Published online Jul 19, 2025. doi: 10.5498/wjp.v15.i7.105249
Revised: March 28, 2025
Accepted: May 20, 2025
Published online: July 19, 2025
Processing time: 175 Days and 14.1 Hours
Anhedonia, a hallmark symptom of major depressive disorder (MDD), is often resistant to common antidepressants. Preliminary evidence indicates that Pedio
To further assess the efficacy of P. acidilactici CCFM6432 in alleviating anhedonia in patients with MDD, using a combination of objective and subjective assessment tools.
Adult patients with MDD exhibiting anhedonic symptoms were enrolled and randomly assigned to two treatment groups: One receiving standard antidepressant therapy plus P. acidilactici CCFM6432, and the other receiving standard antidepressant treatment along with a placebo, for 30 days. Assessments were conducted at baseline and post-intervention using the Hamilton Depression Rating Scale (HAMD), Temporal Experience of Pleasure Scale (TEPS), and synchronous electroencephalography (EEG) during a "Doors Guessing Task." Changes in both clinical outcomes and EEG biomarkers, specifically the stimulus-preceding negativity (SPN) and feedback-related nega
Of the 92 screened participants, 71 were enrolled and 55 completed the study (CCFM6432 group: n = 27; Placebo group: n = 28). No baseline differences were noted between the groups in terms of demographics, clinical assessments, or EEG metrics. A mixed-design analysis of variance revealed that the CCFM6432 group showed significantly greater improvements in both HAMD and TEPS scores compared to the Placebo group. Moreover, the CCFM6432 group demonstrated a significant increase in SPN amplitudes, which were inversely correlated with the improvements observed in HAMD scores. No such changes were observed in the Placebo group.
Adjunctive administration of P. acidilactici CCFM6432 not only augments the therapeutic efficacy of antidepressants but also significantly ameliorates the symptoms of anhedonia in MDD.
Core Tip: Anhedonia, a core symptom of major depressive disorder (MDD), often exhibits poor responsiveness to conventional antidepressants, substantially impairing long-term prognosis. In our randomized controlled trial, adjunctive administration of Pediococcus acidilactici CCFM6432 alongside standard antidepressants demonstrated marked improvements in anhedonia, particularly anticipatory deficits, among patients with MDD. Efficacy was quantified through the Temporal Experience of Pleasure Scale and task-specific electroencephalography biomarkers during the Guessing-Door Task, revealing enhanced reward anticipation neural signatures. These findings highlight the clinical potential of CCFM6432 in addressing anhedonia, with mechanistic implications for gut microbiota-brain axis modulation.
- Citation: Li DX, Hu QM, Xu CC, Yang HY, Liu JK, Sun YF, Wang G, Wang J, Zhou ZH. Efficacy of Pediococcus acidilactici CCFM6432 in alleviating anhedonia in major depressive disorder: A randomized controlled trial. World J Psychiatry 2025; 15(7): 105249
- URL: https://www.wjgnet.com/2220-3206/full/v15/i7/105249.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i7.105249
Major depressive disorder (MDD) is a pervasive and recurrent psychiatric disorder characterized by persistent depressive mood, reduced interest or pleasure in activities, feelings of worthlessness, and a range of physical and cognitive impair
Despite the availability of standard antidepressant treatments, such as selective serotonin reuptake inhibitors (SSRIs), these medications often have limited effects on alleviating anhedonic symptoms[10]. While SSRIs can significantly im
In recent years, the role of gut microbiota in human mental health has gained increasing attention. Increasing evidence suggests that the gut microbiota can influence various physiological processes, such as endocrine responses, autonomic nervous system activity, and immune system functions, through the "gut-brain axis." These interactions, in turn, can impact brain function and behavior[15,16]. Moreover, research has shown that gut microbiota not only regulate myelin formation in the prefrontal cortex[17] but also exert broad effects on brain regions, including the midbrain limbic system[18], which are critical neural substrates involved in the development of MDD and its hallmark symptom of anhedonia[19]. Additionally, animal studies have demonstrated that the absence of gut microbiota can reduce immobility time in the forced swim test in mice, and transplantation of "depressive microbiota" from patients with MDD into germ-free mice induces depressive-like behaviors[20]. Gut dysbiosis, characterized by reduced gut microbial diversity, leads to elevated levels of circulating tight junction proteins such as claudin, endotoxin lipopolysaccharide (LPS), gut-associated systemic inflammatory markers, anti-endotoxin antibodies, and acute-phase proteins, all of which are highly correlated with anhedonia in MDD[21]. This body of evidence provides both theoretical and empirical support for the potential of gut microbiota modulation as a therapeutic approach for improving anhedonic symptoms in MDD.
Probiotic supplements are one of the primary means of modulating gut microbiota and offer advantages such as convenience, safety, and non-toxicity. Studies have demonstrated that various probiotic strains can reduce immune-inflammatory responses, regulate neurotransmitter systems, increase BDNF levels, and enhance neuroplasticity[22,23]. More notably, several animal studies have shown that specific probiotics not only reduce depressive-like behaviors (e.g., decreased immobility time in the forced swim test in chronically stressed mice) but also improve sucrose preference and other pleasure-related behaviors, suggesting their potential to mitigate anhedonic symptoms[24-27]. This compelling preclinical evidence lays a foundation for exploring the biological effects of probiotics on anhedonia in clinical settings. However, to date, there have been limited clinical studies on this subject in patients with MDD, highlighting the importance of further research in this area, which could yield valuable clinical and scientific insights.
Our preliminary studies have demonstrated that certain probiotic formulations, particularly Pediococcus acidilactici (P. acidilactici) CCFM6432 (hereinafter referred to as CCFM6432), can alleviate depressive-like and anxiety-like behaviors in animal models[28,29]. In clinical trials, CCFM6432 has shown potential in reducing the overall severity of depression and improving gastrointestinal symptoms associated with MDD[30,31]. While these findings are promising, empirical evidence specifically linking CCFM6432 to the alleviation of anhedonic symptoms in patients with MDD remains limited.
The current study further investigated the potential of P. acidilactici CCFM6432 as an adjunctive treatment for MDD, with a specific focus on its effects on anhedonic symptoms. Both subjective clinical assessments, such as the Temporal Experience of Pleasure Scale (TEPS), and objective neurophysiological measures, including event-related potentials (ERPs), were used to evaluate these symptoms. We hypothesize that the addition of CCFM6432 to conventional antidepressant therapy will more effectively alleviate anhedonia in patients with MDD, as evidenced by improved TEPS scores and increased amplitude in ERP components, particularly stimulus-preceding negativity (SPN) and feedback-related negativity (FRN), which are associated with reward expectation and feedback processing, respectively[32,33]. Among patients with MDD, both anticipatory (ANT) and consummatory (CON) anhedonia are commonly observed subtypes[34,35]. The measures applied in this study will help further clarify the subtype distribution and corresponding treatment effects.
This study was conducted from March 2023 to June 2024 at the Wuxi Mental Health Center (Jiangsu Province, China), also known as the Affiliated Mental Health Center of Jiangnan University. Prior to its initiation, the study received approval from the Ethics Committee of Wuxi Mental Health Center (WXMHCIRB2023 LLky030) and followed the ethical principles outlined in the Declaration of Helsinki. Additionally, the study was retrospectively registered at the Chinese Clinical Trial Registry (https://www.chictr.org.cn/) under registration number ChiCTR2400093687. Written informed consent was obtained from all participants prior to their inclusion, ensuring their voluntary participation.
This study was a randomized, double-blind, placebo-controlled trial designed to assess the efficacy of P. acidilactici CCFM6432 as an adjunctive treatment for anhedonia in patients with MDD. The general procedure was as follows: Participants were initially screened via the electronic medical system, followed by a face-to-face interview. Eligible individuals were voluntarily recruited based on established inclusion and exclusion criteria. Upon recruitment, baseline clinical assessments and electroencephalography (EEG) measurements were conducted (see Measurements section for details). Participants were then randomly assigned to either the study group (CCFM6432 + standard antidepressant therapy) or the control group (placebo + standard antidepressant therapy) and received their respective treatments for 30 days. Clinical assessments and EEG measurements were repeated within 3 days following the 30-day intervention period. Both the participants and the clinical assessors were blinded to group allocation throughout the trial.
The participants in this study were clinical patients with MDD with anhedonic symptoms. Inclusion criteria were as follows: (1) Diagnosis of MDD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria; (2) A Snaith-Hamilton Pleasure Scale (SHAPS) score of at least 3, indicating the presence of anhedonic symptoms[36]; (3) A stable antidepressant treatment plan prescribed by their psychiatrist, with no changes in antidepressant type expected within the next 30 days; (4) Age between 18 and 65 years and of Chinese Han nationality; (5) Educational level of at least primary school, with no impairments in verbal understanding or motor function; and (6) Voluntary participa
Exclusion criteria were as follows: (1) Comorbid psychiatric conditions such as bipolar disorder or schizophrenia; (2) Uncontrolled severe physical illnesses, including coronary heart disease or thyroid dysfunction; (3) History of head trauma leading to loss of consciousness, epilepsy, or significant gastrointestinal disease or surgery; (4) Significant motor dysfunction of the fingers (e.g., inability to click a mouse); (5) Women who were pregnant, breastfeeding, or planning pregnancy; (6) Use of antibiotics, probiotics, or related products in the past month; and (7) Receipt of modified electroconvulsive therapy (MECT) treatment within the past 6 months due to its potential effects on anhedonia[37].
Eligible participants were assigned to two groups using a simple randomization method, maintaining a 1:1 allocation ratio. The random allocation sequence was meticulously generated by Zhou ZH using the "= RAND()" function in Microsoft Excel. To preserve the integrity of the randomization process and ensure the confidentiality of the sequence until the point of intervention assignment, it was safeguarded in a password-protected Excel file. Zhou ZH was solely responsible for accessing this file, thereby strictly controlling the sequence access. Participants were enrolled by Li DX and Sun YF, who diligently performed the initial screenings and assessments as integral parts of their investigational duties. Wang J, who oversaw project administration, was tasked with assigning participants to their respective interventions. This role was crucial in maintaining the concealment of the allocation sequence until the actual assignment of interventions, further ensuring the study's methodological integrity.
We did not interfere with the participants' ongoing antidepressant therapy, as prescribed by their doctors. The study group, henceforth referred to as the CCFM6432 group, received CCFM6432 in addition to their standard antidepressant regimen. Consistent with our previous studies[31], CCFM6432, developed by the School of Food Science and Technology at Jiangnan University (Jiangsu, China) and produced by the Yangzhou Institute of Food Biotechnology, Jiangnan University, was delivered in sachets containing a minimum of 109 colony-forming units. The control group, henceforth referred to as the Placebo group, received a biologically inactive placebo that was identical to CCFM6432 in terms of packaging, color, weight, odor, and taste. The intervention lasted 30 days, with participants consuming one sachet daily, either directly or dissolved in cold water. Although there was no strict timing requirement, participants were encouraged to take the sachet at a consistent time each day, such as after breakfast or dinner. Throughout the study, participants were instructed to maintain their usual dietary habits and avoid alcohol. Any necessary antibiotic use for clinical reasons had to be reported by the participants. Adherence was monitored by nurses for inpatients, who recorded daily intake, and by family members for outpatients. Outpatients were also asked to retain empty sachets for verification and received regular reminders via phone or text messages.
This study employed a custom-designed information collection form to gather both demographic and clinical data. Demographic data included age, sex, and educational level, while clinical data included the duration of illness, types of antidepressants, and their dosages.
The Chinese version of the TEPS was used to assess the severity of anhedonia, as it distinguishes between ANT and CON pleasure, offering a more nuanced evaluation of anhedonia subtypes compared to the SHAPS, which does not differentiate between them[38,39]. A higher score indicates a greater ability to experience pleasure, reflecting less anhedonia. In this study, SHAPS was employed to screen for the presence of anhedonic symptoms, while TEPS assessed anhedonia severity, as SHAPS lacks the ability to differentiate subtypes and TEPS does not have a strict cutoff for anhedonia. Changes in TEPS total score were used as the primary subjective indicator of interest.
The severity of depression was assessed using the 17-item Hamilton Depression Rating Scale (HAMD), while anxiety symptoms were measured with the Hamilton Anxiety Rating Scale (HAMA). Both the HAMA and HAMD are well-established and widely used tools in psychiatric clinical research and practice, recognized for their strong reliability and validity. Higher scores on these scales reflect greater severity of anxiety and depression symptoms, respectively.
The Chinese versions of the SHAPS, TEPS, HAMA, and HAMD questionnaires have demonstrated robust reliability and validity, affirming their effectiveness for use in clinical studies. These instruments have been rigorously validated, ensuring their reliability and appropriateness for measuring psychological symptoms in the Chinese population[38-43]. Changes in TEPS and its subscale scores were the primary indicators of interest from the subjective evaluation perspective, while changes in HAMA and HAMD scores served as the secondary outcomes.
This study employed a classic experimental paradigm for reward processing, the "Doors Guessing Task"[44], to investigate participants' electrophysiological responses during reward anticipation and following win or loss feedback. The methodology of this paradigm has been detailed in previous reports from our research team[45]. In brief, participants were asked to choose between two identical doors displayed on a computer screen. A monetary gain was awarded if their choice matched the computer's selection, and a loss occurred otherwise. By analyzing ERP indicators during the result-waiting phase and the post-feedback phase, we were able to investigate the neural activities associated with reward expectation and consumption.
EEG signals were simultaneously recorded with participants' behavioral responses during the Doors Guessing Task using a BrainAmp Standard amplifier connected to a 64-channel EasyCap electrode cap (Brain Products GmbH, Gilching, Germany). Although EEG data collection was not confined to a strict fixed time, we standardized the timing across baseline and follow-up sessions to reduce variability. Specifically, if a participant's baseline EEG was recorded in the morning, the follow-up EEG was also scheduled in the morning; likewise, afternoon baseline recordings were followed by afternoon sessions. This approach was adopted to minimize potential circadian influences on EEG signals. Participants were instructed to wash their hair thoroughly the day before EEG collection to ensure good electrode-scalp contact and reduce signal interference caused by oil or dirt. They were also advised to maintain regular sleep the night before and avoid sleep deprivation or fatigue, as these can affect brain electrical activity. In addition, participants were asked to refrain from consuming caffeine-containing products (e.g., coffee, tea) or other stimulants prior to the session to avoid potential interference with EEG signals.
The EEG recording parameters and preprocessing protocols were consistent with those described in our previous report[45]. All EEG data were preprocessed offline using MATLAB (version 2020b; The MathWorks GmbH, München, Germany) in combination with the EEGLAB 2021 software package (https://sccn.ucsd.edu/eeglab/index.php). EEG preprocessing included electrode positioning, bandpass filtering, re-referencing, bad channel interpolation, artifact segment rejection, independent component analysis (ICA), removal of ocular artifacts, data epoching, baseline correction, and outlier removal to ensure data quality and reliability. Specifically, raw EEG data were bandpass filtered between 0.1 Hz and 30 Hz using EEGLAB to eliminate high-frequency noise and low-frequency drift, and a 50 Hz notch filter was applied to remove power line interference. Data were re-referenced to the average of the bilateral mastoids, and bad channels were interpolated using spherical spline interpolation. Prior to ICA decomposition with the runica algorithm, abnormal segments were manually removed through visual inspection. ICA components corresponding to physiological artifacts such as eye movements and muscle activity were identified and excluded. On average, fewer than five trials per participant were rejected due to artifacts. All preprocessing procedures followed the established protocols from our previous studies, which provide further methodological details[45].
Changes in SPN and FRN amplitudes were used as the primary objective indicators of interest. A greater absolute value of SPN amplitude indicates stronger reward anticipation[33,46]. The FRN, measured as the difference wave between the loss and win conditions, reflects the strength of the electrophysiological response to reward feedback[33,47,48]. Following previous reports, the analysis window for SPN was set from -200 ms to 0 ms (relative to the onset of the feedback stimulus), focusing on the average amplitude across right fronto-central regions (FC4, FC6, C4, C6, F4, F6)[49]. For FRN, the analysis window was set from 250 ms to 350 ms post-feedback stimulus, with electrodes located at Fz, FC1, FCz, FC2, and Cz, and the average amplitude within these regions assessed[50].
All statistical analyses were performed using IBM SPSS Statistics version 25 (IBM Corp., Armonk, NY, United States). Normality of continuous variables was assessed using the Shapiro-Wilk test. For normally distributed data, independent-sample t-tests were used for group comparisons, while the Mann-Whitney U test was applied for non-normally distributed data. Categorical data were analyzed using the χ2 test (χ²). For repeated measures data, a 2 × 2 mixed-design analysis of variance (ANOVA) was employed to assess both within-group (time: baseline vs follow-up) and between-group (group: CCFM6432 vs placebo) effects. If the interaction between time and group was significant, simple effects analyses were conducted to further investigate the influence of each factor. When the assumption of sphericity was violated, the Greenhouse-Geisser correction was applied to adjust for violations of this assumption, ensuring the robustness of the results. Post hoc comparisons for significant main effects or interactions were adjusted using the Bonferroni correction to control for multiple testing. Pearson’s correlation analysis was used to explore linear rela
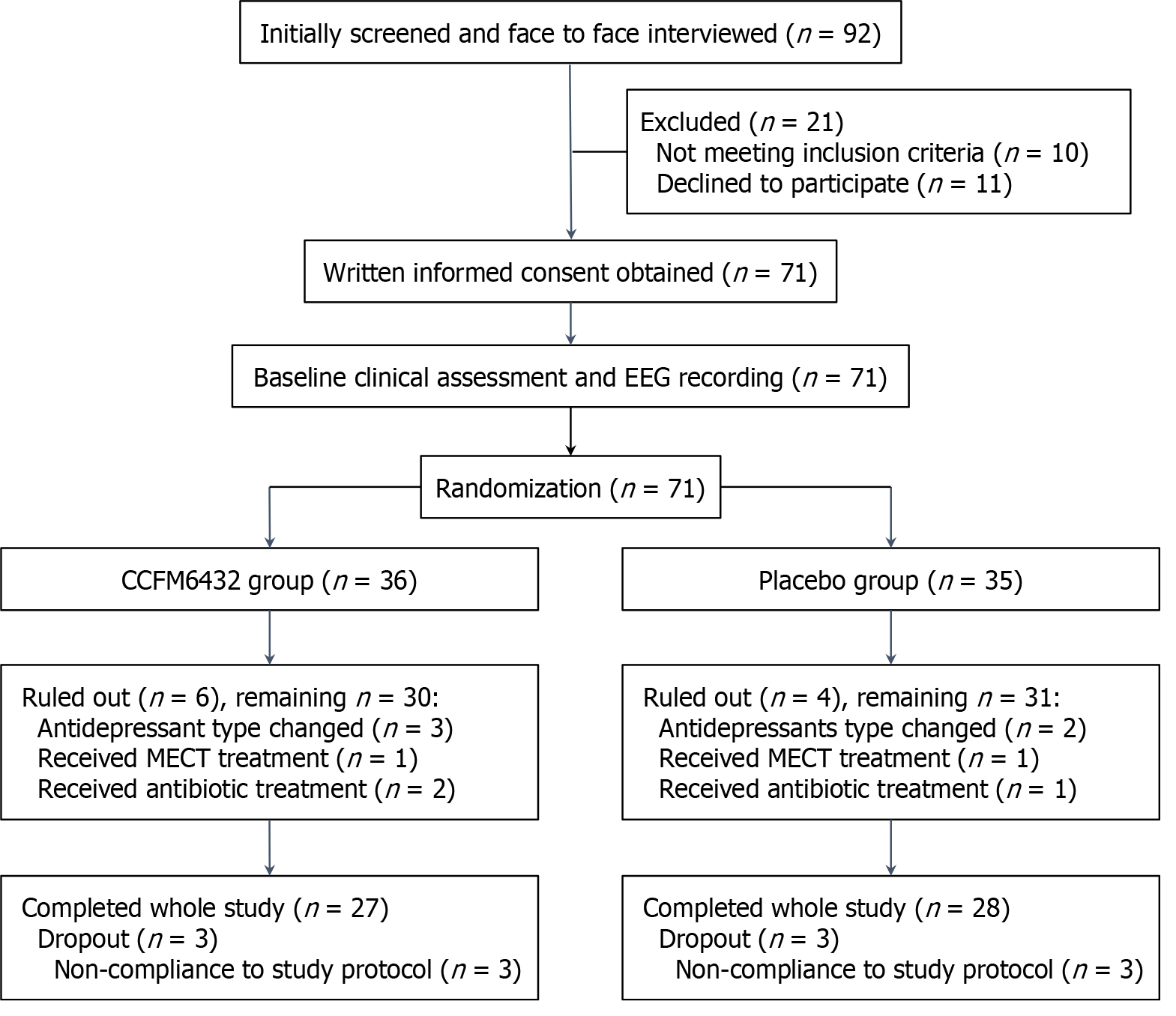
An initial screening of electronic medical records identified 92 potentially eligible patients with MDD who were invited for further interviews. Of these, 21 either did not meet the inclusion criteria or declined to participate. The remaining 71 participants met the criteria, completed the baseline assessment, and were randomly divided into the CCFM6432 group (n = 36) or the placebo group (n = 35). However, 10 participants (6 in the CCFM6432 group and 4 in the placebo group) were ruled out during the study due to changes in antidepressant medications, receipt of MECT, or use of antibiotics. Additionally, 6 participants (3 in each group) were deemed non-compliant in taking either CCFM6432 or the placebo, as reported by their monitors, and were therefore also excluded. Consequently, data from 27 participants in the CCFM6432 group (10 males and 17 females) and 28 participants in the Placebo group (11 males and 17 females) were included in the final analysis. No serious adverse events were observed during the intervention, supporting the good safety profile of CCFM6432. The flowchart of the implementation process is presented in Figure 1.
A power analysis conducted using G-Power software (version 3.1.9.7; Kiel, Germany) indicated that, with a medium effect size (f = 0.25), α = 0.05, and a correlation among repeated measures of 0.5, the current sample size provided statistical power greater than 0.9 when using a mixed-design ANOVA approach.
As shown in Table 1, no significant differences were found between the groups in terms of sex distribution, mean age, body mass index, handedness, or years of education. Similarly, no significant between-group differences were observed in the duration of illness, number of episodes, or equivalent doses of antidepressants, calculated using the defined daily dose method as described in previous studies[51]. Moreover, there were no significant differences in baseline scores on the HAMA, HAMD, or the total and subscale scores of the TEPS (Table 2).
| CCFM6432 (n = 27) | Placebo (n = 28) | Statistics | P value | |
| Sex (M/F) | 10/17 | 11/17 | 0.029 (χ²) | 0.864 |
| Age (years) | 27.30 (6.94) | 31.29 (11.29) | -1.571 (t) | 0.120 |
| BMI | 22.27 (3.45) | 22.37 (3.88) | -0.098 (t) | 0.922 |
| Handedness (R/L/M) | 25/1/1 | 25/2/1 | 0.315 (χ²) | 0.854 |
| Education level (years) | 14.74 (2.36) | 13.96 (3.32) | 0.997 (t) | 0.323 |
| Disease course (years) | 3.74 (4.22) | 5.70 (5.10) | -1.239 (z) | 0.215 |
| Number of episodes | 1.85 (0.91) | 2.29 (1.38) | -1.055 (z) | 0.292 |
| Antidepressant DDD | 1.49 (1.07) | 1.40 (1.29) | -0.606 (z) | 0.544 |
| SSRI/SNRI/NaSSA/SARI | 15/7/2/3 | 16/7/1/4 | 0.490 (χ²) | 0.921 |
| CCFM6432 (n = 27) | Placebo (n = 28) | P value1 | P value2 | P value3 | |
| HAMA_Baseline | 19.07 (7.80) | 18.11 (9.39) | 0.011 | 0.455 | < 0.001 |
| HAMA_Follow-up | 9.04 (5.89) | 12.89 (7.95) | |||
| HAMD_Baseline | 21.74(7.07) | 21.04 (8.69) | 0.013 | 0.492 | < 0.001 |
| HAMD_Follow-up | 9.89 (5.80) | 13.25 (8.72) | |||
| TEPS_Baseline | 70.07 (11.89) | 71.89 (13.87) | 0.041 | 0.645 | 0.007 |
| TEPS_Follow-up | 78.00 (13.92) | 73.07 (15.22) | |||
| ANT_Baseline | 30.56 (6.45) | 33.00 (7.09) | 0.015 | 0.981 | 0.009 |
| ANT_Follow-up | 35.74 (7.15) | 33.21 (8.01) | |||
| CON_Baseline | 39.52 (7.63) | 38.89 (9.33) | 0.293 | 0.486 | 0.031 |
| CON_Follow-up | 42.26 (8.03) | 39.86 (9.16) |
A mixed-design ANOVA of HAMA scores revealed a significant group × time interaction (F(1,53) = 7.009, P = 0.011, ηp² = 0.117). The main effect of group was not significant (F(1,53) = 0.567, P = 0.455, ηp² = 0.011), while there was a significant effect of time (F(1,53) = 70.089, P < 0.001, ηp² = 0.569). Further analysis of simple effects indicated no significant difference in baseline HAMA scores between the groups (F(1,53) = 0.172, P = 0.680, ηp² = 0.003); however, a significant difference was observed in follow-up scores (F(1,53) = 4.154, P = 0.047, ηp² = 0.073), with the CCFM6432 group showing a significantly greater reduction in HAMA scores compared to the Placebo group.
Similar to the HAMA analysis, the mixed-design ANOVA of HAMD scores revealed a significant group × time interaction (F(1,53) = 6.676, P = 0.013, ηp² = 0.112), a significant main effect of Time (F(1,53) = 155.720, P < 0.001, ηp² = 0.746), and no significant effect of Group (F(1,53) = 0.479, P = 0.492, ηp² = 0.009). Further analysis of simple effects showed no significant difference in baseline HAMD scores between the groups (F(1,53) = 0.109, P = 0.743, ηp² = 0.002). Both groups exhibited a notable decrease in HAMD scores post-treatment, with a marginal between-group difference observed at follow-up (F(1,53) = 2.813, P = 0.099, ηp² = 0.050).
For TEPS total scores, a significant group × time interaction was observed (F(1,53) = 4.371, P = 0.041, ηp² = 0.076), along with a significant main effect of Time (F(1,53) = 7.958, P = 0.007, ηp² = 0.131), while no significant main effect of group was found (F(1,53) = 0.215, P = 0.645, ηp² = 0.004). Further analysis of simple effects revealed no significant difference in baseline TEPS scores between the groups (F(1,53) = 0.272, P = 0.604, ηp² = 0.005). Post-intervention, although no significant between-group difference was observed (F(1,53) = 1.567, P = 0.216, ηp² = 0.029), the CCFM6432 group exhibited a significant increase in TEPS total scores (F(1,53) = 11.847, P = 0.001, ηp² = 0.183) whereas the Placebo group showed no significant changes (F(1,53) = 0.272, P = 0.604, ηp² = 0.005).
For the ANT subscale, the mixed-design ANOVA revealed a significant group × time interaction (F(1,53) = 6.275, P = 0.015, ηp² = 0.106), a significant main effect of time (F(1,53) = 7.404, P = 0.009, ηp² = 0.123), and no significant main effect of group (F(1,53) = 0.001, P = 0.981, ηp² = 0.000). Further analysis of simple effects showed no significant between-group difference in baseline ANT subscale scores (F(1,53) = 1.784, P = 0.187, ηp² = 0.033). Post-intervention, although no significant between-group difference was observed (F(1,53) = 1.519, P = 0.223, ηp² = 0.028), the CCFM6432 group showed a significant increase in ANT scores (F(1,53) = 13.411, P = 0.001, ηp² = 0.202) whereas the Placebo group showed no significant changes (F(1,53) = 0.024, P = 0.878, ηp² < 0.001).
For the CON subscale, the mixed-design ANOVA revealed no significant group × time interaction (F(1,53) = 1.126, P = 0.293, ηp² = 0.021) or main effect of group (F(1,53) = 0.492, P = 0.486, ηp² = 0.009), despite the main effect of Time being significant (F(1,53) = 4.897, P = 0.031, ηp² = 0.085). These results indicate that the CON subscale scores changed obviously over time, but there was no significant between-group difference.
In total, no significant between-group differences were observed across all clinical assessments at baseline. However, the CCFM6432 group demonstrated greater improvement than the Placebo group in most variables at follow-up. Table 2 presents the clinical assessment values for both groups at both time points, along with the P values derived from the mixed-design ANOVA.
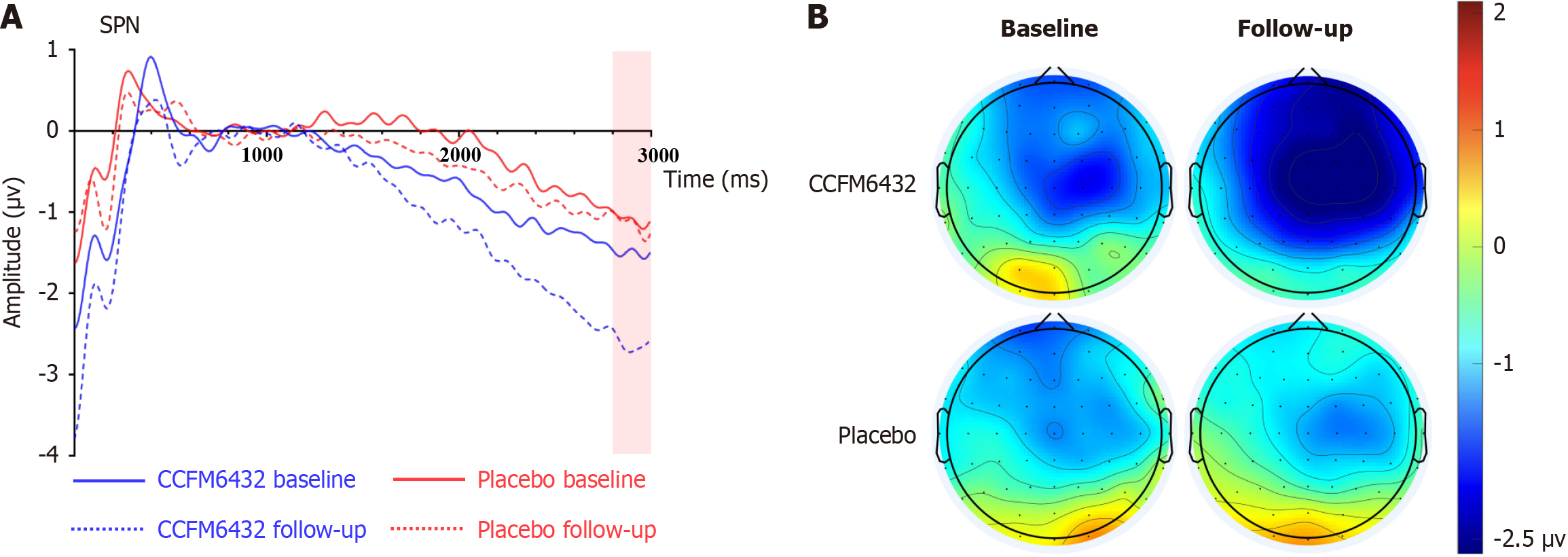
Figure 2 displays the SPN waveforms and topographical maps for both groups at baseline and follow-up. Mixed-design ANOVA for SPN amplitude revealed a significant group × time interaction (F(1,53) = 4.895, P = 0.031, ηp² = 0.085), as well as significant main effects of group (F(1,53) = 4.788, P = 0.033, ηp² = 0.083) and time (F(1,53) = 6.066, P = 0.017, ηp² = 0.103). Simple effects analysis indicated no significant between-group differences at baseline (F(1,53) = 0.705, P = 0.405, ηp² = 0.013), whereas at follow-up, the SPN amplitude was significantly greater in the CCFM6432 group compared to the Placebo group (F(1,53) = 8.765, P = 0.005, ηp² = 0.142). Post-intervention, SPN amplitude significantly increased in the CCFM6432 group (F(1,53) = 10.735, P = 0.002, ηp² = 0.168), with no notable change observed in the Placebo group (F(1,53) = 0.032, P = 0.859, ηp² = 0.001).
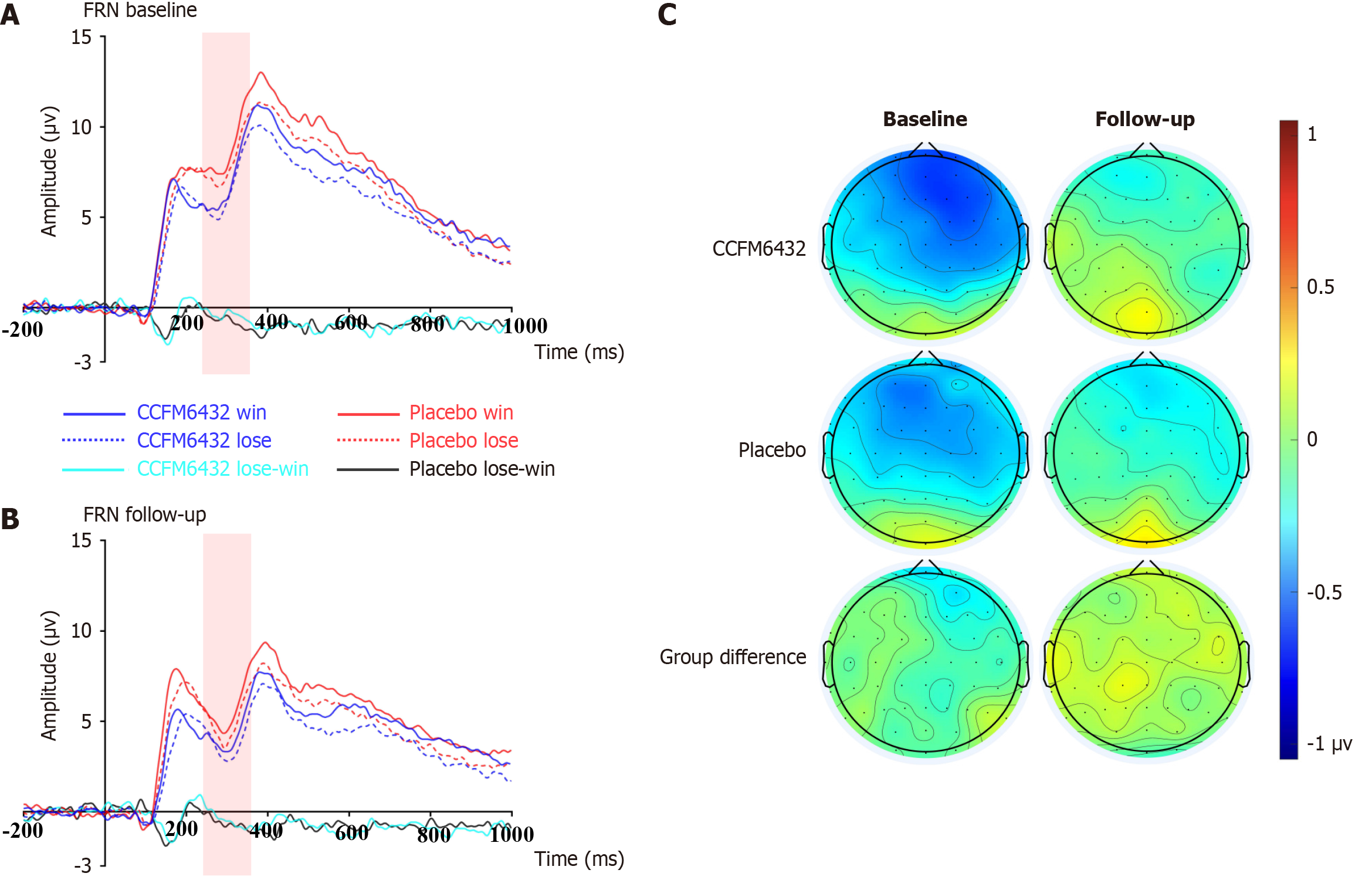
For FRN amplitude, the mixed-design ANOVA revealed no significant group × time interaction (F(1,53) = 0.165, P = 0.686, ηp² = 0.003), nor were there significant main effects for group (F(1,53) = 0.000, P = 0.989, ηp² = 0.000) or time (F(1,53) = 2.143, P = 0.149, ηp² = 0.039). These results suggest that the adjunctive use of CCFM6432 did not significantly impact the improvement of CON pleasure deficit symptoms in patients with MDD, as illustrated in Figure 3.
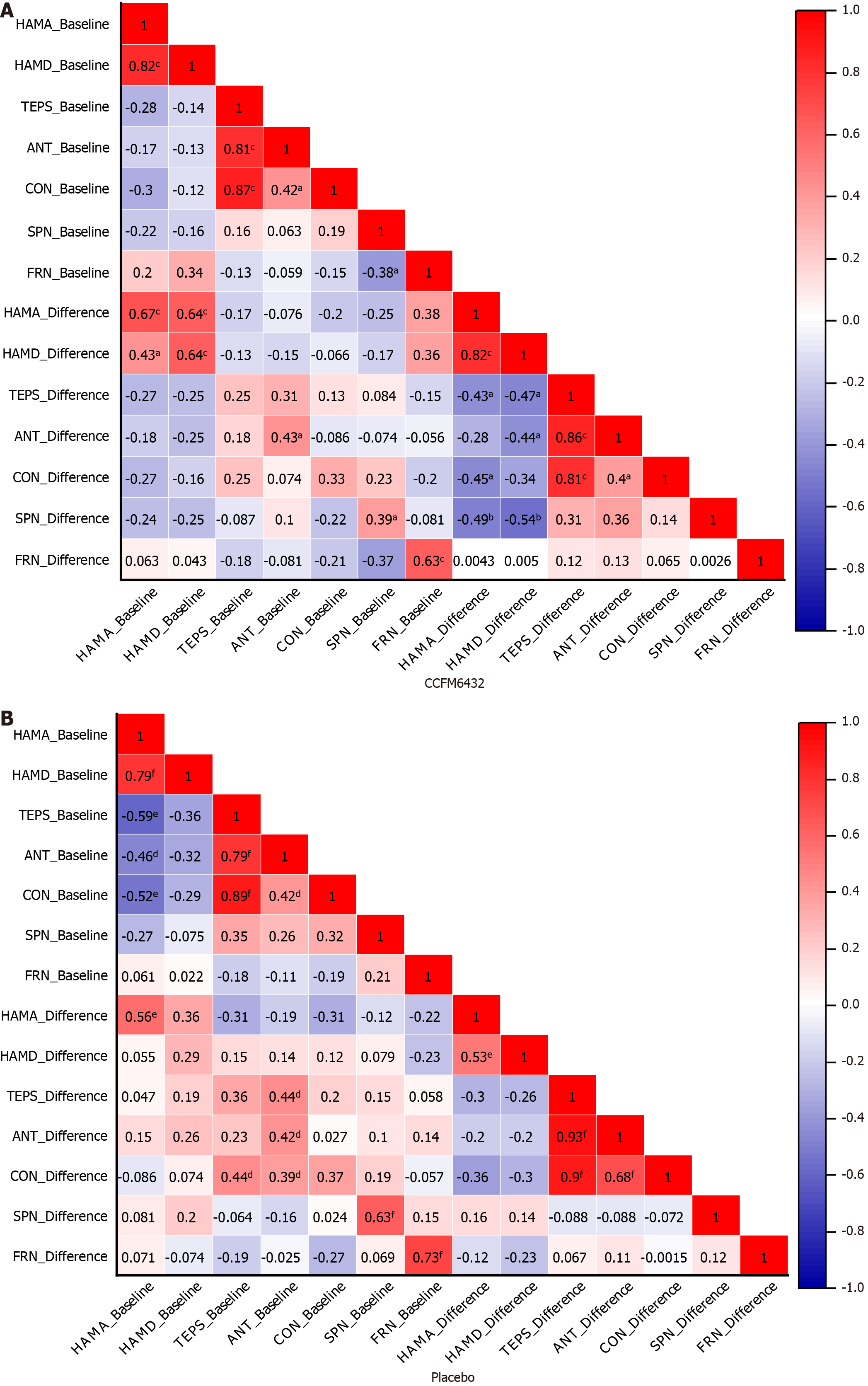
Pearson’s correlation analysis was conducted to explore the interrelations between objective and subjective indicators of depressive and anhedonic symptoms, including baseline levels and their changes before and after intervention (represented by the difference between baseline and follow-up). As shown in Figure 4, although the strength of the correlations varied, the overall pattern remained consistent across both groups. At baseline and in changes from baseline to follow-up, HAMA and HAMD scores consistently exhibited a strong positive correlation (r = 0.53 to 0.82), while TEPS and its subscales generally showed a broad negative correlation with HAMA and HAMD scores (r = -0.12 to -0.47). Notably, the correlation between changes in ERP values and clinical assessment changes post-intervention differed significantly, particularly for SPN. In the CCFM6432 group, post-intervention changes in SPN amplitude were sig
This study evaluated the adjunctive benefits of P. acidilactici CCFM6432, a recently developed probiotic formulation, in patients clinically diagnosed with MDD, with a specific focus on anhedonic symptoms. Our results support its efficacy not only in improving overall depressive symptoms but also in alleviating anhedonia, a critical yet often treatment-resistant symptom of MDD, as assessed through both subjective and objective measures. More specifically, adjunctive treatment with CCFM6432 significantly improved scores on the TEPS, particularly the ANT subscale, and notably increased the amplitude of the SPN, an ERP component associated with reward anticipation. These findings suggest that CCFM6432 effectively reduces anhedonic symptoms in patients with MDD, particularly in relation to ANT pleasure.
The influence of probiotics on gut microbiota and the subsequent modulation of the gut-brain axis provide a physiological basis for their potential impact on neuroendocrine, neurotrophic, and neuroimmune pathways involved in depression. While existing literature has demonstrated that various probiotic formulations can enhance mood without significant side effects[52,53], the findings are not always consistent[51,54,55]. The findings of the present study further substantiate these results, demonstrating that CCFM6432, even as a standalone adjunct to standard antidepressants, can significantly reduce both anxiety and depressive symptoms.
Importantly, this study extends previous observations by demonstrating significant improvements in both subjective assessments and objective ERP components related to pleasure deficits following treatment, providing new evidence of the effectiveness of CCFM6432 in addressing anhedonia in MDD. This is particularly crucial given the limited impact of current antidepressant therapies on this core symptom of MDD, which profoundly influences overall prognosis. To date, few studies have explored the effects of probiotic preparations on anhedonic symptoms in patients with MDD. One study using Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 found significant improvements in anhedonic symptoms, as assessed by the SHAPS, in first-episode, patients with medication-naïve MDD[56]. However, the small sample size, open-label design, and reliance solely on subjective assessments in that study may have introduced biases. In contrast, our study, with a larger sample, more rigorous design, and the inclusion of both subjective and objective measurements, provides stronger evidence supporting CCFM6432’s role in mitigating anhedonia in MDD.
Interestingly, CCFM6432 appeared to primarily affect ANT rather than CON aspects of pleasure. This is reflected in the greater improvements observed in the TEPS ANT subscale scores and more pronounced effects on SPN amplitudes compared to FRN. Our previous research suggested that while patients with MDD exhibit deficits in both CON and ANT pleasure, the neural processing mechanisms underlying reward anticipation and feedback may differ[45], aligning with findings that these subtypes of pleasure are associated with somewhat distinct neural circuits[57]. Previous studies have indicated that CCFM6432's biological effects may be linked to its anti-inflammatory properties, such as reversing abnormal increases in serum LPS levels, downregulating the brain’s Toll-like receptor 4/nuclear factor kappa B signaling pathways, and inhibiting microglial overactivation in the hippocampus[29]. While elevated inflammatory levels have been consistently associated with anhedonia[58], it is noteworthy that recent studies found a significant negative correlation specifically between high peripheral cytokine levels and the ANT subscale of the TEPS, but not with the CON subscale or SHAPS scores[59]. This suggests that the type of anhedonia closely related to high inflammation might predominantly be ANT rather than CON, which may explain why CCFM6432 primarily improved ANT pleasure in our study. Further studies exploring the relationship between CCFM6432’s anti-inflammatory properties and its role in mitigating anhedonia are warranted.
We observed a generally negative correlation between HAMA and HAMD scores and TEPS and its subscales, indicating that increases in anxiety and depression symptoms correspond to exacerbated anhedonic symptoms in patients with MDD. A strong positive correlation between HAMA and HAMD scores was found, with improvements in HAMA scores appearing more pronounced than those in HAMD scores in the CCFM6432 group. This suggests that CCFM6432’s biological effects in mitigating anhedonic symptoms may, in part, be attributed to its efficacy in reducing anxiety symp
Despite these promising findings, this study had several limitations. First, the sample size was modest and derived from a single center, which limits the generalizability of the results. Larger, multicenter studies are necessary to confirm the stability and applicability of these findings. Second, although no statistical differences in medication type and dosage between groups were observed, the potential influences of the medications could not be fully ruled out. Similarly, despite the imposition of dietary restrictions, variations in baseline dietary habits could have influenced the outcomes. However, the real world-like setting of the study might enhance the clinical relevance of the findings compared to those obtained under strict experimental conditions. About 90% of our participants were right-handed, and our sensitivity analysis indicated minimal effects of handedness on our results; however, further research should continue to address handedness to confirm these findings are applicable across different populations. Lastly, while this study primarily described phenomenological observations, it did not elucidate the potential mechanisms by which CCFM6432 alleviates anhedonic symptoms. Future studies are needed to include more in-depth analyses, such as those of fecal and peripheral blood biomarkers, to explore the metabolic, immune, and neurotrophic mechanisms underlying the observed effects.
In summary, our findings not only confirm that P. acidilactici CCFM6432 enhances the efficacy of conventional antidepressants in alleviating both anxiety and depressive symptoms, but also demonstrate its effectiveness in reducing anhe
| 1. | Marx W, Penninx BWJH, Solmi M, Furukawa TA, Firth J, Carvalho AF, Berk M. Major depressive disorder. Nat Rev Dis Primers. 2023;9:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 366] [Reference Citation Analysis (0)] |
| 2. | Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 2894] [Article Influence: 361.8] [Reference Citation Analysis (0)] |
| 3. | Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 926] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 4. | Buckner JD, Joiner TE Jr, Pettit JW, Lewinsohn PM, Schmidt NB. Implications of the DSM's emphasis on sadness and anhedonia in major depressive disorder. Psychiatry Res. 2008;159:25-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Trøstheim M, Eikemo M, Meir R, Hansen I, Paul E, Kroll SL, Garland EL, Leknes S. Assessment of Anhedonia in Adults With and Without Mental Illness: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e2013233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 6. | Ducasse D, Loas G, Dassa D, Gramaglia C, Zeppegno P, Guillaume S, Olié E, Courtet P. Anhedonia is associated with suicidal ideation independently of depression: A meta-analysis. Depress Anxiety. 2018;35:382-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 192] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr Scand. 2001;103:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 203] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Wong S, Le GH, Phan L, Rhee TG, Ho R, Meshkat S, Teopiz KM, Kwan ATH, Mansur RB, Rosenblat JD, McIntyre RS. Effects of anhedonia on health-related quality of life and functional outcomes in major depressive disorder: A systematic review and meta-analysis. J Affect Disord. 2024;356:684-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 9. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. [cited 3 January 2025]. Available from: https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596. |
| 10. | Cao B, Zhu J, Zuckerman H, Rosenblat JD, Brietzke E, Pan Z, Subramanieapillai M, Park C, Lee Y, McIntyre RS. Pharmacological interventions targeting anhedonia in patients with major depressive disorder: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | Nierenberg AA. Residual symptoms in depression: prevalence and impact. J Clin Psychiatry. 2015;76:e1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Pizzagalli DA. Toward a Better Understanding of the Mechanisms and Pathophysiology of Anhedonia: Are We Ready for Translation? Am J Psychiatry. 2022;179:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 13. | Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 811] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 14. | Su YA, Si T. Progress and challenges in research of the mechanisms of anhedonia in major depressive disorder. Gen Psychiatr. 2022;35:e100724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99:1877-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 3215] [Article Influence: 459.3] [Reference Citation Analysis (2)] |
| 16. | Dziedzic A, Maciak K, Bliźniewska-Kowalska K, Gałecka M, Kobierecka W, Saluk J. The Power of Psychobiotics in Depression: A Modern Approach through the Microbiota-Gut-Brain Axis: A Literature Review. Nutrients. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 17. | Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6:e774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 467] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 18. | Yu KB, Hsiao EY. Roles for the gut microbiota in regulating neuronal feeding circuits. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Wang S, Leri F, Rizvi SJ. Anhedonia as a central factor in depression: Neural mechanisms revealed from preclinical to clinical evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2021;110:110289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 20. | Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21:786-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1485] [Article Influence: 148.5] [Reference Citation Analysis (7)] |
| 21. | Safadi JM, Quinton AMG, Lennox BR, Burnet PWJ, Minichino A. Gut dysbiosis in severe mental illness and chronic fatigue: a novel trans-diagnostic construct? A systematic review and meta-analysis. Mol Psychiatry. 2022;27:141-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 22. | Snigdha S, Ha K, Tsai P, Dinan TG, Bartos JD, Shahid M. Probiotics: Potential novel therapeutics for microbiota-gut-brain axis dysfunction across gender and lifespan. Pharmacol Ther. 2022;231:107978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 23. | Suda K, Matsuda K. How Microbes Affect Depression: Underlying Mechanisms via the Gut-Brain Axis and the Modulating Role of Probiotics. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 24. | Gao K, Farzi A, Ke X, Yu Y, Chen C, Chen S, Yu T, Wang H, Li Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022;13:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Guo H, Liu X, Chen T, Wang X, Zhang X. Akkermansia muciniphila Improves Depressive-Like Symptoms by Modulating the Level of 5-HT Neurotransmitters in the Gut and Brain of Mice. Mol Neurobiol. 2024;61:821-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 26. | Tian P, Wang G, Zhao J, Zhang H, Chen W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J Nutr Biochem. 2019;66:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 27. | Zhao L, Li D, Chitrakar B, Li C, Zhang N, Zhang S, Wang X, Wang M, Tian H, Luo Y. Study on Lactiplantibacillus plantarum R6-3 from Sayram Ketteki to prevent chronic unpredictable mild stress-induced depression in mice through the microbiota-gut-brain axis. Food Funct. 2023;14:3304-3318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 28. | Tian P, Chen Y, Qian X, Zou R, Zhu H, Zhao J, Zhang H, Wang G, Chen W. Pediococcus acidilactici CCFM6432 mitigates chronic stress-induced anxiety and gut microbial abnormalities. Food Funct. 2021;12:11241-11249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Yang H, Jiang J, Pei Y, Qian X, Tian P, Wang G, Zhao J. Pediococcus acidilactici CCFM6432 improves depressive behavior in mice by alleviating brain inflammation. Food Ferment Ind. 2023;50:25-32. [DOI] [Full Text] |
| 30. | Tian P, Zou R, Wang L, Chen Y, Qian X, Zhao J, Zhang H, Qian L, Wang Q, Wang G, Chen W. Multi-Probiotics ameliorate Major depressive disorder and accompanying gastrointestinal syndromes via serotonergic system regulation. J Adv Res. 2023;45:117-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 31. | Tian P, Yang H, Hang F, Wang G, Mao X, Jin X, Zhao J. Evaluation of the clinical efficacy of Pediococcus acidilactici CCFM6432 in alleviating depression. Microbiome Res Rep. 2024;3:49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Chen Y, Xu J, Zhou L, Zheng Y. The time course of incentive processing in anticipatory and consummatory anhedonia. J Affect Disord. 2018;238:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Wang Z, Li Q, Nie L, Zheng Y. Neural dynamics of monetary and social reward processing in social anhedonia. Soc Cogn Affect Neurosci. 2020;15:991-1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Wu C, Mu Q, Gao W, Lu S. The characteristics of anhedonia in depression: a review from a clinically oriented perspective. Transl Psychiatry. 2025;15:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 35. | Li Y, Mou X, Jiang W, Yang Z, Shen X, Jin Z, Dai Z, Liu Y, Mao S, Zhang J, Yuan Y. A comparative study of anhedonia components between major depression and schizophrenia in Chinese populations. Ann Gen Psychiatry. 2015;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Ma Y, Guo C, Luo Y, Gao S, Sun J, Chen Q, Lv X, Cao J, Lei Z, Fang J. Altered neural activity in the reward-related circuit associated with anhedonia in mild to moderate Major Depressive Disorder. J Affect Disord. 2024;345:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Zhang T, He K, Bai T, Lv H, Xie X, Nie J, Xie W, Zhu C, Wang K, Tian Y. Altered neural activity in the reward-related circuit and executive control network associated with amelioration of anhedonia in major depressive disorder by electroconvulsive therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Chan RC, Shi YF, Lai MK, Wang YN, Wang Y, Kring AM. The Temporal Experience of Pleasure Scale (TEPS): exploration and confirmation of factor structure in a healthy Chinese sample. PLoS One. 2012;7:e35352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Zhou H, Liu W, Fan J, Xia J, Zhu J, Zhu X. The Temporal Experience of Pleasure Scale (TEPS): Measurement Invariance Across Gender in Chinese University Students. Front Psychol. 2019;10:2130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Liu WH, Wang LZ, Zhu YH, Li MH, Chan RC. Clinical utility of the Snaith-Hamilton-Pleasure scale in the Chinese settings. BMC Psychiatry. 2012;12:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Yong N, Hu H, Fan X, Li X, Ran L, Qu Y, Wang Y, Tan G, Chen L, Zhou J. Prevalence and risk factors for depression and anxiety among outpatient migraineurs in mainland China. J Headache Pain. 2012;13:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Zheng YP, Zhao JP, Phillips M, Liu JB, Cai MF, Sun SQ, Huang MF. Validity and reliability of the Chinese Hamilton Depression Rating Scale. Br J Psychiatry. 1988;152:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 339] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 43. | Fang S, Huang X, Zhang P, He J, Luo X, Zhang J, Xiong Y, Luo F, Wang X, Yao S, Wang X. Factor structure and sex invariance of the temporal experience of pleasure scale (TEPS) in Chinese university students and clinical population. BMC Psychiatry. 2021;21:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 44. | Keren H, O'Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, Pan PM, Meffert L, Kaiser A, Wolke S, Pine DS, Stringaris A. Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. Am J Psychiatry. 2018;175:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 45. | Sun Y, Huang Z, Gao X, Chen L, Wang J, Zhou Z, Zhou H. Neural Correlates of Anhedonia in Major Depressive Disorder: Insights from Concurrent Analysis of Feedback-Related Negativity and Stimulus-Preceding Negativity. Neuropsychiatr Dis Treat. 2023;19:2549-2560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 46. | Ren X, White EJ, Nacke M, Mayeli A, Touthang J, Al Zoubi O, Kuplicki R, Victor TA, Paulus MP, Aupperle RL, Stewart JL. Blunted stimulus-preceding negativity during reward anticipation in major depressive disorder. J Affect Disord. 2024;362:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 47. | Burani K, Klawohn J, Levinson AR, Klein DN, Nelson BD, Hajcak G. Neural Response to Rewards, Stress and Sleep Interact to Prospectively Predict Depressive Symptoms in Adolescent Girls. J Clin Child Adolesc Psychol. 2021;50:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Proudfit GH. The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology. 2015;52:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 680] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 49. | Catalano LT, Wynn JK, Green MF, Gold JM. Reduced neural activity when anticipating social versus nonsocial rewards in schizophrenia: Preliminary evidence from an ERP study. Schizophr Res. 2022;246:7-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Brush CJ, Hajcak G, Bocchine AJ, Ude AA, Muniz KM, Foti D, Alderman BL. A randomized trial of aerobic exercise for major depression: examining neural indicators of reward and cognitive control as predictors and treatment targets. Psychol Med. 2022;52:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 51. | Schaub AC, Schneider E, Vazquez-Castellanos JF, Schweinfurth N, Kettelhack C, Doll JPK, Yamanbaeva G, Mählmann L, Brand S, Beglinger C, Borgwardt S, Raes J, Schmidt A, Lang UE. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: a randomized controlled trial. Transl Psychiatry. 2022;12:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 148] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 52. | Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 53. | Nikolova VL, Cleare AJ, Young AH, Stone JM. Acceptability, Tolerability, and Estimates of Putative Treatment Effects of Probiotics as Adjunctive Treatment in Patients With Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2023;80:842-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 87] [Reference Citation Analysis (0)] |
| 54. | Nadeem I, Rahman MZ, Ad-Dab'bagh Y, Akhtar M. Effect of probiotic interventions on depressive symptoms: A narrative review evaluating systematic reviews. Psychiatry Clin Neurosci. 2019;73:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 55. | Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O, Welsh C, Rieder A, Traynor J, Gregory C, De Palma G, Pigrau M, Ford AC, Macri J, Berger B, Bergonzelli G, Surette MG, Collins SM, Moayyedi P, Bercik P. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology. 2017;153:448-459.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 595] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 56. | Wallace CJK, Milev RV. The Efficacy, Safety, and Tolerability of Probiotics on Depression: Clinical Results From an Open-Label Pilot Study. Front Psychiatry. 2021;12:618279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 57. | Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 777] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 58. | Lucido MJ, Bekhbat M, Goldsmith DR, Treadway MT, Haroon E, Felger JC, Miller AH. Aiding and Abetting Anhedonia: Impact of Inflammation on the Brain and Pharmacological Implications. Pharmacol Rev. 2021;73:1084-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 59. | Costi S, Morris LS, Collins A, Fernandez NF, Patel M, Xie H, Kim-Schulze S, Stern ER, Collins KA, Cathomas F, Parides MK, Whitton AE, Pizzagalli DA, Russo SJ, Murrough JW. Peripheral immune cell reactivity and neural response to reward in patients with depression and anhedonia. Transl Psychiatry. 2021;11:565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/