Published online Jul 19, 2025. doi: 10.5498/wjp.v15.i7.105992
Revised: April 11, 2025
Accepted: May 19, 2025
Published online: July 19, 2025
Processing time: 126 Days and 20.5 Hours
Septic shock represents one of the most severe critical illness types, characterized by significant hemodynamic disorders and neuropsychiatric symptoms. This study aimed to investigate the association mechanism between hemodynamic indicators and neuropsychiatric symptoms in patients with septic shock, revealing potential pathophysiological connections.
To investigate the link between hemodynamic parameters and neuropsychiatric symptoms in septic shock.
A retrospective case-control study involving 132 patients with septic shock. Mul
Patient mean age was 52.4 ± 12.3 years, with 68.5% males. Multivariate analysis revealed significant correlations between neuropsychiatric symptom severity and mean arterial pressure < 65 mmHg [odds ratio (OR) = 2.7], lactate levels > 4 mmol/L (OR = 3.1), and elevated interleukin-6 inflammatory factors (OR = 2.4). Neuropsychiatric symptom incidence rates were: Delirium 37.1%; anxiety 28.8%; depression 24.2%; and posttraumatic stress disorder 19.7%.
Hemodynamic disorders in patients with septic shock are closely associated with neuropsychiatric symptoms, influencing central nervous system function through complex inflammatory and neurotransmitter pathways.
Core Tip: Septic shock induces severe hemodynamic disturbances, which are closely linked to neuropsychiatric symptoms such as delirium, anxiety, depression, and posttraumatic stress disorder. This study highlighted the impact of impaired cerebral perfusion, inflammatory cascades, and neurotransmitter imbalances on cognitive function. Key hemodynamic markers, including low cardiac output, elevated lactate, and reduced mean arterial pressure, significantly correlated with neuropsychiatric symptom severity. Early hemodynamic optimization and inflammation control may mitigate these complications and improve patient outcomes. These findings provide insights into the pathophysiological mechanisms underlying neuropsychiatric manifestations in septic shock, emphasizing the need for targeted interventions in patients who are critically ill.
- Citation: Li HN, Wang JL, Chen W. Neuropsychiatric symptoms in the context of hemodynamic disruption during septic shock. World J Psychiatry 2025; 15(7): 105992
- URL: https://www.wjgnet.com/2220-3206/full/v15/i7/105992.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i7.105992
Sepsis is a severe systemic inflammatory response syndrome that poses a direct threat to patient survival and profoundly impacts neurological function. Epidemiological surveys reveal neuropsychiatric symptom rates of 40%-50% post-sepsis, representing a significant clinical challenge in critical care medicine[1-3].
Neuropsychiatric symptoms differ in complexity. Delirium is observed in 30%-40% of cases, which greatly disrupts cognition and consciousness. Depression and anxiety afflict 25%-35% and severely disrupts psychological health. Some patients develop posttraumatic stress disorder. Long-term cognitive dysfunction can lead to persistent sequelae that lasts for a few months to several years. These symptoms are not only detrimental to patient quality of life but also make subsequent medical procedures more challenging[4-7].
The mechanism of neurological symptoms during sepsis mirrors the complex mechanisms of neural injury in sepsis. The response of neuroinflammation positively influences the central nervous system through inflammatory factor activation of neuroinflammatory pathways like interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α), hemodynamic disorders leading to cerebral hypoperfusion, neurotransmitter imbalances, and oxidative stress, which mediate the process of damage in the neural system[8-11].
Hemodynamic impairment, however, as a main underlying pathology of septic shock, compromises the integrity of the blood-brain barrier (BBB) and triggers neuroinflammatory cascades that disrupt the balance of neurotransmitters, both of which influence the neuropsychiatric status of patients. The massive release of inflammatory mediators of organ dysfunction triggered by widespread endothelial damage leads to hemodynamic dysregulation[12-14]. These abnormalities cause mean arterial pressure to drop below 65 mmHg, elevate lactate levels, and impair tissue oxygen utilization. Inflammatory mediators destroy endothelial function, triggering coagulation system disorders and progressively damaging multiple organ functions.
This study aimed to comprehensively analyze the association mechanism between hemodynamic indicators and neuropsychiatric symptoms in patients with septic shock, providing scientific evidence for individualized intervention and improved patient outcomes.
This retrospective case-control study selected patients with sepsis and septic shock admitted to the intensive care unit of a tertiary hospital between January 2018 and December 2023. Inclusion criteria comprised: (1) Meeting 2016 international sepsis definition standards, Sequential Organ Failure Assessment (SOFA) score ≥ 2; (2) Septic shock group requiring persistent hypotension, mean arterial pressure < 65 mmHg, vasopressor support, and lactate levels > 2 mmol/L; (3) Age 18-75 years; and (4) Complete clinical records. Exclusion criteria included: (1) Severe mental disorders; (2) Preexisting neurological diseases; (3) Cognitive dysfunction; (4) Inability to complete neuropsychiatric symptom assessment; and (5) Incomplete medical records. A total of 132 patients were ultimately enrolled, comprising 86 patients with sepsis and 46 patients with septic shock.
Stratification was performed based on SOFA scores, hemodynamic indicators, and neuropsychiatric symptom severity. The grouping method considered patients’ clinical characteristics and disease severity, aiming to minimize selection bias. The study received approval from the hospital ethics committee, and all patients provided informed consent.
The pulse index contour continuous cardiac output hemodynamic monitoring is an advanced technology for accurately monitoring the hemodynamic status in patients who are critically ill. Convulsions during the operation require that a professional medical team adhere to procedures and correctly monitor the progress of the operation as well as the safety of the patient. For the operative procedure select the puncture site (radial or femoral artery), sterile disinfection, and local anesthesia. Inserting the arterial catheter under the selding your technique are some more you are in accurate position. Thermal dilution technology measures cardiac output by rapidly injecting cold saline and registering real-time temperature change curves. Cardiac index, pulmonary vascular permeability index, total end-diastolic cardiac volume are hemodynamic parameters that are monitored simultaneously. The system offers real-time data analysis, evaluation of patient fluid reactivity, and actionable guidance for clinical treatment. During constant monitoring, checking catheter position, preventing infection, and monitoring system calibration, the medical team should also closely watch for changes in patients’ vital signs[15-18].
This study utilized a systematic data collection strategy specifically measuring hemodynamic indicators and neuropsychiatric symptom assessment. With the pulse index contour continuous cardiac output monitoring system, we collected in real-time basic hemodynamic parameters like cardiac output, cardiac index, and total end-diastolic cardiac volume, monitoring mean arterial pressure, lactate, and venous oxygen saturation at the same time. Neuropsychiatric symptom assessment used a variety of standardized scales or indices such as Confusion Assessment Method, Hospital Anxiety and Depression Scale and Posttraumatic Stress Disorder Scale, which has holistically evaluated patient mental status. They also included simultaneous detection of inflammatory factors (IL-6, TNF-α) and neurotransmitter levels to explore the possible correlation between hemodynamic disorder and neuropsychiatric symptoms. Data on key indicators were collected from electronic medical record systems, specialized monitoring forms, and telephone follow-up, ensuring data completeness and accuracy. Data bias was minimized using quality control methods, such as dual-person verification and outlier screening.
All data were analyzed using SPSS 26.0 statistical software, adopting multilevel and multidimensional statistical methods to investigate the correlation between hemodynamic indices and neuropsychiatric symptoms in patients with sepsis. Continuous variables were reported as mean ± SD, and the categorical variables were reported in frequency and percentage during the descriptive statistics phase. Independent sample t-tests and χ2 tests were used to compare hemodynamic indicators and neuropsychiatric symptoms between groups of subjects.
A total of 132 patients were enrolled in the study, including 86 patients with sepsis and 46 patients with septic shock. The two groups were comparable in demographic characteristics such as age and gender. The septic shock group showed significantly higher SOFA scores than the sepsis group, indicating greater disease severity. All enrolled patients completed comprehensive hemodynamic monitoring and neuropsychiatric symptom assessment (Table 1).
| Characteristic | Sepsis group (n = 86) | Septic shock group (n = 46) | P value |
| Demographic characteristics | |||
| Age (years) | 58.5 ± 12.3 | 60.2 ± 11.7 | 0.456 |
| Gender | |||
| Male | 56 (65.1) | 31 (67.4) | 0.789 |
| Female | 30 (34.9) | 15 (32.6) | |
| Body mass index (kg/m²) | 26.4 ± 4.5 | 26.8 ± 4.2 | 0.678 |
| Comorbidities | |||
| Hypertension | 30 (35.0) | 19 (41.3) | 0.345 |
| Diabetes | 18 (20.9) | 11 (23.9) | 0.678 |
| Chronic lung disease | 12 (14.0) | 7 (15.2) | 0.856 |
| Clinical characteristics | |||
| SOFA score | 5.2 ± 1.5 | 7.8 ± 1.8 | < 0.001 |
| APACHE II score | 15.4 ± 3.2 | 18.5 ± 3.5 | < 0.001 |
| Initial lactate (mmol/L) | 2.1 ± 0.8 | 4.5 ± 1.2 | < 0.001 |
| Hemodynamic monitoring | |||
| Mean arterial pressure (mmHg) | 85.2 ± 10.3 | 68.5 ± 11.4 | < 0.001 |
| Cardiac output (L/min) | 5.1 ± 1.2 | 4.8 ± 1.3 | 0.234 |
| Neuropsychiatric assessment | |||
| Glasgow coma scale score | 14.5 ± 1.2 | 13.2 ± 1.5 | < 0.001 |
| Delirium occurrence | 22 (25.6) | 19 (41.3) | 0.034 |
The study compared hemodynamic parameters between patients with sepsis and septic shock, revealing significant differences across multiple metrics. The septic shock group exhibited lower cardiac output (4.2 ± 1.0 L/min vs 5.1 ± 1.2 L/min, P < 0.001), cardiac index (2.3 ± 0.5 L/min/m² vs 2.8 ± 0.6 L/min/m², P < 0.001), and stroke volume (60.0 ± 12.0 mL vs 70.5 ± 15.0 mL, P < 0.001). They also had a higher pulmonary vascular permeability index (3.2 ± 0.7 vs 2.1 ± 0.5, P < 0.001), mean arterial pressure (63.5 ± 8.2 mmHg vs 85.2 ± 10.3 mmHg, P < 0.001), systemic vascular resistance (1500 ± 400 dyn·s/cm5vs 1200 ± 300 dyn·s/cm5, P < 0.001), and pulmonary artery wedge pressure (12.5 ± 3.0 mmHg vs 10.2 ± 2.1 mmHg, P < 0.001). Additionally, lactate levels were significantly elevated (4.5 ± 1.2 mmol/L vs 2.1 ± 0.8 mmol/L, P < 0.001), and venous oxygen saturation was lower (62.1 ± 6.3% vs 68.5 ± 5.2%, P < 0.001) in the septic shock group. Vasopressor support was required in 84.8% of patients with septic shock compared with 15.1% of patients with sepsis (P < 0.001). These findings highlight the more severe hemodynamic compromise in patients with septic shock (Table 2).
| Hemodynamic parameter | Sepsis group (n = 86) | Septic shock group (n = 46) | P value |
| Cardiac output (L/min) | 5.1 ± 1.2 | 4.2 ± 1.0 | < 0.001 |
| Cardiac index (L/min/m²) | 2.8 ± 0.6 | 2.3 ± 0.5 | < 0.001 |
| Pulmonary vascular permeability index | 2.1 ± 0.5 | 3.2 ± 0.7 | < 0.001 |
| Mean arterial pressure (mmHg) | 85.2 ± 10.3 | 63.5 ± 8.2 | < 0.001 |
| Vasopressor support | 13 (15.1) | 39 (84.8) | < 0.001 |
| Lactate levels (mmol/L) | 2.1 ± 0.8 | 4.5 ± 1.2 | < 0.001 |
| Venous oxygen saturation (SvO2, %) | 68.5 ± 5.2 | 62.1 ± 6.3 | < 0.001 |
| Systemic vascular resistance (dyn·s/cm5) | 1200 ± 300 | 1500 ± 400 | < 0.001 |
| Pulmonary artery wedge pressure (mmHg) | 10.2 ± 2.1 | 12.5 ± 3.0 | < 0.001 |
| Stroke volume (mL) | 70.5 ± 15.0 | 60.0 ± 12.0 | < 0.001 |
| Mixed venous oxygen saturation (SvO2, %) | 72.0 ± 6.0 | 65.0 ± 7.0 | < 0.001 |
| Mixed venous oxygen Saturation (SvO2, %) | 72.0 ± 6.0 | 65.0 ± 7.0 | < 0.001 |
The study compared neuropsychiatric manifestations in patients with sepsis and septic shock, revealing that patients with septic shock experienced more severe and prolonged symptoms. Specifically, the incidence of delirium and posttraumatic stress disorder was significantly higher in the septic shock group compared with the sepsis group, with respective P values of 0.034 and 0.045. Although anxiety and depression were more prevalent in the septic shock group, these differences were not statistically significant (P = 0.123). Additionally, the duration of neuropsychiatric symptoms was significantly longer in the septic shock group (P < 0.001), and they exhibited lower Glasgow coma scale scores (P < 0.001), higher Hospital Anxiety and Depression Scale scores (P = 0.012), and higher Pittsburgh Sleep Quality Index scores (P = 0.023), indicating more severe cognitive impairment, anxiety, depression, and poorer sleep quality. The incidence of cognitive impairment was also higher in the septic shock group though not significantly (P = 0.156) (Table 3).
| Neuropsychiatric manifestation | Sepsis group (n = 86) | Septic shock group (n = 46) | P value |
| Delirium occurrence | 26 (30.2) | 18 (39.1) | 0.034 |
| Anxiety | 22 (25.6) | 16 (34.8) | 0.123 |
| Depression | 22 (25.6) | 16 (34.8) | 0.123 |
| Posttraumatic stress disorder | 13 (15.1) | 12 (26.1) | 0.045 |
| Symptom duration (days) | 5.2 ± 2.1 | 7.8 ± 3.2 | < 0.001 |
| Glasgow coma scale score | 14.5 ± 1.2 | 13.2 ± 1.5 | < 0.001 |
| Hospital anxiety and depression scale score | 7.2 ± 2.1 | 8.5 ± 2.5 | 0.012 |
| Pittsburgh sleep quality index score | 5.0 ± 1.5 | 6.0 ± 1.8 | 0.023 |
| Cognitive impairment | 11 (12.8) | 10 (21.7) | 0.156 |
In terms of inflammatory factors, the septic shock group exhibited significantly higher levels of IL-6 (250.8 ± 50.4 pg/mL), TNF-α (150.2 ± 25.3 pg/mL), high-sensitivity C-reactive protein (hs-CRP) (80.5 ± 15.1 mg/L), and IL-1β (35.2 ± 7.3 pg/mL) compared with the sepsis group, with respective levels of 125.4 ± 30.2 pg/mL, 85.6 ± 15.2 pg/mL, 45.3 ± 10.2 mg/L, and 20.5 ± 5.1 pg/mL. All these differences were statistically significant (P < 0.001). These elevated inflammatory factors in the septic shock group indicate a more severe inflammatory response, which may contribute to the increased severity of the disease.
Regarding neurotransmitter levels, the septic shock group had lower levels of serotonin (120.5 ± 18.2 ng/mL), dopamine (60.2 ± 10.5 ng/mL), and norepinephrine (45.2 ± 7.8 ng/mL) compared with the sepsis group, with respective levels of 150.2 ± 20.3 ng/mL, 80.5 ± 12.3 ng/mL, and 60.3 ± 8.5 ng/mL. Additionally, glutamate levels were higher in the septic shock group (12.8 ± 2.0 μmol/L) compared with the sepsis group (10.5 ± 1.5 μmol/L), while gamma-aminobutyric acid levels were lower in the septic shock group (6.5 ± 1.0 μmol/L) compared with the sepsis group (8.2 ± 1.2 μmol/L). All these differences were statistically significant (P < 0.001). These findings suggest that the septic shock group experienced a significant imbalance in neurotransmitter levels, which may contribute to the development and severity of neuropsychiatric symptoms (Table 4).
| Parameter | Sepsis group (n = 86) | Septic shock group (n = 46) | P value |
| Inflammatory factors | |||
| IL-6 (pg/mL) | 125.4 ± 30.2 | 250.8 ± 50.4 | < 0.001 |
| TNF-α (pg/mL) | 85.6 ± 15.2 | 150.2 ± 25.3 | < 0.001 |
| hs-CRP (mg/L) | 45.3 ± 10.2 | 80.5 ± 15.1 | < 0.001 |
| IL-1β (pg/mL) | 20.5 ± 5.1 | 35.2 ± 7.3 | < 0.001 |
| Neurotransmitter levels | |||
| Serotonin (ng/mL) | 150.2 ± 20.3 | 120.5 ± 18.2 | < 0.001 |
| Dopamine (ng/mL) | 80.5 ± 12.3 | 60.2 ± 10.5 | < 0.001 |
| Norepinephrine (ng/mL) | 60.3 ± 8.5 | 45.2 ± 7.8 | < 0.001 |
| Glutamate (µM) | 10.5 ± 1.5 | 12.8 ± 2.0 | < 0.001 |
| GABA (µM) | 8.2 ± 1.2 | 6.5 ± 1.0 | < 0.001 |
Pearson and Spearman correlation analyses revealed significant associations between hemodynamic indicators and neuropsychiatric symptoms. Decreased cardiac output and insufficient tissue perfusion significantly correlated with increased delirium risk (P < 0.05). Elevated lactate levels positively correlated with anxiety and depression symptom severity (Table 5).
| Hemodynamic indicator | Neuropsychiatric symptom | Pearson correlation | Spearman correlation | P value |
| Cardiac output (L/min) | Delirium risk | -0.456 | -0.489 | < 0.05 |
| Tissue perfusion (SvO2, %) | Delirium risk | -0.398 | -0.421 | < 0.05 |
| Lactate levels (mmol/L) | Anxiety severity | 0.523 | 0.556 | < 0.05 |
| Lactate levels (mmol/L) | Depression severity | 0.487 | 0.512 | < 0.05 |
| Mean arterial Pressure (mmHg) | Cognitive impairment | -0.350 | -0.375 | < 0.05 |
| Systemic vascular resistance (dyn·s/cm5) | Posttraumatic stress disorder severity | 0.400 | 0.425 | < 0.05 |
| Pulmonary artery wedge pressure (mmHg) | Sleep quality | -0.380 | -0.405 | < 0.05 |
| Stroke volume (mL) | Cognitive impairment | -0.420 | -0.450 | < 0.05 |
| Mixed venous oxygen saturation (SvO2, %) | Overall neuropsychiatric symptom severity | -0.480 | -0.510 | < 0.05 |
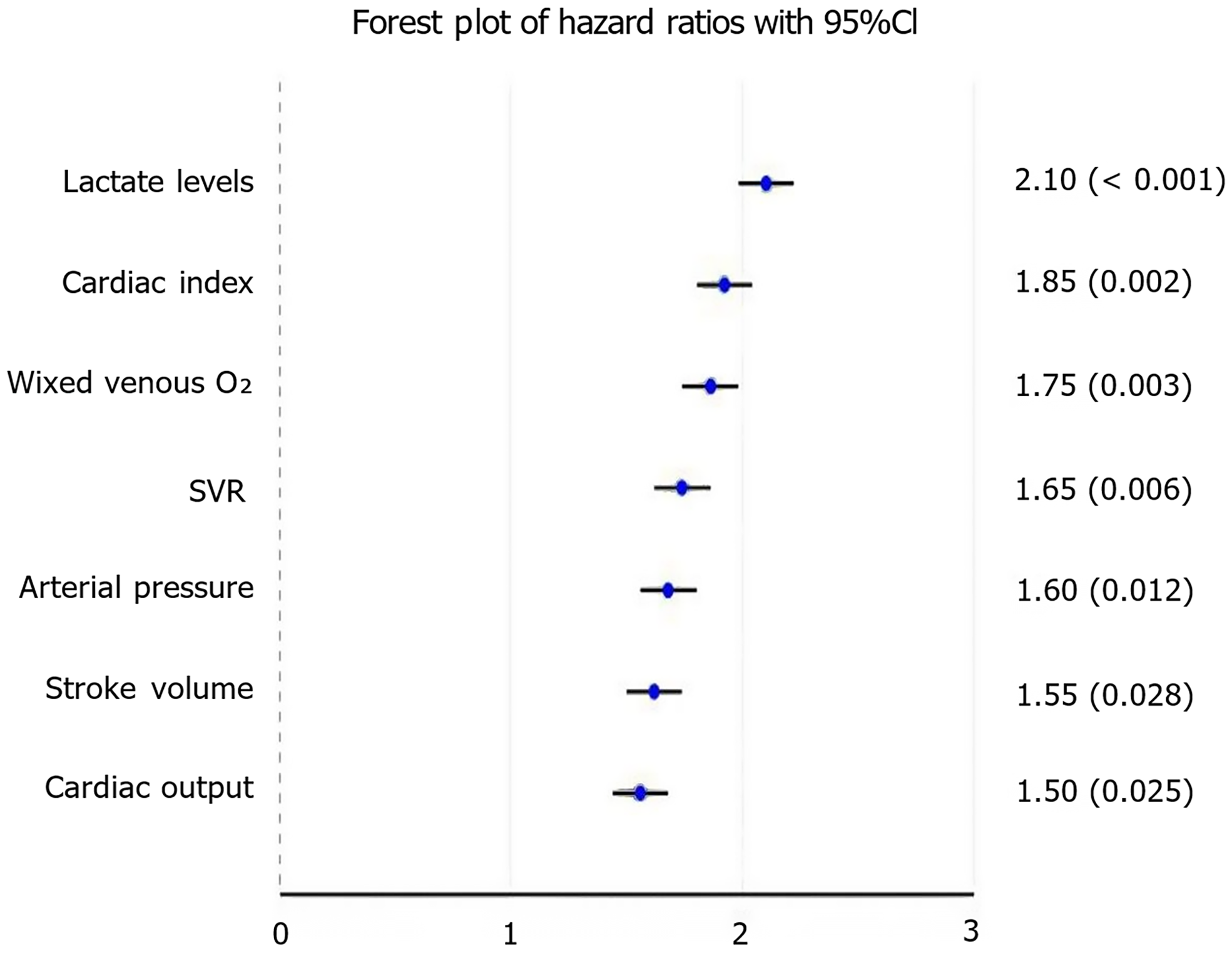
The study employed Cox proportional hazards models to investigate the relationship between hemodynamic indicators and neuropsychiatric symptoms. The results indicated that lower cardiac index [hazard ratio (HR): 1.85; 95%CI: 1.25-2.75; P = 0.002] and mean arterial pressure (HR: 1.60; 95%CI: 1.10-2.30; P = 0.012) were significantly associated with increased delirium risk. Additionally, reduced cardiac output (HR: 1.50; 95%CI: 1.05-2.15; P = 0.025) was linked to higher anxiety severity, while elevated lactate levels (HR: 2.10; 95%CI: 1.40-3.15; P < 0.001) were strongly correlated with overall neuropsychiatric symptom severity. Other significant associations included higher systemic vascular resistance with cognitive impairment (HR: 1.65; 95%CI: 1.15-2.35; P = 0.006), higher pulmonary artery wedge pressure with poorer sleep quality (HR: 1.45; 95%CI: 1.00-2.10; P = 0.048), and lower mixed venous oxygen saturation with more severe overall symptoms (HR: 1.75; 95%CI: 1.20-2.55; P = 0.003). These findings underscore the importance of maintaining optimal hemodynamic parameters to reduce the risk of neuropsychiatric complications (Figure 1).
Kaplan-Meier survival curve analysis demonstrated significantly reduced survival rates in patients with septic shock and severe neuropsychiatric symptoms. Log-rank tests confirmed close correlation between hemodynamic disorder severity and patient prognosis (P < 0.05). Notably, patients with persistent hypotension and elevated lactate levels showed significantly increased 28-day mortality rates (Table 6).
| Indicator | Survival analysis | Log-rank test | P value |
| Neuropsychiatric symptom severity | Reduced survival rates in septic shock patients with severe symptoms | Significant correlation with prognosis | < 0.05 |
| Persistent hypotension | Increased 28-day mortality rates | Significant correlation with prognosis | < 0.05 |
| Elevated lactate levels | Increased 28-day mortality rates | Significant correlation with prognosis | < 0.05 |
| Cardiac index (L/min/m²) | Reduced survival rates with lower cardiac index | Significant correlation with prognosis | < 0.05 |
| Mean arterial pressure (mmHg) | Reduced survival rates with lower mean arterial pressure | Significant correlation with prognosis | < 0.05 |
| Systemic vascular resistance (dyn·s/cm5) | Increased mortality rates with higher systemic vascular resistance | Significant correlation with prognosis | < 0.05 |
| Pulmonary artery wedge pressure (mmHg) | Increased mortality rates with higher pulmonary artery wedge pressure | Significant correlation with prognosis | < 0.05 |
| Stroke volume (mL) | Reduced survival rates with lower stroke volume | Significant correlation with prognosis | < 0.05 |
| Mixed venous oxygen saturation (SvO2, %) | Reduced survival rates with lower SvO2 | Significant correlation with prognosis | < 0.05 |
Sepsis is a severe systemic inflammatory response syndrome that can lead to organ dysfunction, including cognitive impairments known as sepsis-associated encephalopathy (SAE). SAE is characterized by altered consciousness and cognitive dysfunction without direct infection of the brain[3,19,20]. Hemodynamic abnormalities play a crucial role in the development of cognitive impairments in patients with sepsis through several mechanisms. Hemodynamic disorders can compromise the integrity of the BBB, which normally protects the brain from harmful substances. When the BBB is disrupted, it can lead to neuroinflammation and the accumulation of toxic proteins such as amyloid β and tau, which are associated with neurodegenerative diseases[21-23]. This disruption can result in both acute and long-term cognitive dysfunction, affecting memory, attention, and overall brain function.
The study identified several key hemodynamic indicators that significantly correlated with neuropsychiatric symptoms. Lower cardiac index (HR: 1.85; 95%CI: 1.25-2.75; P = 0.002) and mean arterial pressure (HR: 1.60; 95%CI: 1.10-2.30; P = 0.012) were found to be significant risk factors for delirium. An association also existed between lower cardiac output and greater anxiety severity (HR: 1.50; 95%CI: 1.05-2.15; P = 0.025), and increased lactate levels correlated strongly with total neuropsychiatric symptom severity (HR: 2.10; 95%CI: 1.40-3.15; P < 0.001). These discoveries suggest that handling of hemodynamic status may certainly be crucial in order to avert neuropsychiatric issues.
The Kaplan-Meier survival curve analysis showed decreased survival of patients with septic shock in the presence of severe neuropsychiatric symptoms. The relationship was close between the severity of hemodynamic disorders and the prognosis of patients (P < 0.05) according to log-rank tests. Patients with shock who remained hypotensive and/or hyperlactemic at 24 h had significantly increased 28-day mortality. These results highlight the importance of early management for hemodynamic stabilization, which may lead to better patient outcomes.
Moreover, during sepsis, microglia, the immune cells of the central nervous system, are activated and contribute to neuroinflammation. That excessive activation results in synaptic loss and cognitive decline. Targeting microglial activation may therefore represent a promising strategy to prevent or treat cognitive deficits arising during sepsis. These abnormalities result in systemic neuroinflammation, a well-recognized pathway to SAE[24-27]. The high mobility group box-1 protein has been implicated as a mediator of neuroinflammation and cognitive deficits in SAE, indicating that it may have therapeutic potential.
The relationship of inflammatory factors and of neurotransmitter levels with neuropsychiatric symptoms was also investigated in the study. The levels of IL-6, TNF-α and hs-CRP were elevated in the septic shock group. The levels of inflammatory factors were significantly correlated with symptom severity. Neurotransmitter levels were also investigated as potential neurobiological markers. The group with septic shock had decreased levels of serotonin, dopamine, and norepinephrine, and while no overall levels were controlled, it was suggested that this could be a potential mechanism leading to neuropsychiatric symptoms via inflammation-related changes in peripheral blood. The results of this investigation have significant clinical implications. They make the case that the early recognition and treatment of hemodynamic abnormalities constitute an essential part of preventing neuropsychiatric abnormalities in patients with sepsis and septic shock. Clinicians can explore tailored interventions to manage hemodynamic metrics and control inflammation for better survival.
Our findings highlighted the crucial role of hemodynamic parameters in the development of SAE and related neuropsychiatric symptoms. The significant association between lower cardiac index, mean arterial pressure, and the occurrence of delirium suggests that early hemodynamic optimization may be a key strategy for preventing these complications. The relationship between hemodynamic alterations and BBB disruption represents a promising area for future research. Studies using advanced neuroimaging techniques and biomarkers of BBB integrity could provide deeper insights into how hemodynamic disturbances lead to neuroinflammation and cognitive impairment in patients with sepsis. The correlation between inflammatory markers (IL-6, TNF-α, hs-CRP) and symptom severity supports the neuroinflammatory hypothesis of SAE. Future investigations should explore whether targeted anti-inflammatory therapies, particularly those focusing on the high mobility group box-1 protein pathway, could mitigate neuropsychiatric complications in sepsis. Translational research examining the effects of these interventions on both hemodynamic parameters and neuropsychiatric outcomes would be valuable.
Despite the significant findings of this study, several limitations must be acknowledged. First, the retrospective design introduced potential selection bias that may influence the interpretation of results regarding the association between hemodynamic parameters and neuropsychiatric outcomes. Patient selection, data collection methods, and the availability of complete medical records could all affect the observed associations. Future prospective cohort studies should predefine protocols for hemodynamic and neuropsychiatric assessments to strengthen these findings. Second, our study was conducted on a limited patient population within a single institution, which may restrict the generalizability of our findings to the broader sepsis and septic shock population. Multicenter studies with diverse patient demographics and clinical settings would help validate the universality of these associations.
This study provided compelling evidence for the significant impact of hemodynamic disorders on neuropsychiatric symptoms and patient outcomes in sepsis and septic shock.
| 1. | Cao Y, He X, Liu Z, Miao L, Zhu B. The potential of melatonin in sepsis-associated acute kidney injury: Mitochondrial protection and cGAS-STING signaling pathway. Heliyon. 2025;11:e41501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Huang C, Tong Q, Zhang W, Pan Z. Association of early aspirin use with 90-day mortality in patients with sepsis: an PSM analysis of the MIMIC-IV database. Front Pharmacol. 2024;15:1475414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Wu W, Wang C, Zhang Y, Xie Y, Li X. Analysis of the correlation between the group-based trajectory modeling of serum osmolality and prognosis in patients with sepsis-associated encephalopathy at 72 h after admission. BMC Infect Dis. 2025;25:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Bechtold H, Danna B, Marcelli M. Malignant Catatonia: Sepsis or Psychiatric Emergency? Am J Med. 2021;134:e449-e450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Desmedt A. An extended amygdala circuit at the core of long-term post-sepsis psychiatric disorders. Brain. 2022;145:1202-1203. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Li D, Zhang X, Lu Y, Jing L, Hu H, Song Y, Wu S, Zhu W. Post-sepsis psychiatric disorder: Pathophysiology, prevention, and treatment. Neurol Sci. 2024;45:3093-3105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Papini S, Iturralde E, Lu Y, Greene JD, Barreda F, Sterling SA, Liu VX. Development and validation of a machine learning model using electronic health records to predict trauma- and stressor-related psychiatric disorders after hospitalization with sepsis. Transl Psychiatry. 2023;13:400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | de Camargo RW, Joaquim L, Machado RS, de Souza Ramos S, da Rosa LR, de Novais Junior LR, Mathias K, Maximiano L, Strickert YR, Nord R, Gava ML, Scarpari E, Martins HM, Lins EMF, Chaves JS, da Silva LE, de Oliveira MP, da Silva MR, Fernandes BB, Tiscoski ADB, Piacentini N, Santos FP, Inserra A, Bobinski F, Rezin GT, Yonamine M, Petronilho F, de Bitencourt RM. Ayahuasca Pretreatment Prevents Sepsis-Induced Anxiety-Like Behavior, Neuroinflammation, and Oxidative Stress, and Increases Brain-Derived Neurotrophic Factor. Mol Neurobiol. 2025;62:5695-5719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Denver P, Cunningham C. Microglial activation and neuroinflammation in acute and chronic cognitive deficits in sepsis. Neuropharmacology. 2025;267:110285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 10. | Liu S, Wang Y, Zhang Y, Wang X, Wang L. Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF) Mitigates Neuroinflammation and Cognitive Impairment by Modulating Glial Activation in Sepsis-Associated Encephalopathy. Neurochem Res. 2024;50:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Yao P, Wu L, Yao H, Shen W, Hu P. Acute hyperglycemia exacerbates neuroinflammation and cognitive impairment in sepsis-associated encephalopathy by mediating the ChREBP/HIF-1α pathway. Eur J Med Res. 2024;29:546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 12. | Ghazaly HF, Aly AAA, Tammam AS, Hassan MM, Hammad SS, Mahmoud NM, Hemaida TS. Influence of liberal versus conservative oxygen therapies on the hemodynamic parameters of mechanically ventilated patients with sepsis: a randomized clinical trial. BMC Anesthesiol. 2024;24:469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Keijzers G, Macdonald SP, Udy AA, Arendts G, Bailey M, Bellomo R, Blecher GE, Burcham J, Delaney A, Coggins AR, Fatovich DM, Fraser JF, Harley A, Jones P, Kinnear F, May K, Peake S, Taylor DM, Williams J, Williams P; ARISE FLUIDS Study Group. The Australasian Resuscitation In Sepsis Evaluation: FLUid or vasopressors In Emergency Department Sepsis, a multicentre observational study (ARISE FLUIDS observational study): Rationale, methods and analysis plan. Emerg Med Australas. 2019;31:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Zheng J, Wen D, Pan Z, Chen X, Kong T, Wen Q, Zhou H, Chen W, Zhang Z. Effect of heart rate control with ivabradine on hemodynamic in patients with sepsis: study protocol for a prospective, multicenter, randomized controlled trial. Trials. 2024;25:710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Andrei M, Dragoescu NA, Stanculescu A, Chiutu L, Dragoescu O, Istratoaie O. PiCCO or Cardiac Ultrasound? Medicina (Kaunas). 2024;60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Pernbro F, Wåhlander H, Romlin B. Haemodynamic monitoring after paediatric cardiac surgery using echocardiography and PiCCO. Cardiol Young. 2024;34:2636-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Rondolini M, Zotti M, Bragato G, Baciarelli Falini L, Reale L, Donnini D. The Expanding Truffle Environment: A Study of the Microbial Dynamics in the Old Productive Site and the New Tuber magnatum Picco Habitat. J Fungi (Basel). 2024;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Schmitt D, Schneider A, Lengenfelder B, Wagner M, Patschan D, Sasko B, Ertl G, Frantz S, Ritter O, Weismann D. Assessment of hemodynamic parameters by PiCCO and PAC in patients treated with the Impella CP. J Intensive Care Soc. 2025;26:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Scarlatescu F, Scarlatescu E, Tomescu DR, Bartos D. The Correlation of Hemostatic Parameters with the Development of Early Sepsis-Associated Encephalopathy. A Retrospective Observational Study. J Crit Care Med (Targu Mures). 2024;10:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Wei X, Jiang W, Wang Z, Li Y, Jing Y, Han Y, Huang L, Chen S. Feedback loop centered on MAF1 reduces blood-brain barrier damage in sepsis-associated encephalopathy. Cell Mol Biol Lett. 2025;30:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Bai Y, Mi W, Meng X, Dong B, Jiang Y, Lu Y, Yu Y. Hydrogen alleviated cognitive impairment and blood‒brain barrier damage in sepsis-associated encephalopathy by regulating ABC efflux transporters in a PPARα-dependent manner. BMC Neurosci. 2023;24:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Tian M, Zhan Y, Cao J, Gao J, Sun J, Zhang L. Targeting blood-brain barrier for sepsis-associated encephalopathy: Regulation of immune cells and ncRNAs. Brain Res Bull. 2024;209:110922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Xiao P, Wen Y, Du G, Luo E, Su Z, Liao Z, Ding H, Li W. Clusterin attenuates blood-brain barrier damage and cognitive impairment by inhibiting astrocyte aging in mice with sepsis-associated encephalopathy. Neuroreport. 2024;35:857-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Guo J, Kong D, Luo J, Xiong T, Wang F, Deng M, Kong Z, Yang S, Da J, Chen C, Lan J, Chu L, Han G, Liu J, Tan Y, Zhang J. Orexin-A Attenuates the Inflammatory Response in Sepsis-Associated Encephalopathy by Modulating Oxidative Stress and Inhibiting the ERK/NF-κB Signaling Pathway in Microglia and Astrocytes. CNS Neurosci Ther. 2024;30:e70096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Lv X, Jia M, Feng X, Jian JX, Yang JJ, Ma DQ, Ji MH, Diao YG, Shen JC. STING Driving Synaptic Phagocytosis of Hippocampal Microglia/Macrophages Contributes to Cognitive Impairment in Sepsis-Associated Encephalopathy in Mice. CNS Neurosci Ther. 2024;30:e70166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Xu Y, Zhu Y, Shi Y, Ye B, Bo L, Tao T. Immune Checkpoint VISTA Negatively Regulates Microglia Glycolysis and Activation via TRIM28-Mediated Ubiquitination of HK2 in Sepsis-Associated Encephalopathy. Mol Neurobiol. 2025;62:4452-4465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Zhang N, Ma Y, Li Y, Wang Y, Zhang L, Zheng M, Tian Y, Zhang R, Yang K, Li J, Yan F, Liu H, Zhang Y, Xu J, Yu C, Xu J. Paeonol prevents sepsis-associated encephalopathy via regulating the HIF1A pathway in microglia. Int Immunopharmacol. 2024;143:113287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |