Published online Jul 19, 2025. doi: 10.5498/wjp.v15.i7.107524
Revised: April 23, 2025
Accepted: May 19, 2025
Published online: July 19, 2025
Processing time: 102 Days and 2.8 Hours
Fear-related disorders, such as post-traumatic stress disorder (PTSD), significantly impact patients and families. Exposure therapy is a common treatment, but imp
To investigate the role of DNA methylation in the extinction of fear memory, with the goal of identifying potential strategies to enhance the efficacy of exposure therapy for fear-related disorders.
This study investigated the role of DNA methylation in fear memory extinction in mice. DNA methylation was manipulated using N-phthalyl-L-tryptophan (RG108) to reduce methylation and L-methionine injections to enhance it. Neuronal activity, and dendritic spine density was measured following extinction training.
RG108 suppressed extinction, reduced spine density, and inhibited neuronal activity. Methionine injections facilitated extinction.
DNA methylation is crucial for fear memory extinction. Enhancing methylation may improve the efficacy of exposure therapy, offering a potential strategy to treat fear-related disorders.
Core Tip: Reduced methylation by RG108 suppresses extinction, reduces spine density, and inhibits neuronal activity; enhancing methylation by L-methionine improves the efficacy of exposure therapy, offering a potential strategy to treat fear-related disorders.
- Citation: Jiang L, Ma RX, He ES, Zheng XY, Peng X, Ma WH, Li Y, li HW, Zhang XY, Ji JY, Li YJ, Qu SL, Li LJ, Gong ZT. DNA methylation regulates the extinction of fear memory. World J Psychiatry 2025; 15(7): 107524
- URL: https://www.wjgnet.com/2220-3206/full/v15/i7/107524.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i7.107524
Fear-related mental disorders such as post-traumatic stress disorder (PTSD) presents serious health problems for humans. Behavioral interventions are commonly used to treat fear-related disorders, with exposure therapy being a key therapy[1]. However, the effectiveness of exposure therapy varies among patients, as does the duration of treatment[2,3]. Therefore, studying the mechanisms of exposure therapy and developing more stable and effective therapy are significant.
Fear conditioning and extinction are common methods utilized to study exposure therapy in rodents. Rodents undergo extinction training after fear conditioning. Successful extinction training can reduce fear memory in rodents. Many factors can impact the effectiveness of extinction, including extinction protocols[4,5], protein synthesis[6], animal age and sex[7], epigenetic factors[8], neuro activity[9], etc. For example, protein synthesis inhibitors can inhibit the extinction of fear memory[10,11]. DNA methylation plays a role in learning and memory[12]. In our previous research, we found differential expression levels of DNA methyltransferase-3A (Dnmt3a) in animals of different ages[13], and Dnmt3a in the mouse hippocampal dentate gyrus affects the renewal of fear memory[14]. Therefore, we hypothesized that DNA methylation levels play a role in the process of fear memory extinction.
RG108 is a novel non-nucleoside small-molecule DNMT inhibitor designed to target DNA methyltransferase-1 (Dnmt1)[15]. However, it can inhibit the activity of DNA methyltransferase-3B (Dnmt3b)[16] and Dnmt3a[17]. Studies on snails have shown that RG108 can disrupt context memory[18]. Additionally, research in rodents has found that acute RG108 injection affects pattern separation but does not produce long-term effects[19]. These findings suggest that RG108 plays a role in memory regulation; however, its impact on the extinction of fear memory remains unclear.
In this study, we used RG108 to inhibit DNA methylation and found that fear memory extinction was disrupted, accompanied by reduced dendritic spines. Additionally, we demonstrated the correlation between DNA methylation and neuronal activity through chemical genetics and optical fiber recordings. Finally, we found that increasing methylation levels could enhance fear memory extinction. Our results suggest that DNA methylation may regulate the extinction of fear memory by modulating neuronal activity. Drugs that increase DNA methylation, such as folic acid and B vitamins, may be beneficial in enhancing the effectiveness of exposure therapy.
C57BL/6J mice were obtained from SPF (Beijing) Biotechnology Co., Ltd. The mice were housed under standard laboratory conditions at a temperature of 22 ± 1 °C and a 12-hour light/dark cycle (lights on at 8 AM) at Dali University Laboratory Animal Center. DNA methylation varies with animal age and sex[20]. Male mice aged 8 to 12 weeks were selected to eliminate the influence of sex and age. Each experimental group consisted of mice from the same batch and age. All behavioral experiments were conducted between 9 AM and 6 PM. All animal experiments were performed in accordance with the ARRIVE guidelines on the Care and Use of Experimental Animals, approved by the Dali University Animal Care and Use Committee No. 2022-P2-119; all methods were performed in accordance with the relevant guidelines and regulations.
We modified our previously published protocols[14]. The following three contexts were used. In Context A, a Super FCS conditioning chamber was used. Lighting was obtained from Super FCS (Xinruan Information Technology Co., Ltd., Shanghai, China), and 75% alcohol was used as a cleaning agent. In Context B, mice were placed in a rectangular Plexiglas box (20 cm × 35 cm × 20 cm), with black walls and a white floor. The box was cleaned with 1% acetic acid between animals, and illumination was provided by an LED light covered with a yellow shade. In Context B’, the same Super FCS conditioning chamber was used, but with a key modification: the standard metal shock grid floor was replaced with a white acrylic board to create a distinguishable environment from Context A. The chamber was cleaned with the 1% acetic acid between each animal.
Mice explored the chamber for 180 s before the first tone and 120 s after the last tone. After completing all tests, mice were first placed in a temporary holding cage before being returned to their home cages. Conditioning took place in Context A, a 20-s tone (80 dB; 2700 Hz) was presented and co-terminated with a 2-s foot shock (0.7 mA); a total of four pairings were conducted at an interval of 120 s. Recall took place in Context B and B’. Twenty-second tones (80 dB; 2700 Hz) were presented four times each at an interval of 40 s. Regular extinction (Ext) took place in Context B. A 20-s tone was presented 20 times at 40 s intervals. For extinction in the reconsolidation window (Rec + Ext), mice were first placed in Context B’ and exposed to four 20-second tone presentations, each separated by a 40-second intertrial interval. This reactivation session was immediately followed by a 1–2-hour delay, during which the mice were transferred to a new holding box, not their home cage. After the delay, mice were reintroduced to Context B’ for the full extinction session. A 20-s tone was presented 20 times each at an interval of 40 s. Only after all mice in the same cage completed the entire Rec + Ext procedure were they returned to their home cage.
Freezing levels were analyzed using a Super FCS analysis system (Xinruan Information Technology Co., ltd., Shanghai, China) on a computer, and freezing threshold was set to at least more than 1 s without any movement.
In the RG108 experiment, mice received intraperitoneal injections of RG108 (N-phthalyl-L-tryptophan, 0.2 mg/kg; R8279; Sigma-Aldrich Company, United States). RG108 was dissolved in dimethyl sulfoxide (D2438-5x10ML; Sigma-Aldrich) at a concentration of 10 mg/mL, stored at -20 °C, and diluted 50 times in vehicle for intraperitoneal injection. The intraperitoneal injections were administered 2 hours before the fear extinction experiment.
In the methionine (L-methionine, L-M) experiment, mice were intraperitoneally injected twice daily with L-M (5.2 mmol/kg, M9625, Sigma-Aldrich, United States) for 7 days, the behavior test commenced 2 hours after the injection.
We utilized coordinates from our previously published work[14]. All surgeries were conducted under stereotactic guidance. Mice were anesthetized with isoflurane. The virus was administered using a 5-microliter micro syringe (Hamilton) equipped with a 33-G needle. The injection speed was controlled by a micro syringe pump controller (KD Scientific). Recombinant adeno-associated virus (rAAV) was introduced into the dorsal dentate gyrus (dDG) using the following coordinates (from Bregma): Anteroposterior (AP) = -2.1 mm, mediolateral (ML) = ± 1.4 mm, dorsoventral (DV) = -2.1 mm. The injection speed was set at 180 nL/min. The injection needle remained in place for at least 300 seconds to allow sufficient diffusion.
Mice were sacrificed 2 hours after i.p. injection of RG108 (0.2 mg/kg) or methionine injection and perfused with phosphate-buffered saline (PBS) solution. The hippocampus was isolated from the brain, total DNA was extracted using a nucleic acid purification kit (Axygen, United States), and relative DNA methylation level was measured using a Methyl Flash Methylated DNA 5-mC Quantification Kit (Colorimetric) (Epigentek, United States).
Mice were anesthetized with pentobarbital sodium, perfused with 4% paraformaldehyde and PBS through the heart. Slices were cut at 30 μm. Brain sections were treated with 0.5% Triton X-100, 10% goat serum and 0.2% skim milk powder in PBS for 1 hour at room temperature. The section was incubated with primary antibody [anti-Dnmt3a (Rabbit), 1:500-1:1000, CST, #3598] overnight at room temperature, washed with PBS three times (each for 10 minutes), incubated with secondary antibody (goat anti-Rabbit 488, A11008, 1:400;) for 1 hour in the dark at room temperature, and washed three times in PBS (each for 10 minutes). Finally, sections were incubated with Hoechst for 5 min and mounted on glass slides. Images were taken using an inverted microscope with 10X Objective (ix73; Olympus, Japan). Co-localization of Dnmt3a positive cells were counted using ImageJ.
Golgi staining was performed using the FD Rapid GolgiStainTM Kit (PK401/PK401A). Images were taken using an Upright microscope with 10X, 40X (Zeiss imager.A2; Germany).
The virus was obtained from OBiO Technology (Shanghai, China) Corp., Ltd. (pGP-AAV-syn-jGCaMP7s-WPRE, H11264). The titers were approximately 3.45 × 1013 genome copies per mL, and the virus was used after a 50-fold dilution.
For in vivo fiber recording, mice underwent a single surgery. Mice were deeply anesthetized with isoflurane and injected with virus prior to implanting the ceramic ferrule containing the optical fiber [200 μm O.D., 0.37 numerical aperture (NA); Inper, China] over the injection site. Thereafter, 300 nL of rAAV-syn-jGCaMP7s-WPRE virus (OBiO Technology, China) was injected using a micro syringe pump. After virus injection, two skull screws were inserted around the implantation site, and a ceramic ferrule was slowly lowered into dorsal dentate gyrus (dDG) (-2.1 AP, ± 1.4 mL, -2.0 DV; from Bregma) and fixed to the skull with dental acrylic. Imaging experiments were conducted 4 weeks after this procedure. An optical fiber (200 μm O.D., NA = 0.37, 200 cm long) was used. To minimize bleaching, laser power at the tip of optical fiber was set to Blue 20–40 μW, Purple 10–20 μW. We used optical fiber recording set-up (Dual-Channel Fiber Photometry, Inper, Hangzhou, Zhejiang, China) to record fluorescence signals from jGCaMP7s. Light from a 470-nm LED was bandpass filtered, collimated, reflected by dichroic mirrors, and focused by a 20× objective. A 470-nm LED light was delivered at a power of 20–40 μW on the tip of the fiber optic cannula. The end of the fiber was imaged at a frame rate of 50 fps, with Inper Studio, and the mean value of a region of interest of an end-face of the fiber was calculated using Inper Analysis software. To serve as an isosbestic control channel, 410 nm LED light was delivered alternately with 470 nm LED light.
Behavioral data were recorded and analyzed using the Super FCS system software (Shanghai, China). All mice that received AAV injections were perfused, and their brains were extracted and cryosectioned at the end of the experiment to examine viral expression. Mice without any virus expression in the dDG were excluded from the data analysis and removed from the dataset. Statistical analysis was performed using paired/unpaired t-test and two-way analysis of variance (GraphPad Prism), as noted. Results are expressed as mean ± SEM. Statistical significance was set at P < 0.05.
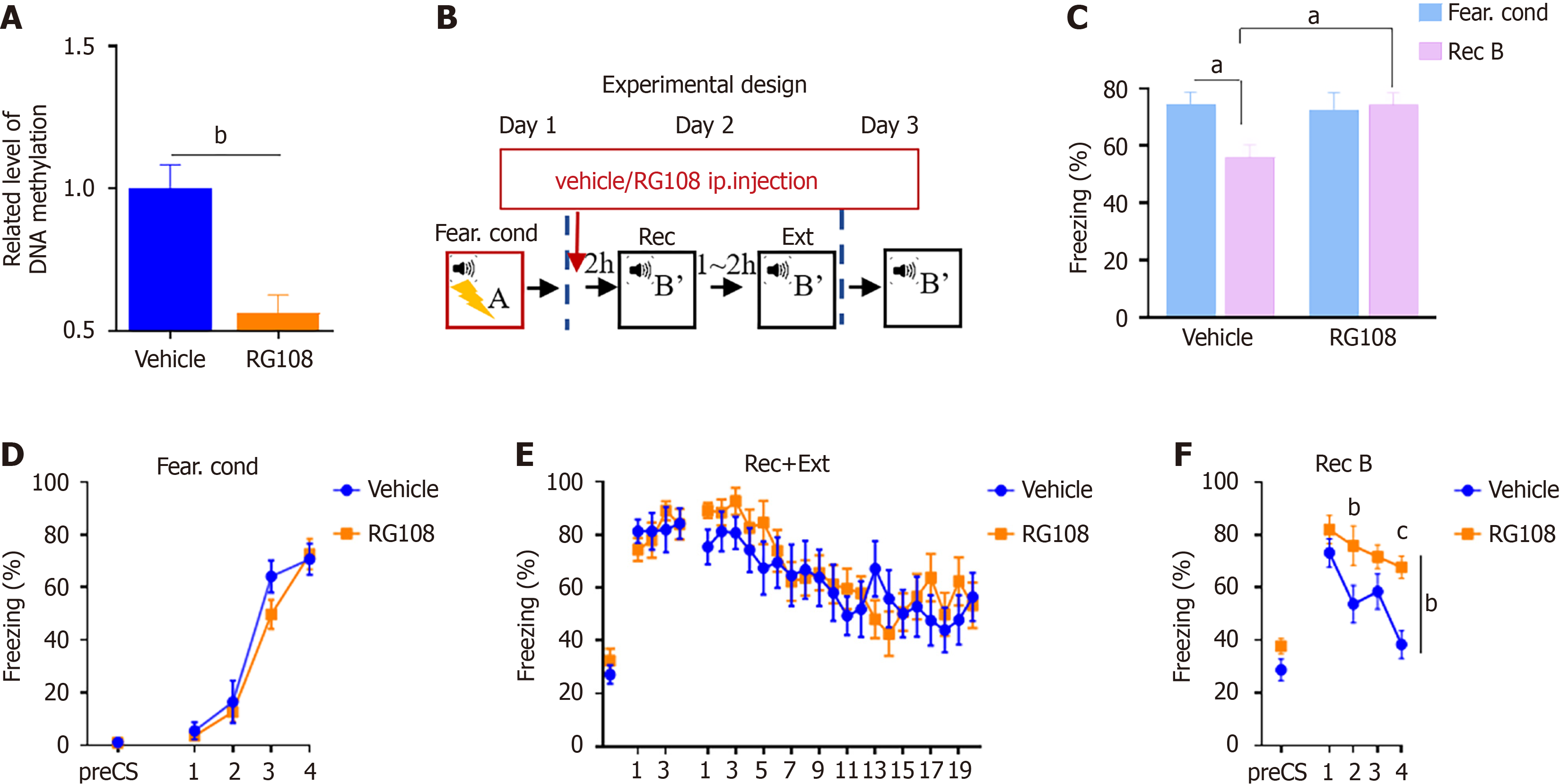
To investigate the correlation between DNA methylation and fear memory extinction, we modulated the levels of DNA methylation using RG108. Initially, we administered RG108 through intraperitoneal injection (0.2 mg/kg) and, after 2 hours, isolated the hippocampus to extract DNA for methylation analysis. The results revealed a reduction in DNA methylation levels in the mouse brain after RG108 injection (Figure 1A; unpaired t-test, T9 = 4.291, P < 0.01).
In our previous research, we observed that extinction within the reconsolidation time window significantly increased the Dnmt3a expression in the dDG region[14]. In this study, we also used extinction training within the reconsolidation time window (termed Rec + Ext), to examine the role of RG108 in fear memory extinction. To assess whether changes in DNA methylation affect extinction training within the reconsolidation time window (Rec + Ext), on Day 1, fear conditioning; on Day 2, mice were divided into two groups: Mice received RG108/vehicle injection 2 hours before Rec + Ext. On Day 3, both groups underwent recall (Figure 1B). The results indicated no difference in fear memory establishment between the two groups on Day 1 (Figure 1D) and during the extinction training on the second day within Rec + Ext (Figure 1E). In the recall test on the third day, the RG108 group showed significantly higher freezing levels compared to the control group (Figure 1F; two-way RM ANOVA, F(1,20) = 9.063, P < 0.01). Additionally, when comparing freezing levels during recall to freezing values during the highest freezing level of fear conditioning in Day 1, the vehicle group exhibited a significant decrease, while the RG108 group showed no significant change (Figure 1C; two-way RM ANOVA, F. cond vs Rec B, vehicle, T20 = 2.564, P < 0.05; RG108, T20 = 0.2606, P = 0.7971).
These results indicate that inhibiting DNA methylation levels with RG108 disrupts fear memory extinction, and the inhibitory effect of RG108 is more pronounced during extinction training within the reconsolidation window (Rec + Ext).
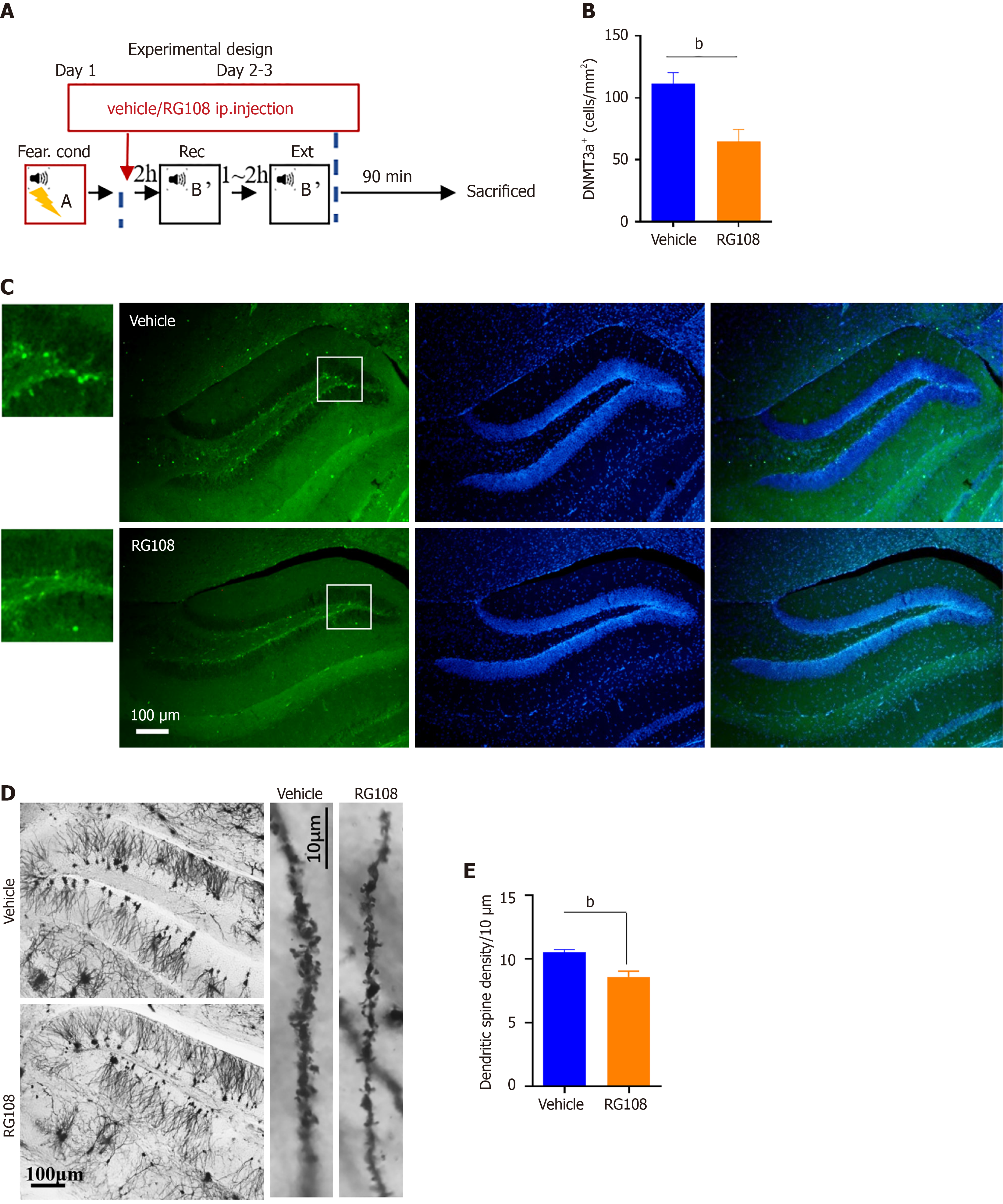
To investigate the mechanism through which RG108 inhibits fear memory extinction, we performed Dnmt3a and Golgi staining on mice injected with RG108/vehicle. On Day 1, fear conditioning; on Day 2–3, RG108 or vehicle was injected 2 hours before Rec + Ext. After the completion of Rec + Ext, brains were collected 1.5 hours later for staining (Figure 2A). Staining results revealed a significant reduction in the expression of Dnmt3a in the RG108 group compared to the vehicle group (Figure 2B and C; unpaired t-test, T6 = 3.617, P < 0.05). Golgi staining indicated a significantly lower dendritic spine density in the dentate gyrus of the hippocampus in the RG108 group compared to the vehicle group (Figure 2D and E; unpaired test, T16 = 3.844, P < 0.01). As spine density was related to neuronal activity[21], these results suggest that changes in DNA methylation and Dnmt3a expression may be associated with neuronal activity.
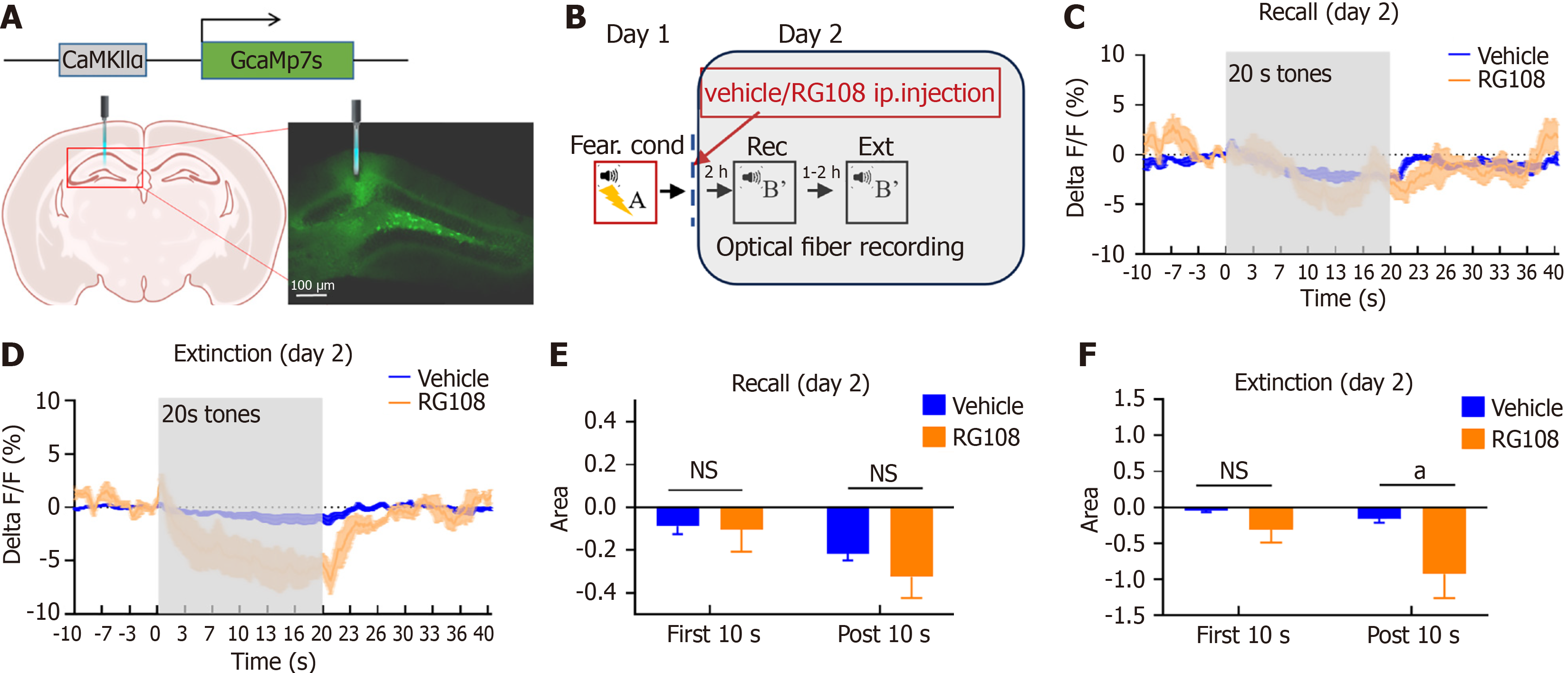
Given our previous findings correlating neural activity in the dDG region with fear memory extinction and renewal[9], and the influence of RG108 on extinction was more significant in the Rec + Ext protocol, we conducted fiber photometry recordings during the Rec + Ext process after RG108 or vehicle injection (Figure 3A and B). We observed no significant differences between the two groups during the recall phase; however, during the extinction phase, neuronal activity within the 20 s auditory stimulus range significantly decreased in the RG108 group, gradually recovering after the stimulus ended (Figure 3C and D). In contrast, the vehicle group showed no significant changes (Figure 3C and D). Further analysis of the stimulus, divided into the first 10 s and the post 10 s revealed a significant decrease in the RG108 group during the post 10 s compared to the vehicle group during the extinction process but not the recall process (Figure 3E and F; Figure 3F extinction, two-way RM ANOVA, F(1,4) = 3.888, P = 0.1199; first 10 s, T8 = 0.9554, P = 0.3674; post 10 s, T8 = 2.800, P < 0.05). These results indicate that RG108, while reducing methylation and Dnmt3a expression, disrupts fear memory extinction by suppressing neuronal activity during the extinction process, and that RG108 may disrupt the extinction of fear memory by inhibiting neuronal activity, and inhibiting neuronal activity also suppresses the expression of Dnmt3a.
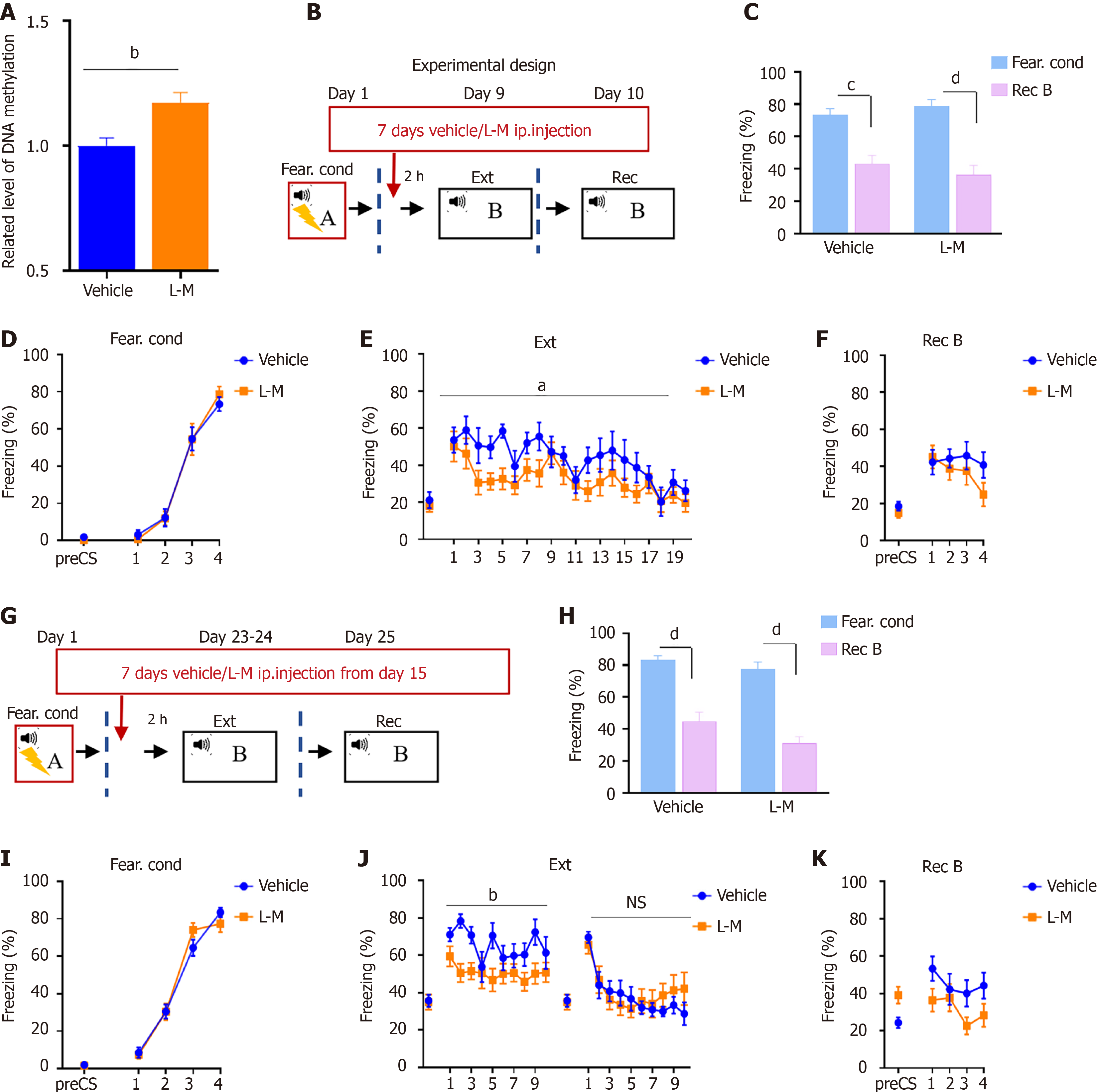
To further validate the role of DNA methylation in fear memory extinction, we explored whether increasing methylation could promote fear memory extinction. In our previous research, we found that the expression of Dnmt3a significantly increased after Rec + Ext training compared to Ext training[14]. To minimize behavioral-induced changes in DNA methylation levels, we utilized regular 20-cycle conditioned stimulus (CS) (Ext) training for assessment. L-M injections for 7 consecutive days increased DNA methylation levels in the mouse hippocampal region (Figure 4A; unpaired t-test, T8 = 3.406, P < 0.01). Therefore, we initiated 7 days of L-M or vehicle injections after fear conditioning, followed by continuous extinction training (Ext) starting 2 hours after the injection (Figure 4B). The behavioral results are shown in Figure 4C-F. Compared to the control group, mice did not exhibit significant differences during recall (Figure 4C and F), but the speed of fear memory extinction was significantly faster than that of the control group (Figure 4E; two-way RM ANOVA, F(1, 17) = 5.353, P < 0.05).
Since exposure therapy typically begins long after the establishment of fear memory in real-world conditions, we also conducted fear memory extinction tests for long-term memory. We initiated 7 days of L-M or vehicle injections 15 days after fear conditioning, followed by continuous extinction training (Ext) starting 2 hours after the injection (Figure 4G). The results were similar to short-term memory extinction training (Figure 4H-K), showing that the L-M group exhibited a faster rate of fear memory extinction than the vehicle group, with a significant difference on the first day of extinction training (Figure 4H; two-way RM ANOVA, F(1, 22) = 8.798, P < 0.01), but no differences were observed on the second day and during recall. These results indicate that increased methylation can facilitate the process of fear memory extinction training but did not affect recall after fear memory extinction.
In our previous studies, we observed an increase in Dnmt3a expression in the dorsal hippocampal dentate gyrus (dDG) region after extinction training within the consolidation window (Rec + Ext), indicating a potential relationship between Dnmt3a expression and the reconsolidation time window[14]. In this study, we observed that inhibiting DNMTs during Rec + Ext had a significant impact on the outcome of extinction. Additionally, we found that RG108 can inhibit neuronal activity in the dDG. Furthermore, we employed pharmacological methods to enhance DNA methylation, which stabilized methylation changes and facilitated fear memory extinction. This approach was effective for both short-term and long-term memory. These findings suggest that increasing DNA methylation can promote the extinction of fear memory, whereas inhibiting DNA methylation can impede this process.
Although the dDG is more commonly associated with context memory[22,23]. The role of the dDG in auditory fear memory remains debated, as some studies suggest that the dDG does not play a significant role in auditory fear conditioning. In this study, we found that RG108 can influence neural activity in the dDG and hippocampal DNA methylation levels, thereby affecting the outcome of fear memory recall 24 hours after extinction training. In our previous research, we observed that Dnmt3a is predominantly expressed in the dDG region of adult mice[13]. Regulation of DNA methylation levels via RG108 and L-M resulted in significant changes in hippocampal DNA methylation. Therefore, we propose that DNA methylation primarily affects the hippocampus. Consequently, subsequent experiments, including fiber recording and staining, focused on the dDG region, where we detected notable changes. Although the hippocampus is not traditionally considered critical for the formation of context fear memory, it plays a functional role during extinction training. In our prior research, we demonstrated that enhancing dDG activity using chemogenetic methods during extinction training could facilitate the extinction of fear memory and suppress its renewal[9]. Additionally, Zhang et al[24] found that increased dDG activity following fear memory extinction reduces the expression of fear memory after extinction training. This finding aligns with the current study, where RG108 suppressed dDG activity and inhibited fear memory extinction. The hippocampus is believed to play a pivotal role in the transition from short-term to long-term memory[25,26]. Thus, the effect of RG108 was not observed during extinction training, but became evident during recall 24 hours later. However, since RG108 was administered intraperitoneally, we cannot rule out its potential effects on other brain regions. It is plausible that RG108 also influenced areas such as the amygdala and prefrontal cortex, thereby contributing to its impact on fear memory extinction.
RG108 can inhibit the activity of Dnmt1, Dnmt3b, and Dnmt3a. Our findings indicate that RG108 significantly reduces DNA methylation levels in the mouse hippocampus within 2 hours of injection. Furthermore, histological analysis conducted after the extinction training revealed that RG108 reduces the expression of Dnmt3a and decreases dendritic spine density. In a study using endometrial cancer Ishikawa cell lines, RG108 downregulated Dnmt3b expression[16]. Additionally, RG108 was shown to improve the expression levels of TET1, TET2, TET3, and 5 hmC, promoting passive DNA demethylation through the remarkably inhibited expression of DNMT1, DNMT3A, and 5 mC in embryos derived from fetal fibroblasts (FFs)[27]. Moreover, TET enzymes, including TET1, play a role in regulating fear memory. Overexpression of TET1 in the hippocampus can disrupt the formation of fear memories[28]. To date, no studies have reported the regulatory effects of RG108 on DNMT expression in the nervous system. However, based on the aforementioned findings, we hypothesize that RG108 may also suppress Dnmt3a expression through the regulation of TET-related genes. Given that DNMTs play a crucial role in synapse formation[29,30], RG108 may influence dendritic spine density by modulating the expression of DNMTs, including Dnmt3a.
How do DNMTs regulate memory? The use of RG108 during the Rec + Ext process can inhibit neuronal activity. After increasing the methylation level before extinction training, we found that the speed of fear memory extinction increased, but the freezing value reached during the recall process did not show a significant change. This result is consistent with the effect of chemogenetically activating neuronal activity in the dDG area[9]. Additionally, the results of injecting RG108 were consistent with the results obtained by chemogenetically inhibiting neuronal activity in the dDG area[9]. These results indicate a reciprocal relationship between DNMTs and neuronal activity.
How do DNMTs and neuronal activity mutually regulate each other? In 2007, Miller et al[31] published an article in Neuron, after discovering that DNMTs influence the formation of memory by affecting the methylation of the memory-related gene PP1, which plays a crucial role in synaptic formation. In September 2010, LaPlant et al[32] found that the DNMTs inhibitor RG108 can reduce dendritic spine density in the nucleus accumbens (NAc) neurons induced by cocaine, leading to a significant decrease in functional synapses and a reduction in the frequency of miniature excitatory postsynaptic currents. Moreover, overexpression of Dnmt3a in the NAc region can increase dendritic spine density[32]. In 2015, Meadows et al[30]. conducted studies on cultured neurons and found that DNMTs regulate glutamatergic synaptic homeostasis by modulating the expression of downstream genes. In 2016, researchers in Chile[33,34] found that inhibiting the activity of DNMTs with the inhibitor 5-aza-2-deoxycytidine can impair synaptic plasticity in the hippocampal CA1 region of rats. Additionally, Nelson et al[35] found that conditional knockout of Dnmt1 and Dnmt3a disrupts long-term potentiation and affects dendritic spine formation. In our project, we also found that the DNMTs inhibitor RG108 can reduce dendritic spine density and neuroactivity. These studies suggest that DNMTs may regulate neuronal activity by modulating downstream gene expression and synaptic plasticity.
N-methyl-D-aspartate (NMDA) receptor-dependent synaptic activity can increase DNA methylation[35]. During the activation of NMDA receptors, CaM is activated, leading to the activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII). Activated CaMKII can phosphorylate cAMP-response element binding protein (CREB), and phosphorylated CREB can regulate gene expression, causing long-term changes in neurons[36]. Yang et al[37] found that CREB can participate in paclitaxel-induced neuropathic pain through the activation of Dnmt3a. Ashok et al[38]. also found through ChIP methods that CREB can act as a transcription factor regulating the expression of Dnmt3b. In our previous study, we found that most Dnmt3a-positive neurons are also CaMKII-positive neurons[14]. These studies suggest that increased neuronal activity may increase the expression of DNMTs through the activation of the CaMKII/CREB pathway.
In summary, we found that DNA methylation plays a crucial role in the extinction of fear memory. Inhibiting DNA methylation can suppress extinction and reduce dendritic spine density and neuronal activity, while promoting methylation can facilitate the extinction of fear memory. There is a reciprocal relationship between neuronal activity and DNA methylation. Our research suggests that combining exposure therapy with drugs that promote methylation, such as B vitamins, may enhance the effectiveness of exposure therapy.
DNA methylation plays a critical role in memory extinction. Enhancing methylation may improve the efficacy of exposure therapy, offering a potential strategy for treating fear-related disorders.
We acknowledge the Experimental Animal Center of Dali University for its assistance in the care of the animals.
| 1. | Curtiss JE, Levine DS, Ander I, Baker AW. Cognitive-Behavioral Treatments for Anxiety and Stress-Related Disorders. Focus (Am Psychiatr Publ). 2021;19:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Huang T, Li H, Tan S, Xie S, Cheng Q, Xiang Y, Zhou X. The efficacy and acceptability of exposure therapy for the treatment of post-traumatic stress disorder in children and adolescents: a systematic review and meta-analysis. BMC Psychiatry. 2022;22:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | DeRubeis RJ, Crits-Christoph P. Empirically supported individual and group psychological treatments for adult mental disorders. J Consult Clin Psychol. 1998;66:37-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Quirk GJ, Paré D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Erasing fear memories with extinction training. J Neurosci. 2010;30:14993-14997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 754] [Cited by in RCA: 703] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 6. | Penha Farias C, Guerino Furini CR, Godfried Nachtigall E, Kielbovicz Behling JA, Silva de Assis Brasil E, Bühler L, Izquierdo I, de Carvalho Myskiw J. Extinction learning with social support depends on protein synthesis in prefrontal cortex but not hippocampus. Proc Natl Acad Sci U S A. 2019;116:1765-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | McCormick CM, Mongillo DL, Simone JJ. Age and adolescent social stress effects on fear extinction in female rats. Stress. 2013;16:678-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Marshall PR, Bredy TW. Neuroepigenetic mechanisms underlying fear extinction: emerging concepts. Psychopharmacology (Berl). 2019;236:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Gong Z, Wang Z, Jiang L, Wang X, Zhang B, Vashisth MK, Zhou Q. Neuronal activity in the dorsal dentate gyrus during extinction regulates fear memory extinction and renewal. Exp Neurol. 2022;358:114224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1785] [Cited by in RCA: 1868] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 11. | Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704-5710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 375] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 12. | Morris MJ, Monteggia LM. Role of DNA methylation and the DNA methyltransferases in learning and memory. Dialogues Clin Neurosci. 2014;16:359-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Wang X, Jiang L, Ma W, Zheng X, He E, Zhang B, Vashisth MK, Gong Z. Maternal separation affects anxiety-like behavior beginning in adolescence and continuing through adulthood and related to Dnmt3a expression. J Neurophysiol. 2022;128:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 14. | Gong Z, Zhou Q. Dnmt3a in the dorsal dentate gyrus is a key regulator of fear renewal. Sci Rep. 2018;8:5093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Lyko F, Brown R. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J Natl Cancer Inst. 2005;97:1498-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 369] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 16. | Yang L, Hou J, Cui XH, Suo LN, Lv YW. RG108 induces the apoptosis of endometrial cancer Ishikawa cell lines by inhibiting the expression of DNMT3B and demethylation of HMLH1. Eur Rev Med Pharmacol Sci. 2017;21:5056-5064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Lyu TJ, Qiu X, Wang Y, Zhang L, Dai Y, Wang X, Zhao S, Xiang M, Cui L, Cheng S, Liu Y, Gu H, Jiang Y, Meng X, Wang Y, Zhao X, Wang X, Li Q, Wang M, Jiang Y, Xu Z, Huang X, Li H, Wang Y, Li Z. DNMT3A dysfunction promotes neuroinflammation and exacerbates acute ischemic stroke. MedComm (2020). 2024;5:e652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Zuzina AB, Vinarskaya AK, Balaban PM. DNA Methylation Inhibition Reversibly Impairs the Long-Term Context Memory Maintenance in Helix. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Argyrousi EK, de Nijs L, Lagatta DC, Schlütter A, Weidner MT, Zöller J, van Goethem NP, Joca SRL, van den Hove DLA, Prickaerts J. Effects of DNA methyltransferase inhibition on pattern separation performance in mice. Neurobiol Learn Mem. 2019;159:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Shealy EP, Schwartz TS, Cox RM, Reedy AM, Parrott BB. DNA methylation-based age prediction and sex-specific epigenetic aging in a lizard with female-biased longevity. Sci Adv. 2025;11:eadq3589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Segal M. Dendritic spines, synaptic plasticity and neuronal survival: activity shapes dendritic spines to enhance neuronal viability. Eur J Neurosci. 2010;31:2178-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Dees RL, Kesner RP. The role of the dorsal dentate gyrus in object and object-context recognition. Neurobiol Learn Mem. 2013;106:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Fournier NM, Duman RS. Illuminating hippocampal control of fear memory and anxiety. Neuron. 2013;77:803-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Wang Z, Ju J, Liao J, Zhou Q. Elevated activity in the dorsal dentate gyrus reduces expression of fear memory after fear extinction training. J Psychiatry Neurosci. 2021;46:E390-E401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Olsen RK, Moses SN, Riggs L, Ryan JD. The hippocampus supports multiple cognitive processes through relational binding and comparison. Front Hum Neurosci. 2012;6:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Slotnick SD. The hippocampus and long-term memory. Cogn Neurosci. 2022;13:113-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 27. | Zhai Y, Zhang Z, Yu H, Su L, Yao G, Ma X, Li Q, An X, Zhang S, Li Z. Dynamic Methylation Changes of DNA and H3K4 by RG108 Improve Epigenetic Reprogramming of Somatic Cell Nuclear Transfer Embryos in Pigs. Cell Physiol Biochem. 2018;50:1376-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Kaas GA, Zhong C, Eason DE, Ross DL, Vachhani RV, Ming GL, King JR, Song H, Sweatt JD. TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron. 2013;79:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 29. | Fan XY, Yang JY, Dong YX, Hou Y, Liu S, Wu CF. Oxytocin inhibits methamphetamine-associated learning and memory alterations by regulating DNA methylation at the Synaptophysin promoter. Addict Biol. 2020;25:e12697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Meadows JP, Guzman-Karlsson MC, Phillips S, Holleman C, Posey JL, Day JJ, Hablitz JJ, Sweatt JD. DNA methylation regulates neuronal glutamatergic synaptic scaling. Sci Signal. 2015;8:ra61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 881] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 32. | LaPlant Q, Vialou V, Covington HE 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iñiguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolaños CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 524] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 33. | Muñoz P, Estay C, Díaz P, Elgueta C, Ardiles ÁO, Lizana PA. Inhibition of DNA Methylation Impairs Synaptic Plasticity during an Early Time Window in Rats. Neural Plast. 2016;2016:4783836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 861] [Cited by in RCA: 790] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 35. | Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 36. | Blanke ML, VanDongen AMJ. Activation Mechanisms of the NMDA Receptor. In: Biology of the NMDA Receptor. Boca Raton (FL): CRC Press/Taylor & Francis; 2009–. [PubMed] |
| 37. | Yang Y, Wen J, Zheng B, Wu S, Mao Q, Liang L, Li Z, Bachmann T, Bekker A, Tao YX. CREB Participates in Paclitaxel-Induced Neuropathic Pain Genesis Through Transcriptional Activation of Dnmt3a in Primary Sensory Neurons. Neurotherapeutics. 2021;18:586-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Ashok C, Selvam M, Ponne S, Parcha PK, Raja KMP, Baluchamy S. CREB acts as a common transcription factor for major epigenetic repressors; DNMT3B, EZH2, CUL4B and E2F6. Med Oncol. 2020;37:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/