Published online Jun 22, 2016. doi: 10.5498/wjp.v6.i2.215
Peer-review started: October 14, 2015
First decision: January 15, 2016
Revised: January 28, 2016
Accepted: March 14, 2016
Article in press: March 16, 2016
Published online: June 22, 2016
Processing time: 250 Days and 22.6 Hours
AIM: To evaluate antidepressant-like effect of memantine in a rat model.
METHODS: Male Wistar rats were treated intraperitoneally with either vehicle, memantine (10 mg/kg) or imipramine (20 mg/kg), for 3 wk. Twenty-four hour after the last treatment animals were challenged with quinpirole (0.3 mg/kg s.c.) and tested for motor activity. After 1 h habituation to the motility cages, the motor response was recorded for the following 45-min and the data were collected in 5-min time bins.
RESULTS: As expected, chronic treatment with imipramine potentiated the locomotor stimulant effect of quinpirole. On the contrary, chronic memantine administration failed to induce the behavioral supersensitivity to the dopamine agonist.
CONCLUSION: The results show that memantine, at variance with antidepressant treatments, fails to induce dopaminergic behavioral supersensitivity. This observation is consistent with the results of preclinical and clinical studies suggesting that memantine does not have an acute antidepressant action but does have an antimanic and mood-stabilizing effect.
Core tip: Memantine at variance with virtually all antidepressant treatments, fails to induce dopaminergic behavioral supersensitivity. This observation is consistent with the results of preclinical and clinical studies suggesting that memantine does not have an acute antidepressant action but does have an antimanic and mood-stabilizing effect.
- Citation: Demontis F, Serra G. Failure of memantine to “reverse” quinpirole-induced hypomotility. World J Psychiatr 2016; 6(2): 215-220
- URL: https://www.wjgnet.com/2220-3206/full/v6/i2/215.htm
- DOI: https://dx.doi.org/10.5498/wjp.v6.i2.215
The blockade of serotonin and noradrenaline reuptake by tricyclic antidepressants or the inhibition of monoaminoxidase (MAO) by MAO inhibitors has been considered responsible for the therapeutic action of the first generation of antidepressant drugs. On the contrary, the role of dopamine in the mechanism of action of these drugs has been neglected for long time. However, in 1979[1] we first reported that chronic treatment with antidepressant drugs activates dopaminergic transmission.
In the last few decades numerous studies have confirmed that virtually all antidepressant (AD) treatments (including electroconvulsive shock and REM-sleep deprivation) increase the motor-stimulant effect of dopamine receptor agonists by sensitizing D2 dopamine receptors in the mesolimbic system[2-9].
In these last few years, it has been hypothesized that glutamate and NMDA receptors play a role in the neurobiology of depression[10-14]. Moreover, it has been reported that the NMDA receptor blocker, memantine, reduces immobility time in the forced swimming test (FST), a widely used animal model of depression, suggesting that it could have a potential antidepressant effect[15-17].
However, it has been recently reported that memantine fails to reduce immobility time in the FST after chronic treatment[18], and to reverse anhedonia in the chronic mild stress model of depression[19], suggesting that the antidepressant-like effect observed in the FST after acute treatment should be considered a “false positive”[20].
These observations are consistent with clinical studies that failed to find an acute antidepressant effect of memantine in humans[21-29].
Moreover, we have recently reported preclinical and clinical evidence suggesting that memantine has an antimanic and mood-stabilizing action[18,30-39].
Thus, to further clarify the effect of memantine in animal models of depression, we compared the effect of chronic administration of memantine and imipramine on the locomotor response to the dopamine D2-like receptor agonist quinpirole, as a measure of dopamine D2-like receptor sensitivity[40].
As expected, chronic administration of imipramine potentiated the locomotor stimulant effect of quinpirole, while memantine failed to affect the quinpirole action.
The present study was carried out in accordance with Italian law, which allows experiments on laboratory animals only after submission of a research project to the competent authorities, and in accordance with the “Guide for the Care and Use of Laboratory Animals” 8th Edition (National Research Council of Academies, The National Academies Press, Washington DC, 2011).
Male Wistar rats (Harlan, Italy), weighing initially 125-149 g, were housed in groups of 2 per cage in controlled environmental condition (temperature 22-24 °C, humidity 50%-60%; light on at 8:00, off at 20:00), with free access to food and water.
The animals (n = 60) were divided into three groups (n = 20) and treated with vehicle (distilled water) controls, memantine HCl (Ebixa sol. Lundbeck Italy s.p.a) and imipramine HCl (Sigma, Haldrich) for 3 wk.
They were challenged with quinpirole and tested for motor activity 24 h after the end of this treatment. Imipramine HCl and quinpirole HCl (Sigma, Haldrich) were dissolved in distilled water. Memantine and imipramine were administered intraperitoneally in daily injections, at the dose of 10 mg/kg and 20 mg/kg, respectively, in a volume of 1 mL/kg. Quinpirole was administered subcutaneously at the dose of 0.30 mg/kg in a volume of 1 mL/kg.
Motor activity was measured by an apparatus consisting of a mobile rack (height 180 cm, width 100 cm and depth 60 cm) with eight compartments (height 40 cm, width 45 cm, depth 50 cm), into which a transparent perspex cage (height 19 cm, floor area 23 cm2× 33 cm2) was placed (Imetronic, Pessac, France). Motor activity was detected by a system of photocell infrared beams, dividing the cage area into two sectors, rear and front sector. In particular, the interruption of two photocell beams belonging to two different sectors was recorded as a “long movement” motility count. The interruption of two photocell beams belonging to the same sector was recorded as a “short movement” motility count. A “barrier” of infrared photocell beams, placed at the height of 15 cm, detected rearing activity. The apparatus was connected to a personal computer by an electronic interface. Experiments were performed between 0900 and 1500 h. After 1-h habituation to the motility cages, the rats were divided into 2 groups and treated s.c. with control vehicle (n = 30) and quinpirole (n = 30).
The motor response was recorded for the following 45 min and data were collected in 5-min time bins.
The results were analysed by analysis of variance, supplemented by F tests for contrasts. Habituation and quinpirole challenge data were analysed separately. All data are presented as mean ± SEM; P < 0.05 is considered to be statistically significant.
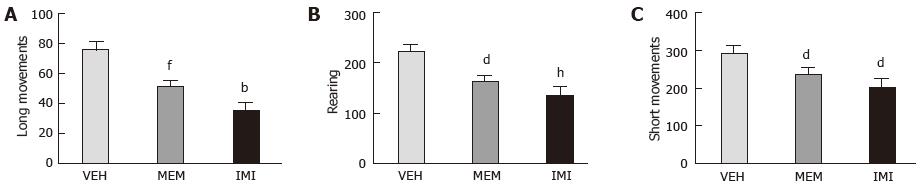
As shown in Figure 1, during 1 h of habituation to the motility cage, animals chronically treated with imipramine and memantine showed a significant reduction of motor activity, measured as long movements, rearing activity and short movements.
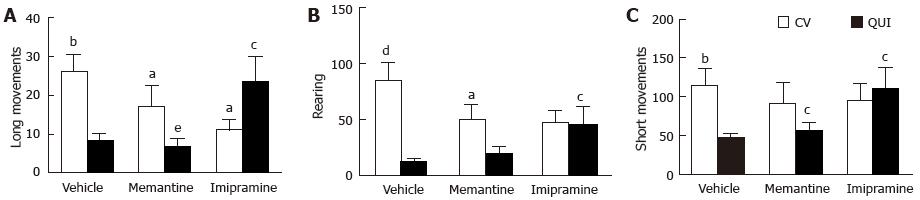
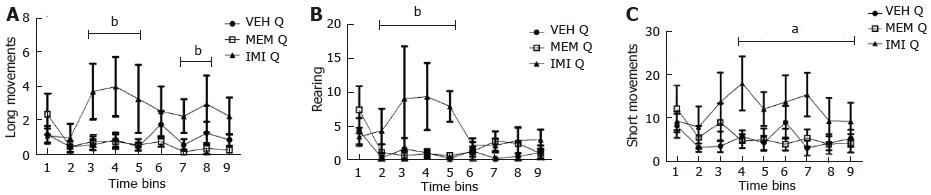
Figure 2 shows that quinpirole reduced the locomotor activity, assessed as long movements, rearing activity and short movements, in control and memantine-treated rats, On the contrary, in imipramine-treated animals quinpirole stimulated locomotor activity (long movements and short movements) or prevented its sedative effect (rearing). Figure 3 shows the time course of quinpirole effect. Imipramine, but not memantine, stimulates locomotor activity induced by the dopamine agonist.
The present results confirm that chronic treatment with imipramine potentiated the locomotor response to the selective dopamine D2 receptor agonist quinpirole.
Quinpirole, as well as other dopamine agonists, has a biphasic effect on locomotor activity. A low doses it stimulates dopamine D2 autoreceptors mediating sedation/reduced motor activity, while at relatively high doses stimulates post-synaptic dopamine D2 receptors and and increases motor activity. In the present experiment the used dose of quinpirole reduces motor activity by stimulating dopamine autoreceptors in control animals. Imipramine, but not memantine, reverses this effect (i.e., increases locomotor activity) because the stimulation of the supersensitive post-synaptic receptors overcomes the sedative effect due to the stimulation of autoreceptors (this issue has been extensively addressed in Serra et al[6,41,42]).
These findings are consistent with the large body of studies that strongly suggest that virtually all antidepressant treatments sensitize dopamine D2 receptors in the mesolimbic system[2-4,6,8,9,41,42]. On the contrary, memantine fails to stimulate the locomotor response to quinpirole, suggesting that, at variance with antidepressant treatments, it does not sensitize D2 receptors. This observation provides further support to our hypothesis[30,31,42] that the effect observed in the FST is a “false positive” and is in keeping with the failure of clinical studies to demonstrate an acute antidepressant action of memantine in depressed subjects[21-29]. Moreover, the results are consistent with our preclinical and clinical observations that strongly suggest that memantine has an antimanic and mood-stabilizing effect in patients with bipolar mood disorders. Indeed, we found that memantine prevents the dopamine D2 receptor sensitization induced by imipramine[30], that has been suggested to underly antidepressant-induced mania in humans[43,44]. Moreover, Gao et al[45] have reported an antimanic-like effect of memantine in two widely used animal models of mania. In addition we found that memantine prevents the bipolar-like behavior (mania followed by depression) induced by imipramine[18] suggesting that the drug may have a mood-stabilizing effect (i.e., the ability to prevent mania/hypomania and depression episodes in manic depressive illness). This effect is the opposite to that observed with antidepressant drugs, which have been defined as “mood destabilizers”[46] because of their ability to induce mania in humans suffering from mood disorders.
Finally, the results are consistent with our[34-39] and Keck et al[47] reports of an acute antimanic effect of memantine and our observations of a long-lasting and progressive mood-stabilizing action of memantine in severely ill patients with bipolar disorder[34-39].
The authors thank Denis Greenan for his important contribution to the drafting of this report.
Chronic antidepressant treatments, including electroconvulsive shock and REM sleep deprivation, potentiate the locomotor activity induced by dopamine D2 receptor agonists, suggesting that they sensitize dopamine D2 receptors in the mesolimbic system.
Preclinical and clinical evidence suggests that memantine has an acute antimanic and a long-lasting mood stabilizing effect. On the contrary, while has been reported an antidepressant-like effect of the drug in the forced swimming test (FST), the administration of the compound in depressed patients appears to be ineffective.
The authors found that memantine, at variance with virtually all antidepressant treatments, fails to sensitize dopamine D2 receptors, suggesting that the antidepressant-like effect observed in the FST should be considered a “false positive”. This observation is consistent with the clinical reports of the lack of antidepressant action of memantine in depressed patients.
The authors’ observation further support the suggestion to use memantine, as well as lithium, as an acute antimanic and a long-term mood stabilizing treatment.
Memantine as a new mood stabilizer for the long-term prophylaxis of bipolar disorders.
A well-written paper, which adds to the evidence for the use of memantine in bipolar disorder.
| 1. | Serra G, Argiolas A, Klimek V, Fadda F, Gessa GL. Chronic treatment with antidepressants prevents the inhibitory effect of small doses of apomorphine on dopamine synthesis and motor activity. Life Sci. 1979;25:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 166] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Collu M, Poggiu AS, Devoto P, Serra G. Behavioural sensitization of mesolimbic dopamine D2 receptors in chronic fluoxetine-treated rats. Eur J Pharmacol. 1997;322:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | D’Aquila PS, Collu M, Devoto P, Serra G. Chronic lithium chloride fails to prevent imipramine-induced sensitization to the dopamine D(2)-like receptor agonist quinpirole. Eur J Pharmacol. 2000;395:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Gershon AA, Vishne T, Grunhaus L. Dopamine D2-like receptors and the antidepressant response. Biol Psychiatry. 2007;61:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Spyraki C, Fibiger HC. Behavioural evidence for supersensitivity of postsynaptic dopamine receptors in the mesolimbic system after chronic administration of desipramine. Eur J Pharmacol. 1981;74:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 185] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Serra G, Collu M, D’Aquila PS, De Montis GM, Gessa GL. Possible role of dopamine D1 receptor in the behavioural supersensitivity to dopamine agonists induced by chronic treatment with antidepressants. Brain Res. 1990;527:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Serra G, Collu M, D’Aquila PS, De Montis GM, Gessa GL. Chronic imipramine «reverses» B-HT 920-induced hypomotility in rats. J Neural Transm Gen Sect. 1991;84:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Serra G, Collu M, D’Aquila PS, Gessa GL. Role of the mesolimbic dopamine system in the mechanism of action of antidepressants. Pharmacol Toxicol. 1992;71 Suppl 1:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 867] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 10. | Sills MA, Loo PS. Tricyclic antidepressants and dextromethorphan bind with higher affinity to the phencyclidine receptor in the absence of magnesium and L-glutamate. Mol Pharmacol. 1989;36:160-165. [PubMed] |
| 11. | Reynolds IJ, Miller RJ. Tricyclic antidepressants block N-methyl-D-aspartate receptors: similarities to the action of zinc. Br J Pharmacol. 1988;95:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 140] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Prikhozhan AV, Kovalev GI, Raevskiĭ KS. Effects of antidepressive agents on glutamatergic autoregulatory presynaptic mechanism in the rat cerebral cortex. Biull Eksp Biol Med. 1990;110:624-626. [PubMed] |
| 13. | Bouron A, Chatton JY. Acute application of the tricyclic antidepressant desipramine presynaptically stimulates the exocytosis of glutamate in the hippocampus. Neuroscience. 1999;90:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Gołembiowska K, Zylewska A. Effect of antidepressant drugs on veratridine-evoked glutamate and aspartate release in rat prefrontal cortex. Pol J Pharmacol. 1999;51:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Skuza G, Rogóz Z. The synergistic effect of selective sigma receptor agonists and uncompetitive NMDA receptor antagonists in the forced swim test in rats. J Physiol Pharmacol. 2006;57:217-229. [PubMed] |
| 16. | Rogóz Z, Skuza G, Maj J, Danysz W. Synergistic effect of uncompetitive NMDA receptor antagonists and antidepressant drugs in the forced swimming test in rats. Neuropharmacology. 2002;42:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Moryl E, Danysz W, Quack G. Potential antidepressive properties of amantadine, memantine and bifemelane. Pharmacol Toxicol. 1993;72:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Demontis F, Falconi M, Canu D, Serra G. Memantine prevents “bipolar-like” behavior induced by chronic treatment with imipramine in rats. Eur J Pharmacol. 2015;752:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Papp M, Gruca P, Lason-Tyburkiewicz M, Willner P. Antidepressant, anxiolytic and procognitive effects of rivastigmine and donepezil in the chronic mild stress model in rats. Psychopharmacology (Berl). 2016;233:1235-1243. [PubMed] |
| 20. | Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl). 1988;94:147-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 730] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 21. | Strzelecki D, Tabaszewska A, Barszcz Z, Józefowicz O, Kropiwnicki P, Rabe-Jabłońska J. A 10-week memantine treatment in bipolar depression: a case report. Focus on depressive symptomatology, cognitive parameters and quality of life. Psychiatry Investig. 2013;10:421-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Smith EG, Deligiannidis KM, Ulbricht CM, Landolin CS, Patel JK, Rothschild AJ. Antidepressant augmentation using the N-methyl-D-aspartate antagonist memantine: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2013;74:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Stevens J, Bies RR, Shekhar A, Anand A. Bayesian model of Hamilton Depression Rating Score (HDRS) with memantine augmentation in bipolar depression. Br J Clin Pharmacol. 2013;75:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Anand A, Gunn AD, Barkay G, Karne HS, Nurnberger JI, Mathew SJ, Ghosh S. Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: a double-blind, randomized, placebo-controlled trial. Bipolar Disord. 2012;14:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Lenze EJ, Skidmore ER, Begley AE, Newcomer JW, Butters MA, Whyte EM. Memantine for late-life depression and apathy after a disabling medical event: a 12-week, double-blind placebo-controlled pilot study. Int J Geriatr Psychiatry. 2012;27:974-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Zdanys K, Tampi RR. A systematic review of off-label uses of memantine for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1362-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Ferguson JM, Shingleton RN. An open-label, flexible-dose study of memantine in major depressive disorder. Clin Neuropharmacol. 2007;30:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Zarate CA, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK, Charney DS. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 29. | Omranifard V, Shirzadi E, Samandari S, Afshar H, Maracy MR. Memantine add on to citalopram in elderly patients with depression: A double-blind placebo-controlled study. J Res Med Sci. 2014;19:525-530. [PubMed] |
| 30. | Serra G, Demontis F, Serra F, De Chiara L, Spoto A, Girardi P, Vidotto G, Serra G. Memantine: New prospective in bipolar disorder treatment. World J Psychiatry. 2014;4:80-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Sani G, Serra G, Kotzalidis GD, Romano S, Tamorri SM, Manfredi G, Caloro M, Telesforo CL, Caltagirone SS, Panaccione I. The role of memantine in the treatment of psychiatric disorders other than the dementias: a review of current preclinical and clinical evidence. CNS Drugs. 2012;26:663-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Koukopoulos A, Reginaldi D, Serra G, Koukopoulos A, Sani G, Serra G. Antimanic and mood-stabilizing effect of memantine as an augmenting agent in treatment-resistant bipolar disorder. Bipolar Disord. 2010;12:348-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Koukopoulos A, Serra G, Koukopoulos AE, Reginaldi D, Serra G. The sustained mood-stabilizing effect of memantine in the management of treatment resistant bipolar disorders: findings from a 12-month naturalistic trial. J Affect Disord. 2012;136:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Serra G, Koukopoulos AE, Demontis F, Koukopoulos A. Memantine: A New Mood Stabilizer for Treatment-Resistant Bipolar Disorders. In: Bipolar Disorder - A Portrait of a Complex Mood Disorder 2012; 99-120. |
| 35. | Serra G, D’Aquila PS, Demontis F, De Chiara L, Koukopoulos AE, Galistu A, Koukopoulos A, Serra G. Memantine in the long-term prophylaxis of treatment-resistant bipolar mood disorders. Bipolard Disorder: Symptoms, Management and Risk Factor. New York: Nova Science Publisher Inc 2013; 145-164. |
| 36. | Serra G, De Chiara L, Koukopoulos A, Serra G. Antimanic and long-lasting mood stabilizing effect of memantine in bipolar I mood disorder: two case reports. J Clin Psychopharmacol. 2013;33:715-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Serra G, De Chiara L, Manfredi G, Koukopoulos AE, Sani G, Girardi P, Koukopoulos A, Serra G. Memantine in the management of affective recurrences of bipolar disorders after the discontinuation of long-term lithium treatment: three case histories. Ther Adv Psychopharmacol. 2014;4:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Serra G, DE Chiara L, Koukopoulos AE, Koukopoulos A, Serra G, Kahn DA. Memantine in the treatment and prophylaxis of bipolar II disorder and comorbid fibromyalgia: a case report. J Psychiatr Pract. 2014;20:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Serra G, Koukopoulos A, De Chiara L, Koukopoulos AE, Tondo L, Girardi P, Baldessarini RJ, Serra G. Three-year, naturalistic, mirror-image assessment of adding memantine to the treatment of 30 treatment-resistant patients with bipolar disorder. J Clin Psychiatry. 2015;76:e91-e97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | D’Aquila PS, Collu M, Gessa GL, Serra G. The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol. 2000;405:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Serra G. Uso della memantina peril trattamento dei disturbi dell׳umore. Italian patent IT MI20090174. 2009;Feb 11. |
| 42. | Serra G. Memantine for treating bipolar mood disorders resistant to conventional treatments. European patent EP 2 218 450 A1. 2010;Jan 22. |
| 43. | Baldessarini RJ, Faedda GL, Offidani E, Vázquez GH, Marangoni C, Serra G, Tondo L. Antidepressant-associated mood-switching and transition from unipolar major depression to bipolar disorder: a review. J Affect Disord. 2013;148:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 44. | Gessa GL, Pani L, Serra G, Fratta W. Animal models of mania. Adv Biochem Psychopharmacol. 1995;49:43-66. [PubMed] |
| 45. | Gao Y, Payne RS, Schurr A, Hougland T, Lord J, Herman L, Lei Z, Banerjee P, El-Mallakh RS. Memantine reduces mania-like symptoms in animal models. Psychiatry Res. 2011;188:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Ghaemi SN. Treatment of rapid-cycling bipolar disorder: are antidepressants mood destabilizers? Am J Psychiatry. 2008;165:300-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Keck PE, Hsu HA, Papadakis K, Russo J. Memantine efficacy and safety in patients with acute mania associated with bipolar I disorder: a pilot evaluation. Clin Neuropharmacol. 2009;32:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chakrabarti S, Marzuillo P, Muneoka K S- Editor: Qiu S L- Editor: A E- Editor: Wu HL