reduced glucose metabolism in the posterior cingulate gyrus. H2150-PET analysis of patients with AD also showed reduced perfusion in the posterior cingulate gyrus[5], indicating that this technique can be used not only for the evaluation of brain metabolism but also for brain perfusion. 3D-SSP was first applied to SPECT by Bartenstein et al[6].
The present study aimed to evaluate whether statistical analysis of brain perfusion SPECT images by 3D-SSP was useful for distinguishing between AD and depression.
This study included 43 patients who presented with both depressive symptoms and memory disturbance (13 men and 30 women with a mean age of 67.7 years). All were clinically diagnosed with depression or AD and were ambulatory patients at the Department of Psychiatry, Juntendo University Shizuoka Hospital, Shizuoka Japan. The presence or absence of depressive symptoms was checked using the 2-item Patient Health Questionnaire depression module[7]. Depressive symptoms were considered present when either the patient or a family member reported that the patient had at least one of the symptoms suggesting “depressed mood” or “loss of interest or joy”. Memory disturbance was considered present when at least one of the signs on the observation list for early signs of dementia[8] was noted. Patients showing evident signs of non-AD type dementia (vascular dementia, dementia with Lewy body, fronto-temporal lobar degeneration, etc.), according to clinical symptoms or diagnostic imaging findings, were excluded from the study. The study was approved by the Juntendo University Shizuoka Hospital Ethics Committee. All participants provided written informed consent.
Each patient was evaluated with following: (1) the Minimal Mental State Examination (MMSE); (2) the Hamilton Rating Scale for Depression (HAM-D); (3) Clinical Global Impression-Severity scale (CGI-S) (for evaluating AD); and (4) N-isopropyl-p-[I-123]iodine amphetamine (I-123I IMP) SPECT images with 3D-SSP. Using the CGI-S, AD severity was rated on a 7-point scale: 1 (normal), 2 (borderline), 3 (mild), 4 (moderate), 5 (moderately severe), 6 (severe), and 7 (most severe).
Imaging was carried out while each patient lay still in a supine position, awake but with eyes closed. 111 MBq [3 mCi] of 123I-IMP (Perfusamin® Injection: IMP) was intravenously injected via the right cubital vein, followed by SPECT imaging 15 min later using SYMBIA-E (Siemens) with a Low-Medium Energy General Purpose collimator. The data were projected with the following parameters: Energy window, 159 KeV ± 15%; acquisition mode, step and shoot; acquisition time, 30 s × 36 Views (18 min); acquisition angle, 10°/view; rotation radius, 13.5 cm; matrix size, 128 × 128; magnification ratio, × 1.45; and pixel size, 3.3 mm.
Tomographic data were pre-processed with a Butterworth filter (order, 8; cut-off, 0.26 Nyquist) and then reconstructed from the 128 × 128 matrix of the transverse section. The Chang method was used for attenuation correction. The attenuation coefficient was set at μ = 0.06. Scatter correction was not used. Filtered back-projection by a Gaussian filter was employed for image reconstruction.
The SPECT images were statistically analysed by 3D-SSP and stereotactic extraction estimation (SEE)[9].
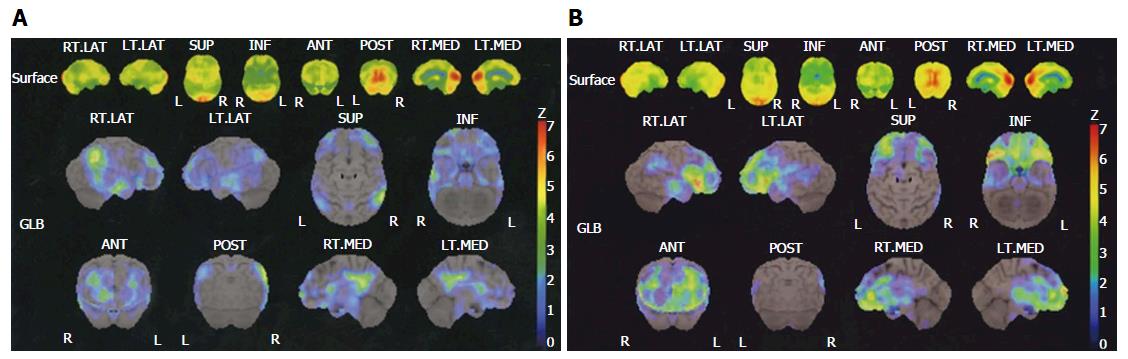
3D-SSP: 3D-SSP is a technique that is well-established in usefulness for the statistical analysis of images, as shown by previous studies[3,4,10] and the Society of Nuclear Medicine. Transaxial images were selected from the transaxial, coronal, and sagittal SPECT images for statistical analysis using NEUROSTAT® brain image analysis software. Image conversion was performed using standard brain coordinates followed by extraction of functional information using brain surface projection to determine the Z-score relative to the normal database (NDB).
The results of analysis with 3D-SSP can vary markedly depending on the NDB that is used. The NDB for this study was derived from data for healthy volunteers aged 50-79 years old; the data were collected at facilities equipped with the same type of SPECT system that we use. The construction of NDB was approval by ethics committee of each facility, and each participant provided written informed consent. Individuals with MMSE scores of 27 or higher and MRI/magnetic resonance angiography findings corresponding to their age were considered healthy. The SPECT device and the settings for image acquisition and reconstruction used to create the NDB were identical to those used in the present study.
SEE: SEE[9] classifies 3D-SSP brain surface projection data to regions based on neuroanatomy and Brodmann’s classification. This procedure involves the assignment of anatomical and functional information to each pixel in a given image. The coordinate system for the brain surface projection data is identical to the Talairach brain coordinates[11]. Anatomical information was assigned to the 3D-SSP brain surface projection data by applying the “Talairach Daemon”, which is designed to assign anatomical information to each pixel in accordance with this coordinate system[12,13]. The statistical standard deviation (Z-score) was determined for each region.
AD-associated and depression-associated regions were defined as the brain regions that exhibit characteristic perfusion reductions in the presence of AD[3-6] and depression[14] (AD-associated regions: Superior parietal lobule, inferior parietal lobule, precuneus, and posterior cingulate gyrus; depression-associated regions: Superior frontal gyrus, middle frontal gyrus, and inferior frontal gyrus).
Spearman correlation coefficients (two tailed) were used to evaluate whether the maximum Z-scores of the AD-associated and depression-associated regions correlated with the HAM-D, MMSE, or CGI-S scores.
Principal axis factor analysis with varimax rotation (df = 21) was performed for the following variables: Age, sex, HAM-D score, MMSE score, CGI-S score, and the maximum Z-scores of the AD-associated and depression-associated regions. The statistical analyses were performed in PASW® version 18 for Windows.
Patients with HAM-D scores of 10 or higher were considered to have depressive symptoms (HAM-D-positive). Patients with MMSE scores of 24 or lower were considered to have symptoms of dementia (MMSE-positive). In evaluating the SPECT Z-score with 3D-SSP, the rating “AD-positive” was made in cases with reduction of perfusion that were 2 standard deviations (SDs) or greater (Z-score ≥ 2.00) in at least one of the AD-associated regions. Similarly, a “depression-positive” rating was given to cases showing 2 SDs or greater reductions of perfusion in at least one of the depression-associated regions.
MMSE-positive was confirmed in 19 cases and MMSE-negative in 24. HAM-D-positive in 14 cases and HAM-D-negative in 29 was also confirmed. The CGI-S score was 1 in 3 cases, 2 in 11 cases, 3 in 13 cases, 4 in 13 cases, 5 in 2 cases, and 6 in 1 case. In the SPECT 3D-SSP analysis, AD-positive in 9 cases and AD-negative in 34, and depression-positive in 13 cases and depression-negative in 30 was observed. The 3D-SSP results of two representative cases are presented in Figure 1.
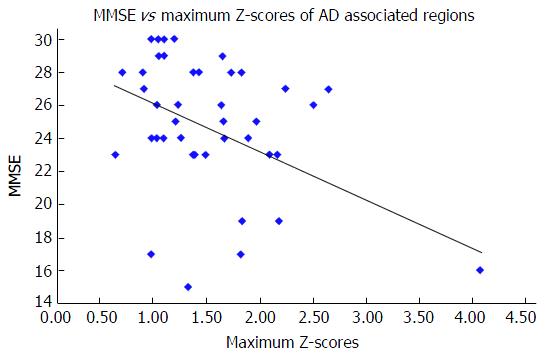
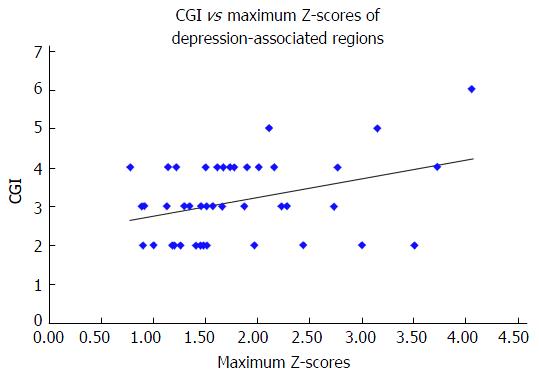
The maximum Z-scores of AD-associated regions correlated significantly with MMSE (Spearman r = -0.333, n = 43, P = 0.029) (Figure 2), but not with the HAM-D score (Spearman r = 0.014, n = 43, P = 0.928) or the CGI-S score (Spearman r = 0.275, n = 43, P = 0.074). The maximum Z-scores of depression-associated regions correlated significantly with the CGI-S score (Spearman r = 0.309, n = 43, P = 0.043) (Figure 3), but not with the MMSE score (Spearman r = -0.189, n = 43, P = 0.224) or HAM-D score (Spearman r = 0.047, n = 43, P = 0.763).
Three significant factors with eigenvalues over 1 were identified (Table 1). Factor 1, a tendency for AD, i.e., negative correlation with MMSE and positive correlations with CGI-S and the Z-scores of AD-associated and depression-associated regions; Factor 2, a tendency for pseudo-dementia, i.e., more marked in women, positive correlations with age and HAM-D and a negative correlation with MMSE; and Factor 3, a depressive tendency, i.e., negative correlation with age and positive correlations with HAM-D and MMSE (Table 2).
| Total of explained variance | |||||||||
| Factors | Initial eigenvalues | Sums of squares of loadings after sampling | Sums of squares of loadings after rotation | ||||||
| Total | Proportion of explained variance (%) | Cumulative proportion of explained variance (%) | Total | Proportion of explained variance (%) | Cumulative proportion of explained variance (%) | Total | Proportion of explained variance (%) | Cumulative proportion of explained variance (%) | |
| Factor 1 | 2.422 | 34.603 | 34.603 | 1.961 | 28.017 | 28.017 | 1.805 | 25.780 | 25.780 |
| Factor 2 | 1.223 | 17.478 | 52.081 | 0.982 | 14.025 | 42.041 | 1.067 | 15.248 | 41.029 |
| Factor 3 | 1.166 | 16.656 | 68.738 | 0.484 | 6.920 | 48.961 | 0.555 | 7.932 | 48.961 |
| Factor 4 | 0.805 | 11.502 | 80.240 | ||||||
| Factor 5 | 0.598 | 8.538 | 88.778 | ||||||
| Factor 6 | 0.500 | 7.144 | 95.922 | ||||||
| Factor 7 | 0.285 | 4.078 | 100.000 | ||||||
| Factors | Factor 1 | Factor 2 | Factor 3 |
| Age | 0.103 | 0.171 | -0.504 |
| Sex | 0.061 | 0.960 | -0.107 |
| HAM-D | 0.135 | 0.241 | 0.350 |
| MMSE | -0.679 | -0.178 | 0.399 |
| CGI | 0.763 | -0.082 | -0.048 |
| AD regions | 0.674 | 0.078 | 0.063 |
| Depression regions | 0.523 | 0.119 | 0.042 |
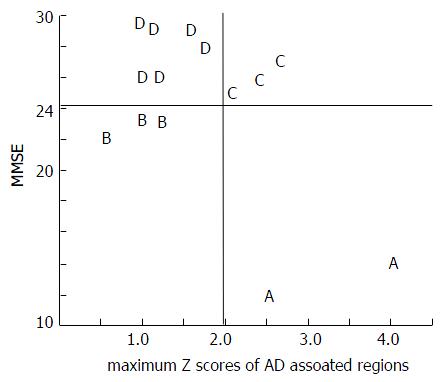
In the 14 HAM-D-positive cases in which it was difficult to distinguish between AD and depression, we investigated the use of a four-type classification system (2 × 2 = 4), based on the AD rating (positive/negative) and the MMSE rating (positive/negative). The maximum Z scores of depression-associated regions correlated strongly with Factor 1 (tendency for AD) but only weakly with Factor 3 (tendency for depression). Thus, the maximum Z score of depression-associated regions was not adopted as a criterion for evaluation (Figure 4).
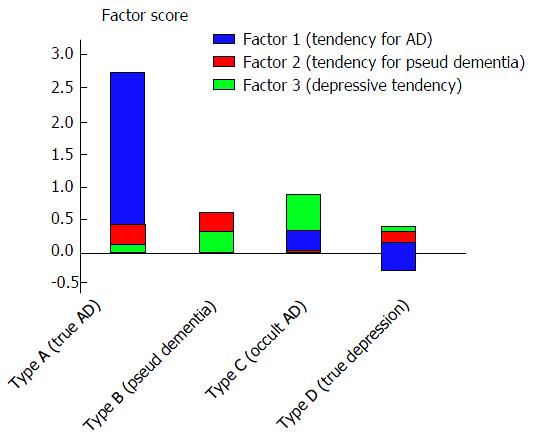
Each type was interpreted as follows. Type A (2 cases, both women) was interpreted as being “true AD accompanied by depressive symptoms” because both AD and MMSE were positive. Type B (3 cases, all women) was interpreted as being “pseudo-dementia” because AD was negative and MMSE was positive. Type C (3 cases; 2 women and 1 man) was interpreted as being “occult AD” because AD was positive and MMSE was negative. Type D (6 cases; 3 women and 3 men) was interpreted as being “true depression (non-AD)” because both AD and MMSE were negative (Figure 4). In the analysis of the score for each factor for each type, Type A (true AD) had high scores for Factor 1 (tendency for AD), type B (pseudo-dementia) had high scores for Factor 2 (tendency for pseudo-dementia), and type D (true depression) had high scores for Factor 3 (depressive tendency). This finding seemed to validate the use of this classification system. All of the Type B cases were women and Factor 2 loaded the variable “sex” to a greater amount (0.96), indicating that women have a higher tendency for pseudo-dementia. Type C cases (occult AD) had high scores for Factors 1 and 3, suggesting that Type C cases have a strong tendency for AD while also presenting with a depressive tendency (Figure 5).
The results of the present study indicate that the Z-scores of AD-associated regions have high sensitivity for the diagnosis of AD, while the Z scores of depression-associated regions correlated only weakly with the depressive tendency in factor analysis and lacked specific sensitivity for depressive symptoms.
This study had some limitations, and more work needed. First, only 14 patients were included in the classification analysis, so this classification system needs to be tested in a larger number of patients. There were differences between sexes, and this, too, merits further study in a larger population. Second, we excluded patients with non-AD dementia according to their clinical symptoms or diagnostic imaging findings. A more detailed structured interview or more sophisticated evaluation for differential diagnosis would help more rigorously rule out non-AD dementia. Third, concerning the AD-associated and depression-associated regions, it would be better to conduct a more detailed analysis of the individual areas. The definition of depression-associated regions needs to be reviewed because the Z-scores of these regions failed to show specific sensitivity. The validity of the definition of these regions for distinguishing between AD and depression must be established based on future evaluations or meta-analyses of larger samples. Kang et al[15] used SPECT and found that AD patients with clinically significant depression had significantly lower perfusion in the right orbitofrontal and inferior frontal gyri than non-depressive AD patients, whereas AD patients with clinically significant apathy had had significantly lower perfusion in the right amygdala, temporal, posterior cingulate, right superior frontal, postcentral, and left superior temporal gyri than non-apathetic AD patients[15]. Thus, there may be some overlaps between AD- and depression-associated regions. On the other hand, Terada et al[16] investigated the cerebral blood flow of AD patients with depressive symptoms, excluding the effect of apathy and anxiety. They found that the dorsolateral prefrontal area was significantly involved in the pathogenesis of depressive symptoms in AD, and that the area on the left side in particular may be closely related to depressive symptoms[16]. In the future, AD- and depression-associated regions should be better defined to differentiate between depression and anxiety/apathy. Fourth, the use of this classification system to identify Types A-D requires validation by prospective observation. Fifth, although clinicians often find that cognitive impairment symptoms respond to treatment for depression, a substantial proportion of patients with pseudo-dementia will develop dementia during follow-up. Therefore, the use of SPECT findings to distinguish between AD and depression should be viewed with caution in terms of the clinical implications.
This study found that MMSE, HAM-D, and CGI-S findings correlated significantly with the Z-scores of AD-associated and depression-associated regions as determined using SPECT imaging with 3D-SSP analysis. Factor analysis identified three significant factors: (1) Factor 1, a tendency for AD; (2) Factor 2, a tendency for pseudo-dementia; and (3) Factor 3, a depressive tendency. Our results indicated that patients presenting with both depressive symptoms and memory disturbance could be divided into four types: (1) Type A, true AD; (2) Type B, pseudo-dementia; (3) Type C, occult AD; and (4) Type D, true depression. The scores for the three factors validated the identifications of these types. Thus, statistical analysis of I-123 IMP perfusion SPECT images using 3D-SSP shows great promise for distinguishing between depression and the depressed mood that is characteristic of early stage AD.
In clinical practice, it is often difficult to distinguish between depression and the depressed mood seen in the early stage of Alzheimer’s disease (AD). Among the different imaging modalities, brain perfusion single photon emission computed tomography (SPECT) has been introduced clinically for making a differential diagnosis of early stage AD because it can detect brain function abnormalities before the appearance of organic changes in the brain better than other techniques.
Three-dimensional stereotactic surface projection (3D-SSP) is a technique used for the statistical analysis of SPECT images.
The present study aimed to evaluate whether statistical analysis of brain perfusion SPECT images by 3D-SSP was useful for distinguishing between AD and depression. As results, factor analysis identified three significant factors. Of these, Factor 1 could be interpreted as favouring a tendency for AD, Factor 2 as favouring a tendency for pseudo-dementia, and Factor 3 as favouring a depressive tendency. Furthermore, the authors investigated whether these patients could be categorized as types: Type A (true AD), Type B (pseudo-dementia), Type C (occult AD), and Type D (true depression). The factor scores in factor analysis supported the validity of this classification.
The authors’ results suggest that SPECT with 3D-SSP is highly useful for distinguishing between depression and depressed mood in the early stage of AD.
3D-SSP: Three-dimensional stereotactic surface projection is a technique used for the statistical analysis of SPECT images. This technique converts images of the brains of patients with inter-individual variances in morphological features using standard brain coordinates to extract functional information (projected onto the brain surface), followed by statistical processing of the data for visual representation of the extent and severity of the reduction in brain metabolism or brain perfusion.
This is an interesting study on the use of SPECT in differentiating depression from dementia.
| 1. | Bonte FJ, Harris TS, Hynan LS, Bigio EH, White CL. Tc-99m HMPAO SPECT in the differential diagnosis of the dementias with histopathologic confirmation. Clin Nucl Med. 2006;31:376-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Dougall NJ, Bruggink S, Ebmeier KP. Systematic review of the diagnostic accuracy of 99mTc-HMPAO-SPECT in dementia. Am J Geriatr Psychiatry. 2004;12:554-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 3. | Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238-1248. [PubMed] |
| 4. | Minoshima S, Foster NL, Kuhl DE. Posterior cingulate cortex in Alzheimer’s disease. Lancet. 1994;344:895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 191] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Ishii K, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, Mori E. Demonstration of decreased posterior cingulate perfusion in mild Alzheimer’s disease by means of H215O positron emission tomography. Eur J Nucl Med. 1997;24:670-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Bartenstein P, Minoshima S, Hirsch C, Buch K, Willoch F, Mösch D, Schad D, Schwaiger M, Kurz A. Quantitative assessment of cerebral blood flow in patients with Alzheimer’s disease by SPECT. J Nucl Med. 1997;38:1095-1101. [PubMed] |
| 7. | Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3238] [Cited by in RCA: 4589] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 8. | Hopman-Rock M, Tak EC, Staats PG. Development and validation of the Observation List for early signs of Dementia (OLD). Int J Geriatr Psychiatry. 2001;16:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Mizumura S, Kumita S, Cho K, Ishihara M, Nakajo H, Toba M, Kumazaki T. Development of quantitative analysis method for stereotactic brain image: assessment of reduced accumulation in extent and severity using anatomical segmentation. Ann Nucl Med. 2003;17:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 1222] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 11. | Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thiem Medical Publishers, New York. 1988;. |
| 12. | Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 14. | Navarro V, Gastó C, Lomeña F, Mateos JJ, Marcos T. Frontal cerebral perfusion dysfunction in elderly late-onset major depression assessed by 99MTC-HMPAO SPECT. Neuroimage. 2001;14:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kang JY, Lee JS, Kang H, Lee HW, Kim YK, Jeon HJ, Chung JK, Lee MC, Cho MJ, Lee DS. Regional cerebral blood flow abnormalities associated with apathy and depression in Alzheimer disease. Alzheimer Dis Assoc Disord. 2012;26:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Terada S, Oshima E, Sato S, Ikeda C, Nagao S, Hayashi S, Hayashibara C, Yokota O, Uchitomi Y. Depressive symptoms and regional cerebral blood flow in Alzheimer’s disease. Psychiatry Res. 2014;221:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chakrabarti S, Szekely A S- Editor: Ji FF L- Editor: A E- Editor: Li D