Published online Jul 19, 2025. doi: 10.5498/wjp.v15.i7.106761
Revised: April 23, 2025
Accepted: May 22, 2025
Published online: July 19, 2025
Processing time: 112 Days and 19.9 Hours
Mild cognitive impairment (MCI) is a high-risk precursor to Alzheimer’s disease characterized by declining memory or other progressive cognitive functions with
To investigate the efficacy of repetitive transcranial magnetic stimulation (rTMS) in patients with MCI.
This retrospective analysis involved 180 patients with MCI who were admitted to The First Hospital of Shanxi Medical University from January 2021 to June 2023. Participants were allocated into the research (n = 98, receiving rTMS) and control groups (n = 82, receiving sham stimulation). Memory tests, cognitive function assessments, event-related potential–P300 tests, and electroencephalogram (EEG) examinations were conducted pre-treatment and post-treatment. Further, memory quotient (MQ), cognitive function scores, and EEG grading results were compa
Pre-treatment MQ scores, long-term and short-term memory, as well as imme
Patients with MCI receiving rTMS therapy demonstrated improved memory and cognitive functions and EEG grading and exhibited high safety with fewer adverse reactions.
Core Tip: Mild cognitive impairment falls short of meeting the diagnostic criteria for dementia and is generally considered a transitional phase between normal aging and dementia. Repetitive transcranial magnetic stimulation is currently considered an emerging treatment for early cognitive rehabilitation. This study will investigate the effectiveness and safety of the treatment from neuropsychological and neurophysiological perspectives using the memory quotient and the electroencephalogram as the subjects.
- Citation: Fu HX. Effects of repetitive transcranial magnetic stimulation on electroencephalogram and memory function in patients with mild cognitive impairment. World J Psychiatry 2025; 15(7): 106761
- URL: https://www.wjgnet.com/2220-3206/full/v15/i7/106761.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i7.106761
Mild cognitive impairment (MCI) represents a syndrome characterized by a significant decline in cognitive abilities, ma
Repetitive transcranial magnetic stimulation (rTMS) is currently recognized as a therapeutic approach for early cognitive dysfunction rehabilitation. Multiple domestic and international studies have revealed the positive impact of rTMS on improving the cognitive function of patients with dementia, primarily by exerting a remodeling effect on the cerebral cortex[7]. Research into cognitive function improvements in AD with high-frequency rTMS predominantly centers on prefrontal cortex stimulation[8]. This therapy has gained wider acceptance in the mental, neurological, and rehabilitation fields. Furthermore, the literature supports the feasibility of rTMS in treating patients with MCI[9,10]. With its non-invasiveness, ease of operation, and reliability, rTMS is widely applied in clinical settings, especially as an adju
This retrospective analysis involved 180 patients with MCI treated at The First Hospital of Shanxi Medical University from January 2021 to June 2023. Based on different treatment approaches, they were assigned to the research (n = 98) and control groups (n = 82).
Inclusion criteria: (1) MCI diagnosis based on Petersen’s diagnostic criteria for MCI included self-reported memory decline corroborated by family members or informants, preserved general cognitive function, normal overall activities of daily living; (2) Objective evidence of memory impairment, such as memory test performance of ≤ 1.5 SDs below age-matched and education-matched controls; (3) Age 50–70 years; (4) No prior pharmacotherapy for cognitive impairment; and (5) Complete clinical data.
Exclusion criteria: (1) History of epilepsy; (2) Neuroimaging-confirmed neurological disorders (e.g., cerebral hemorrhage, cerebral infarction, intracranial space-occupying lesions) causing memory impairment; (3) Severe depression, anxiety, or other pseudodementia conditions; (4) Contraindications to rTMS treatment; (5) Immunodeficiency, coagulation abnorma
A MagPro-R30 magnetic stimulator (Tonica, Denmark) was used in the rTMS treatment of the research group. First, the resting motor threshold (RMT) was identified. Patients were seated with surface electrodes placed on the abductor pollicis brevis muscle, whereas the reference electrode was positioned at the metacarpophalangeal joint of the thumb. The stimulation site targeted the contralateral cortical motor area corresponding to the thumb. The stimulation coil was repeatedly adjusted over the scalp until reproducible, clear-waveform motor-evoked potentials (MEPs) were obtained. The stimulation intensity was then gradually reduced while maintaining the coil position until MEPs were elicited in 3 out of 5 consecutive stimuli, which define the RMT. Bilateral prefrontal areas received high-frequency stimulation at 80% RMT intensity (10 Hz per session) during rTMS treatment. Patients underwent 40 high-frequency treatment sessions (30 minute/session), consisting of 50 pulses per train (50 trains total) at 30-second intervals. Treatments were administered once daily, five times a week. The control group received sham stimulation with an inactive coil that mimicked the same stimulation parameters without delivering effective magnetic pulses.
Memory function: The Wechsler Memory Scale–Revised Chinese version (WMS–RC) was employed to measure changes in patients’ memory quotient (MQ). The WMS–RC includes three major test categories: (1) Long-term memory; (2) Short-term memory; and (3) Immediate memory tests. The long-term memory test covers personal experiences, temporal and spatial memory, and digit span sequencing tasks (forward sequencing 1–100, backward sequencing 100–1, and cumulative calculations). The short-term memory test includes picture recall, visual recognition, visual reproduction, associative learning, tactile tests, and comprehension memory. The immediate memory test involves forward and backward digit spans. In this study, the primary observation items include WMS–RC subtest scores for digital span sequencing, picture recall, visual recognition, visual reproduction, associative learning, comprehension memory, and immediate memory, as well as the overall MQ. The MQ reflects the participant’s overall memory level and is derived from WMS–RC measurements. It is calculated by summing up the raw scores from each WMS–RC subtest to obtain a total score. The total score is then adjusted with an age-specific weighting factor based on the participant’s age. Finally, the weighted total score is converted to the MQ using standardized reference tables.
Cognitive function: This was assessed using the Montreal Cognitive Assessment (MoCA) to evaluate overall cognitive performance. The MoCA assesses cognitive domains, consisting of visuospatial and executive function, naming, attention, language, abstraction, delayed recall, calculation, and orientation. The total score is 30 points, with higher scores indicating better cognitive function. The scoring criteria are > 26 points (indicating normal cognitive function), 18–26 points (MCI), 10–17 points (moderate cognitive impairment), and < 10 points (severe cognitive impairment). The cut-off scores for cognitive impairment are illiterate at ≤ 13 points; primary school education at ≤ 19 points; secondary school education and above at ≤ 24 points, respectively.
Event-related potential-P300 test: The P300 test was conducted using the Neuroscan 64-channel event-related potential (ERP) system (United States). A visual “oddball” paradigm was used to elicit P300 responses. The latency and amplitude of the P300 component in response to target stimuli were measured.
Electroencephalogram examination: Electroencephalogram (EEG) was performed with an EEG machine with scalp electrodes placed on the patient. Recordings were taken while the patient was awake and in a quiet state, using both monopolar and bipolar montages. A hyperventilation test was conducted for 25–30 minutes, and EEG results were categorized as normal, mildly abnormal, moderately abnormal, or severely abnormal. EEG grading criteria include (1) Grade I (normal): Dominant α rhythm; (2) Grade II (mildly abnormal): Predominant θ waves; (3) Grade III (moderately abnormal): Predominant δ and θ waves with occasional α waves; (4) Grade IV (severely abnormal): Diffuse δ waves with scattered δ waves in some leads, accompanied by electrical silence in other regions; and (5) Grade V (extremely abnor
Adverse reactions: The incidence of adverse reactions, including mild dizziness, nausea and vomiting, and diarrhea, was documented.
Statistical Package for the Social Sciences version 25.0 was used for statistical data analyses. Measurement data were expressed as mean ± SD. Intergroup comparisons for normally distributed measurement data were conducted using the t-test. The non-parametric Mann–Whitney U rank-sum test was used instead in cases where the data did not follow a normal distribution. Enumeration data were expressed as percentages and intergroup comparisons were conducted using the χ² test. A P-value of < 0.05 indicated statistical significance.
The research group exhibited a male-to-female ratio of 49:49, with an age range of 50–70 years (64.17 years ± 4.33 years). The average body mass index (BMI) was 23.33 kg/m² ± 1.31 kg/m², and the average disease duration ranged from 1 year to 4 years (2.32 years ± 0.96 years). The duration of education varied from 6 years to 46 years (9.81 years ± 1.77 years). Among these participants, 37 participants were unmarried/divorced, whereas 61 participants were married. Comorbi
| Control group (n = 82) | Research group (n = 98) | t/χ² value | P value | |
| Gender (male/female) | 46/36 | 49/49 | 0.666 | 0.414 |
| Age (years) | 63.57 ± 3.59 | 64.17 ± 4.33 | 1.001 | 0.318 |
| Body mass index (kg/m2) | 23.03 ± 1.21 | 23.33 ± 1.31 | 1.583 | 0.115 |
| Disease duration (years) | 2.38 ± 1.01 | 2.32 ± 0.96 | 0.408 | 0.684 |
| Duration of education (years) | 9.93 ± 1.49 | 9.81 ± 1.77 | 0.486 | 0.627 |
| Marital status | 0.030 | 0.862 | ||
| Married | 50 | 61 | ||
| Unmarried/divorced | 32 | 37 | ||
| Hypertension | 31 | 40 | 0.170 | 0.681 |
| Diabetes | 28 | 35 | 0.0482 | 0.826 |
| Hyperlipidemia | 35 | 37 | 0.452 | 0.502 |
The control group demonstrated no marked differences in MQ scores or memory scores post-treatment compared with pre-treatment values (P > 0.05). In contrast, the research group exhibited significant improvements in MQ scores as well as long- and short-term memory scores post-treatment compared with pre-treatment values (P < 0.05), whereas no significant difference was observed in immediate memory scores (P > 0.05). The research group demonstrated statistically significant net increases in MQ scores, long-term memory scores, and short-term memory scores (P < 0.05), except for immediate memory, compared with the control group (Table 2).
| Memory quotient | Long-term memory | Short-term memory | Immediate memory | ||
| Before treatment | Control group (n = 82) | 75.41 ± 11.83 | 19.74 ± 6.92 | 26.95 ± 10.52 | 4.59 ± 1.78 |
| Research group (n = 98) | 75.66 ± 10.01 | 19.51 ± 6.79 | 27.63 ± 8.32 | 4.60 ± 2.38 | |
| t value | 0.154 | 0.224 | 0.484 | 0.031 | |
| P value | 0.878 | 0.822 | 0.629 | 0.975 | |
| After treatment | Control group (n = 82) | 75.51 ± 10.00 | 20.73 ± 6.13 | 28.27 ± 8.19 | 4.67 ± 2.09 |
| Research group (n = 98) | 86.53 ± 9.34a | 23.62 ± 5.52a | 33.93 ± 9.06a | 5.12 ± 1.85 | |
| t value | 7.633 | 3.326 | 4.359 | 1.596 | |
| P value | < 0.0001 | 0.001 | < 0.0001 | 0.112 | |
| Net increase | Control group (n = 82) | 0.13 ± 11.96 | 0.99 ± 9.27 | 0.63 ± 9.25 | 0.09 ± 2.84 |
| Research group (n = 98) | 10.87 ± 7.53 | 4.11 ± 8.03 | 5.09 ± 11.77 | 0.52 ± 2.68 | |
| t value | 7.324 | 2.419 | 2.786 | 1.043 | |
| P value | < 0.0001 | 0.017 | 0.005 | 0.298 |
The research group showed significant improvements in the following WMS–RC subscale scores compared to pre-treatment values: (1) Forward digit span (1–100); (2) Backward digit span (100–1); (3) Cumulative calculations; (4) Visual recognition; (5) Visual reproduction; (6) Associative learning; (7) Comprehension memory; and (8) Digit span, with all improvements being statistically significant (P < 0.05). In contrast, the control group demonstrated no significant changes in any of these subscale scores (P > 0.05). Further, all post-treatment subscale scores were statistically higher in the research group than in the control group (P < 0.05) (Table 3).
| Control group (n = 82) | Research group (n = 98) | |||||
| Before treatment | After treatment | Net increase | Before treatment | After treatment | Net increase | |
| Forward digit span (1–100) | 7.05 ± 1.02 | 7.13 ± 1.92 | 0.09 ± 2.18 | 7.20 ± 2.58 | 7.93 ± 2.35a,b | 0.72 ± 3.30 |
| Backward digit span (100–1) | 5.22 ± 1.86 | 5.49 ± 1.73 | 0.27 ± 2.46 | 5.43 ± 2.13 | 6.55 ± 1.76a,b | 1.12 ± 2.05b |
| Cumulative calculations | 7.03 ± 1.65 | 7.38 ± 2.11 | 0.35 ± 2.62 | 7.09 ± 2.32 | 8.00 ± 1.75a,b | 0.91 ± 2.67 |
| Visual recognition | 6.09 ± 1.74 | 6.50 ± 1.95 | 0.41 ± 2.41 | 6.14 ± 2.17 | 7.09 ± 2.10a,b | 0.95 ± 3.04 |
| Visual reproduction | 3.65 ± 1.50 | 3.74 ± 2.00 | 0.10 ± 2.57 | 3.59 ± 1.64 | 4.54 ± 2.06a,b | 0.95 ± 2.58b |
| Associative learning | 4.35 ± 1.96 | 4.78 ± 1.85 | 0.43 ± 2.66 | 4.43 ± 1.98 | 5.42 ± 1.77a,b | 1.00 ± 2.66 |
| Comprehension memory | 5.50 ± 1.23 | 5.46 ± 1.37 | -0.04 ± 1.97 | 5.46 ± 1.66 | 6.18 ± 1.47a,b | 0.72 ± 2.12b |
| Digit span | 4.78 ± 1.75 | 4.91 ± 1.81 | 0.13 ± 2.25 | 5.01 ± 1.82 | 5.82 ± 1.95a,b | 0.81 ± 2.52 |
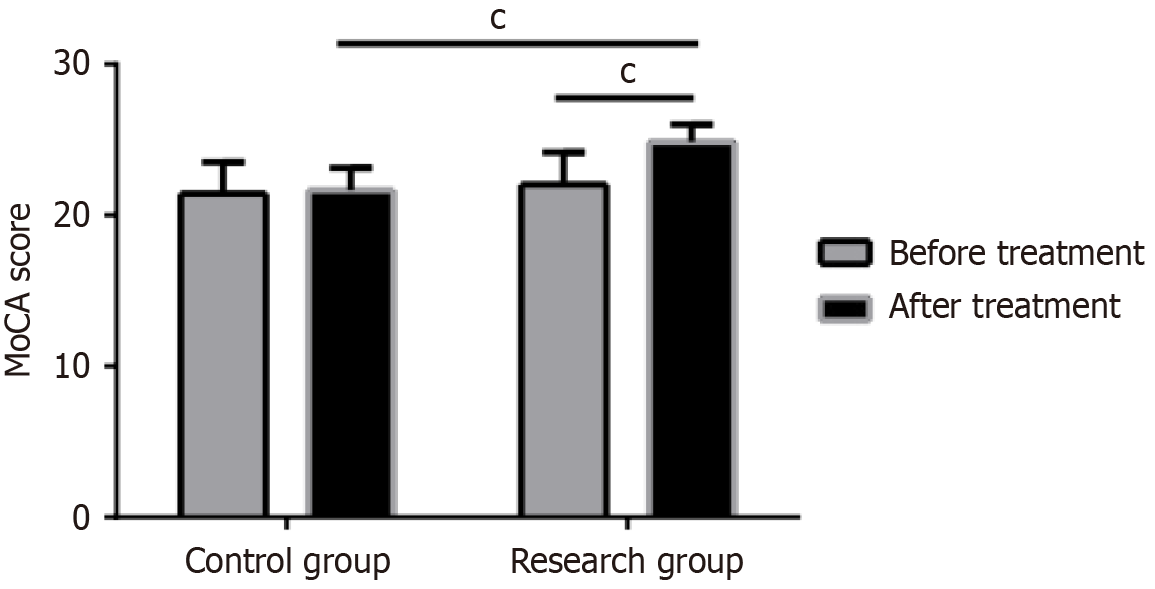
At baseline, no evident differences were found in MoCA scores between the two groups. Post-treatment, the research group demonstrated a significant increase in both MoCA scores compared to baseline values (P < 0.05), whereas the control group showed no significant changes (P > 0.05). Figure 1 illustrates detailed comparisons.
At baseline, no significant differences in P300 latency and amplitude were found between the groups (latency: t = 1.103, amplitude: t = 0.201, P > 0.05). Post-treatment, the research group demonstrated a significant decrease in P300 latency and an evident increase in amplitude compared to baseline measurements (t = 13.267, t = 13.816, P < 0.0001). In contrast, the control group demonstrated no significant changes in either P300 latency or amplitude (t = 1.100, t = 1.101, P > 0.05). Moreover, the research group showed significantly shorter P300 latency and higher amplitude post-treatment compared with the control group (t = 12.831, t = 11.006, P < 0.0001) (Table 4).
| Latency (ms) | t value | P value | Amplitude (uv) | t value | P value | |||
| Before treatment | After treatment | Before treatment | After treatment | |||||
| Control group (n = 82) | 338.31 ± 18.79 | 341.34 ± 16.42 | 1.100 | 0.273 | 3.45 ± 0.99 | 3.63 ± 1.27 | 1.101 | 0.313 |
| Research group (n = 98) | 341.21 ± 16.47 | 312.18 ± 14.07 | 13.267 | < 0.0001 | 3.48 ± 1.00 | 5.66 ± 1.20 | 13.816 | < 0.0001 |
| t value | 1.103 | 12.831 | 0.201 | 11.006 | ||||
| P value | 0.271 | < 0.0001 | 0.841 | < 0.0001 | ||||
At baseline, no noticeable differences were found in EEG grading between the two groups (χ² = 0.209, P = 0.901). Post-treatment, the research group demonstrated significant improvement in EEG grading compared with baseline (χ² = 6.242, P = 0.044). Mild abnormal EEGs (II) usually indicate a slight deviation in the form, frequency, amplitude, or distribution of brain waves. Normal people can demonstrate mild abnormal EEGs under emotional stress and poor rest conditions, and they generally do not have a significant impact on the patient’s health. Post-treatment, the results revealed that the research group’s EEG grading was significantly superior to that of the control group (χ² = 23.752, P < 0.0001) (Table 5).
| I | II | III | IV | V | ||
| Before treatment | Control group (n = 82) | 13 | 57 | 12 | 0 | 0 |
| Research group (n = 98) | 17 | 65 | 16 | 0 | 0 | |
| χ² value | 0.209 | |||||
| P value | 0.901 | |||||
| After treatment | Control group (n = 82) | 15 | 37 | 30 | 0 | 0 |
| Research group (n = 98) | 28 | 63 | 7 | 0 | 0 | |
| χ² value | 23.752 | |||||
| P value | < 0.0001 | |||||
Adverse reactions include diarrhea in 3 patients, nausea and vomiting in 6 patients, and mild dizziness in 7 patients of the control group, and diarrhea in 1 patient, nausea and vomiting in 4 patients, and mild dizziness in 5 patients of the research group. The total incidence of adverse reactions in the research group was 8.2%, which was significantly lower than the 19.5% observed in the control group (χ² = 4.976, P = 0.026) (Table 6).
| Diarrhea | Nausea and vomiting | Mild dizziness | Total incidence | |
| Control group (n = 82) | 3 | 6 | 7 | 16 (19.5) |
| Research group (n = 98) | 1 | 4 | 3 | 8 (8.2) |
| χ² value | 4.976 | |||
| P value | 0.026 |
MCI is a clinically prevalent condition, classified into two types based on the extent of cognitive impairment: (1) Non-amnestic MCI; and (2) Amnestic MCI. Non-amnestic MCI presents with a decline in cognitive functions other than memory and may progress to frontotemporal dementia, whereas amnestic MCI typically develops toward AD, with worsening symptoms significantly affecting daily life[14,15]. RTMS is a neurophysiological technique developed from transcranial magnetic stimulation. It generates a time-varying magnetic field that causes an electric field in the cerebral cortex, producing induced currents. These currents act on brain tissue, which stimulate neuronal depolarization and generate evoked potentials, thereby modulating the excitability of local cortical regions[16]. RTMS at different frequencies exerts varying effects on the cerebral cortex. In particular, high-frequency rTMS promotes the release of excitatory neurotransmitters, such as dopamine and glutamate, improves neural conduction velocity, and stimulates cortical exci
In this study, we initially investigated MQ alterations of the two patient groups post-treatment. The results revealed that the MQ score, along with long-term and short-term memory scores, in the research group increased after treatment compared to pre-treatment levels; however, no significant change was found in immediate memory. In contrast, the control group demonstrated no statistically significant differences in any of these parameters pre-treatment and post-treatment. The research group demonstrated significant improvements in scores for 1–100 forward digit span, 100–1 backward digit span, cumulative scores, visual recognition, visual reproduction, associative learning, comprehension memory, and digit span through the WMS-RC assessment. MCI is characterized by impaired short-term memory, with immediate memory remaining relatively intact. According to research, the most prominent and earliest-emerging cognitive impairments in patients with MCI and early AD are associated with explicit memory, such as associative learning, recall, and reproduction, with significant impairments found in experience and orientation abilities[18]. The short-term memory impairment in patients was notably alleviated after rTMS treatment, aligning with most clinical reports. Further, rTMS treatment caused improved MoCA scores, signifying enhancements in patients’ cognitive func
Extant research has established a correlation between the extent of EEG abnormalities and the degree of cognitive impairment[24]. EEG detects subtle declines in brain function before the manifestation of structural and metabolic ab
However, this study is subject to several limitations, including a relatively simplistic design, a limited sample size, and a short follow-up duration. The results indicate that rTMS improved therapeutic outcomes, alleviated clinical symptoms, and enhanced daily functioning in patients with MCI; however, the potential for bias in the study cannot be disregarded, which may be caused by limited sample size, limited observational indicators, etc. Thus, randomized controlled trials with larger cohorts and extended observation periods are warranted to identify optimal stimulation parameters and protocols, thereby maximizing the therapeutic efficacy of rTMS for cognitive impairment. Further, in clinical practice, we can use rTMS as an adjunctive physical stimulation therapy in the rehabilitation process, in addition to the patient’s medication for cyclic rTMS. Concurrently, rTMS-induced inter-epileptic seizures are the biggest side effect of rTMS, whether or not to induce inter-epileptic seizures is mainly associated with the stimulation intensity, frequency, stimu
In summary, rTMS represents a safe and effective non-pharmacological intervention for MCI and demonstrates the potential to mitigate memory decline, particularly as evidenced by EEG slowing, and to improve cognitive function to a measurable extent.
| 1. | Dunne RA, Aarsland D, O'Brien JT, Ballard C, Banerjee S, Fox NC, Isaacs JD, Underwood BR, Perry RJ, Chan D, Dening T, Thomas AJ, Schryer J, Jones AM, Evans AR, Alessi C, Coulthard EJ, Pickett J, Elton P, Jones RW, Mitchell S, Hooper N, Kalafatis C, Rasmussen JGC, Martin H, Schott JM, Burns A. Mild cognitive impairment: the Manchester consensus. Age Ageing. 2021;50:72-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 2. | Deright J. Mild Cognitive Impairment. Essential Neuropsychology: A Concise Handbook for Adult Practitioners. Cham: Springer, 2022. [DOI] [Full Text] |
| 3. | Pike KE, Cavuoto MG, Li L, Wright BJ, Kinsella GJ. Subjective Cognitive Decline: Level of Risk for Future Dementia and Mild Cognitive Impairment, a Meta-Analysis of Longitudinal Studies. Neuropsychol Rev. 2022;32:703-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 4. | Muhammad T, Govindu M, Srivastava S. Relationship between chewing tobacco, smoking, consuming alcohol and cognitive impairment among older adults in India: a cross-sectional study. BMC Geriatr. 2021;21:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Talwar P, Kushwaha S, Chaturvedi M, Mahajan V. Systematic Review of Different Neuroimaging Correlates in Mild Cognitive Impairment and Alzheimer's Disease. Clin Neuroradiol. 2021;31:953-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Bai W, Chen P, Cai H, Zhang Q, Su Z, Cheung T, Jackson T, Sha S, Xiang YT. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a meta-analysis and systematic review of epidemiology studies. Age Ageing. 2022;51:afac173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 140] [Reference Citation Analysis (0)] |
| 7. | Aloizou AM, Pateraki G, Anargyros K, Siokas V, Bakirtzis C, Sgantzos M, Messinis L, Nasios G, Peristeri E, Bogdanos DP, Doskas TK, Tzeferakos G, Dardiotis E. Repetitive Transcranial Magnetic Stimulation in the Treatment of Alzheimer's Disease and Other Dementias. Healthcare (Basel). 2021;9:949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Leblhuber F, Geisler S, Ehrlich D, Steiner K, Kurz K, Fuchs D. High Frequency Repetitive Transcranial Magnetic Stimulation Improves Cognitive Performance Parameters in Patients with Alzheimer's Disease - An Exploratory Pilot Study. Curr Alzheimer Res. 2022;19:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Sharbafshaaer M, Gigi I, Lavorgna L, Esposito S, Bonavita S, Tedeschi G, Esposito F, Trojsi F. Repetitive Transcranial Magnetic Stimulation (rTMS) in Mild Cognitive Impairment: Effects on Cognitive Functions-A Systematic Review. J Clin Med. 2023;12:6190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 10. | Jiang L, Cui H, Zhang C, Cao X, Gu N, Zhu Y, Wang J, Yang Z, Li C. Repetitive Transcranial Magnetic Stimulation for Improving Cognitive Function in Patients With Mild Cognitive Impairment: A Systematic Review. Front Aging Neurosci. 2020;12:593000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Tavakoli H, Heidarpanah A. Literature Review of the Efficacy of Repetitive Transcranial Magnetic Stimulation on Epilepsy. Iran J Child Neurol. 2023;17:9-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Krogh S, Aagaard P, Jønsson AB, Figlewski K, Kasch H. Effects of repetitive transcranial magnetic stimulation on recovery in lower limb muscle strength and gait function following spinal cord injury: a randomized controlled trial. Spinal Cord. 2022;60:135-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Luo J, Feng Y, Hong Z, Yin M, Zheng H, Zhang L, Hu X. High-frequency repetitive transcranial magnetic stimulation promotes neural stem cell proliferation after ischemic stroke. Neural Regen Res. 2024;19:1772-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 14. | Cotelli M, Manenti R, Brambilla M, Gobbi E, Ferrari C, Binetti G, Cappa SF. Cognitive telerehabilitation in mild cognitive impairment, Alzheimer's disease and frontotemporal dementia: A systematic review. J Telemed Telecare. 2019;25:67-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, Nixon RA, Jones DT. Alzheimer disease. Nat Rev Dis Primers. 2021;7:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 1565] [Article Influence: 313.0] [Reference Citation Analysis (1)] |
| 16. | Yang S, Chang MC. Effect of Repetitive Transcranial Magnetic Stimulation on Pain Management: A Systematic Narrative Review. Front Neurol. 2020;11:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Turi Z, Lenz M, Paulus W, Mittner M, Vlachos A. Selecting stimulation intensity in repetitive transcranial magnetic stimulation studies: A systematic review between 1991 and 2020. Eur J Neurosci. 2021;53:3404-3415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Joubert S, Gardy L, Didic M, Rouleau I, Barbeau EJ. A Meta-Analysis of Semantic Memory in Mild Cognitive Impairment. Neuropsychol Rev. 2021;31:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Rektorova I, Megova S, Bares M, Rektor I. Cognitive functioning after repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia: a pilot study of seven patients. J Neurol Sci. 2005;229-230:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Wang X, Mao Z, Ling Z, Yu X. Repetitive transcranial magnetic stimulation for cognitive impairment in Alzheimer's disease: a meta-analysis of randomized controlled trials. J Neurol. 2020;267:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Lin Y, Jiang WJ, Shan PY, Lu M, Wang T, Li RH, Zhang N, Ma L. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer's disease: A systematic review and meta-analysis. J Neurol Sci. 2019;398:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Chou YH, Ton That V, Sundman M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2020;86:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 23. | Demirayak P, Kıyı İ, İşbitiren YÖ, Yener G. Cognitive load associates prolonged P300 latency during target stimulus processing in individuals with mild cognitive impairment. Sci Rep. 2023;13:15956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 24. | Roohi-Azizi M, Azimi L, Heysieattalab S, Aamidfar M. Changes of the brain's bioelectrical activity in cognition, consciousness, and some mental disorders. Med J Islam Repub Iran. 2017;31:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Moore HC, Parsons MW, Yue GH, Rybicki LA, Siemionow W. Electroencephalogram power changes as a correlate of chemotherapy-associated fatigue and cognitive dysfunction. Support Care Cancer. 2014;22:2127-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Jackson CE, Snyder PJ. Electroencephalography and event-related potentials as biomarkers of mild cognitive impairment and mild Alzheimer's disease. Alzheimers Dement. 2008;4:S137-S143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Tateishi H, Mizoguchi Y, Monji A. Is the Therapeutic Mechanism of Repetitive Transcranial Magnetic Stimulation in Cognitive Dysfunctions of Depression Related to the Neuroinflammatory Processes in Depression? Front Psychiatry. 2022;13:834425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Yuan LQ, Zeng Q, Wang D, Wen XY, Shi Y, Zhu F, Chen SJ, Huang GZ. Neuroimaging mechanisms of high-frequency repetitive transcranial magnetic stimulation for treatment of amnestic mild cognitive impairment: a double-blind randomized sham-controlled trial. Neural Regen Res. 2021;16:707-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/