Published online Feb 19, 2026. doi: 10.5498/wjp.v16.i2.114736
Revised: November 6, 2025
Accepted: December 9, 2025
Published online: February 19, 2026
Processing time: 125 Days and 17.8 Hours
Depression is frequently accompanied by gastrointestinal disturbances, reflecting the close interplay between emotional regulation and gut physiology. The central amygdala (CeA), particularly its GABAergic neurons, has been identified as a critical hub within the brain-gut axis. However, the mechanisms by which CeA inhibitory neurons mediate comorbid depressive and gastrointestinal symptoms remain poorly understood, and their potential as therapeutic targets has not been fully explored.
To investigate CeA GABAergic mechanisms linking mood and gastric function and to appraise the efficacy of electroacupuncture (EA).
A mouse model of chronic unpredictable mild stress (CUMS) was used to induce depressive-like behavior and gastric dysfunction. Behavioral tests, gastric motility assays, and fluorescent gastric emptying imaging were performed. CeA GABAergic activity was examined using immunofluorescence, calcium imaging, and chemo
CUMS mice exhibited reduced exploration, anhedonia, increased immobility, impaired gastric motility, and de
EA attenuates depressive-like and gastric deficits in CUMS mice, with evidence implicating CeA GABAergic neu
Core Tip: This study reveals the pivotal role of central amygdala (CeA) GABAergic neurons in mediating depressive-like behavior and gastrointestinal dysfunction in mice subjected to chronic unpredictable mild stress. Chemogenetic manipulation confirmed that CeA GABAergic hyperactivation drives these comorbidities, while inhibition alleviates them. Electroacupuncture reduced depressive symptoms and gastric impairment by modulating CeA GABAergic activity. These findings highlight electroacupuncture as a promising therapy for depression with gastrointestinal comorbidity, offering new mechanistic insights into brain-gut interactions.
- Citation: Ma HK, Zhu SL, Li XY, Yuan Y, Huang QY, Wang H, Shen GM, Wang XY. Electroacupuncture alleviates depression and gastrointestinal dysfunction by rebalancing GABAergic activity in the central amygdala. World J Psychiatry 2026; 16(2): 114736
- URL: https://www.wjgnet.com/2220-3206/full/v16/i2/114736.htm
- DOI: https://dx.doi.org/10.5498/wjp.v16.i2.114736
Depression is a highly prevalent psychiatric disorder that affects millions of people worldwide, yet its underlying mechanisms remain incompletely understood. Increasing evidence suggests that brain regions involved in both mood regulation and physiological homeostasis, such as the amygdala, play key roles in the pathophysiology of depression[1,2]. For instance, chronic-stress-induced reductions in GABAergic inhibition within the basolateral amygdala have been shown to disrupt inhibitory signaling and promote anxiety-like and depression-like behavior in mice[3].
The central amygdala (CeA) has attracted growing attention for its involvement in stress responses, emotional re
Electroacupuncture (EA) has emerged as a promising therapeutic strategy for modulating these neural circuits. Preclinical studies show that EA alleviates depressive-like behavior and restores gastrointestinal function through mul
Here, we investigated the role of CeA GABAergic neurons in mediating the comorbidity of depression and gastroin
Male C57BL/6J mice (6-8 weeks; 20-25 g) were used. Animals were group-housed (five per cage) under controlled conditions (25 ± 2 °C; 50% ± 5% relative humidity) on a 12-hour light/dark cycle with ad libitum access to food and water. Males were selected to limit variance from estrous-cycle-related hormonal fluctuations in behavioral and gastric readouts and to ensure continuity with prior datasets. All procedures complied with the institutional animal care standards of Anhui University of Chinese Medicine and were authorized by the Animal Ethics Committee (No. AHUCM-mouse-2024216).
CUMS was used to induce depressive-like behavior. Mice were exposed to two or three stressors per day for 28 con
Body weight was recorded every 4 days at 09:00.
Mice were fasted for 12 hours prior to the test and individually housed with free access to water. A weighed portion of standard chow was provided, and after 1 hour the residual food was weighed. Food intake (g) was calculated as: Food intake = initial weight - residual weight.
Mice received EA under 1% isoflurane using needles placed at ST36 (3-4 mm depth) and CV12 (approximately 1.5 mm depth)[11], connected to a HANS-200A stimulator (2/100 Hz alternating sparse-dense waveform, 20 minutes), once daily for 7 days. Based on validated rodent protocols and pilot titrations, current intensity was set to 2 mA at ST36 (titrated to a slight local muscle twitch without distress)[12] and 1 mA at CV12 (perceptible abdominal fasciculation without guarding)[13,14]. EA in the hM3D (Gq) + clozapine N-oxide (CNO) group began 40 minutes post-CNO.
Open-field test: Locomotor activity was measured in a 45 cm × 45 cm × 45 cm chamber using the SMART 2.0 video-tracking system. After 1-minute adaptation, behavior was recorded for 10 minutes. Total distance traveled and time/distance in the central area were quantified. The chamber was cleaned with 75% ethanol between trials[12].
Elevated plus maze: The maze was configured as a plus sign with two open and two enclosed arms meeting at 90°. Each mouse was placed on the central platform facing a closed arm and allowed to explore for 7 minutes. SMART 2.0 recorded arm entries and time spent in each arm. The apparatus was cleaned with 75% ethanol after each session.
Sucrose preference test: Mice were given two identical bottles of water for 6 hours, followed by two bottles of 1% sucrose solution for 6 hours. After 12-hour water and food deprivation, mice were presented with one bottle of water and one of sucrose solution for 6 hours, with bottle positions switched midway. Consumption was calculated, and sucrose prefe
Tail suspension test: Mice were suspended by the tail 50 cm above the surface using adhesive tape. After 1-minute adaptation, immobility time during the 6-minute test was recorded[17].
Forced swim test: Mice were tested singly in a transparent Plexiglas cylinder (30 cm high, 19 cm diameter) containing water at 24 ± 1 °C to a depth of 25 cm. After 1-minute adaptation, immobility time during the 6-minute test was recorded[17].
Free feeding test: Mice were placed in a behavioral chamber (50 cm × 25 cm × 30 cm) for 20 minutes/day over 2 days with food pellets and non-edible objects placed in opposite corners. All assays took place in the light cycle. During the 1-hour test, SMART 2.0 tracked movement. The percentage of time in the food zone was computed as: (Time in food zone ÷ total time) × 100[18]. The apparatus was swabbed with 75% ethanol after each session.
Gastric motility: Following overnight fasting, mice were anesthetized with 1.5%-3% isoflurane. After incisions at the scapular and abdominal regions, a strain gauge (120 Ω, 3 mm × 2 mm) was affixed to the gastric body with tissue adhesive. Wires were tunneled subcutaneously to the scapular incision and connected to a PowerLab system. Gastric motility was recorded for at least 20 minutes, and data were analyzed with LabChart software (AD Instruments) to determine the frequency and amplitude of gastric contractions[18].
Gastric emptying (in vivo imaging system): Mice were fasted for 24 hours, with water withheld during the final 2 hours, then gavaged with 100 μL methylcellulose (10 mg/mL) containing 0.01 mg Super Fluor 750 (Xingye, China). Animals were anesthetized with isoflurane and imaged every 3 minutes using the IVIS Lumina III system (excitation/emission: 745/800 nm). Regions of interest were placed over the stomach, and fluorescence was quantified as average photon flux/cm² using Living Image software[18].
Stereotaxic injection: Under isoflurane anesthesia, mice were placed in a stereotaxic frame with heating support (36 °C). After skull exposure and leveling, coordinates for the CeA were targeted (AP: -1.02 mm; ML: -2.77 mm; DV: -4.55 mm). Viral vectors were injected at 35 nL/minute using a microinjection pump. The needle remained for 10 minutes before withdrawal to minimize backflow. Chemogenetic tools (rAAV-VGAT1-hM3Dq-mCherry, “hM3Dq”, BrainVTA Cat PT-0489; rAAV-VGAT1-hM4Di-mCherry, “hM4Di”, BrainVTA Cat PT-0488) were injected bilaterally into the CeA of C57BL/6J mice.
Viral targeting and histology: Coronal sections were registered to the Paxinos and Franklin atlas to assign Bregma levels. CeA boundaries were traced in Image J by a rater blinded to group, using atlas landmarks and on-image calibration. A second independent rater reviewed a random 20% subset before unblinding, and disagreements were resolved by consensus. Prespecified exclusions were: (1) Off-target expression beyond CeA (e.g., > 10% outside CeA on any level or spread into adjacent nuclei across ≥ 2 consecutive levels); (2) Fiber/implant misplacement (> 200 μm from target); and (3) Insufficient expression or major imaging artifacts.
Behavioral/physiological assays for chemogenetic activation or inhibition commenced 3 weeks post-delivery. CNO (5 mg/kg, MedChemExpress Cat HY-17366) or saline was administered intraperitoneally for 7 consecutive days[18].
The calcium indicator virus rAAV-VGAT1-GCaMP6s-WPRE-hGH (BrainVTA Cat PT-5608) was injected into the unilateral CeA. After 3 weeks, an optical fiber was implanted. Single-channel fiber photometry was performed 1 week later. Neurons were excited at 470 nm (30-50 μW), with 405 nm (20 μW) used as a control for motion artifacts. Ca2+ signals (ΔF/F) were calculated as (F-F0)/F0. Animals were acclimated for 1 hour before recording, and spontaneous CeA GABA neuron activity was analyzed 20 minutes after test initiation.
Mice were deeply anesthetized with isoflurane, perfused with 25 mL saline followed by 25 mL 4% paraformaldehyde. Brains were post-fixed in 4% paraformaldehyde overnight (4 °C).
Frozen (40 μm, free-floating): Cryoprotected in 20%-30% sucrose. The brain tissue was embedded into optimal cutting temperature compound, and coronal sections were cut to a thickness of 40 μm using a cryostat (CM1860, Leica). Block/permeabilize 1 hour at room temperature (1% bovine serum albumin/0.3% Triton X-100), incubate overnight at 4 °C with primary antibodies (c-Fos, Synaptic Systems Cat 226308, 1:200; GABA, Sigma Cat a2052, 1:250; GAD67, Abcam Cat AB213508, 1:100), wash, apply Alexa Fluor 488/594 secondaries (1:500, 1 hour, room temperature/37 °C), DAPI, mount with antifade.
Paraffin (5 μm, slide-mounted): Dehydrate/embed, the brain tissue was embedded into dehydrated paraffin and cut into 5 μm-thick sections, section to charged slides, deparaffinize/rehydrate, heat antigen retrieval (citrate pH 6.0, 10-15 minutes), permeabilize (0.1% Triton X-100, 10 minutes), block (1% bovine serum albumin + 5% normal donkey serum, 1 hours), stain as above, DAPI, mount.
CeA verification: In virus-injected brains, assess co-localization of reporter (mCherry/GFP) with GAD67 within CeA.
Data were tested for normality. Normally or log-normally distributed data were analyzed with Student’s t test; if distributions deviated, Mann-Whitney U tests were applied. For multiple groups, one-way analysis of variance was used. Repeated measures (e.g., body weight, food intake, gastric emptying) were analyzed with two-way analysis of variance. Data are expressed as mean ± SE, with 95%CI where applicable. Statistical significance was set at P < 0.05, P < 0.01, and P < 0.001. Analyses and figures were generated in Prism 9.5.0 (GraphPad Software). All assessments were performed blind to group allocation.
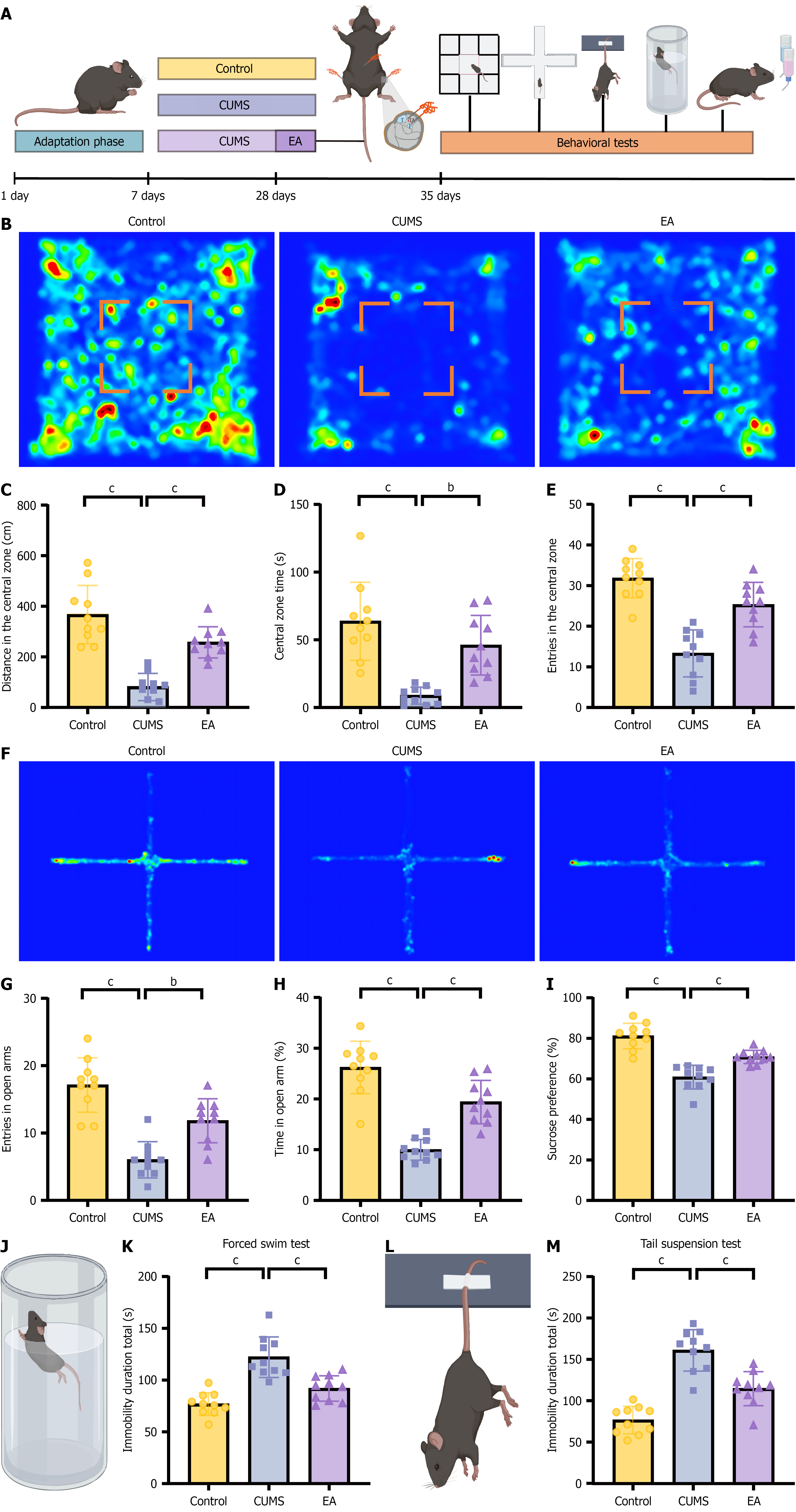
Behavioral testing provides a reliable assessment of depression-like phenotypes in rodents. A CUMS model was established by exposing mice to three distinct stressors daily for 28 consecutive days (Figure 1A). After modeling, the open-field test (Figure 1B-E) and elevated plus maze test (Figure 1F-H) revealed reduced exploratory activity and increased depressive-like behavior in CUMS mice compared with controls. The sucrose preference test (Figure 1I) showed a marked reduction in sucrose consumption, reflecting anhedonia. The forced swim test (Figure 1J and K) and tail suspension test (Figure 1L and M) demonstrated significantly increased immobility, indicating enhanced despair-like behavior. All these findings confirm the successful induction of depression-like behavior in the CUMS model. EA significantly reversed these behavioral abnormalities. Thus, EA effectively alleviated depression-like behavior induced by chronic stress.
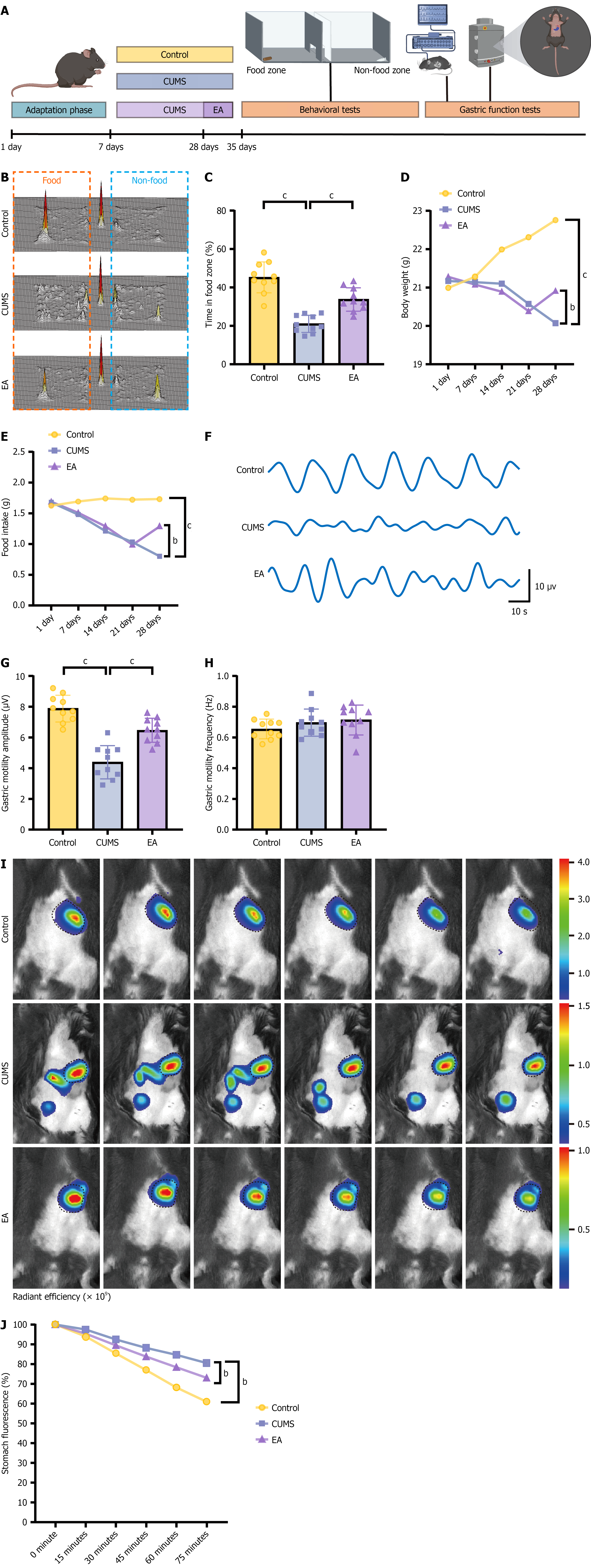
Beyond behavioral deficits, CUMS produced marked gastric dysfunction (Figure 2A). In the free-feeding test (Figure 2B and C), CUMS mice showed reduced food exploration; body-weight tracking (Figure 2D) and food-intake measurements (Figure 2E) also declined. Electrogastrography/contractile recordings (Figure 2F-H) revealed a significant reduction in contraction amplitude, while the dominant gastric motility frequency was unchanged (Figure 2H). In vivo small-animal imaging (Figure 2I and J) confirmed delayed gastric emptying. EA improved all readouts of gastric function. These data indicate that EA not only restores mood-related behavior but also normalizes CUMS-induced gastric dysfunction.
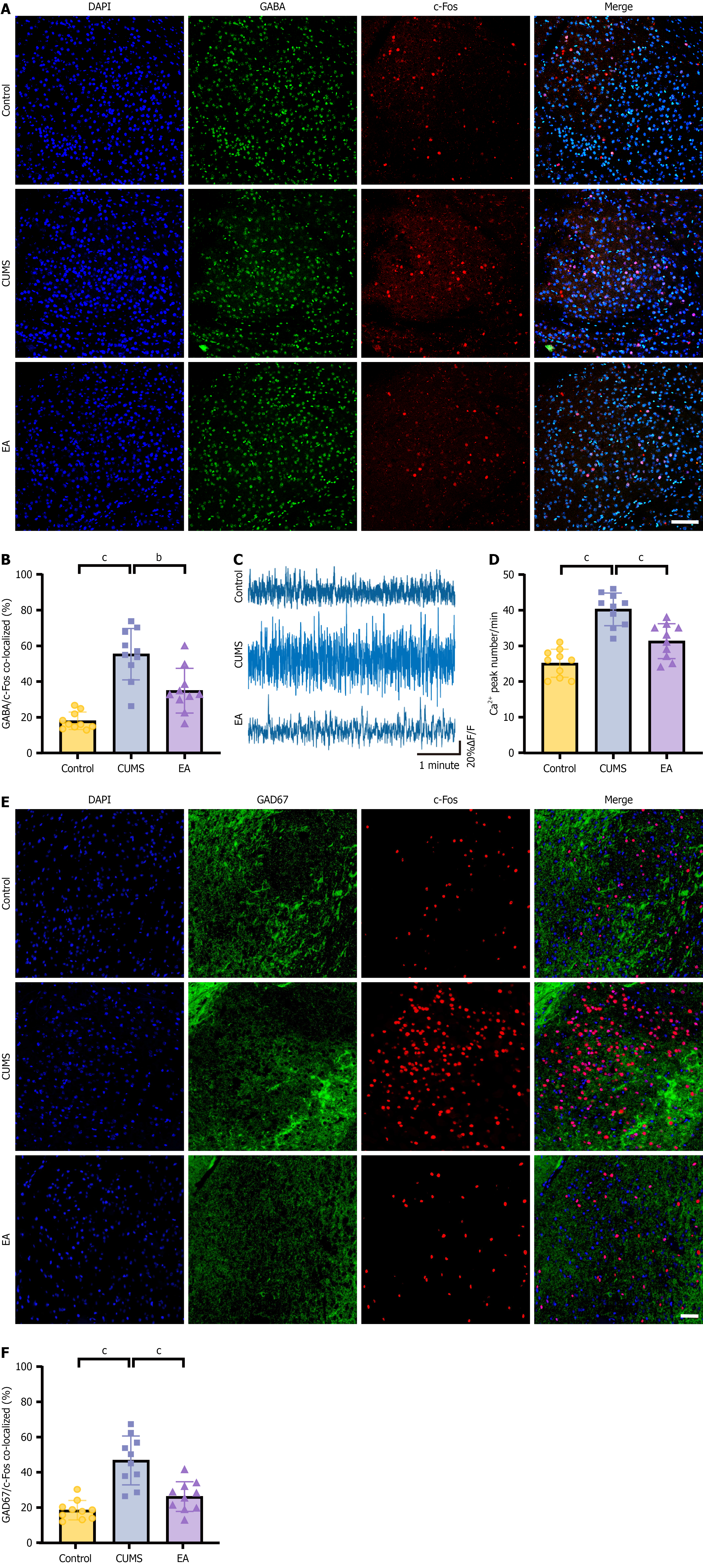
Immunofluorescence revealed a marked increase in GABA/c-Fos co-localized neurons in the CeA of CUMS mice com
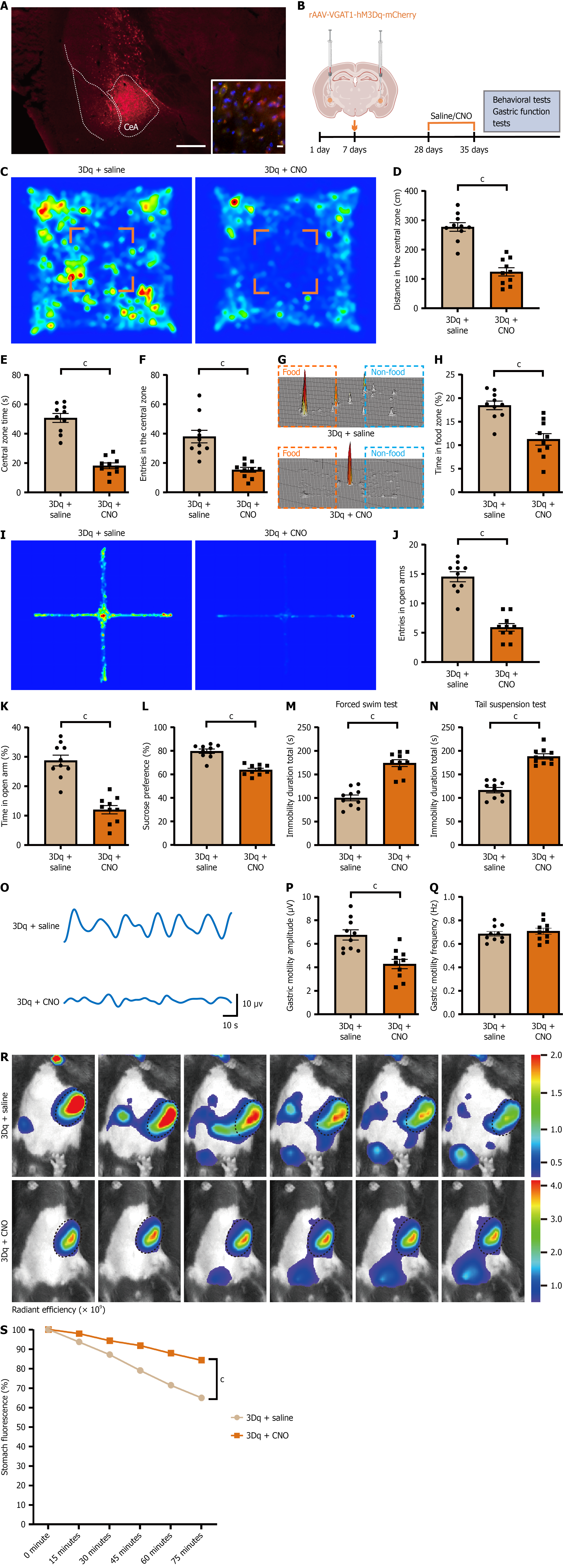
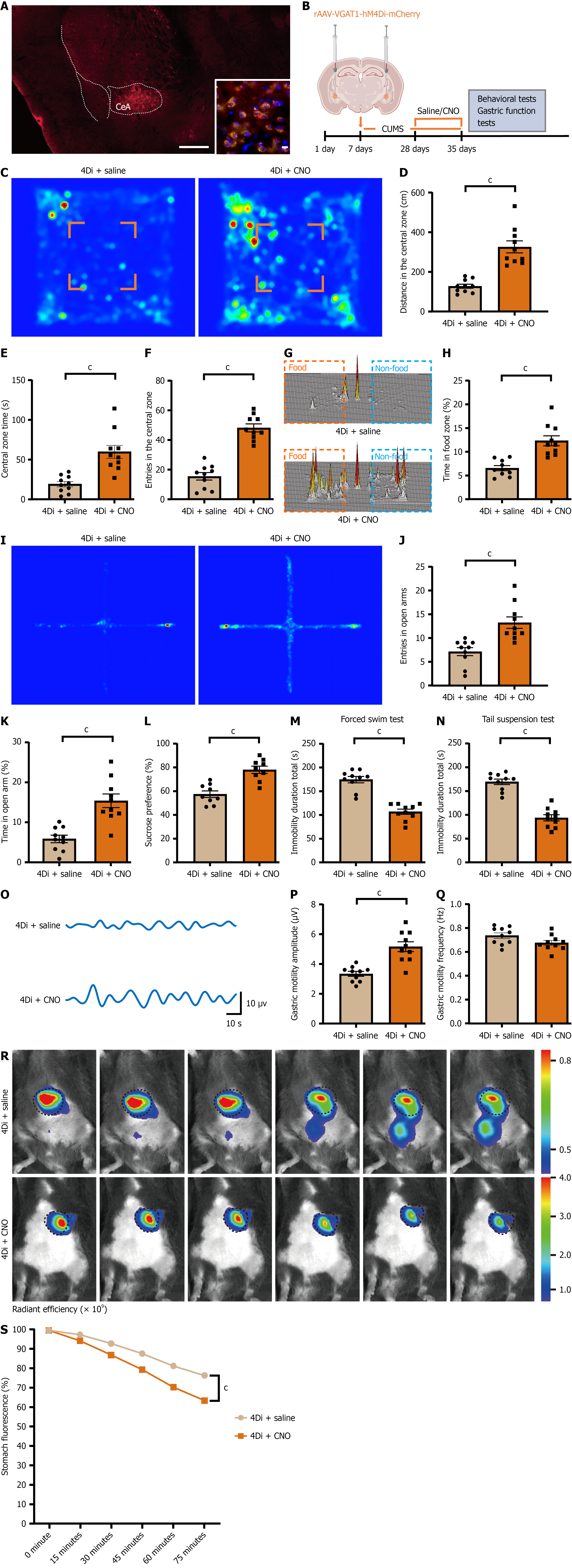
The CeA is a key hub regulating emotion and gastrointestinal function, with GABAergic neurons representing the predominant neuronal type. To assess whether hyperactivation of these neurons drives pathological outcomes, we used chemogenetic activation. To validate GABAergic targeting, rAAV-VGAT1-hM3Dq-mCherry was injected bilaterally into the CeA of C57BL/6J mice; subsequent immunofluorescence quantified mCherry/GAD67 co-labeling in the CeA. Twenty-eight days later, behavioral and gastric function assessments were conducted 40 minutes after CNO administration. Controls consisted of hM3Dq-expressing mice receiving saline (vehicle) (Figure 4A and B). Following intraperitoneal CNO injection, activated mice displayed reduced locomotion, entries, and time spent in the center zone during the open-field test (Figure 4C-F); diminished food-zone preference in the free-feeding test (Figure 4G and H); fewer open-arm entries and shorter open-arm dwell time in the elevated plus maze (Figure 4I-K); marked reduction in sucrose consumption in the sucrose preference test (Figure 4L); and prolonged immobility in the forced swim and tail suspension tests (Figure 4M and N). Compared with the saline control group, gastric motility amplitude was significantly reduced, while frequency showed no significant difference (Figure 4O-Q). Gastric emptying rate was significantly decreased (Figure 4R and S). These results indicate that activation of CeA GABAergic neurons is sufficient to induce both depressive-like behavior and gastric dysfunction, reiterating key features of the CUMS phenotype.
To test the converse, we bilaterally injected rAAV-VGAT1-hM4Di-mCherry into the CeA of CUMS mice to achieve chemogenetic inhibition (Figure 5A and B). Twenty-eight days later, behavioral testing was performed 40 minutes after CNO administration. Twenty-eight days later, behavioral testing was conducted 40 minutes after CNO administration. Controls consisted of hM4Di-expressing mice receiving saline (vehicle). Relative to control, CNO-treated hM4Di mice showed increased locomotion, center entries, and center time in the open field (Figure 5C-F); greater preference for the feeding area in the free-feeding test (Figure 5G and H); enhanced open-arm exploration in the elevated plus maze (Figure 5I-K); higher sucrose preference in the sucrose preference test (Figure 5L); and reduced immobility in the forced-swim and tail-suspension tests (Figure 5M and N). Compared with the saline control group, gastric motility amplitude was significantly reduced, while frequency showed no significant difference (Figure 5O-Q). Gastric emptying rate was significantly reduced (Figure 5R and S). Thus, chemogenetic inhibition of CeA GABAergic neurons alleviates depressive-like behavior and restores gastric function in CUMS mice.
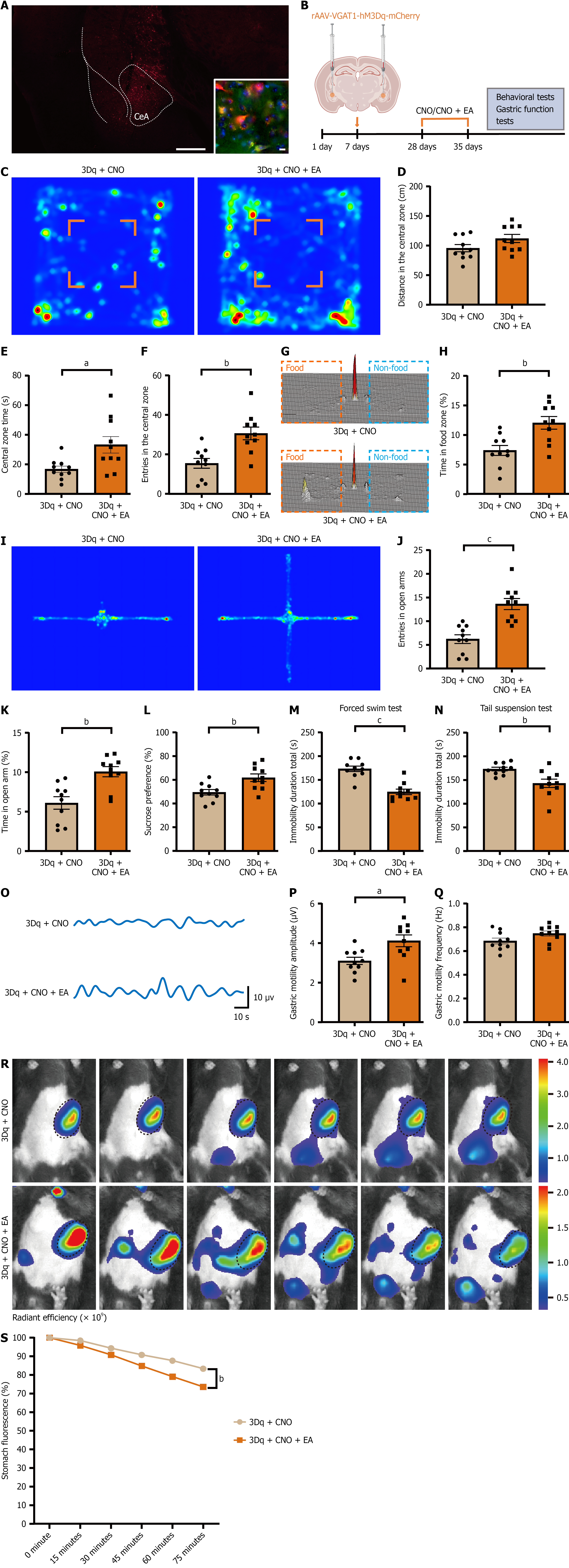
To test whether EA acts via CeA GABAergic neurons, we applied EA in mice with chemogenetic activation of CeA GABA neurons. After 21 days of viral expression, mice received CNO and were assigned to CNO or CNO + EA. Two control conditions were included to rule out nonspecific effects: (1) The hM3Dq-expressing mice injected with saline (vehicle); and (2) The mCherry control-virus mice injected with CNO (Figure 6A and B). Primary analysis focused on CNO vs
Depression commonly co-occurs with gastrointestinal dysfunction, yet the circuit basis linking these domains remains incompletely defined. The CeA is a nexus for stress, emotion, and autonomic control, with GABAergic neurons forming its predominant neuronal population. Chronic stress is thought to weaken GABAergic inhibition, producing network hyperexcitability, negative affect, and gastrointestinal dysmotility[3,19]. Our results align with this framework: (1) CUMS increased c-Fos and calcium activity in CeA GABA neurons; and (2) Chemogenetic activation reproduced depressive-like behavior and gastric dysfunction. Conversely, inhibition attenuated both phenotypes. In the same animals, EA reduced CeA hyperactivity and restored behavioral and gastric outcomes. These data position CeA GABAergic neurons as a shared integrator of mood and visceral function and identify a circuit node that is sensitive to EA. In the CUMS model, EA improved affective behavior and gastric motility through engagement of CeA GABAergic neurons. Beyond behavioral rescue, EA restored gastric motility and emptying, indicating coordinated benefits across the gut-brain axis. Convergent evidence from c-Fos mapping, VGAT-defined fiber photometry, and chemogenetic manipulations supports a role for CeA GABAergic neurons as a critical substrate through which EA exerts antidepressant and promotility effects.
Anatomical and physiological evidence indicate robust CeA communication with autonomic nuclei that control vagal output. The CeA projects to the dorsal vagal complex (DVC), including the dorsal motor nucleus of the vagus (DMV) and nucleus of the solitary tract (NTS)[20,21]. CeA stimulation modulates DVC firing, particularly inhibiting NTS neurons[22], while vagal afferents arising from the gastrointestinal tract reciprocally influence CeA activity[23]. Situated within this bidirectional architecture, our causal manipulations support a model in which CeA inhibitory tone integrates emotional state with vagal autonomic output to shape gastric function. This model parsimoniously explains why normalizing CeA activity via EA yields parallel improvements in affective behavior and motility.
Prior work suggests that EA ameliorates stress-induced pathology through diverse mechanisms, including modulation of inflammatory pathways in the prefrontal cortex, prevention of astrocytic atrophy, and TRPV1-dependent signaling in the gut[24-26]. Clinical signals such as shortened duration of postoperative ileus-indicate translational potential, albeit in different indications[27]. Our study complemented these observations by localizing a CeA-specific route: Even when CeA GABA neurons were chemogenetically overactivated, EA partially restored behavioral and gastric function, consistent with circuit-level rebalancing of inhibitory tone. The partial rescue observed under chemogenetic activation suggests that EA engages a multi-target, multi-pathway network beyond rebalancing CeA GABAergic tone. In parallel with direct CeA modulation, EA likely enhances vagal efferent output via DVC circuits (NTS/DMV) and alters hypothalamic stress in
Heterogeneity in stress paradigms and stimulation parameters likely underlies divergent findings. Relative to acute stress, CUMS better reflects chronic depression and produces region-specific GABAergic adaptations[7,37,38]. Although certain CUMS stressors (e.g., temperature or restraint) may transiently influence gastrointestinal motility, all stressors were delivered intermittently at moderate intensity, and measurements were obtained after completion of the full stress cycle rather than immediately post-stressor, minimizing acute confounding. Although the tail suspension test/forced swim test and open-field test/elevated plus maze partially overlap in assessing depressive and anxiety-like behaviors, re
Collectively, the data support a model in which EA restores inhibitory balance within CeA-autonomic circuits, ac
In conclusion, our findings indicate that EA alleviates depressive-like behavior and gastric dysmotility, at least in part, via modulation of CeA GABAergic neurons - a critical node linking mood regulation and gastric function. Convergent evidence from chemogenetic manipulations and calcium imaging supports a model in which EA reduces aberrant CeA activity and helps re-establish homeostasis within the brain-gut axis. These results suggest a circuit-level mechanism underlying EA’s bidirectional effects and point to therapeutic opportunities for comorbid affective and gastrointestinal disorders.
We thank all the experimenters of Anhui University of Chinese Medicine for their support of this study.
| 1. | Zhang WH, Zhang JY, Holmes A, Pan BX. Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol Psychiatry. 2021;89:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 2. | Ma C, Zhang RZ, Lu HY, Dou TT, Li L, Yan XK. The Effect of Acupuncture on the Morphology and Neural Coding Damage of the Central Amygdala in Mice with Chronic Inflammatory Pain and Depression. J Inflamm Res. 2025;18:3255-3268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Jie F, Yin G, Yang W, Yang M, Gao S, Lv J, Li B. Stress in Regulation of GABA Amygdala System and Relevance to Neuropsychiatric Diseases. Front Neurosci. 2018;12:562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | He X, Ji P, Guo R, Ming X, Zhang H, Yu L, Chen Z, Gao S, Guo F. Regulation of the central amygdala on intestinal motility and behavior via the lateral hypothalamus in irritable bowel syndrome model mice. Neurogastroenterol Motil. 2023;35:e14498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Han RT, Kim YB, Park EH, Kim JY, Ryu C, Kim HY, Lee J, Pahk K, Shanyu C, Kim H, Back SK, Kim HJ, Kim YI, Na HS. Long-Term Isolation Elicits Depression and Anxiety-Related Behaviors by Reducing Oxytocin-Induced GABAergic Transmission in Central Amygdala. Front Mol Neurosci. 2018;11:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Guo Y, Wei W, Chen JD. Effects and mechanisms of acupuncture and electroacupuncture for functional dyspepsia: A systematic review. World J Gastroenterol. 2020;26:2440-2457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (2)] |
| 7. | Wang J, Zhu H, Song X, Zhao J, Zhang J, Zhang J, Li S, Rong P. Electroacupuncture regulates gut microbiota to reduce depressive-like behavior in rats. Front Microbiol. 2024;15:1327630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 8. | Chen Y, Shen P, Li Q, Ong SS, Qian Y, Lu H, Li M, Xu T. Electroacupuncture and Tongbian decoction ameliorate CUMS-induced depression and constipation in mice via TPH2/5-HT pathway of the gut-brain axis. Brain Res Bull. 2025;221:111207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Chen B, Jin K, Dong J, Cheng S, Kong L, Hu S, Chen Z, Lu J. Hypocretin-1/Hypocretin Receptor 1 Regulates Neuroplasticity and Cognitive Function through Hippocampal Lactate Homeostasis in Depressed Model. Adv Sci (Weinh). 2024;11:e2405354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 10. | He H, He H, Mo L, You Z, Zhang J. Priming of microglia with dysfunctional gut microbiota impairs hippocampal neurogenesis and fosters stress vulnerability of mice. Brain Behav Immun. 2024;115:280-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 11. | Liu S, Wang Z, Su Y, Qi L, Yang W, Fu M, Jing X, Wang Y, Ma Q. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 2021;598:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 480] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 12. | Dong S, Zhao L, Liu J, Sha X, Wu Y, Liu W, Sun J, Su Y, Zhuang Z, Chen J, Dong Y, Xie B, Zhou A, Ji H, Wang Y, Deng X, Jing X, Ma Q, Wang N, Liu S. Neuroanatomical organization of electroacupuncture in modulating gastric function in mice and humans. Neuron. 2025;113:3243-3259.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Wang XY, Chen XQ, Wang GQ, Cai RL, Wang H, Wang HT, Peng XQ, Zhang MT, Huang S, Shen GM. A neural circuit for gastric motility disorders driven by gastric dilation in mice. Front Neurosci. 2023;17:1069198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Su YS, He W, Wang C, Shi H, Zhao YF, Xin JJ, Wang XY, Shang HY, Hu L, Jing XH, Zhu B. "Intensity-response" effects of electroacupuncture on gastric motility and its underlying peripheral neural mechanism. Evid Based Complement Alternat Med. 2013;2013:535742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Verharen JPH, de Jong JW, Zhu Y, Lammel S. A computational analysis of mouse behavior in the sucrose preference test. Nat Commun. 2023;14:2419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Zhu YJ, Huang J, Chen R, Zhang Y, He X, Duan WX, Zou YL, Sun MM, Sun HL, Cheng SM, Wang HC, Zhang H, Wu WN. Autophagy dysfunction contributes to NLRP1 inflammasome-linked depressive-like behaviors in mice. J Neuroinflammation. 2024;21:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 17. | Liu D, Zheng X, Hui Y, Xu Y, Du J, Du Z, Che Y, Wu F, Yu G, Zhang J, Gong X, Guo G. Lateral hypothalamus orexinergic projection to the medial prefrontal cortex modulates chronic stress-induced anhedonia but not anxiety and despair. Transl Psychiatry. 2024;14:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Dong WY, Zhu X, Tang HD, Huang JY, Zhu MY, Cheng PK, Wang H, Wang XY, Wang H, Mao Y, Zhao W, Zhang Y, Tao WJ, Zhang Z. Brain regulation of gastric dysfunction induced by stress. Nat Metab. 2023;5:1494-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Ding X, Lin Y, Chen C, Yan B, Liu Q, Zheng H, Wu Y, Zhou C. DNMT1 Mediates Chronic Pain-Related Depression by Inhibiting GABAergic Neuronal Activation in the Central Amygdala. Biol Psychiatry. 2023;94:672-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 20. | Danielsen EH, Magnuson DJ, Gray TS. The central amygdaloid nucleus innervation of the dorsal vagal complex in rat: a Phaseolus vulgaris leucoagglutinin lectin anterograde tracing study. Brain Res Bull. 1989;22:705-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Takeuchi Y, Matsushima S, Matsushima R, Hopkins DA. Direct amygdaloid projections to the dorsal motor nucleus of the vagus nerve: a light and electron microscopic study in the rat. Brain Res. 1983;280:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Zhang X, Cui J, Tan Z, Jiang C, Fogel R. The central nucleus of the amygdala modulates gut-related neurons in the dorsal vagal complex in rats. J Physiol. 2003;553:1005-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Krieger JP, Asker M, van der Velden P, Börchers S, Richard JE, Maric I, Longo F, Singh A, de Lartigue G, Skibicka KP. Neural Pathway for Gut Feelings: Vagal Interoceptive Feedback From the Gastrointestinal Tract Is a Critical Modulator of Anxiety-like Behavior. Biol Psychiatry. 2022;92:709-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Pang F, Yang Y, Huang S, Yang Z, Zhu Z, Liao D, Guo X, Zhou M, Li Y, Tang C. Electroacupuncture Alleviates Depressive-like Behavior by Modulating the Expression of P2X7/NLRP3/IL-1β of Prefrontal Cortex and Liver in Rats Exposed to Chronic Unpredictable Mild Stress. Brain Sci. 2023;13:436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 25. | Lin SS, Zhou B, Chen BJ, Jiang RT, Li B, Illes P, Semyanov A, Tang Y, Verkhratsky A. Electroacupuncture prevents astrocyte atrophy to alleviate depression. Cell Death Dis. 2023;14:343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 26. | Zhong X, Zhang Z, Li J, Liu D, Ma C, Wang G. Effects of Electroacupuncture on Gastrointestinal Motility Function, Pain, and Inflammation via Transient Receptor Potential Vanilloid 1 in a Rat Model after Colonic Anastomoses. Dis Markers. 2022;2022:5113473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Yang JW, Yan SY, Lu Y, Han JG, Pei W, Zhao JJ, Li ZK, Zhou H, Yang NN, Wang LQ, Yang YC, Liu CZ. Electroacupuncture vs Sham Electroacupuncture in the Treatment of Postoperative Ileus After Laparoscopic Surgery for Colorectal Cancer: A Multicenter, Randomized Clinical Trial. JAMA Surg. 2023;158:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 28. | Wang H, Liu WJ, Wang XY, Chen XQ, Cai RL, Zhang MT, Wang HT, He GW, Zhang Z, Shen GM. A central amygdala input to the dorsal vagal complex controls gastric motility in mice under restraint stress. Front Physiol. 2023;14:1074979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 29. | Zheng J, Wang Y, Zhang C, Zhang A, Zhou Y, Xu Y, Yu J, Tian Z. Electroacupuncture negatively regulates the Nesfatin-1/ERK/CREB pathway to alleviate HPA axis hyperactivity and anxiety-like behaviors caused by surgical trauma. Chin Med. 2024;19:108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 30. | Rinaman L, Banihashemi L, Koehnle TJ. Early life experience shapes the functional organization of stress-responsive visceral circuits. Physiol Behav. 2011;104:632-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Sammons M, Popescu MC, Chi J, Liberles SD, Gogolla N, Rolls A. Brain-body physiology: Local, reflex, and central communication. Cell. 2024;187:5877-5890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 32. | Torres-Rodriguez JM, Wilson TD, Singh S, Torruella-Suárez ML, Chaudhry S, Adke AP, Becker JJ, Neugebauer B, Lin JL, Martinez Gonzalez S, Soler-Cedeño O, Carrasquillo Y. The parabrachial to central amygdala pathway is critical to injury-induced pain sensitization in mice. Neuropsychopharmacology. 2024;49:508-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 33. | Huo R, Han SP, Liu FY, Shou XJ, Liu LY, Song TJ, Zhai FJ, Zhang R, Xing GG, Han JS. Responses of Primary Afferent Fibers to Acupuncture-Like Peripheral Stimulation at Different Frequencies: Characterization by Single-Unit Recording in Rats. Neurosci Bull. 2020;36:907-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Uchida S, Kagitani F, Sato-Suzuki I. Somatoautonomic reflexes in acupuncture therapy: A review. Auton Neurosci. 2017;203:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Yong CY, Chen S, Chen H, Chu X, Zhang C, Tan C, Ye L, Li JS. Central neuromechanisms underlying control of intragastric pressure through acupuncture at Zusanli (ST36) in rats: the upper cervical cord is the key link between the ascending and descending pathways. Neural Regen Res. 2016;11:971-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 36. | Guo Z, Wei N, Ye R, Sun T, Qiu S, Shao X, Ge X, Guan L, Fang J, Fang J, Du J. Map activation of various brain regions using different frequencies of electroacupuncture ST36, utilizing the FosCreER strategy. Acupunct Herb Med. 2024;4:386-398. [DOI] [Full Text] |
| 37. | Yang Y, Yu H, Babygirija R, Shi B, Sun W, Zheng X, Zheng J. Electro-Acupuncture Attenuates Chronic Stress Responses via Up-Regulated Central NPY and GABA (A) Receptors in Rats. Front Neurosci. 2020;14:629003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Liang F, Liu S, Zhang H, Xiang R, Xie M, He X, Wang S, Wu S, Li J. Effects of chronic unpredictable mild stress on gut sensation and function in male mice. Stress. 2024;27:2374768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Chang X, Wang L, Sun H, Wang Z, Yang Z, Chen S. Electroacupuncture at different frequencies improves visceral pain in IBS rats through different pathways. Neurogastroenterol Motil. 2024;36:e14874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 40. | Wang Q, Li Z, Nie D, Mu X, Wang Y, Jiang Y, Zhang Y, Lu Z. Low-frequency electroacupuncture exerts antinociceptive effects through activation of POMC neural circuit induced endorphinergic input to the periaqueductal gray from the arcuate nucleus. Mol Pain. 2024;20:17448069241254201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/