Published online Jul 19, 2025. doi: 10.5498/wjp.v15.i7.106062
Revised: March 20, 2025
Accepted: April 14, 2025
Published online: July 19, 2025
Processing time: 145 Days and 22.9 Hours
Sepsis, a life-threatening condition, can lead to acute skin failure characterized by extensive skin damage. This is often due to decreased blood flow, inflammation, and increased susceptibility to infection. Acute skin failure in people with sepsis is often associated with sleep disturbances, anxiety, and poor mood. Inflammatory markers and lactate levels correlate with these psychiatric symptoms, suggesting a link between skin and brain function. The skin and the central nervous system (CNS) have bidirectional communication. The CNS is also in close contact with the digestive tract. The gut, skin, and brain influence each other’s functions thr
Core Tip: Acute skin failure tends to cause insomnia and anxiety. The skin, along with the gut, is an organ that functions as a barrier, forming the gut-skin-brain axis. The gut-skin-brain axis is a bidirectional relationship mediated by the immune system, hormones, and the autonomic nervous system. Studying the gut-skin-brain axis holds immense promise for understanding mental illness due to the intricate and bidirectional communication network it represents. In essence, the gut-skin-brain axis provides a framework for understanding how interactions between the gut, skin, and brain can influence mental health, opening new avenues for prevention and treatment.
- Citation: Nagamine T. Gut-skin-brain axis in people suffering from sepsis with acute skin failure. World J Psychiatry 2025; 15(7): 106062
- URL: https://www.wjgnet.com/2220-3206/full/v15/i7/106062.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i7.106062
Sepsis has a profound effect on mental function. Sepsis accompanied by acute skin failure can cause physical and mental complications[1]. One suggested mechanism is that the immune response affects mental function, as lactate levels and cytokines are correlated with anxiety and sleep disturbances. The reason why acute skin failure due to sepsis affects mental function is that there is bidirectional communication between the skin and the brain, the skin-brain axis. The skin is an important organ that protects the internal environment from the external world. In this article, acute skin failure and mental function in people with sepsis are discussed.
Sepsis is a life-threatening condition that occurs when the body’s response to an infection injures its own tissues and organs. It can cause a variety of symptoms, including skin lesions. The types of skin lesions that can occur with sepsis vary. Some common types include petechiae, purpura, ecchymosis, bullae, and necrosis[2]. Petechiae are small, pinpoint red or purple spots on the skin caused by bleeding under the skin. Purpura are larger, bruise-like spots on the skin, also caused by subcutaneous bleeding. Ecchymosis is a larger area of skin discoloration caused by bleeding under the skin. Bullae are large, fluid-filled blisters on the skin. Skin necrosis is the death of skin tissue, which can result in open sores or ulcers.
Monitoring skin lesions in sepsis is critical for several reasons, as they can provide valuable insight into the patient’s condition and guide treatment strategies. Skin lesions can be one of the early signs of sepsis, especially with certain types of infections. Early detection of these lesions can lead to earlier diagnosis and treatment, significantly improving the patient’s chances of survival. This is particularly true for conditions such as meningococcal sepsis, for which a characteristic rash can be a hallmark. The appearance and location of skin lesions can sometimes help identify the source of the infection. For example, lesions near a surgical site may indicate a postoperative infection, while those in a particular pattern may indicate a specific type of pathogen. The type and extent of skin lesions may reflect the severity of sepsis. Widespread or rapidly progressing lesions may indicate more severe infection and a poorer prognosis. For example, the presence of bullae (large blisters) or skin necrosis often indicates a more critical condition. Severe skin dysfunction is observed in severe cases of sepsis and is collectively referred to as acute skin failure[3].
Acute skin failure is a serious complication that can occur in people with sepsis. Symptoms of acute skin failure can vary depending on the underlying cause and severity of the condition. However, common signs and symptoms include widespread skin redness and blistering, skin peeling or flaking, severe pain, fever and chills, and signs of infection[4]. Diagnosis of acute skin failure includes a thorough physical examination, review of medical history, and laboratory testing. Treatment typically focuses on treating the underlying cause, supporting the patient’s overall condition, and managing the skin damage. The prognosis for acute skin failure depends on the underlying cause, the severity of the condition, and the patient’s overall health. In some cases, acute skin failure can be life-threatening, especially if it is associated with serious infection or other complications. However, with prompt and appropriate treatment, many patients can recover from acute skin failure.
The concept of “acute skin failure” is an evolving one, and there is not a single, universally agreed-upon set of diagnostic criteria. Skin failure is essentially the skin’s inability to perform its vital functions, such as barrier protection, thermoregulation, and fluid balance. Acute skin failure refers to this dysfunction occurring rapidly, often in the context of severe illness. Diagnostic challenges include that unlike other organ failures, there aren’t readily available blood tests or imaging studies to definitively diagnose skin failure. Differentiating skin failure from pressure injuries or other dermatological conditions can be challenging. It’s important to note that research is ongoing to further refine the definition and diagnostic criteria for acute skin failure.
Sepsis can cause a drop in blood pressure and reduced blood flow to the skin. This can deprive the skin of oxygen and nutrients, leading to tissue damage and breakdown. Sepsis triggers a strong inflammatory response throughout the body. This inflammation can damage the skin, making it more vulnerable to injury. Patients with sepsis have an increased risk of developing skin infections. These infections can further damage the skin and contribute to skin breakdown.
The exact reasons why some people with sepsis develop skin lesions and others do not are not fully understood, but several factors are thought to play a role. Some types of infections are more likely to cause skin lesions than others. For example, infections with certain bacteria, such as Staphylococcus aureus, are more likely to cause skin lesions than infections with other bacteria. People with more severe sepsis are more likely to develop skin lesions than people with less severe sepsis. The body’s immune response to infection can vary from person to person. Some people may have a stronger inflammatory response that can lead to skin lesions. People with certain underlying health conditions, such as diabetes or a weakened immune system, may be more likely to develop skin lesions with sepsis.
Psychotic symptoms may occur in individuals with acute skin failure, although they are not as common as other symptoms such as confusion, disorientation, and delirium. The exact cause of psychotic symptoms in these patients is not fully understood, but it is thought to be related to a combination of factors. Sepsis can cause inflammation and damage to the brain, which can lead to a variety of neurological and psychiatric symptoms, including psychosis[5]. Some medications used to treat sepsis and acute skin failure can cause psychotic side effects. Patients with pre-existing mental illness may be more likely to experience psychotic symptoms when critically ill with sepsis and acute skin failure. The prognosis for psychological and psychotic problems in individuals with acute skin failure due to sepsis depends on the severity of the sepsis, the extent of skin damage, and the overall health of the patient. In some cases, these symptoms may resolve with treatment of the underlying condition. In others, they may persist or become chronic.
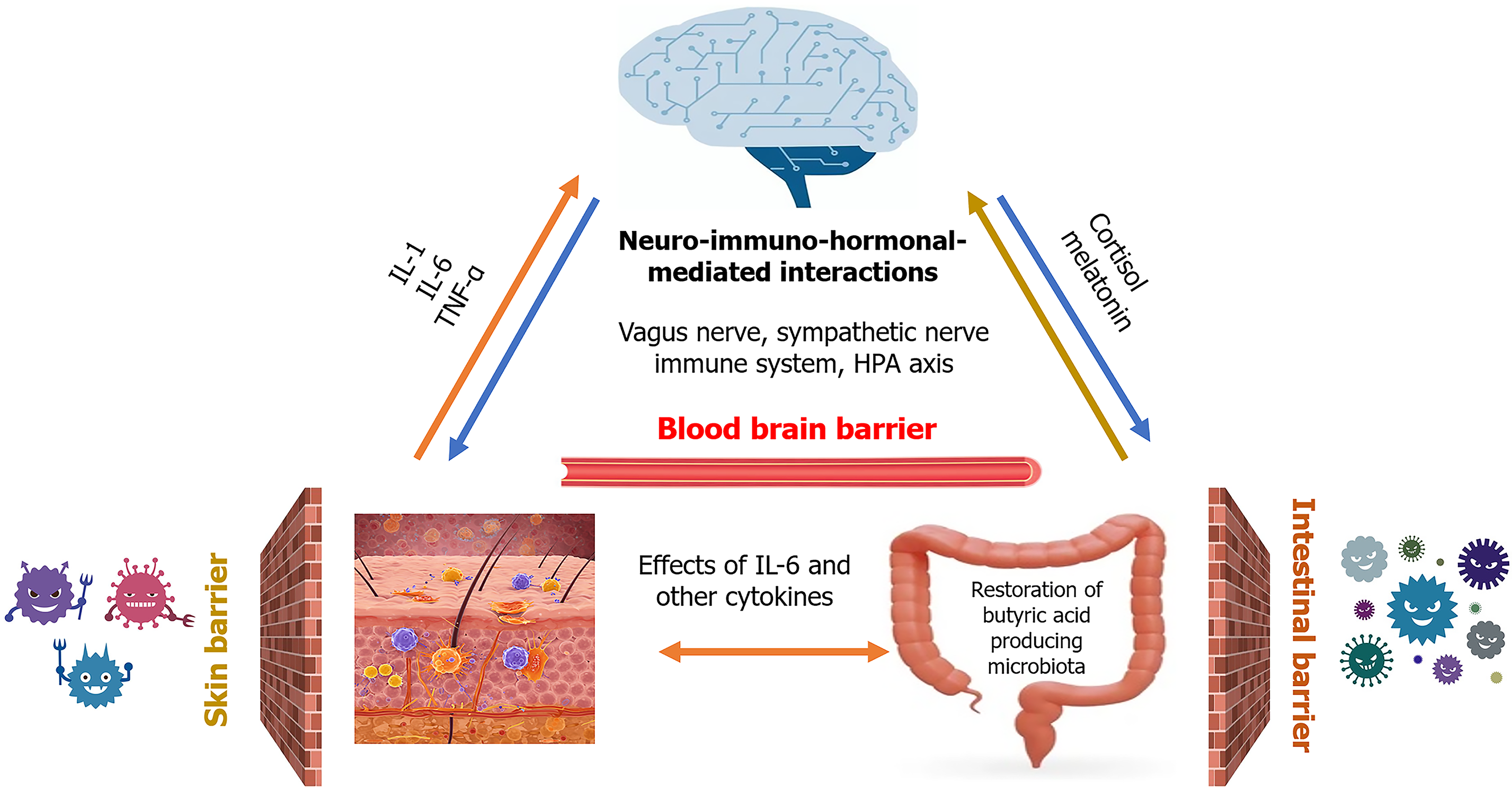
Acute skin failure is related to mental function because the skin communicates with the central nervous system (CNS). Whether or not acute skin failure occurs in sepsis is related to the communication between the CNS and the skin. The skin and the CNS are in constant communication and influence each other’s functions in various ways[6]. This intricate interaction plays a critical role in maintaining overall health and well-being and is referred to as the skin-brain axis. The skin-brain axis is a complex, bidirectional communication system that plays a vital role in health and disease. It involves intricate interactions between the skin, the nervous system, and the immune system. The skin is rich in immune cells that can release cytokines, signaling molecules that influence both local and systemic responses. Stress, inflammation, or injury in the skin can trigger the release of pro-inflammatory cytokines like interleukin 1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α). These cytokines can activate sensory nerve endings in the skin, transmitting signals to the CNS. Additionally, cytokines can enter the bloodstream and cross the blood-brain barrier to directly affect brain function, the hypothalamic-pituitary-adrenal axis, leading to the release of stress hormones such as cortisol.
The skin is equipped with a vast network of sensory receptors that detect various stimuli such as touch, temperature, pain, and pressure. These receptors transmit signals to the CNS via sensory nerves. Sensory nerve fibers carry information from the skin to the spinal cord, which then transmits it to the brain. The brain interprets these signals and initiates appropriate responses.
The autonomic nervous system, part of the CNS, controls involuntary functions such as heart rate, breathing, and digestion. It also innervates the skin, regulating blood flow, sweating, and hair follicle activity. The CNS, particularly the hypothalamus and pituitary gland, release hormones that can affect skin function. For example, stress hormones can increase sebum production and contribute to acne. The CNS interacts with the immune system, which plays a role in skin health. Stress can suppress the immune system, making the skin more susceptible to infection.
One of the mechanisms by which the skin-brain axis causes mental illness, including anxiety and depression, is T cell-mediated immunity. T cells and related cytokines play a key role in the pathogenesis of psychiatric disorders and are critical components of the skin-brain axis[7]. In addition to directly damaging the blood-brain barrier, T cells and secreted cytokines may interact with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system to exacerbate skin diseases or mental disorders. For example, clozapine, a drug used to treat treatment-resistant schizophrenia, is also known to affect the immune system. Excessive salience is thought to be one of the pathological conditions of schizophrenia, and animal models have shown that clozapine reduces this salience. Clozapine acts on the skin-brain axis to dose-dependently reduce emotional hyperthermia activated by excessive salience[8]. Clozapine’s effects on adrenergic and cholinergic receptors can influence the autonomic nervous system, which plays a crucial role in the skin-brain axis. By modulating autonomic activity, clozapine may indirectly affect skin function and its communication with the CNS. Given the role of inflammation in the skin-brain axis, clozapine’s anti-inflammatory properties could potentially influence this communication pathway. Clozapine’s complex pharmacological actions likely involve multiple pathways, including those related to the skin-brain axis. While further research is needed to fully elucidate these interactions, clozapine’s ability to modulate neurotransmitter activity, inflammation, and stress responses provides a plausible explanation for its effectiveness in reducing emotional hyperactivity. Understanding the interaction between the skin and the CNS is critical to the diagnosis and treatment of various skin and neurological disorders.
The skin is a barrier organ, but there is another barrier organ in the human body: The digestive tract. Bacteria that reside in both the skin and the digestive tract express genes at many times the rate of human cells and maintain barrier function. The skin-brain axis is also related to the gut microbiota. People with sepsis also have reduced blood flow to the intestine, making them more susceptible to intestinal dysbiosis[9]. In addition, the antibiotics used in treatment affect the gut microbiota. Patients with sepsis may also have reduced barrier function in the gut and skin. In essence, antibiotic treatment in sepsis, while necessary to combat infection, can have unintended consequences on the gut-skin-brain axis. Understanding these mechanisms is crucial for developing strategies to mitigate these effects and improve patient outcomes.
The gut has its own nervous system (the enteric nervous system) that communicates with the brain through the vagus nerve[10]. This allows signals from the gut to influence brain activity and vice versa. The skin also has an extensive network of nerves that connect it to the brain. The gut microbiota can influence the production of hormones that affect both skin and brain function. For example, it plays a role in the production of serotonin, a neurotransmitter critical for mood regulation. The gut microbiome influences the immune system, which in turn can affect skin health and brain function. An imbalance in the gut microbiota, or dysbiosis, can lead to inflammation that manifests in both the skin (e.g., eczema, psoriasis) and the brain (e.g., anxiety, depression). The trillions of microorganisms (bacteria, fungi, viruses) that live in the gut. They play a vital role in digestion, immunity, and even produce neurochemicals that can affect the brain.
The connection between the gut, skin, and brain has recently received attention as the gut-skin-brain axis (Figure 1). The brain receives signals from both the gut and the skin and can affect their function through neural, hormonal, and immune pathways. Autonomic nervous system, comprising the sympathetic and parasympathetic nervous systems, allows the brain to control gut motility, secretion, and blood flow. It also influences skin blood flow, sweating, and piloerection. These neurons control muscle contractions, which can affect gut motility and skin reflexes. The brain can then alter the gut biome, the gut lining, and skin function through these efferent signals. The vagus nerve is a major bidirectional communication highway. From the gut, it transmits information about gut microbiota composition, nutrient levels, and inflammatory signals to the brainstem. From the skin, sensory nerves transmit signals about temperature, touch, pain, and itch. These nerves carry sensory information from the skin and gut to the spinal cord, which then relays signals to the brain.
The gut-skin-brain axis is an active area of research, with scientists exploring the specific mechanisms by which these systems interact and how to target them for therapeutic purposes[11]. This could lead to new treatments for a variety of skin, mental health and neurological conditions. Recent reports have shown that administration of probiotics in psoriasis can be effective in improving skin and mental health symptoms, suggesting that there is inter-organ cooperation via the gut-skin-brain axis and that the gut microbiota is one of the therapeutic targets[12]. Gut microbiota, particularly fiber-fermenting bacteria, produce short chain fatty acids (SCFAs) such as acetate, propionate, butyrate as a byproduct of carbohydrate fermentation. SCFAs, particularly butyrate, strengthen the gut barrier by enhancing tight junction protein expression. A healthy gut barrier prevents the leakage of bacterial components (e.g., lipopolysaccharide) into the bloodstream, which triggers systemic inflammation. SCFAs bind to G protein-coupled receptors on immune cells, such as T cells and macrophages. This binding can inhibit pro-inflammatory cytokine production (e.g., TNF-α, IL-6), and promote the development of regulatory T cells, which suppress inflammation. Moreover, butyrate, in particular, has been shown to inhibit histone deacetylases, leading to increased expression of anti-inflammatory genes. By understanding the intricate connections within the gut-skin-brain axis, we can appreciate the importance of a holistic approach to health that considers the interplay between these three vital systems.
In summary, sepsis is a phenomenon in which bacteria breach the barrier function and enter the body. The two main barriers the body has against foreign invaders are the skin and the gut. Sepsis with acute skin failure is a pathology in which the barrier function is weakened. Changes in microbiota composition, metabolite profiles, and gene expression can provide objective measures of treatment response. Identifying specific microbial metabolites or signaling pathways involved in the gut-skin-brain axis could lead to the development of novel therapeutic targets. For example, drugs that modulate SCFA production or signaling could be used to treat mental health disorders. By identifying individual variations in gut microbiota composition and metabolite profiles, clinicians can tailor interventions to address specific needs. This could involve personalized probiotic or prebiotic therapies, dietary modifications, or fecal microbiota transplantation. Technological advancements may enable early detection of gut dysbiosis and other biomarkers associated with increased risk of mental illness in sepsis. These technologies can be used to monitor the effectiveness of interventions targeting the gut-skin-brain axis. This could facilitate preventive interventions to mitigate the development of psychiatric symptoms. By applying these technologies to elucidate the pathology of the gut-skin-brain axis, various factors that affect mental function can be detected, which is likely to be useful in the treatment of mental illness.
| 1. | Liu YF, Cong W, Zhou CM, Yu Y, Zhang XJ. Relationship between inflammatory factors, lactic acid levels, acute skin failure, bad mood, and sleep quality. World J Psychiatry. 2025;15: 102763. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Pulido-Pérez A, Bergón-Sendín M, Suárez-Fernández R, Muñoz-Martín P, Bouza E. Skin and sepsis: contribution of dermatology to a rapid diagnosis. Infection. 2021;49:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 3. | Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:e157-e166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Dalgleish L, Campbell J, Finlayson K, Barakat-Johnson M, Beath A, Ingleman J, Parker C, Coyer F. Understanding Skin Failure: A Scoping Review. Adv Skin Wound Care. 2021;34:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Pan S, Lv Z, Wang R, Shu H, Yuan S, Yu Y, Shang Y. Sepsis-Induced Brain Dysfunction: Pathogenesis, Diagnosis, and Treatment. Oxid Med Cell Longev. 2022;2022:1328729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 90] [Reference Citation Analysis (0)] |
| 6. | Fregoso DR, Hadian Y, Gallegos AC, Degovics D, Maaga J, Keogh CE, Kletenik I, Gareau MG, Isseroff RR. Skin-brain axis signaling mediates behavioral changes after skin wounding. Brain Behav Immun Health. 2021;15:100279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Yang J, Zhang S, Wu Q, Chen P, Dai Y, Long J, Wu Y, Lin Y. T cell-mediated skin-brain axis: Bridging the gap between psoriasis and psychiatric comorbidities. J Autoimmun. 2024;144:103176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 8. | Blessing WW, Blessing EM, Mohammed M, Ootsuka Y. Clozapine, chlorpromazine and risperidone dose-dependently reduce emotional hyperthermia, a biological marker of salience. Psychopharmacology (Berl). 2017;234:3259-3269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Blessing W, McAllen R, McKinley M. Control of the Cutaneous Circulation by the Central Nervous System. Compr Physiol. 2016;6:1161-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Kandpal M, Indari O, Baral B, Jakhmola S, Tiwari D, Bhandari V, Pandey RK, Bala K, Sonawane A, Jha HC. Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | Ferraretto A, Donetti E, García-Mena J, Pacheco-López G. Editorial: The gut-skin-brain axis in human health and disease. Front Nutr. 2023;10:1155614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Abdi A, Oroojzadeh P, Valivand N, Sambrani R, Lotfi H. Immunological aspects of probiotics for improving skin diseases: Influence on the Gut-Brain-Skin Axis. Biochem Biophys Res Commun. 2024;702:149632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/