Published online Jan 19, 2026. doi: 10.5498/wjp.v16.i1.111010
Revised: July 29, 2025
Accepted: October 23, 2025
Published online: January 19, 2026
Processing time: 193 Days and 22.1 Hours
Non-right-handedness (NRH), encompassing left-handedness and mixed-han
Core Tip: Despite prior studies on non-right-handedness and psychiatric disorders, this review uniquely integrated epidemiological, genetic, and neurobiological evidence. It revealed higher non-right-handedness prevalence in schizophrenia, post-traumatic stress disorder, autism spectrum disorder, etc. It is linked to early developmental disruptions, genetic variants, and abnormal brain lateralization. Bidirectional causality is proposed. The review offered a novel framework and highlights fu
- Citation: Wang QQ, Sun ZS, Wang JS. Non-right-handedness and psychiatric disorders: A synthesis of epidemiological, genetic, and neurobiological evidence. World J Psychiatry 2026; 16(1): 111010
- URL: https://www.wjgnet.com/2220-3206/full/v16/i1/111010.htm
- DOI: https://dx.doi.org/10.5498/wjp.v16.i1.111010
Handedness is defined as the preferential use of one hand over the other and is a consistent behavioral characteristic observed in the majority of humans, with 9.33% of the population identifying as left-handed, 9.49% exhibiting mixed-han
This observed association between NRH and psychiatric disorders prompts further investigation into their underlying connections. Does NRH merely reflect underlying neurodevelopmental perturbations that also predispose individuals to psychopathology? Or might NRH itself play a contributory role in shaping psychiatric risk? To address these questions, this review adopted an integrated, narrative approach that thematically organized current findings across three interconnected perspectives. First, we examined epidemiological patterns to identify which psychiatric conditions show elevated NRH prevalence and explored their shared developmental features. Second, we considered developmental and genetic mechanisms that may simultaneously influence handedness and psychiatric vulnerability, such as early life disruptions or common genetic architectures. Third, we reviewed neurobiological pathways, focusing on how alterations in brain asymmetry and interhemispheric connectivity might contribute to the observed associations between NRH and psychopathology.
To guide this synthesis, we prioritized high-quality, empirically grounded studies, including meta-analyses, genome-wide association studies (GWAS), and neuroimaging work, selected for their conceptual relevance and methodological rigor. We emphasized converging trends rather than isolated findings and reflected on areas of consistency, contradiction, and uncertainty across disciplines. Importantly, while we do not attempt to provide definitive causal answers, we proposed a working framework to contextualize NRH within the broader neurodevelopmental landscape of psychiatric disorders. By bridging epidemiology, genetics, and neurobiology, we sought to clarify the possible mechanisms behind this behavioral-neuropsychiatric link, to evaluate its clinical or developmental relevance, and to highlight promising avenues for future research and translational application.
The majority of studies have reported elevated rates of NRH, including both left-handedness and mixed-handedness, across a range of psychiatric disorders. Among psychiatric conditions, schizophrenia has been most consistently associated with increased NRH. Indeed, an earlier review by Satz and Green[4] in 1999 concluded that elevated NRH is consistently observed among patients with schizophrenia, potentially linking this to specific clinical symptoms and neuropsychological characteristics, including symptom severity and cognitive or language deficits. These conclusions were further supported by recent meta-analyses, showing approximately 1.5 to 1.8 times more likely to be NRH than the general population[3,5-7].
Beyond schizophrenia, elevated NRH has also been documented in other disorders. A recent meta-analysis of PTSD summarized an overall odds ratio (OR) of 1.8 with mixed-handedness showing an even stronger association (OR = 2.4) than left-handedness alone[8]. However, other disorders that also present with internalizing symptoms, such as de
Some studies (n > 200) reported elevated performance-related anxiety among university students with NRH[12,13]. However, higher anxiety among individuals with NRH does not imply an increased NRH proportion in anxiety disorders. Indeed, a systematic review concluded that anxiety disorders showed no consistent elevation in NRH[14]. Si
By comparison, neurodevelopmental disorders constitute another subgroup that shows a markedly elevated pre
A report has shown that individuals with dyslexia are slower overall in completing pegboard tasks; thus, the increase in NRH may be associated with their poorer two-handed coordination[20]. Similarly, individuals with developmental coordination disorder (DCD) demonstrate higher-than-average rates of left-handedness with some studies reporting prevalence rates of 14.7%-30.6%[21,22]. However, ADHD, one of the most common neurodevelopmental disorders that frequently co-occurs with DCD and shares similar motor-coordination challenges[23], shows an ambiguous relationship with NRH. For instance, a community study of 520 school-age children found no significant association between handedness and ADHD traits[24], whereas a recent meta-analysis (n > 2000) reported a borderline increase in left-handedness (P = 0.09) and a significant elevation in overall NRH (P = 0.02) compared with healthy controls[25].
Large-scale studies, particularly meta-analyses, have demonstrated that schizophrenia, PTSD, ASD, dyslexia, DLD, ID, DCD, and ADHD exhibit increased rates of NRH compared with the general population, with similar conclusions reported in a recent second-order meta-analysis examining handedness across multiple psychiatric disorders[3] (Table 1). Upon further examination, it is evident that these conditions share common characteristics, including early developmen
| Psychiatric disorder | OR or effect size | Ref. |
| Schizophrenia | OR = 1.5-1.8 | [5-7] |
| PTSD | OR = 1.8 (mixed: OR = 2.4) | [8] |
| Depression | Inconsistent (meta-analysis: No difference) | [9-11] |
| Anxiety disorder | Inconsistent | [9,14] |
| Bipolar disorder | No increase | [15] |
| ASD | OR = 3.5 | [16,17] |
| ID | Moderate increase | [17] |
| Dyslexia | OR = 1.2 | [18] |
| DLD | OR = 1.2 | [19] |
| DCD | Not always reported | [21,22] |
| ADHD | OR = 1.1-1.2 (borderline) | [25] |
Given the elevated prevalence of NRH observed in psychiatric disorders discussed above, one potential explanation is that early developmental disruptions might simultaneously affect NRH and vulnerability to these disorders. Multiple studies have already indicated that early disruptions, particularly those occurring during the prenatal and perinatal pe
For several decades, scientific investigations have explored the relationship between handedness and early-life con
The observed correlation between low birth weight and NRH may indeed indicate the impact of underlying prenatal or perinatal factors on the development of handedness. Research has identified several factors, such as preterm birth, intrauterine growth restriction, maternal stress, and hormonal exposure, that are associated with higher rates of NRH. Preterm birth has been associated with over a 2-fold increase in NRH risk in extremely preterm samples[45], a pattern also supported by large cohort data[1]. Intrauterine growth restriction, like polyembryony, has also been associated with elevated NRH prevalence[40]. Furthermore, severe maternal stress during pregnancy may increase NRH rates by about 30%[46,47]. Hormonal exposure, especially elevated prenatal testosterone, has been hypothesized to delay left-hemisphere development and shift functional dominance as described in the Geschwind-Galaburda hypothesis[48,49]. Reciprocally, these prenatal or perinatal influences are also well-established risk factors for various neurodevelopmental disorders, including ASD[50], ADHD[50], schizophrenia[51], and DLD[52], suggesting that these early developmental influences may underlie the elevated rates of NRH observed in these conditions.
Taken together, a growing body of research suggests that prenatal or perinatal influences, such as low birth weight, prematurity, intrauterine growth restriction, maternal stress, and atypical hormonal exposure, may jointly contribute to both elevated NRH and increased vulnerability to psychiatric disorders. These findings align with recent second-order meta-analytic evidence indicating that disorders with early developmental onset and those involving language dys
Beyond early developmental disruptions, another prominent explanation for the NRH-psychiatric disorder association is that they share common genetic underpinnings. Although handedness is a stable and commonly considered heritable trait, its heritability estimated from genetic studies is relatively modest. Twin studies estimate that the heritability of handedness is approximately 25%[53] while single-nucleotide polymorphism-based heritability from large-scale GWAS is lower, typically around 3%-6%, and narrow-sense heritability is 11.9%[54,55]. This suggests that while handedness is genetically influenced, it is not strongly determined by genetic variation alone. In contrast, many psychiatric disorders, such as schizophrenia, ASD, and ADHD, show considerably higher heritability, often exceeding 70%[56-58]. Given the relatively low heritability of handedness compared with most psychiatric disorders, the genetic overlap between NRH and these conditions is likely constrained, and its contribution to their phenotypic association remains unclear.
Early candidate gene-based genetic studies have reported that some specific loci might be associated with both left-handedness and mixed-handedness as well as an elevated risk of psychiatric disorders. For example, variants in leucine rich repeat transmembrane neuronal 1, an imprinted gene on chromosome 2p12, were initially reported to be associated with both left-handedness in families with dyslexia and schizophrenia susceptibility[59,60]. Similarly, variants of pro
However, these associations have largely failed to replicate in subsequent GWAS, questioning the validity of early candidate gene findings[55]. A recent large-scale meta-analytic GWAS study involving over 1.7 million individuals identified 41 loci associated with left-handedness and 7 with mixed-handedness[55]. Importantly, several handedness-associated loci also showed genetic correlations with schizophrenia and bipolar disorder, with specific variants (e.g., rs6224, rs13107325, and rs45527431) demonstrating concordant effects on both traits[55]. Beyond specific loci, tissue, and pathway enrichment analyses consistently highlight central nervous system structures and developmental pathways that may contribute to psychiatric disorder vulnerability, such as microtubule regulation, axonogenesis, and cerebral cortex morphology[55,63].
In particular, multiple handedness-associated genes, including tubulin beta class I (TUBB), TUBB3, TUBB4A, microtubule-associated protein 2, and N-myc downstream regulates gene-1, encode proteins involved in microtubule dynamics and neuronal development, which are essential processes during early brain organization and hemispheric specialization[63]. These same genes have also been implicated in psychiatric disorders, such as schizophrenia[64] and ASD[65]. This convergence suggests that microtubule-related genetic pathways may influence both cerebral asymmetry and neural circuit formation, thereby linking handedness with psychopathology at the neurodevelopmental level. However, it is crucial to note that these limited genetic associations have small effect sizes with individual variants typically explaining < 0.1% of the liability variance in handedness. Given such limited genetic determination, the shared genetic component likely accounts for only a small portion of the NRH-psychiatric disorders association. Moreover, recent exome-wide analyses have shown that rare protein-altering variants also contributed minimally to handedness heritability[54]. Therefore, although shared genetic underpinnings represent the most intuitive hypothesis, their actual explanatory power remains highly questionable.
From a neurobiological perspective, another potential explanation for the handedness-psychiatric disorder association lies in brain asymmetry patterns. Different handedness preferences reflect underlying cerebral lateralization differences that may be related to psychiatric disorders that have been widely documented to exhibit various forms of brain asymmetry abnormalities.
Most individuals who are right-handed exhibit dominant left-hemisphere control for fine motor tasks, supported by characteristic structural and functional brain asymmetries[66]. Neuroimaging studies have confirmed that left-handers are more likely to exhibit atypical (e.g., bilateral or rightward) hemispheric dominance compared with right-handers[67,68]. Beyond motor lateralization, handedness is also closely linked to language lateralization[67-69]. While the majority of individuals exhibited left-hemisphere dominance for language-related brain regions, individuals with NRH are more likely to demonstrate atypical language lateralization, often characterized by rightward shifts that mirror their atypical motor-related lateralization[67-69]. This evidence suggests that individuals with NRH may exhibit reduced structural and functional brain asymmetry in relevant regions, leading to less pronounced hemispheric dominance for specific cognitive and motor functions compared with those who are right-handed.
Reciprocally, reduced brain asymmetry has also been documented across multiple psychiatric disorders. The ENIGMA consortium has systematically examined brain asymmetry patterns in several handedness-related psychiatric conditions through large-scale meta-analyses. For instance, in ADHD pediatric patients demonstrated reduced rightward asym
Despite these convergent findings, the relationship between reduced brain asymmetry, handedness, and psychiatric disorders appears more complex than once thought. One challenge lies in the fact that studies simultaneously investigating the influence of both NRH and psychiatric disorders on brain asymmetry are still quite limited. For instance, one study investigating brain asymmetry with both schizophrenia and NRH (n = 100 per group) identified an NRH-associated rightward shift in grey matter volume of the superior temporal gyrus but failed to detect a significant inte
Another point of consideration is that large-scale brain asymmetry studies of psychiatric disorders (such as those from the ENIGMA consortium) have generally not observed a significant influence of handedness on disease-related brain asymmetry changes (a finding potentially limited by the low prevalence of left-handedness). These studies at least par
In addition to lateralization patterns, interhemispheric connectivity, particularly via the corpus callosum (CC), has been implicated in the neurobiology of handedness[76-78]. The CC is the largest white matter tract in the brain and plays a critical role in facilitating interhemispheric communication[79]. Earlier studies reported that individuals with NRH exhibit larger CC volumes compared to right-handers[80]. However, a recent meta-analysis found no significant differences in overall CC size across different handedness groups[81]. This result may be partly explained by another study that suggested a negative correlation between CC thickness and the degree of handedness lateralization rather than its direction[82], implying that studies based on a dichotomous classification of handedness might not readily observe such subtle variations.
Furthermore, beyond gross callosal anatomy, some studies suggest that microstructural differences within the CC may also vary with handedness[76,83], although these investigations typically involve small sample sizes. Moreover, tissue enrichment analyses of handedness-associated genetic loci have also highlighted the CC as a highly relevant brain region[55]. While human studies on overall CC size yield mixed results, animal models also provide evidence that agenesis or disruption of the CC in rodents leads to a reduction in paw preference, underscoring that callosal dysfunction impairs the establishment of lateralized motor behavior[84,85]. However, it is crucial to distinguish this from human mixed-handedness; in rodents, it refers to inconsistent use of a single paw in a specific task, whereas in humans, mixed-handedness is defined by the preferential use of different hands for different tasks.
Regarding psychiatric disorders, multiple conditions are frequently associated with CC abnormalities. However, contrary to the prevailing notion that NRH is associated with larger CC, reduced CC is frequently observed in psychiatric disorders, including schizophrenia[86-88], ASD[89,90], and ADHD[91,92]. Nevertheless, opposite effects have also been reported. For example, a study of patients with neuroleptic-naïve recent-onset schizophrenia revealed a significant inc
Abnormalities of the CC have been proposed to reflect changes in interhemispheric connectivity that may be associated with both handedness and psychiatric disorders. In line with this, a study reported that individuals who are right-handed have stronger interhemispheric functional connectivity between the left primary motor cortex and the contralateral right premotor cortex compared with those who are left-handed[95]. Furthermore, as suggested by Innocenti et al[96], these CC changes may also reflect reduced thalamic input. Notably, a recent multivariate GWAS revealed significant heritability in right thalamic connections and identified a positive correlation with the polygenic score for left-handedness (r = 0.07, P = 1.74 × 10-31)[78]. In summary, cerebral lateralization and interhemispheric communications, particularly via the CC, may play crucial roles in the association between handedness and psychiatric disorders.
Current evidence suggests a consistent association between elevated rates of NRH and several psychiatric disorders, while the underlying mechanism for these associations is still largely unclarified. The early development interruption, shared genetic underpinnings, and atypical brain asymmetry may partially explain such associations. However, existing evidence from these potential mechanisms remains insufficient to fully elucidate the interactive effects between NRH and psychiatric disorders. Specifically, it remains unclear whether NRH and psychiatric disorders represent unrelated parallel consequences of shared factors (e.g., prenatal disruptions), whether they stem from common underlying mechanisms (e.g., shared genetic vulnerabilities) or whether a direct causal relationship exists between them.
One prevalent hypothesis posits that NRH as a stable and heritable trait serves as a risk factor for psychiatric disorders[75]. This is often attributed to NRH-associated brain lateralization changes, such as weak asymmetry, which are hypothesized to increase the risk of these disorders[68]. Furthermore, genetic variations influencing brain lateralization are often considered co-risk factors for both conditions[63]. However, such hypotheses currently lack sufficient multilevel empirical support. First, it remains uncertain whether observed brain lateralization changes related to NRH are a cause or a consequence of NRH. Second, the debate continues regarding whether altered brain lateralization genuinely increases the risk of psychiatric disorders. For instance, Bishop[97] proposed that weak asymmetry might be a consequence of dyslexia/specific language impairment-related learning impairment rather than its cause. Lastly, even the potential causal direction between NRH and psychiatric disorders remains ambiguous.
Given that handedness is widely considered to reflect innate and stable neurobiological organization, researchers have often hypothesized that NRH might influence psychiatric disorders based on the typical onset sequence. Some resear
Furthermore, an adoption study found no significant differences in handedness between individuals influenced by biological parents vs adopted parents when adoption occurred in the infant period[102]. These findings collectively un
While these discussions shed light on the multifaceted nature of the NRH-psychiatric disorder association, a clearer understanding is still hindered by critical limitations in the field. Specifically, the neuropsychological mechanisms underlying both handedness and psychiatric disorders, particularly regarding handedness itself, remain incompletely understood. Despite decades of research, the developmental, genetic, and neurobiological determinants of handedness remain poorly characterized.
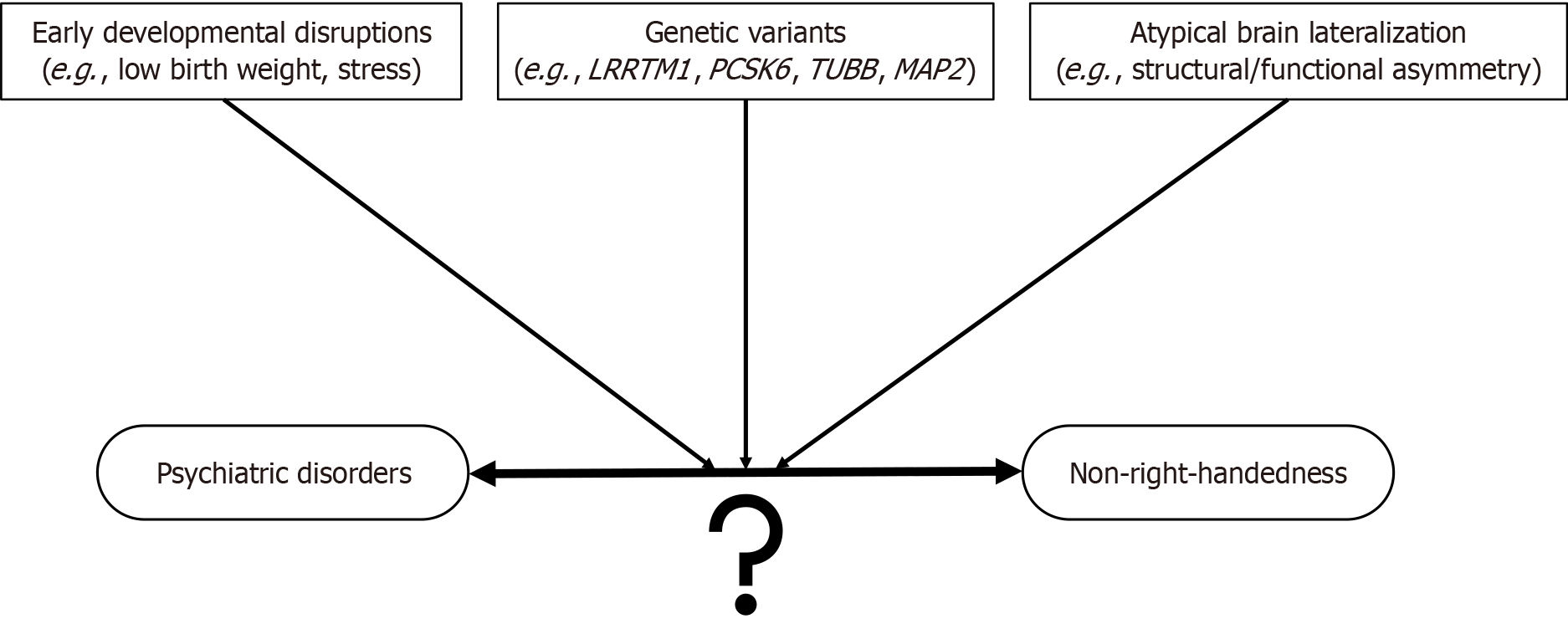
NRH is overrepresented in several psychiatric disorders, especially those with early onset and cognitive or motor impairments. Although early developmental disruption, overlapping genetic architecture, and atypical brain lateralization are proposed contributors to this pattern, their relative contributions and interactions remain unclear (Figure 1). NRH may precede, accompany, or result from emerging psychopathology. For instance, although handedness emerges prenatally and consolidates in early childhood, neurodevelopmental disruptions or early symptoms of psychiatric disorders could interfere with its typical establishment. Conversely, NRH could act as a proxy marker for subtle brain anomalies that predispose to mental illness.
Beyond theoretical implications, these findings hold potential applied relevance. NRH, as a non-invasive and easily observable trait, may serve as a developmental risk marker for neuropsychiatric conditions when considered alongside other early indicators such as language or motor delays. Although it lacks diagnostic specificity, routine assessment of handedness in pediatric or clinical settings may help inform early screening frameworks or individualized neurodevelopmental monitoring protocols. Moreover, atypical handedness may provide a behavioral window into cerebral lateralization profiles, which could inform neuropsychological evaluation strategies.
To advance both scientific understanding and translational utility, future research should prioritize longitudinal and mechanistic approaches. Large-scale, prospective cohort studies beginning in infancy and incorporating handedness trajectories, neuroimaging, cognitive profiling, and genetic data will be crucial for clarifying the temporal dynamics and causal relationships between NRH and psychiatric outcomes. Parallel development of animal models capable of manipulating lateralized behavior will also enable direct testing of mechanistic hypotheses. Ultimately, these efforts will help determine whether NRH can be leveraged as an early, low-cost signal to guide risk stratification and early intervention in vulnerable populations.
| 1. | Papadatou-Pastou M, Ntolka E, Schmitz J, Martin M, Munafò MR, Ocklenburg S, Paracchini S. Human handedness: A meta-analysis. Psychol Bull. 2020;146:481-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 286] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 2. | Brandler WM, Paracchini S. The genetic relationship between handedness and neurodevelopmental disorders. Trends Mol Med. 2014;20:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Packheiser J, Borawski J, Berretz G, Merklein SA, Papadatou-Pastou M, Ocklenburg S. Handedness in mental and neurodevelopmental disorders: A systematic review and second-order meta-analysis. Psychol Bull. 2025;151:476-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Satz P, Green MF. Atypical handedness in schizophrenia: some methodological and theoretical issues. Schizophr Bull. 1999;25:63-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry. 2001;178:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 313] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Dragovic M, Hammond G. Handedness in schizophrenia: a quantitative review of evidence. Acta Psychiatr Scand. 2005;111:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Hirnstein M, Hugdahl K. Excess of non-right-handedness in schizophrenia: meta-analysis of gender effects and potential biases in handedness assessment. Br J Psychiatry. 2014;205:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Borawski J, Papadatou-Pastou M, Packheiser J, Ocklenburg S. Handedness in post-traumatic stress disorder: A meta-analysis. Neurosci Biobehav Rev. 2023;145:105009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 9. | Logue DD, Logue RT, Kaufmann WE, Belcher HM. Psychiatric disorders and left-handedness in children living in an urban environment. Laterality. 2015;20:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Denny K. Handedness and depression: evidence from a large population survey. Laterality. 2009;14:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Packheiser J, Schmitz J, Stein CC, Pfeifer LS, Berretz G, Papadatou-Pastou M, Peterburs J, Ocklenburg S. Handedness and depression: A meta-analysis across 87 studies. J Affect Disord. 2021;294:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Thomas CL, Fitch SB. Handedness and test anxiety: An examination of mixed-handed and consistent-handed students. Exp Results. 2023;4:e15. [DOI] [Full Text] |
| 13. | Wright L, Hardie SM. Left-handers look before they leap: handedness influences reactivity to novel Tower of Hanoi tasks. Front Psychol. 2015;6:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Ocklenburg S, Borawski J, Mundorf A, Riedel K, Lischke A. Handedness and anxiety: a review. Laterality. 2023;28:336-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Mundorf A, Borawski J, Ocklenburg S. Behavioral lateralization in bipolar disorders: a systematic review. Int J Bipolar Disord. 2023;11:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Markou P, Ahtam B, Papadatou-Pastou M. Elevated Levels of Atypical Handedness in Autism: Meta-Analyses. Neuropsychol Rev. 2017;27:258-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Samadi SA. Handedness in autism spectrum disorders and intellectually disabled children and adolescents - Contrasting caregivers' reports with assessments of hand preference. Heliyon. 2024;10:e25935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Eglinton E, Annett M. Handedness and dyslexia: a meta-analysis. Percept Mot Skills. 1994;79:1611-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Abbondanza F, Dale PS, Wang CA, Hayiou-Thomas ME, Toseeb U, Koomar TS, Wigg KG, Feng Y, Price KM, Kerr EN, Guger SL, Lovett MW, Strug LJ, van Bergen E, Dolan CV, Tomblin JB, Moll K, Schulte-Körne G, Neuhoff N, Warnke A, Fisher SE, Barr CL, Michaelson JJ, Boomsma DI, Snowling MJ, Hulme C, Whitehouse AJO, Pennell CE, Newbury DF, Stein J, Talcott JB, Bishop DVM, Paracchini S. Language and reading impairments are associated with increased prevalence of non-right-handedness. Child Dev. 2023;94:970-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Stoodley CJ, Stein JF. A processing speed deficit in dyslexic adults? Evidence from a peg-moving task. Neurosci Lett. 2006;399:264-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Darvik M, Lorås H, Pedersen AV. The Prevalence of Left-Handedness Is Higher Among Individuals With Developmental Coordination Disorder Than in the General Population. Front Psychol. 2018;9:1948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Goez H, Zelnik N. Handedness in patients with developmental coordination disorder. J Child Neurol. 2008;23:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Lee T, Lim J, Kim S, Kim J, Park KJ, Joung YS, Kim HW. The association between symptoms of developmental coordination disorder and neuropsychological characteristics in children with and without ADHD. Front Psychiatry. 2024;15:1441102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Niederhofer H. Hand preference in attention deficit hyperactivity disorder. Percept Mot Skills. 2005;101:808-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Nastou E, Ocklenburg S, Hoogman M, Papadatou-Pastou M. Handedness in ADHD: Meta-Analyses. Neuropsychol Rev. 2022;32:877-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Bedford R, Pickles A, Lord C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Res. 2016;9:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Viholainen H, Ahonen T, Lyytinen P, Cantell M, Tolvanen A, Lyytinen H. Early motor development and later language and reading skills in children at risk of familial dyslexia. Dev Med Child Neurol. 2006;48:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Müürsepp I, Ereline J, Gapeyeva H, Pääsuke M. Motor performance in 5-year-old preschool children with developmental speech and language disorders. Acta Paediatr. 2009;98:1334-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Flapper BC, Schoemaker MM. Developmental coordination disorder in children with specific language impairment: co-morbidity and impact on quality of life. Res Dev Disabil. 2013;34:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Athanasiadou A, Buitelaar JK, Brovedani P, Chorna O, Fulceri F, Guzzetta A, Scattoni ML. Early motor signs of attention-deficit hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2020;29:903-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Filatova S, Koivumaa-Honkanen H, Hirvonen N, Freeman A, Ivandic I, Hurtig T, Khandaker GM, Jones PB, Moilanen K, Miettunen J. Early motor developmental milestones and schizophrenia: A systematic review and meta-analysis. Schizophr Res. 2017;188:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Torrisi R, Arnautovic E, Pointet Perizzolo VC, Vital M, Manini A, Suardi F, Gex-Fabry M, Rusconi Serpa S, Schechter DS. Developmental delay in communication among toddlers and its relationship to caregiving behavior among violence-exposed, posttraumatically stressed mothers. Res Dev Disabil. 2018;82:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | McLaughlin KA, Koenen KC, Bromet EJ, Karam EG, Liu H, Petukhova M, Ruscio AM, Sampson NA, Stein DJ, Aguilar-Gaxiola S, Alonso J, Borges G, Demyttenaere K, Dinolova RV, Ferry F, Florescu S, de Girolamo G, Gureje O, Kawakami N, Lee S, Navarro-Mateu F, Piazza M, Pennell BE, Posada-Villa J, Ten Have M, Viana MC, Kessler RC. Childhood adversities and post-traumatic stress disorder: evidence for stress sensitisation in the World Mental Health Surveys. Br J Psychiatry. 2017;211:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 34. | Pratchett LC, Yehuda R. Foundations of posttraumatic stress disorder: does early life trauma lead to adult posttraumatic stress disorder? Dev Psychopathol. 2011;23:477-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Odintsova VV, van Dongen J, van Beijsterveldt CEM, Ligthart L, Willemsen G, de Geus EJC, Dolan CV, Boomsma DI. Handedness and 23 Early Life Characteristics in 37,495 Dutch Twins. Twin Res Hum Genet. 2023;26:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Satz P. Pathological left-handedness: an explanatory model. Cortex. 1972;8:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 205] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | O'Callaghan MJ, Tudehope DI, Dugdale AE, Mohay H, Burns Y, Cook F. Handedness in children with birthweights below 1000 g. Lancet. 1987;1:1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Rodriguez A, Waldenström U. Fetal origins of child non-right-handedness and mental health. J Child Psychol Psychiatry. 2008;49:967-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Heikkilä K, Van Beijsterveldt CEM, Haukka J, Iivanainen M, Saari-Kemppainen A, Silventoinen K, Boomsma DI, Yokoyama Y, Vuoksimaa E. Triplets, birthweight, and handedness. Proc Natl Acad Sci U S A. 2018;115:6076-6081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | de Kovel CGF, Carrión-Castillo A, Francks C. A large-scale population study of early life factors influencing left-handedness. Sci Rep. 2019;9:584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 41. | Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 729] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 42. | Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 442] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 43. | Zwicker JG, Yoon SW, Mackay M, Petrie-Thomas J, Rogers M, Synnes AR. Perinatal and neonatal predictors of developmental coordination disorder in very low birthweight children. Arch Dis Child. 2013;98:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Holsti L, Grunau RV, Whitfield MF. Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr. 2002;23:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Domellöf E, Johansson AM, Rönnqvist L. Handedness in preterm born children: a systematic review and a meta-analysis. Neuropsychologia. 2011;49:2299-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Glover V, O'Connor TG, Heron J, Golding J; ALSPAC Study team. Antenatal maternal anxiety is linked with atypical handedness in the child. Early Hum Dev. 2004;79:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and mixed-handedness. Pediatr Res. 2007;62:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Galaburda AM. The testosterone hypothesis: Assessment since Geschwind and Behan, 1982. Ann Dyslexia. 1990;40:18-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | Stoyanov Z, Nikolova P, Pashalieva I. Season of birth, Geschwind and Galaburda hypothesis, and handedness. Laterality. 2011;16:607-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Doi M, Usui N, Shimada S. Prenatal Environment and Neurodevelopmental Disorders. Front Endocrinol (Lausanne). 2022;13:860110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 51. | Davies C, Segre G, Estradé A, Radua J, De Micheli A, Provenzani U, Oliver D, Salazar de Pablo G, Ramella-Cravaro V, Besozzi M, Dazzan P, Miele M, Caputo G, Spallarossa C, Crossland G, Ilyas A, Spada G, Politi P, Murray RM, McGuire P, Fusar-Poli P. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta-analysis. Lancet Psychiatry. 2020;7:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 52. | Tomblin JB, Smith E, Zhang X. Epidemiology of specific language impairment: prenatal and perinatal risk factors. J Commun Disord. 1997;30:325-43; quiz 343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 104] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Pfeifer LS, Schmitz J, Papadatou-Pastou M, Peterburs J, Paracchini S, Ocklenburg S. Handedness in twins: meta-analyses. BMC Psychol. 2022;10:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Schijven D, Soheili-Nezhad S, Fisher SE, Francks C. Exome-wide analysis implicates rare protein-altering variants in human handedness. Nat Commun. 2024;15:2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Cuellar-Partida G, Tung JY, Eriksson N, Albrecht E, Aliev F, Andreassen OA, Barroso I, Beckmann JS, Boks MP, Boomsma DI, Boyd HA, Breteler MMB, Campbell H, Chasman DI, Cherkas LF, Davies G, de Geus EJC, Deary IJ, Deloukas P, Dick DM, Duffy DL, Eriksson JG, Esko T, Feenstra B, Geller F, Gieger C, Giegling I, Gordon SD, Han J, Hansen TF, Hartmann AM, Hayward C, Heikkilä K, Hicks AA, Hirschhorn JN, Hottenga JJ, Huffman JE, Hwang LD, Ikram MA, Kaprio J, Kemp JP, Khaw KT, Klopp N, Konte B, Kutalik Z, Lahti J, Li X, Loos RJF, Luciano M, Magnusson SH, Mangino M, Marques-Vidal P, Martin NG, McArdle WL, McCarthy MI, Medina-Gomez C, Melbye M, Melville SA, Metspalu A, Milani L, Mooser V, Nelis M, Nyholt DR, O'Connell KS, Ophoff RA, Palmer C, Palotie A, Palviainen T, Pare G, Paternoster L, Peltonen L, Penninx BWJH, Polasek O, Pramstaller PP, Prokopenko I, Raikkonen K, Ripatti S, Rivadeneira F, Rudan I, Rujescu D, Smit JH, Smith GD, Smoller JW, Soranzo N, Spector TD, Pourcain BS, Starr JM, Stefánsson H, Steinberg S, Teder-Laving M, Thorleifsson G, Stefánsson K, Timpson NJ, Uitterlinden AG, van Duijn CM, van Rooij FJA, Vink JM, Vollenweider P, Vuoksimaa E, Waeber G, Wareham NJ, Warrington N, Waterworth D, Werge T, Wichmann HE, Widen E, Willemsen G, Wright AF, Wright MJ, Xu M, Zhao JH, Kraft P, Hinds DA, Lindgren CM, Mägi R, Neale BM, Evans DM, Medland SE. Genome-wide association study identifies 48 common genetic variants associated with handedness. Nat Hum Behav. 2021;5:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 56. | Wray NR, Gottesman II. Using summary data from the danish national registers to estimate heritabilities for schizophrenia, bipolar disorder, and major depressive disorder. Front Genet. 2012;3:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 57. | Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, Gillan N, Hallett V, Lietz S, Garnett T, Ronald A, Plomin R, Rijsdijk F, Happé F, Bolton P. Heritability of Autism Spectrum Disorder in a UK Population-Based Twin Sample. JAMA Psychiatry. 2015;72:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 58. | Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24:562-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 680] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 59. | Leach EL, Prefontaine G, Hurd PL, Crespi BJ. The imprinted gene LRRTM1 mediates schizotypy and handedness in a nonclinical population. J Hum Genet. 2014;59:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Francks C, Maegawa S, Laurén J, Abrahams BS, Velayos-Baeza A, Medland SE, Colella S, Groszer M, McAuley EZ, Caffrey TM, Timmusk T, Pruunsild P, Koppel I, Lind PA, Matsumoto-Itaba N, Nicod J, Xiong L, Joober R, Enard W, Krinsky B, Nanba E, Richardson AJ, Riley BP, Martin NG, Strittmatter SM, Möller HJ, Rujescu D, St Clair D, Muglia P, Roos JL, Fisher SE, Wade-Martins R, Rouleau GA, Stein JF, Karayiorgou M, Geschwind DH, Ragoussis J, Kendler KS, Airaksinen MS, Oshimura M, DeLisi LE, Monaco AP. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol Psychiatry. 2007;12:1129-1139, 1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 61. | Robinson KJ, Hurd PL, Read S, Crespi BJ. The PCSK6 gene is associated with handedness, the autism spectrum, and magical ideation in a non-clinical population. Neuropsychologia. 2016;84:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Scerri TS, Brandler WM, Paracchini S, Morris AP, Ring SM, Richardson AJ, Talcott JB, Stein J, Monaco AP. PCSK6 is associated with handedness in individuals with dyslexia. Hum Mol Genet. 2011;20:608-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 63. | Ocklenburg S, Mundorf A, Peterburs J, Paracchini S. Genetics of human handedness: microtubules and beyond. Trends Genet. 2025;41:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 64. | Grubisha MJ, Sun X, MacDonald ML, Garver M, Sun Z, Paris KA, Patel DS, DeGiosio RA, Lewis DA, Yates NA, Camacho C, Homanics GE, Ding Y, Sweet RA. MAP2 is differentially phosphorylated in schizophrenia, altering its function. Mol Psychiatry. 2021;26:5371-5388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | Chang Q, Yang H, Wang M, Wei H, Hu F. Role of Microtubule-Associated Protein in Autism Spectrum Disorder. Neurosci Bull. 2018;34:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Tomasi D, Volkow ND. Associations between handedness and brain functional connectivity patterns in children. Nat Commun. 2024;15:2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 67. | Sha Z, Pepe A, Schijven D, Carrión-Castillo A, Roe JM, Westerhausen R, Joliot M, Fisher SE, Crivello F, Francks C. Handedness and its genetic influences are associated with structural asymmetries of the cerebral cortex in 31,864 individuals. Proc Natl Acad Sci U S A. 2021;118:e2113095118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 68. | Wiberg A, Ng M, Al Omran Y, Alfaro-Almagro F, McCarthy P, Marchini J, Bennett DL, Smith S, Douaud G, Furniss D. Handedness, language areas and neuropsychiatric diseases: insights from brain imaging and genetics. Brain. 2019;142:2938-2947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 69. | Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123 Pt 12:2512-2518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1008] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 70. | Postema MC, Hoogman M, Ambrosino S, Asherson P, Banaschewski T, Bandeira CE, Baranov A, Bau CHD, Baumeister S, Baur-Streubel R, Bellgrove MA, Biederman J, Bralten J, Brandeis D, Brem S, Buitelaar JK, Busatto GF, Castellanos FX, Cercignani M, Chaim-Avancini TM, Chantiluke KC, Christakou A, Coghill D, Conzelmann A, Cubillo AI, Cupertino RB, de Zeeuw P, Doyle AE, Durston S, Earl EA, Epstein JN, Ethofer T, Fair DA, Fallgatter AJ, Faraone SV, Frodl T, Gabel MC, Gogberashvili T, Grevet EH, Haavik J, Harrison NA, Hartman CA, Heslenfeld DJ, Hoekstra PJ, Hohmann S, Høvik MF, Jernigan TL, Kardatzki B, Karkashadze G, Kelly C, Kohls G, Konrad K, Kuntsi J, Lazaro L, Lera-Miguel S, Lesch KP, Louza MR, Lundervold AJ, Malpas CB, Mattos P, McCarthy H, Namazova-Baranova L, Nicolau R, Nigg JT, Novotny SE, Oberwelland Weiss E, O'Gorman Tuura RL, Oosterlaan J, Oranje B, Paloyelis Y, Pauli P, Picon FA, Plessen KJ, Ramos-Quiroga JA, Reif A, Reneman L, Rosa PGP, Rubia K, Schrantee A, Schweren LJS, Seitz J, Shaw P, Silk TJ, Skokauskas N, Soliva Vila JC, Stevens MC, Sudre G, Tamm L, Tovar-Moll F, van Erp TGM, Vance A, Vilarroya O, Vives-Gilabert Y, von Polier GG, Walitza S, Yoncheva YN, Zanetti MV, Ziegler GC, Glahn DC, Jahanshad N, Medland SE; ENIGMA ADHD Working Group, Thompson PM, Fisher SE, Franke B, Francks C. Analysis of structural brain asymmetries in attention-deficit/hyperactivity disorder in 39 datasets. J Child Psychol Psychiatry. 2021;62:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 71. | Postema MC, van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Filho GB, Calderoni S, Calvo R, Daly E, Deruelle C, Di Martino A, Dinstein I, Duran FLS, Durston S, Ecker C, Ehrlich S, Fair D, Fedor J, Feng X, Fitzgerald J, Floris DL, Freitag CM, Gallagher L, Glahn DC, Gori I, Haar S, Hoekstra L, Jahanshad N, Jalbrzikowski M, Janssen J, King JA, Kong XZ, Lazaro L, Lerch JP, Luna B, Martinho MM, McGrath J, Medland SE, Muratori F, Murphy CM, Murphy DGM, O'Hearn K, Oranje B, Parellada M, Puig O, Retico A, Rosa P, Rubia K, Shook D, Taylor MJ, Tosetti M, Wallace GL, Zhou F, Thompson PM, Fisher SE, Buitelaar JK, Francks C. Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nat Commun. 2019;10:4958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 72. | Schijven D, Postema MC, Fukunaga M, Matsumoto J, Miura K, de Zwarte SMC, van Haren NEM, Cahn W, Hulshoff Pol HE, Kahn RS, Ayesa-Arriola R, Ortiz-García de la Foz V, Tordesillas-Gutierrez D, Vázquez-Bourgon J, Crespo-Facorro B, Alnæs D, Dahl A, Westlye LT, Agartz I, Andreassen OA, Jönsson EG, Kochunov P, Bruggemann JM, Catts SV, Michie PT, Mowry BJ, Quidé Y, Rasser PE, Schall U, Scott RJ, Carr VJ, Green MJ, Henskens FA, Loughland CM, Pantelis C, Weickert CS, Weickert TW, de Haan L, Brosch K, Pfarr JK, Ringwald KG, Stein F, Jansen A, Kircher TTJ, Nenadić I, Krämer B, Gruber O, Satterthwaite TD, Bustillo J, Mathalon DH, Preda A, Calhoun VD, Ford JM, Potkin SG, Chen J, Tan Y, Wang Z, Xiang H, Fan F, Bernardoni F, Ehrlich S, Fuentes-Claramonte P, Garcia-Leon MA, Guerrero-Pedraza A, Salvador R, Sarró S, Pomarol-Clotet E, Ciullo V, Piras F, Vecchio D, Banaj N, Spalletta G, Michielse S, van Amelsvoort T, Dickie EW, Voineskos AN, Sim K, Ciufolini S, Dazzan P, Murray RM, Kim WS, Chung YC, Andreou C, Schmidt A, Borgwardt S, McIntosh AM, Whalley HC, Lawrie SM, du Plessis S, Luckhoff HK, Scheffler F, Emsley R, Grotegerd D, Lencer R, Dannlowski U, Edmond JT, Rootes-Murdy K, Stephen JM, Mayer AR, Antonucci LA, Fazio L, Pergola G, Bertolino A, Díaz-Caneja CM, Janssen J, Lois NG, Arango C, Tomyshev AS, Lebedeva I, Cervenka S, Sellgren CM, Georgiadis F, Kirschner M, Kaiser S, Hajek T, Skoch A, Spaniel F, Kim M, Kwak YB, Oh S, Kwon JS, James A, Bakker G, Knöchel C, Stäblein M, Oertel V, Uhlmann A, Howells FM, Stein DJ, Temmingh HS, Diaz-Zuluaga AM, Pineda-Zapata JA, López-Jaramillo C, Homan S, Ji E, Surbeck W, Homan P, Fisher SE, Franke B, Glahn DC, Gur RC, Hashimoto R, Jahanshad N, Luders E, Medland SE, Thompson PM, Turner JA, van Erp TGM, Francks C. Large-scale analysis of structural brain asymmetries in schizophrenia via the ENIGMA consortium. Proc Natl Acad Sci U S A. 2023;120:e2213880120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 73. | Querne L, Berquin P, Vernier-Hauvette MP, Fall S, Deltour L, Meyer ME, de Marco G. Dysfunction of the attentional brain network in children with Developmental Coordination Disorder: a fMRI study. Brain Res. 2008;1244:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 74. | de Guibert C, Maumet C, Jannin P, Ferré JC, Tréguier C, Barillot C, Le Rumeur E, Allaire C, Biraben A. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia). Brain. 2011;134:3044-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 75. | Deep-Soboslay A, Hyde TM, Callicott JP, Lener MS, Verchinski BA, Apud JA, Weinberger DR, Elvevåg B. Handedness, heritability, neurocognition and brain asymmetry in schizophrenia. Brain. 2010;133:3113-3122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, Schweiger E, Wittling W. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res Cogn Brain Res. 2004;21:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 77. | Cowell P, Gurd J. Handedness and the Corpus Callosum: A Review and Further Analyses of Discordant Twins. Neuroscience. 2018;388:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Sha Z, Schijven D, Fisher SE, Francks C. Genetic architecture of the white matter connectome of the human brain. Sci Adv. 2023;9:eadd2870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 79. | Roland JL, Snyder AZ, Hacker CD, Mitra A, Shimony JS, Limbrick DD, Raichle ME, Smyth MD, Leuthardt EC. On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proc Natl Acad Sci U S A. 2017;114:13278-13283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 80. | Raaf N, Westerhausen R. Hand preference and the corpus callosum: Is there really no association? Neuroimage Rep. 2023;3:100160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Westerhausen R, Papadatou-Pastou M. Handedness and midsagittal corpus callosum morphology: a meta-analytic evaluation. Brain Struct Funct. 2022;227:545-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 82. | Luders E, Cherbuin N, Thompson PM, Gutman B, Anstey KJ, Sachdev P, Toga AW. When more is less: associations between corpus callosum size and handedness lateralization. Neuroimage. 2010;52:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 83. | Westerhausen R, Walter C, Kreuder F, Wittling RA, Schweiger E, Wittling W. The influence of handedness and gender on the microstructure of the human corpus callosum: a diffusion-tensor magnetic resonance imaging study. Neurosci Lett. 2003;351:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 84. | Ribeiro AS, Eales BA, Lloyd-Price J, Biddle FG. Predictability and randomness of paw choices are critical elements in the behavioural plasticity of mouse paw preference. Anim Behav. 2014;98:167-176. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Ribeiro AS, Eales BA, Biddle FG. Short-term and long-term memory deficits in handedness learning in mice with absent corpus callosum and reduced hippocampal commissure. Behav Brain Res. 2013;245:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Vermeulen CL, du Toit PJ, Venter G, Human-Baron R. A morphological study of the shape of the corpus callosum in normal, schizophrenic and bipolar patients. J Anat. 2023;242:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 87. | Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, Lobaugh N, Pollock BG, Mulsant BH. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry. 2013;70:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 88. | Türk Y, Ercan I, Sahin I, Erdemli Gursel B, Uzunoglu A, Öge C, Beyazyüz E, Albayrak Y. Corpus callosum in schizophrenia with deficit and non-deficit syndrome: a statistical shape analysis. Gen Psychiatr. 2021;34:e100635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 89. | Valenti M, Pino MC, Mazza M, Panzarino G, Di Paolantonio C, Verrotti A. Abnormal Structural and Functional Connectivity of the Corpus Callosum in Autism Spectrum Disorders: a Review. Rev J Autism Dev Disord. 2020;7:46-62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 90. | Minnigulova A, Davydova E, Pereverzeva D, Sorokin A, Tyushkevich S, Mamokhina U, Danilina K, Dragoy O, Arutiunian V. Corpus callosum organization and its implication to core and co-occurring symptoms of Autism Spectrum Disorder. Brain Struct Funct. 2023;228:775-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 91. | Yu M, Gao X, Niu X, Zhang M, Yang Z, Han S, Cheng J, Zhang Y. Meta-analysis of structural and functional alterations of brain in patients with attention-deficit/hyperactivity disorder. Front Psychiatry. 2022;13:1070142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 92. | Parlatini V, Itahashi T, Lee Y, Liu S, Nguyen TT, Aoki YY, Forkel SJ, Catani M, Rubia K, Zhou JH, Murphy DG, Cortese S. White matter alterations in Attention-Deficit/Hyperactivity Disorder (ADHD): a systematic review of 129 diffusion imaging studies with meta-analysis. Mol Psychiatry. 2023;28:4098-4123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 93. | John JP, Shakeel MK, Jain S. Corpus callosal area differences and gender dimorphism in neuroleptic-naïve, recent-onset schizophrenia and healthy control subjects. Schizophr Res. 2008;103:11-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C, Botteron KN, Elison JT, Dager SR, Estes AM, Hazlett HC, Schultz RT, Zwaigenbaum L, Piven J; IBIS Network. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain. 2015;138:2046-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 95. | Pool EM, Rehme AK, Eickhoff SB, Fink GR, Grefkes C. Functional resting-state connectivity of the human motor network: differences between right- and left-handers. Neuroimage. 2015;109:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 96. | Innocenti GM, Ansermet F, Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatry. 2003;8:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 97. | Bishop DV. Cerebral asymmetry and language development: cause, correlate, or consequence? Science. 2013;340:1230531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 98. | Hepper PG, Shahidullah S, White R. Origins of fetal handedness. Nature. 1990;347:431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, Il Shin J, Kirkbride JB, Jones P, Kim JH, Kim JY, Carvalho AF, Seeman MV, Correll CU, Fusar-Poli P. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27:281-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1834] [Cited by in RCA: 1900] [Article Influence: 475.0] [Reference Citation Analysis (1)] |
| 100. | Nelson EL, Campbell JM, Michel GF. Unimanual to bimanual: tracking the development of handedness from 6 to 24 months. Infant Behav Dev. 2013;36:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 101. | Campbell JM, Marcinowski EC, Latta J, Michel GF. Different assessment tasks produce different estimates of handedness stability during the eight to 14 month age period. Infant Behav Dev. 2015;39:67-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Saudino K, Mcmanus IC. Handedness, footedness, eyedness and earedness in the Colorado Adoption Project. British J of Dev Psycho. 1998;16:167-174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Dawson G, Rieder AD, Johnson MH. Prediction of autism in infants: progress and challenges. Lancet Neurol. 2023;22:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 104. | Mollon J, David AS, Zammit S, Lewis G, Reichenberg A. Course of Cognitive Development From Infancy to Early Adulthood in the Psychosis Spectrum. JAMA Psychiatry. 2018;75:270-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 105. | Johnston DW, Nicholls MER, Shah M, Shields MA. Handedness, health and cognitive development: Evidence from children in the National Longitudinal Survey of Youth. J R Stat Soc Ser A Stat Soc. 2013;176:841-860. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/