Published online Jul 19, 2025. doi: 10.5498/wjp.v15.i7.107721
Revised: April 22, 2025
Accepted: June 4, 2025
Published online: July 19, 2025
Processing time: 103 Days and 18.4 Hours
Major depressive disorder (MDD), one of the most prevalent mental illnesses, is characterized by anhedonia, the inability to experience pleasure from rewarding activities. This minireview examines the complex relationship between music, anhedonia, and neural activity from neuroimaging and neuroelectrophysiological perspectives. It synthesizes the latest advances in music neuroscience, exploring music's potential to modulate emotional responses and alleviate anhedonia in depressed individuals. Anhedonia has been linked to dysfunctional brain reward circuits. Functional magnetic resonance imaging studies have revealed that the potential mechanism by which music exerts its anti-depressive effect may involve the reactivation of the anterior cingulate cortex, while electroencephalographic studies have revealed that oscillatory network dysfunction significantly impairs music perception engagement in patients with MDD. Musical chills, representing intense emotional peaks during musical experiences, can evoke profound plea
Core Tip: Although several reviews have explored the relationship between music and neural activity, there remains a notable gap in comprehensive reviews specifically examining the association of music with anhedonia in major depressive disorder (MDD). To the best of our knowledge, this minireview provides the first systematic synthesis of music’s effects on reward function in MDD from both neuroimaging and neuroelectrophysiological perspectives, while proposing novel directions for future research.
- Citation: Sun YF, Zhang Q, Wang J, Zhou ZH. Neuroimaging and neuroelectrophysiological features of music's effects on anhedonia in major depressive disorder: A minireview. World J Psychiatry 2025; 15(7): 107721
- URL: https://www.wjgnet.com/2220-3206/full/v15/i7/107721.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i7.107721
Major depressive disorder (MDD) is a severe mental illness that significantly impairs both physical and psychological health. MDD causes profound psychological distress, often leading to hopeless, and in severe cases, suicidal behavior. Its widespread impact poses significant challenges to economic development and social stability[1,2]. Anhedonia, one of the core symptoms of MDD, is primarily characterized by diminished motivation to seek pleasure and a reduced capacity to experience joy[3]. The limited efficacy of conventional treatments for anhedonia has prompted researchers to explore new intervention strategies.
Music is an integral aspect of human culture. It brings joy, reduces anxiety and sadness, stimulates movement, and promotes social connections. Beyond its emotional and behavioral effects, music may also enhance brain and cognitive development, improve functional well-being, optimize quality of life, and potentially mitigate symptoms of various diseases. As a non-pharmacological intervention, music therapy demonstrates unique advantages in improving mood and cognitive function. Multiple meta-analyses have confirmed music’s positive effects on MDD[4,5]. A recent meta-analysis (n = 421) demonstrated that music therapy, when combined with conventional treatment, produced significant short-term improvements in anxiety-related and depressive symptoms compared to conventional treatment alone[6]. These benefits may enhance motivation, emotional regulation, and interpersonal functioning in patients with MDD[6]. However, the mechanisms underlying music’s influence on brain activity remain unclear. Studies utilizing advanced neuroscientific techniques (e.g., electroencephalography [EEG] and functional magnetic resonance imaging [fMRI]) have yielded inconsistent results. These discrepancies reflect the inherent complexity of music’s impact on the brain.
Sound waves that reach the tympanic membrane initiate a series of complex mechanical, chemical, and neural events in the cochlea, brainstem, midbrain nuclei, and cortex to generate auditory perception[7]. Music represents a structured arrangement of sound in time, space, and intensity[8] that can stimulate emotional changes, physiological arousal (autonomic and endocrine changes), motor expressions of emotion (smiling), and action tendencies (dancing, singing, playing instruments, foot-tapping, and clapping)[9]. The complex effects of music on human physiology and behavior present significant challenges when investigating music's impact on anhedonia.
Music can elicit strong pleasurable sensations. Many individuals report experiencing chills or “goosebumps”—referred to as musical chills—when listening to emotionally evocative passages. This phenomenon is associated with increased activity in the brain's reward system[10]. Unlike primary (e.g., food, sex) or secondary (e.g., money, power) rewards, music is thought to engage the reward network through distinct neural pathways[11,12]. By contrast, individuals with musical anhedonia exhibit intact auditory perception yet report no pleasure from musical stimuli, despite normal hedonic responses to non-musical rewards[13]. Significant progress has been made in musical anhedonia research over the past decade[12-15]. One seminal study identified a cohort of healthy individuals without depression or general anhedonia who, despite normal music perception capabilities, derived no subjective pleasure from enjoyable music while maintaining intact hedonic responses to monetary rewards[16]. A meta-analysis revealed distinct activation patterns: The right ventromedial striatum, right superior temporal gyrus (STG), and prefrontal cortex (PFC) showed more reliable activation to music, whereas the left insula, bilateral putamen, and right amygdala were more consistently activated by food rewards[11]. These findings collectively suggest that music engages reward functions through pathways distinct from those activated by other reward stimuli. This growing body of evidence provides novel therapeutic insights for remitting anhedonia in patients with MDD.
This minireview synthesizes existing evidence on the effects of music intervention on anhedonia in MDD from neuroimaging and neuroelectrophysiological perspectives, providing novel insights to inform future research and clinical practice.
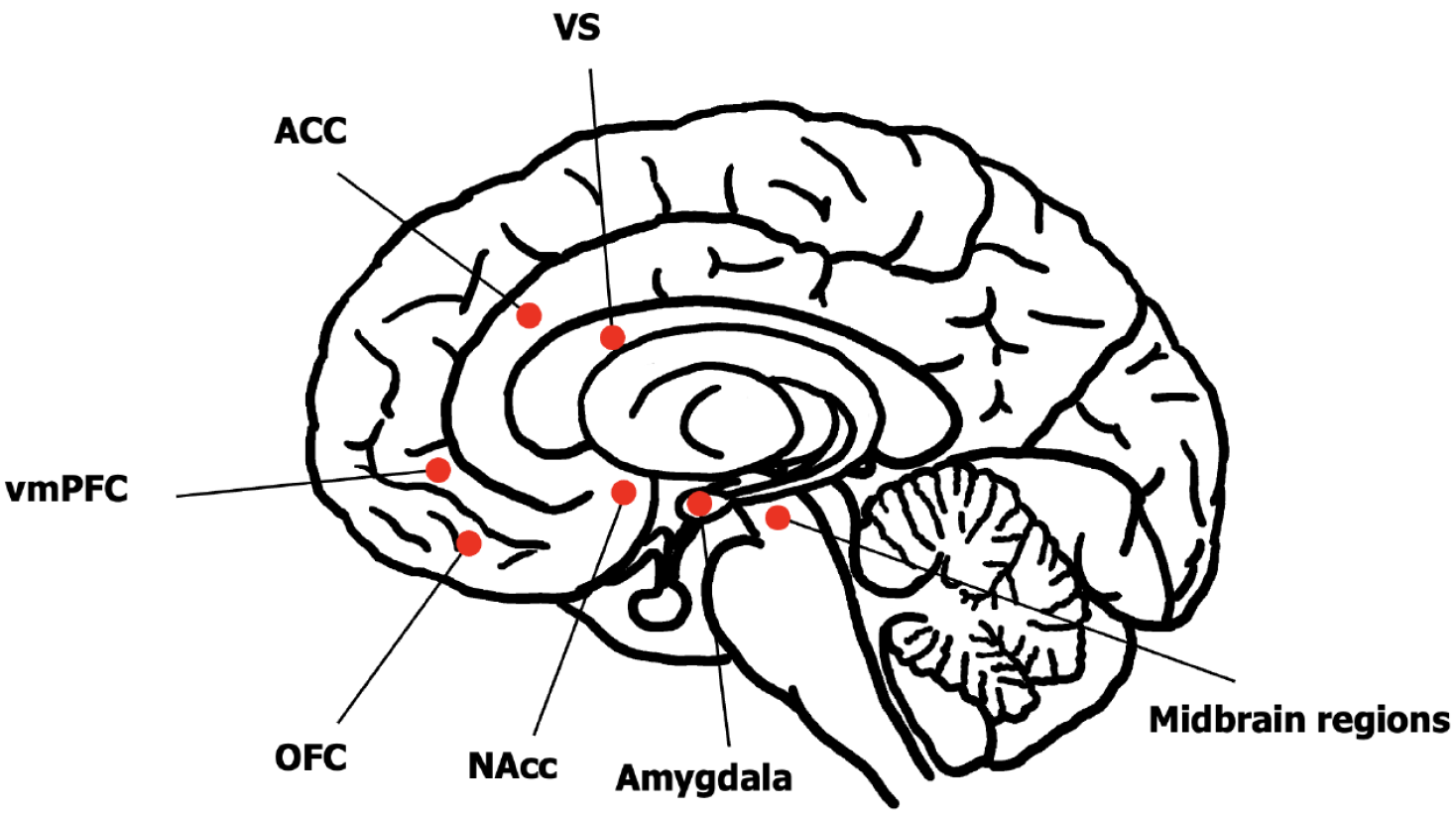
Anhedonia is closely associated with reward processing, involving both structural and functional aspects of the dopaminergic mesolimbic reward circuit[3]. The dopaminergic mesolimbic system originates in the ventral tegmental area and projects to the ventral striatum (VS) including the nucleus accumbens (NAcc) and dorsal striatum (e.g., caudate nucleus, putamen), subsequently extending to various subregions of the orbitofrontal cortex (OFC), dorsolateral PFC, and anterior cingulate cortex (ACC)[17]. Structural neuroimaging studies have revealed volume reductions in multiple brain regions among patients with MDD, including the ventromedial PFC (vmPFC)[18-20], ACC[21], caudate nucleus[22], and amygdala[23,24]. These structural alterations of key reward-related brain regions may contribute to anhedonia.
Functional neuroimaging studies have further revealed abnormalities in neural circuitry among patients with MDD. Although some findings are inconsistent, accumulating evidence demonstrates altered functional connectivity among default mode, salience, and central executive networks in MDD[25-27]. Particularly during reward processing, reduced activation has been observed in key regions including the VS, ACC, and OFC[28], and is closely associated with anhedonia. These neuroimaging findings provide crucial clues for understanding the neural mechanisms underlying anhedonia while also offering objective biomarkers for evaluating the effects of music-based interventions.
Music-based interventions significantly modulate brain activity, particularly in reward-related circuits. fMRI studies demonstrate that listening to pleasurable music robustly activates key components of the reward system, including the VS, midbrain regions, amygdala, OFC, vmPFC, NAcc, and ACC[10,29] (Figure 1). Notably, self-selected music elicits stronger activation in auditory and reward systems compared to experimenter-chosen stimuli[30]. Subjects listening to preferred music exhibit enhanced functional connectivity between the NAcc and STG and increased coupling with bilateral auditory cortices[31]. The degree of activation in these regions positively correlates with subjective pleasure ratings, suggesting that music may alleviate anhedonia by normalizing reward circuit function, enhancing auditory-reward integration, and facilitating positive emotional processing.
Research on music and reward processing has progressed remarkably, yet most studies have evaluated healthy participants, leaving the effects of music on anhedonia in MDD patients relatively understudied. Current evidence suggests that music may exert its anti-depressive effect by reactivating the ACC[32]. An fMRI study employing both positive and negative valence musical and non-musical stimuli revealed heightened ACC activation among patients with MDD, particularly in dorsal regions, in response to negative non-musical stimuli[32]. However, conflicting findings emerged from a similar study demonstrating that both patients with MDD and healthy controls exhibited stronger ACC activation in response to positive vs negative music, with patients with MDD showing no significant difference in ACC activation between negative music and baseline conditions[33]. Additional research has identified specific neural correlates of anhedonia in MDD during musical stimulation. One study found that the severity of anhedonia was associated with deficient activation of the posterior vmPFC and mesolimbic reward system during pleasurable music listening[34]. Jenkins et al[35] reported reduced activation of the left NAcc in patients with MDD compared to healthy controls when exposed to classical music. Furthermore, Deng et al[36] discovered that patients with MDD exhibited decreased functional connectivity in emotional recognition brain regions across different music valences, with statistically significant differences in connectivity patterns between patients and controls particularly evident during negative music stimulation.
Neuroelectrophysiological studies have revealed significant associations between anhedonia and specific event-related potential (ERP) components. Stimulus-preceding negativity (SPN), a right-lateralized frontocentral slow-wave negativity, is implicated not only in anticipatory affective and motivational processing but is also potentially linked to dopaminergic activity during reinforcement learning stages. Another reward-related ERP component, feedback-related negativity (FRN), primarily reflects the evaluation of reward feedback valence (loss vs gain). Reduced amplitudes of both SPN and FRN have been consistently observed in patients with MDD[37,38]. Furthermore, patients with MDD exhibit attenuated P3 and late positive potential components that are positive waves that index attentional resource allocation and higher-order processing of emotional stimuli[39,40]. These ERP components may potentially serve as biomarkers for assessing the severity of anhedonia.
Electrophysiological studies have consistently demonstrated that music elicits distinct neural responses measurable through EEG and ERP techniques. In the frequency domain, theta-band (4-8 Hz) phase synchronization between the right temporal and frontal regions increases proportionally with subjective pleasure ratings during music listening[41]. Additionally, frontal beta (13-30 Hz) and gamma (30-100 Hz) oscillations closely track prediction-error signals in musical reward processing[42].
Time-domain analyses reveal important differences between neural responses to musical vs monetary rewards. Compared to financial stimuli, music evokes smaller P2 amplitudes but larger N2 components, suggesting distinct patterns of early sensory processing and conflict monitoring. Interestingly, FRN shows no significant differences between different valences of music, nor were there significant differences in P3 amplitudes between monetary and musical rewards[43]. Music perception particularly engages specialized ERP components that reflect different levels of auditory processing. Unexpected chord progressions can elicit both early right anterior negativity (ERAN) and mismatch negativity (MMN) components[44-46]. MMN appears to reflect violations of immediate musical patterns (such as rhythmic structures and melodic contours), whereas ERAN is more specifically associated with deviations from learned musical syntax rules, which represent violations of acquired structural knowledge of music[47,48]. Recent evidence robustly confirms that while MMN and ERAN share common predictive coding mechanisms, they represent distinct neural processes[46,49]. This theoretical distinction is crucial as it elucidates how the resolution or violation of musical expectations contribute to emotional responses through tension mechanisms.
EEG-based investigations of music's effects on patients with MDD remain limited. One study found that during music perception, patients with MDD exhibited reduced connectivity patterns in the delta band but increased connectivity in the beta band, along with an absence of the left-hemisphere lateralization effect observed in healthy individuals[50]. Additional studies on neural oscillations have revealed that dysfunctional oscillatory networks may impair engagement with music perception in patients with MDD[51].
Musical chills represent a peak physiological response to intense musical pleasure[10,52], analogous to tearful emotional reactions. During chill episodes, individuals report strong subjective pleasure experiences accompanied by measurable changes in heart rate and skin conductance[12,53]. Neuroimaging studies have disclosed that music chills activate an evolutionarily conserved brain network involving the limbic system, striatum, and midbrain structures[10]. A positron emission tomography imaging study specifically demonstrated a correlation of the intensity of chills with neural activity in the VS, amygdala, and medial PFC[54]. Research by Mori[52] revealed that chill responses can be triggered by violations of rhythmic expectations, while harmonious acoustic spectra may induce tearfulness. These effects emerge from carefully constructed musical tension created through variations in rhythm, dynamics, melody, harmony, and timbre. In tonal music, the tension specifically related to melodic and harmonic motion is termed tonal tension[55]. Psychological studies have established that the resolution of tonal tension or violation of listeners' expectations constitutes a key mechanism for eliciting musical emotions[56,57]. fMRI evidence further shows that increasing musical tension correlates with enhanced blood oxygen level-dependent signals in the left lateral OFC and activates the right superficial amygdala[58].
An EEG study of musical chills revealed distinct theta-band activity in prefrontal regions, with source localization identifying significantly greater activation in the insula, OFC, and supplementary motor area (SMA). These findings provide further evidence for the involvement of bilateral insula, OFC, vmPFC, SMA, and ventral/dorsal striatum in mediating musical chills[59].
These findings collectively demonstrate that musical chills provide an objective, physiologically measurable indicator of peak reward experiences during music listening, with distinct neural correlates in reward and emotional processing circuits. This phenomenon offers valuable insights into understanding the neurobiological basis of aesthetic experiences and may inform music-based therapeutic interventions to mitigate reward processing deficits.
Anhedonia, as one of the core symptoms of MDD, shows limited responsiveness to pharmacological treatments. Music, as an effective non-pharmacological intervention for mood modulation, holds significant potential for alleviating anhedonia. Although the precise mechanisms underlying music's effects on reward processing remain unclear, neuroimaging studies have demonstrated its ability to activate multiple brain regions within the reward circuitry that include the VS, midbrain, amygdala, OFC, vmPFC, NAcc, and ACC. Current research has predominantly focused on healthy populations, with relatively few studies investigating individuals with MDD. Future studies should prioritize examining MDD populations to better understand music's therapeutic potential. While fMRI offers superior spatial resolution, its temporal resolution is relatively limited. Given that reward processing can be subdivided into distinct phases, such as decision, appetitive, consummatory, and learning phases[20] that require precise temporal characterization, future research could employ magnetoencephalography (MEG) to investigate the relationship between music and reward processing, as MEG provides both high temporal and spatial resolution.
Many electrophysiological studies using musical stimuli have adopted stimulus onset (time-zero) at the beginning of musical playback for time-domain analysis. However, unlike discrete stimuli such as images or brief tones (e.g., beeps), music unfolds over time, making onset-locked event marking theoretically problematic. This approach is particularly questionable in valence judgment paradigms, as listeners cannot determine a musical piece's emotional valence solely from its initial notes. Future research should implement more rigorous experimental designs with temporally appropriate event markers that better capture music's dynamic emotional progression.
Musical chills can evoke profound pleasure in healthy individuals. However, whether patients with MDD retain this capacity remains unexplored. Current evidence suggests that distinct neural pathways mediate music-derived pleasure compared to conventional rewards (e.g., money/food). This raises a compelling possibility: If patients with MDD can indeed experience musical chills, such responses might engage alternative reward-processing mechanisms, potentially bypassing the dysfunctional circuits underlying anhedonia to restore hedonic capacity.
Music can activate multiple brain regions. Unlike other reward stimuli, music may engage reward-related brain circuits through unique pathways. This provides new insights for alleviating anhedonia in patients with MDD who exhibit diminished reward responses to money and food. Notably, musical chills—an intensely pleasurable experience—could serve as a potential tool for improving anhedonia in individuals with MDD.
| 1. | Greenberg PE, Fournier AA, Sisitsky T, Simes M, Berman R, Koenigsberg SH, Kessler RC. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39:653-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 511] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 2. | Lu J, Xu X, Huang Y, Li T, Ma C, Xu G, Yin H, Xu X, Ma Y, Wang L, Huang Z, Yan Y, Wang B, Xiao S, Zhou L, Li L, Zhang Y, Chen H, Zhang T, Yan J, Ding H, Yu Y, Kou C, Shen Z, Jiang L, Wang Z, Sun X, Xu Y, He Y, Guo W, Jiang L, Li S, Pan W, Wu Y, Li G, Jia F, Shi J, Shen Z, Zhang N. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2021;8:981-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 522] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 3. | Pizzagalli DA. Toward a Better Understanding of the Mechanisms and Pathophysiology of Anhedonia: Are We Ready for Translation? Am J Psychiatry. 2022;179:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 4. | de Witte M, Pinho ADS, Stams GJ, Moonen X, Bos AER, van Hooren S. Music therapy for stress reduction: a systematic review and meta-analysis. Health Psychol Rev. 2022;16:134-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 5. | Lin TH, Liao YC, Tam KW, Chan L, Hsu TH. Effects of music therapy on cognition, quality of life, and neuropsychiatric symptoms of patients with dementia: A systematic review and meta-analysis of randomized controlled trials. Psychiatry Res. 2023;329:115498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Aalbers S, Fusar-Poli L, Freeman RE, Spreen M, Ket JC, Vink AC, Maratos A, Crawford M, Chen XJ, Gold C. Music therapy for depression. Cochrane Database Syst Rev. 2017;11:CD004517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Peretz I, Zatorre RJ. Brain organization for music processing. Annu Rev Psychol. 2005;56:89-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 347] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Koelsch S. Brain correlates of music-evoked emotions. Nat Rev Neurosci. 2014;15:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 652] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 9. | Koelsch S. Investigating the Neural Encoding of Emotion with Music. Neuron. 2018;98:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98:11818-11823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1465] [Cited by in RCA: 1302] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 11. | Mas-Herrero E, Maini L, Sescousse G, Zatorre RJ. Common and distinct neural correlates of music and food-induced pleasure: A coordinate-based meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2021;123:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Martínez-Molina N, Mas-Herrero E, Rodríguez-Fornells A, Zatorre RJ, Marco-Pallarés J. Neural correlates of specific musical anhedonia. Proc Natl Acad Sci U S A. 2016;113:E7337-E7345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Belfi AM, Loui P. Musical anhedonia and rewards of music listening: current advances and a proposed model. Ann N Y Acad Sci. 2020;1464:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Kathios N, Patel AD, Loui P. Musical anhedonia, timbre, and the rewards of music listening. Cognition. 2024;243:105672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Benson P, Kathios N, Loui P. Predictive coding in musical anhedonia: A study of groove. PLoS One. 2024;19:e0301478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Mas-Herrero E, Zatorre RJ, Rodriguez-Fornells A, Marco-Pallarés J. Dissociation between musical and monetary reward responses in specific musical anhedonia. Curr Biol. 2014;24:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 802] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 18. | Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 519] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 19. | Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 307] [Article Influence: 23.6] [Reference Citation Analysis (11)] |
| 20. | Husain M, Roiser JP. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci. 2018;19:470-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 417] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 21. | Zacková L, Jáni M, Brázdil M, Nikolova YS, Marečková K. Cognitive impairment and depression: Meta-analysis of structural magnetic resonance imaging studies. Neuroimage Clin. 2021;32:102830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 22. | Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 969] [Cited by in RCA: 937] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 23. | Kangas BD, Der-Avakian A, Pizzagalli DA. Probabilistic Reinforcement Learning and Anhedonia. Curr Top Behav Neurosci. 2022;58:355-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Egger K, Schocke M, Weiss E, Auffinger S, Esterhammer R, Goebel G, Walch T, Mechtcheriakov S, Marksteiner J. Pattern of brain atrophy in elderly patients with depression revealed by voxel-based morphometry. Psychiatry Res. 2008;164:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Yang H, Chen X, Chen ZB, Li L, Li XY, Castellanos FX, Bai TJ, Bo QJ, Cao J, Chang ZK, Chen GM, Chen NX, Chen W, Cheng C, Cheng YQ, Cui XL, Duan J, Fang Y, Gong QY, Guo WB, Hou ZH, Hu L, Kuang L, Li F, Li HX, Li KM, Li T, Liu YS, Liu ZN, Long YC, Lu B, Luo QH, Meng HQ, Peng D, Qiu HT, Qiu J, Shen YD, Shi YS, Si TM, Tang YQ, Wang CY, Wang F, Wang K, Wang L, Wang X, Wang Y, Wang YW, Wu XP, Wu XR, Xie CM, Xie GR, Xie HY, Xie P, Xu XF, Yang J, Yao JS, Yao SQ, Yin YY, Yuan YG, Zang YF, Zhang AX, Zhang H, Zhang KR, Zhang L, Zhang ZJ, Zhao JP, Zhou R, Zhou YT, Zhu JJ, Zhu ZC, Zou CJ, Zuo XN, Yan CG. Disrupted intrinsic functional brain topology in patients with major depressive disorder. Mol Psychiatry. 2021;26:7363-7371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 26. | Yan CG, Chen X, Li L, Castellanos FX, Bai TJ, Bo QJ, Cao J, Chen GM, Chen NX, Chen W, Cheng C, Cheng YQ, Cui XL, Duan J, Fang YR, Gong QY, Guo WB, Hou ZH, Hu L, Kuang L, Li F, Li KM, Li T, Liu YS, Liu ZN, Long YC, Luo QH, Meng HQ, Peng DH, Qiu HT, Qiu J, Shen YD, Shi YS, Wang CY, Wang F, Wang K, Wang L, Wang X, Wang Y, Wu XP, Wu XR, Xie CM, Xie GR, Xie HY, Xie P, Xu XF, Yang H, Yang J, Yao JS, Yao SQ, Yin YY, Yuan YG, Zhang AX, Zhang H, Zhang KR, Zhang L, Zhang ZJ, Zhou RB, Zhou YT, Zhu JJ, Zou CJ, Si TM, Zuo XN, Zhao JP, Zang YF. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci U S A. 2019;116:9078-9083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 601] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 27. | Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1418] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 28. | Pizzagalli DA, Roberts AC. Prefrontal cortex and depression. Neuropsychopharmacology. 2022;47:225-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 381] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 29. | Koelsch S, Cheung VKM, Jentschke S, Haynes JD. Neocortical substrates of feelings evoked with music in the ACC, insula, and somatosensory cortex. Sci Rep. 2021;11:10119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Quinci MA, Belden A, Goutama V, Gong D, Hanser S, Donovan NJ, Geddes M, Loui P. Longitudinal changes in auditory and reward systems following receptive music-based intervention in older adults. Sci Rep. 2022;12:11517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 31. | Salimpoor VN, van den Bosch I, Kovacevic N, McIntosh AR, Dagher A, Zatorre RJ. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science. 2013;340:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 354] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 32. | Lepping RJ, Atchley RA, Chrysikou E, Martin LE, Clair AA, Ingram RE, Simmons WK, Savage CR. Neural Processing of Emotional Musical and Nonmusical Stimuli in Depression. PLoS One. 2016;11:e0156859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Naseri P, Alavi Majd H, Tabatabaei SM, Khadembashi N, Najibi SM, Nazari A. Functional Brain Response to Emotional Musical Stimuli in Depression, Using INLA Approach for Approximate Bayesian Inference. Basic Clin Neurosci. 2021;12:95-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Young CB, Chen T, Nusslock R, Keller J, Schatzberg AF, Menon V. Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl Psychiatry. 2016;6:e810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Jenkins LM, Skerrett KA, DelDonno SR, Patrón VG, Meyers KK, Peltier S, Zubieta JK, Langenecker SA, Starkman MN. Individuals with more severe depression fail to sustain nucleus accumbens activity to preferred music over time. Psychiatry Res Neuroimaging. 2018;275:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Deng J, Chen Y, Zeng W, Luo X, Li Y. Brain Response of Major Depressive Disorder Patients to Emotionally Positive and Negative Music. J Mol Neurosci. 2022;72:2094-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Proudfit GH. The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology. 2015;52:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 672] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 38. | Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, Chan RC. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 39. | Santopetro NJ, Mulligan EM, Brush CJ, Hajcak G. Reduced P300 amplitude is consistently associated with trait anhedonia across repeated assessments. Psychophysiology. 2022;59:e14127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Klawohn J, Burani K, Bruchnak A, Santopetro N, Hajcak G. Reduced neural response to reward and pleasant pictures independently relate to depression. Psychol Med. 2021;51:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 41. | Ara A, Marco-Pallarés J. Fronto-temporal theta phase-synchronization underlies music-evoked pleasantness. Neuroimage. 2020;212:116665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Ueno F, Shimada S. Neural Mechanism of Musical Pleasure Induced by Prediction Errors: An EEG Study. Brain Sci. 2024;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Alí Diez I, Fàbrega-Camps G, Parra-Tíjaro J, Marco-Pallarés J. Anticipatory and consummatory neural correlates of monetary and music rewarding stimuli. Brain Cogn. 2024;179:106186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 44. | Koelsch S, Gunter TC, Schröger E, Tervaniemi M, Sammler D, Friederici AD. Differentiating ERAN and MMN: an ERP study. Neuroreport. 2001;12:1385-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Lee DJ, Jung H, Loui P. Attention Modulates Electrophysiological Responses to Simultaneous Music and Language Syntax Processing. Brain Sci. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Ishida K, Nittono H. Relationship between early neural responses to syntactic and acoustic irregularities in music. Eur J Neurosci. 2022;56:6201-6214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 47. | Koelsch S. Music-syntactic processing and auditory memory: similarities and differences between ERAN and MMN. Psychophysiology. 2009;46:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Koelsch S, Vuust P, Friston K. Predictive Processes and the Peculiar Case of Music. Trends Cogn Sci. 2019;23:63-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 49. | Ishida K, Nittono H. Relationship between schematic and dynamic expectations of melodic patterns in music perception. Int J Psychophysiol. 2024;196:112292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Liu W, Zhang C, Wang X, Xu J, Chang Y, Ristaniemi T, Cong F. Functional connectivity of major depression disorder using ongoing EEG during music perception. Clin Neurophysiol. 2020;131:2413-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 51. | Liu W, Wang X, Xu J, Chang Y, Hamalainen T, Cong F. Identifying Oscillatory Hyperconnectivity and Hypoconnectivity Networks in Major Depression Using Coupled Tensor Decomposition. IEEE Trans Neural Syst Rehabil Eng. 2021;29:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Mori K. Decoding peak emotional responses to music from computational acoustic and lyrical features. Cognition. 2022;222:105010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Klepzig K, Horn U, König J, Holtz K, Wendt J, Hamm AO, Lotze M. Brain imaging of chill reactions to pleasant and unpleasant sounds. Behav Brain Res. 2020;380:112417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 843] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 55. | Navarro-Cáceres M, Caetano M, Bernardes G, Sánchez-Barba M, Merchán Sánchez-Jara J. A Computational Model of Tonal Tension Profile of Chord Progressions in the Tonal Interval Space. Entropy (Basel). 2020;22:1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Vuust P, Gebauer LK, Witek MA. Neural underpinnings of music: the polyrhythmic brain. Adv Exp Med Biol. 2014;829:339-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Wu Q, Sun L, Ding N, Yang Y. Musical tension is affected by metrical structure dynamically and hierarchically. Cogn Neurodyn. 2024;18:1955-1976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Lehne M, Rohrmeier M, Koelsch S. Tension-related activity in the orbitofrontal cortex and amygdala: an fMRI study with music. Soc Cogn Affect Neurosci. 2014;9:1515-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 59. | Chabin T, Gabriel D, Chansophonkul T, Michelant L, Joucla C, Haffen E, Moulin T, Comte A, Pazart L. Cortical Patterns of Pleasurable Musical Chills Revealed by High-Density EEG. Front Neurosci. 2020;14:565815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/