Published online May 19, 2025. doi: 10.5498/wjp.v15.i5.102618
Revised: February 22, 2025
Accepted: March 21, 2025
Published online: May 19, 2025
Processing time: 188 Days and 8 Hours
Major depressive disorder (MDD) is characterized by persistent depressed mood and cognitive symptoms. This study aimed to discover biomarkers for MDD, explore its pathological mechanisms, and examine the associations of the identified biomarkers with clinical and psychological variables.
To discover candidate biomarkers for MDD identification and provide insight into the pathological mechanism of MDD.
The current study adopted a single-center cross-sectional case-control design. Serum samples were obtained from 100 individuals diagnosed with MDD and 97 healthy controls (HCs) aged between 18 to 60 years. Metabolomics was performed on an Ultimate 3000 UHPLC system coupled with Q-Exactive MS (Thermo Scien
The study included 100 MDD patients and 97 HCs. Metabolomic profiling identified 35 significantly different metabolites (e.g., cortisol, sebacic acid, and L-glutamic acid). Receiver operating characteristic curve analysis highlighted 8-HETE, 10-HDoHE, cortisol, 12-HHTrE, and 10-hydroxydecanoic acid as top diagnostic biomarkers for MDD. Significant correlations were found between metabolites (e.g., some lipids, steroids, and amino acids) and clinical and psychological variables.
Our study reported metabolites (some lipids, steroids, amino acids, carnitines, and alkaloids) responsible for discriminating MDD patients and HCs. This metabolite profile may enable the development of a laboratory-based diagnostic test for MDD. The mechanisms underlying the association between psychological or clinical variables and differential metabolites deserve further exploration.
Core Tip: Our study reported metabolites (some lipids, steroids, amino acids, carnitines, and alkaloids) responsible for discriminating major depressive disorder (MDD) patients and healthy controls. This metabolite signature may facilitate the development of a laboratory-based diagnostic test for MDD. The mechanisms underlying the association between psychological or clinical variables and differential metabolites deserve further exploration.
- Citation: Cao B, Liu YL, Wang N, Huang Y, Lu CX, Li QY, Zou HY. Alterations of serum metabolic profile in major depressive disorder: A case-control study in the Chinese population. World J Psychiatry 2025; 15(5): 102618
- URL: https://www.wjgnet.com/2220-3206/full/v15/i5/102618.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i5.102618
Major depressive disorder (MDD) is characterized by persistent depressed mood, loss of interest or pleasure in previously enjoyable activities, recurrent thoughts of death, and physical and cognitive symptoms[1], which is highly prevalent and disabling. The Global Burden of Disease Study (GBD 2021) estimated that approximately 4.7% of people worldwide had a depressive episode in the past 12 months. Currently, the diagnosis of MDD still relies on the subjective identification of symptom clusters rather than empirical laboratory tests. The current diagnostic modality results in a considerable error rate. Thus, the diagnosis of MDD is still difficult[2]. A meta-analysis in 2022 indicated that the extremely low detection of depression by primary care clinicians poses a serious threat to scaling up mental healthcare in low and middle-income countries[3]. Consequently, the identification of high-quality depression biomarkers and the development of precise and accessible early diagnostic tools are imperative[4]. However, research in this field has thus far[2] not lived up to its potential[2].
The scientific community concurs that several biological, psychological, and social environmental variables collectively contribute to the pathogenesis of MDD[5]. The biogenic amine (monoamine) hypothesis, neuroendocrine dysregulation, cytokine theory, and hereditary variables[6], are several pathophysiological mechanisms of MDD that are generally agreed upon[7]. Specific to the field of metabolomics, there have been some comparisons between MDD and healthy controls (HCs) in previous studies. A comprehensive meta-analysis revealed 23 differentially expressed metabolites between patients with MDD and controls across 46 studies, encompassing amino acids (L-glutamine, L-serine, L-methionine, and L-tryptophan), lipids [phosphatidylcholine (32:1), linoleic acid, palmitoleic acid, oleic acid, dodecanoic acid, and palmitic acid], carnitines (L-acetylcarnitine), and various other metabolites[8]. Paige et al[9] reported that several fatty acids, glycerol, and γ-aminobutyric acid (GABA) were altered in currently depressed patients when compared with controls. Zheng et al[10] found 17 differentially expressed peripheral blood mononuclear cell metabolites to discriminate patients with MDD from HCs, including amino acids (GABA, homoserine, isoleucine, and valine), lipids (octanoic acid, lanosterol, and γ-tocopherol), and some other metabolites. Ali-Sisto et al[11] indicated that serum concentrations of inosine and guanosine diminished, while the levels of xanthine and adenosine were elevated in participants with MDD compared to non-depressed controls. Despite advancements in metabolomics research concerning MDD, the majority of these studies have produced inconsistent findings, hence limiting their clinical applicability. Previous studies have also demonstrated that psychological variables [including the Snaith-Hamilton Pleasure Scale (SHAPS), the 5-item World Health Organization Well-Being Index (WHO-5), the Hamilton Depression Rating Scale-24 items (HAMD-24), and the Generalized Anxiety Disorder 7-item scale (GAD-7)] and clinical variables [body mass index (BMI)] play roles in regulating differential metabolites. Irregularities in reward processing are significant indicators of MDD and schizophrenia[12]. Specifically, these variables may influence metabolic pathways through mechanisms such as neuroendocrine regulation, inflammation, and oxidative stress, thereby contributing to the metabolic alterations observed in psychiatric disorders. Anhedonia may also affect metabolite production and regulation through neuroendocrine pathways[13]. The WHO-5 is one of the most commonly used questionnaires for evaluating subjective psychological well-being[14]. A systematic study indicated a potential beneficial correlation between the neurotransmitter serotonin and well-being (i.e., hedonic well-being). The evidence on the role of additional small molecules, including metabolites, remains inconclusive[15]. A recent large-scale metabolomics meta-analysis showed that depression is associated with a signature in circulating metabolites[16], suggesting that changes in HAMD-24 scores may indirectly reflect dynamic changes in metabolites. GAD-7 is used to assess the severity of anxiety symptoms. Metabolite association studies examining anxiety in animal models and clinical cohorts have revealed changed concentrations of several metabolites[17]. The BMI is the best available tool for monitoring progress in the campaign against obesity[18]. An increasing body of evidence indicates that metabolic abnormalities arising from central obesity, which contribute to metabolic disorders, may also account for the heightened prevalence of depression in individuals with obesity[19]. Furthermore, renal function levels may indirectly indicate human metabolism and excretion. Evidence indicates that patients with depression frequently exhibit renal function abnormalities[20].
In this study, liquid chromatography coupled with mass spectrometry (LC-MS) was used to identify the differential metabolites in serum samples from patients with MDD and HCs, with an aim to discover candidate biomarkers for MDD identification and provide insight into the pathological mechanism of MDD. We also attempted to explore the metabolic mechanisms of differential metabolites by examining their association with other clinical variables [BMI, blood urea nitrogen (BUN), creatinine (CREA), and uric acid (URIC)] and psychological variables [disease severity, pleasure deficit (SHAPS), anxiety (GAD-7), and well-being (WHO-5)].
This study was approved by the Ethics Committee of Chongqing Ninth People's Hospital (Approval No. IRB-2021-016), and performed conforming to the Declaration of Helsinki. Before sample collection, written informed consent was obtained from all participants.
A cross-sectional patient-control design was employed to compare patients with MDD aged 18 to 60 years to age- and region-matched controls who had never experienced depression, in order to delineate the metabolic profile of MDD patients[2,21]. A total of 197 participants were enrolled in this study, including 100 patients diagnosed with MDD and 97 HCs. All participants were voluntarily recruited from Chongqing Ninth People's Hospital in Chongqing, China between January 2022 and December 2024. The DSM-V diagnostic criteria and the HAMD-24 were evaluated for a singular de
Demographic information from all participants was collected by trained healthcare workers. Information was collected on gender, age, body mass index, education level, smoking, and alcohol consumption. The HAMD-24 was used to assess the severity of mental symptoms, whilst the SHAPS is regarded as the optimal instrument for evaluating the sense of pleasure. The GAD-7 was used to assess different types of anxiety disorders in the general population. Standard clinical tests for renal function include BUN, CREA, and URIC. BMI was employed to measure an individual's degree of obesity.
Approximately 8.5 mL of blood samples were collected after a 12-hour fasting period in the morning (between 7-9 a.m.). Serum was separated by centrifugation at 3000 × g for 4 min at 4 °C, and then aliquoted into labeled 1.5-mL Eppendorf vials and stored at -80 °C until analysis. Metabolites were extracted from the serum samples using liquid-liquid extraction. In brief, 100 μL of serum was extracted by fourfold volume of cold chloroform:methanol (v/v = 2:1). The mixture was centrifuged at 13000 × g for 15 min, and then the upper and lower phases were separately collected and evaporated under vacuum. The dried samples were stored at -80 °C until LC-MS analysis. For LC-MS analysis, the aqueous phase was dissolved in 50 μL water. The organic phase was dissolved in 100 μL chloroform/methanol (1:1), and diluted with 300 μL isopropanol/acetonitrile/water (2:1:1). After centrifugation at 12000 rpm for 15 min, 6 μL of supernatant was injected for LC-MS analysis.
Metabolomics was performed on an Ultimate 3000 UHPLC system coupled with Q-Exactive MS (Thermo Scientific). An Xbridge amide column (100 × 4.6 mm, inner diameter, 3.5 μm; Waters) was used to separate compounds in the water phase (metabolomics) at 30 °C. The mobile phase A consisted of 5 mmol/L ammonium acetate in water with 5% ace
Data-dependent acquisition (DDA) was performed using the Q-Exactive HF MS (Thermo Fisher Scientific, Waltham, MA, United States) as previously described. Each acquisition cycle consisted of one survey scan (MS1) at 60000 resolution from 60 to 900 m/z for hydrophilic metabolites, followed by 10 MS/MS scans in HCD mode at 15000 resolution using step-NCE of 15, 30, and 45. The dynamic exclusion was set to 10 s. Acquisition was performed in positive and negative ion modes separately. The automatic gain control target was set to 5e6 and 2e5 for the MS1 and MS/MS scans, res
Raw data obtained from the DDA-MS were processed with MS-DIAL software v3.6 according to the user guide as previously described[22]. Briefly, the raw MS data was converted into the standardized .abf file format using the Reifycs ABF converter (http://www.reifycs.com/AbfConverter/index.html). Next, MS-DIAL software was employed to perform feature detection, spectra deconvolution, metabolite identification, and peak alignment. Briefly, the MS1 and MS2 spectra-based metabolite identification was performed in MSDIAL by searching the acquired spectra against the MassBank database provided by MSDIAL software, containing information about metabolites. The tolerance for MS1 and MS/MS search was set to 0.01 Da and 0.05 Da, separately. The threshold for the identification score was established at 70%. The remaining parameters used in MS-DIAL were set as default.
Basic information was analyzed using SPSS 28.0 software. Numerical data are presented as the mean ± SD, or median and interquartile range, while categorical variables are summarized using frequencies and proportions [n (%)]. An in
The online software Metaboanalyst 6.0 (https://www.metaboanalyst.ca/) was used to process and analyze the ob
A total of 100 adults diagnosed with MDD, consisting of 44 males and 56 females, were included in the study. Additionally, 97 HCs were recruited, including 28 males and 69 females. No significant differences were seen in age, education level, smoking status, or drinkers between the two participant groups (all P > 0.05). However, it is worth noting that the sex distribution in the MDD group was more varied compared to the HC group. The mean BMI of HCs was significantly higher than that of MDD patients (22.21 ± 4.25 vs 23.91 ± 2.14, P < 0.001). Table 1 displays the clinical and demographic features of the participants. The SHAPS, GAD-7, and HAMD-24 scores of HCs were significantly lower than those of MDD patients (all P < 0.001).
| Variable | MDD patients (n = 100) | HCs (n = 97) | P value |
| Age (years), mean ± SD | 39.07 ± 15.23 | 40.33 ± 6.61 | 0.238 |
| Sex, n (%) | 0.027 | ||
| Male | 44 (44.0) | 28 (28.9) | |
| Female | 56 (56.0) | 69 (71.1) | |
| Education level, n (%) | 0.751 | ||
| Primary school | 19 (19.0) | 22 (22.7) | |
| Secondary school | 48 (48.0) | 41 (42.3) | |
| High school | 23 (23.0) | 21 (21.6) | |
| Undergraduate or above | 10 (10.0) | 13 (13.4) | |
| Drinker, n (%) | 11 (11.0) | 16 (16.5) | 0.262 |
| Smoker, n (%) | 8 (8.0) | 15 (15.5) | 0.103 |
| BMI (kg/m2), mean ± SD | 22.21 ± 4.25 | 23.91 ± 2.14 | 0.001 |
| HAMD-24, mean ± SD | 27.14 ± 6.10 | 2.08 ± 1.63 | < 0.001 |
| SHAPS, mean ± SD | 37.71 ± 4.97 | 18.33 ± 3.51 | < 0.001 |
| GAD-7, mean ± SD | 19.76 ± 3.28 | 7.54 ± 0.93 | < 0.001 |
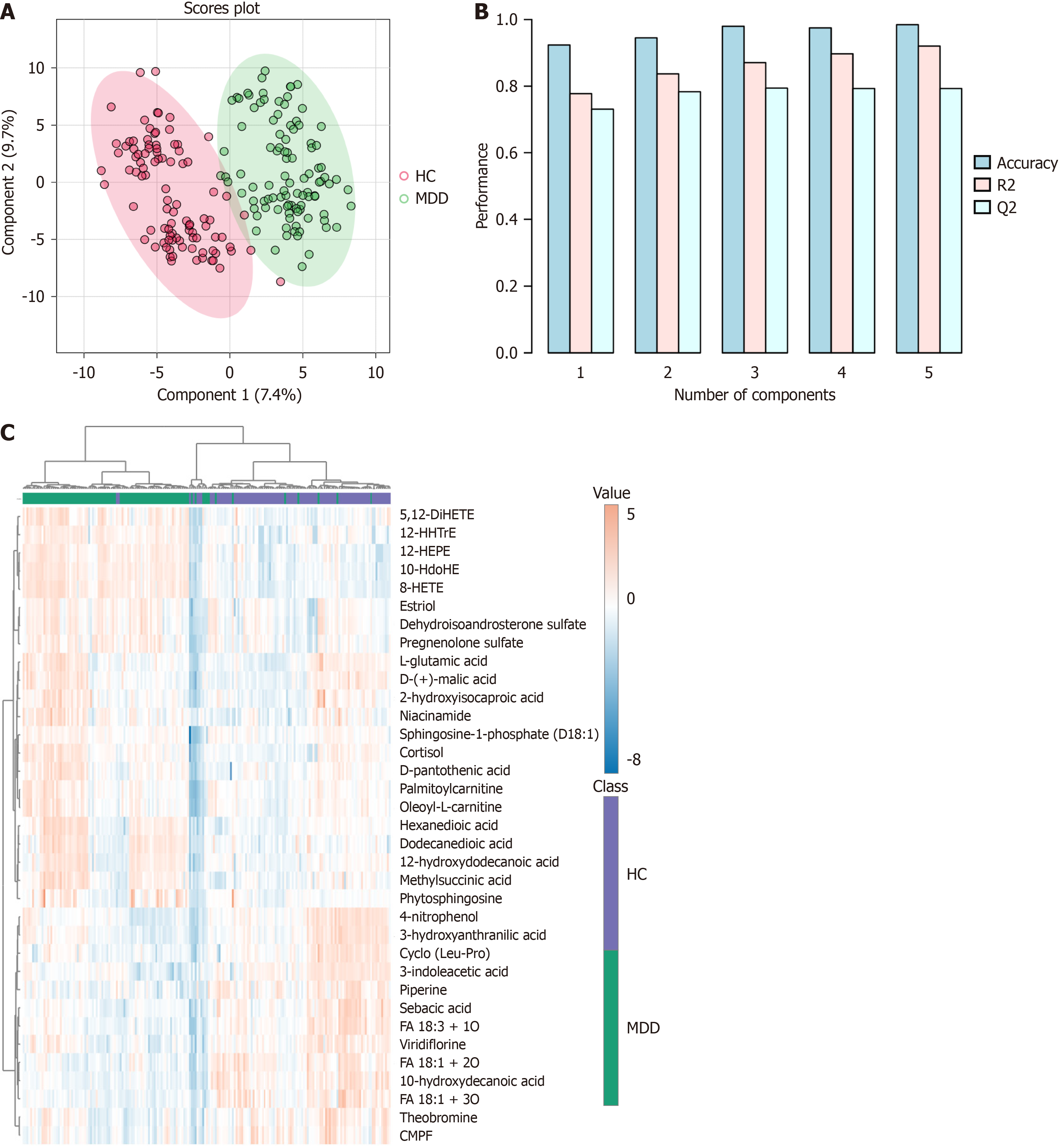
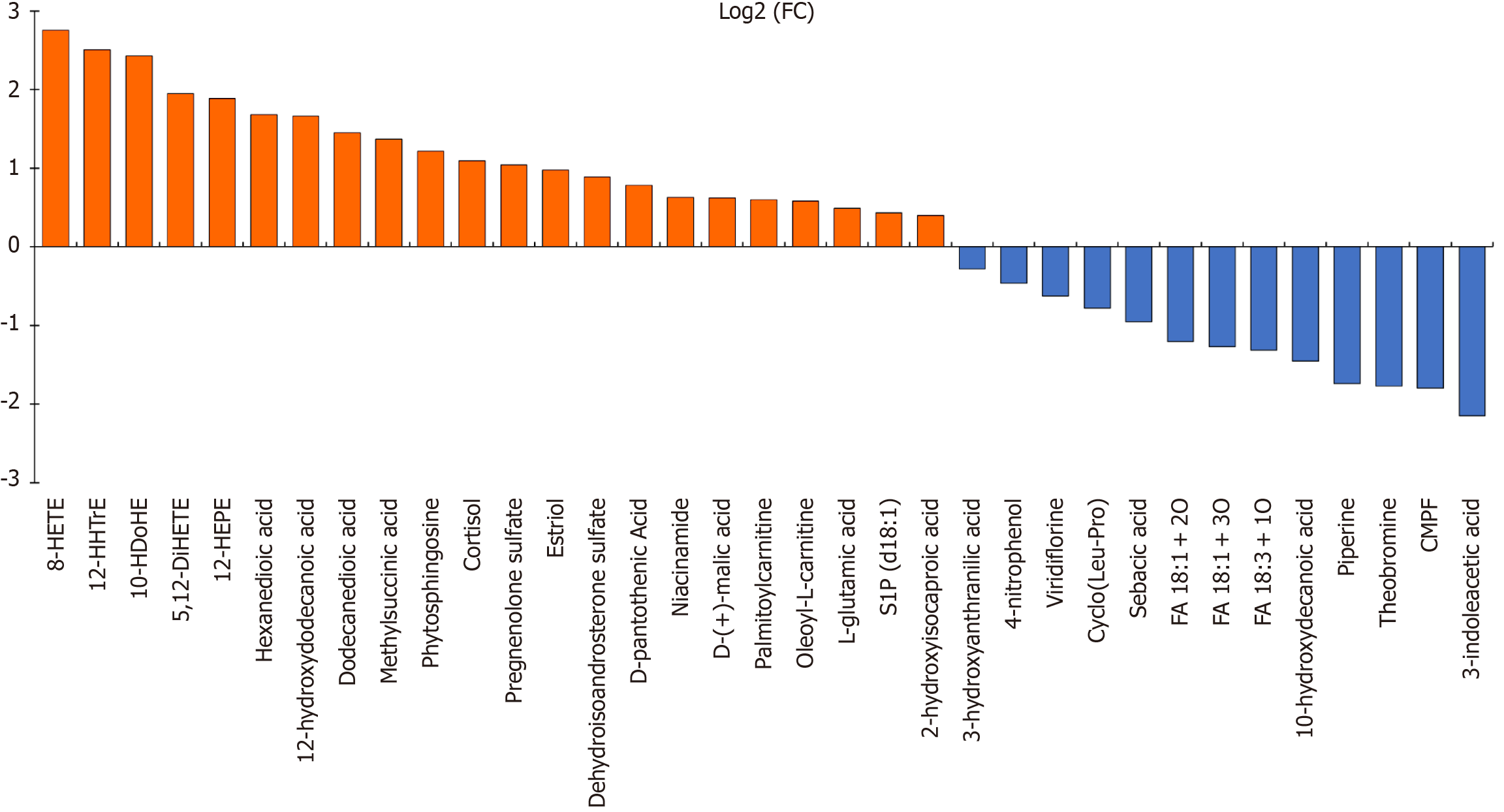
Upon concluding data processing, a total of 197 features were incorporated into the data analysis to identify differences in metabolomic profiles between the MDD group and HC group. The PCA scatter plot (Supplementary Figure 1A) shows a significant separation of groups. The screen plot indicates that the top five PCs accounted for 39.9% of the accumulated variance (Supplementary Figure 1B). The PLS-DA graph reflects remarkable separations of MDD and HCs in Figure 1. Differential metabolites between the HC and MDD groups were selected based on VIP > 1.5, FDR adjusted P < 0.05, and FC > 1.2 or < 0.83. A total of 35 identified metabolites were considered significantly different in serum (Table 2). Compared to HCs, 22 metabolites were raised and 13 were decreased in the MDD group. A heatmap was created to visually depict the relative changes in these metabolites (Figure 1C). For the untargeted metabolomic profiling, a total of 20 positive-mode features were identified, including 2 kinds each of lipids, steroids, amino acids, carnitines, and alkaloids, and 5 kinds of other metabolites. Additionally, 15 negative-mode features were identified, consisting of 13 kinds of lipids, 2 kinds of steroids, and 2 kinds of other metabolites. The majority of the discovered differential metabolites consisted of lipids and steroids, while there were also amino acids, carnitines, and alkaloids. The comprehensive information regarding these metabolites can be found in Table 2. The log2 transformed FC for the 35 metabolites that showed differential expression between individuals with MDD and HCs (Figure 2). The volcano plot demonstrates the significant differences of metabolites between the MDD and HC samples (Supplementary Figure 2). The heatmap of the top 25 features obtained by the t-test is shown in Supplementary Figure 3. R-SVM showed that the error rate of the first six features was only 19.8% (Supplementary Figure 4). Pathway analysis of potential mechanisms of candidate metabolic biomarkers for MDD is shown in Supplementary Figure 5.
| Adduct type | Metabolite | Reference m/z | FC | log2 (FC) | FDR | VIP | Ontology | Trend |
| POS | Phytosphingosine | 318.30 | 2.33 | 2.43 | 3.34E-16 | 2.42 | 1,3-aminoalcohols | ↑ |
| Cortisol | 363.22 | 2.14 | -1.45 | 1.00E+00 | 3.23 | 21-hydroxysteroids | ↑ | |
| Estriol | 289.18 | 1.97 | 1.89 | 2.38E-18 | 2.55 | Estrogens and derivatives | ↑ | |
| D-pantothenic acid | 220.12 | 1.72 | 2.50 | 4.40E-06 | 1.50 | Secondary alcohols | ↑ | |
| Niacinamide | 123.06 | 1.54 | 1.66 | 3.13E-07 | 1.64 | Nicotinamides | ↑ | |
| Palmitoylcarnitine | 400.34 | 1.51 | 0.40 | 1.74E-10 | 1.98 | Acyl carnitines | ↑ | |
| Oleoyl-L-carnitine | 426.36 | 1.50 | -0.28 | 7.24E-07 | 1.60 | Acyl carnitines | ↑ | |
| L-glutamic acid | 146.05 | 1.41 | -2.15 | 3.19E-11 | 2.05 | Glutamic acid and derivatives | ↑ | |
| S1P (d18:1) | 380.26 | 1.35 | -0.46 | 2.32E-16 | 2.43 | Phosphosphingolipids | ↑ | |
| Viridiflorine | 286.20 | 0.65 | 1.95 | 4.77E-18 | 2.53 | Pyrrolizidines | ↓ | |
| Cyclo(Leu-Pro) | 211.14 | 0.58 | 2.76 | 6.45E-09 | 1.83 | Alpha amino acids and derivatives | ↓ | |
| Sebacic acid | 225.11 | 0.52 | -1.80 | 9.56E-09 | 1.81 | Medium-chain fatty acids | ↓ | |
| Piperine | 286.14 | 0.30 | 1.10 | 7.26E-09 | 1.82 | Alkaloids and derivatives | ↓ | |
| Theobromine | 181.02 | 0.29 | -0.78 | 3.13E-07 | 1.64 | Xanthines | ↓ | |
| 3-indoleacetic acid | 176.07 | 0.23 | 0.22 | 1.44E-07 | 1.68 | Indole-3-acetic acid derivatives | ↓ | |
| NEG | 8-HETE | 279.20 | 6.77 | 0.89 | 1.71E-26 | 2.96 | Long-chain fatty acids | ↑ |
| 12-HHTrE | 319.23 | 5.67 | 1.45 | 8.90E-22 | 2.74 | Hydroxyeicosatetraenoic acids | ↑ | |
| 10-HDoHE | 343.23 | 5.38 | 0.78 | 3.68E-21 | 2.71 | Very long-chain fatty acids | ↑ | |
| 5,12-DiHETE | 335.22 | 3.86 | 0.98 | 5.46E-16 | 2.40 | Leukotrienes | ↑ | |
| 12-HEPE | 317.21 | 3.70 | -1.20 | 7.26E-09 | 1.83 | Hydroxyeicosapentaenoic acids | ↑ | |
| Hexanedioic acid | 145.05 | 3.21 | -1.27 | 3.54E-08 | 1.75 | Medium-chain fatty acids | ↑ | |
| 12-hydroxydodecanoic acid | 215.17 | 3.17 | -1.32 | 3.13E-07 | 1.64 | Medium-chain hydroxy acids and derivatives | ↑ | |
| Dodecanedioic acid | 229.14 | 2.73 | 1.68 | 7.04E-15 | 2.33 | Medium-chain fatty acids | ↑ | |
| Methylsuccinic acid | 131.03 | 2.59 | 0.49 | 6.38E-11 | 2.02 | Methyl-branched fatty acids | ↑ | |
| Pregnenolone sulfate | 395.19 | 2.06 | 1.37 | 3.07E-08 | 1.76 | Sulfated steroids | ↑ | |
| Dehydroisoandrosterone sulfate | 367.16 | 1.85 | 0.63 | 6.90E-08 | 1.72 | Sulfated steroids | ↑ | |
| D-(+)-malic acid | 133.01 | 1.54 | 0.59 | 1.02E-12 | 2.17 | Beta hydroxy acids and derivatives | ↑ | |
| 2-hydroxyisocaproic acid | 131.07 | 1.32 | 0.60 | 5.57E-14 | 2.27 | Hydroxy fatty acids | ↑ | |
| 3-hydroxyanthranilic acid | 152.03 | 0.82 | 1.22 | 1.63E-18 | 2.57 | Hydroxybenzoic acid derivatives | ↓ | |
| 4-nitrophenol | 138.02 | 0.73 | -1.74 | 1.57E-09 | 1.90 | Nitrophenols | ↓ | |
| FA 18:1 + 2O | 313.24 | 0.43 | 1.04 | 2.34E-11 | 2.06 | Long-chain fatty acids | ↓ | |
| FA 18:1 + 3O | 329.23 | 0.42 | -0.95 | 1.38E-07 | 1.68 | Long-chain fatty acids | ↓ | |
| FA 18:3 + 1O | 297.24 | 0.40 | 0.43 | 6.45E-09 | 1.83 | Lineolic acids and derivatives | ↓ | |
| 10-hydroxydecanoic acid | 187.14 | 0.37 | -1.77 | 1.93E-09 | 1.89 | Medium-chain hydroxy acids and derivatives | ↓ | |
| CMPF | 239.09 | 0.29 | -0.62 | 1.67E-08 | 1.79 | Furanoid fatty acids | ↓ |
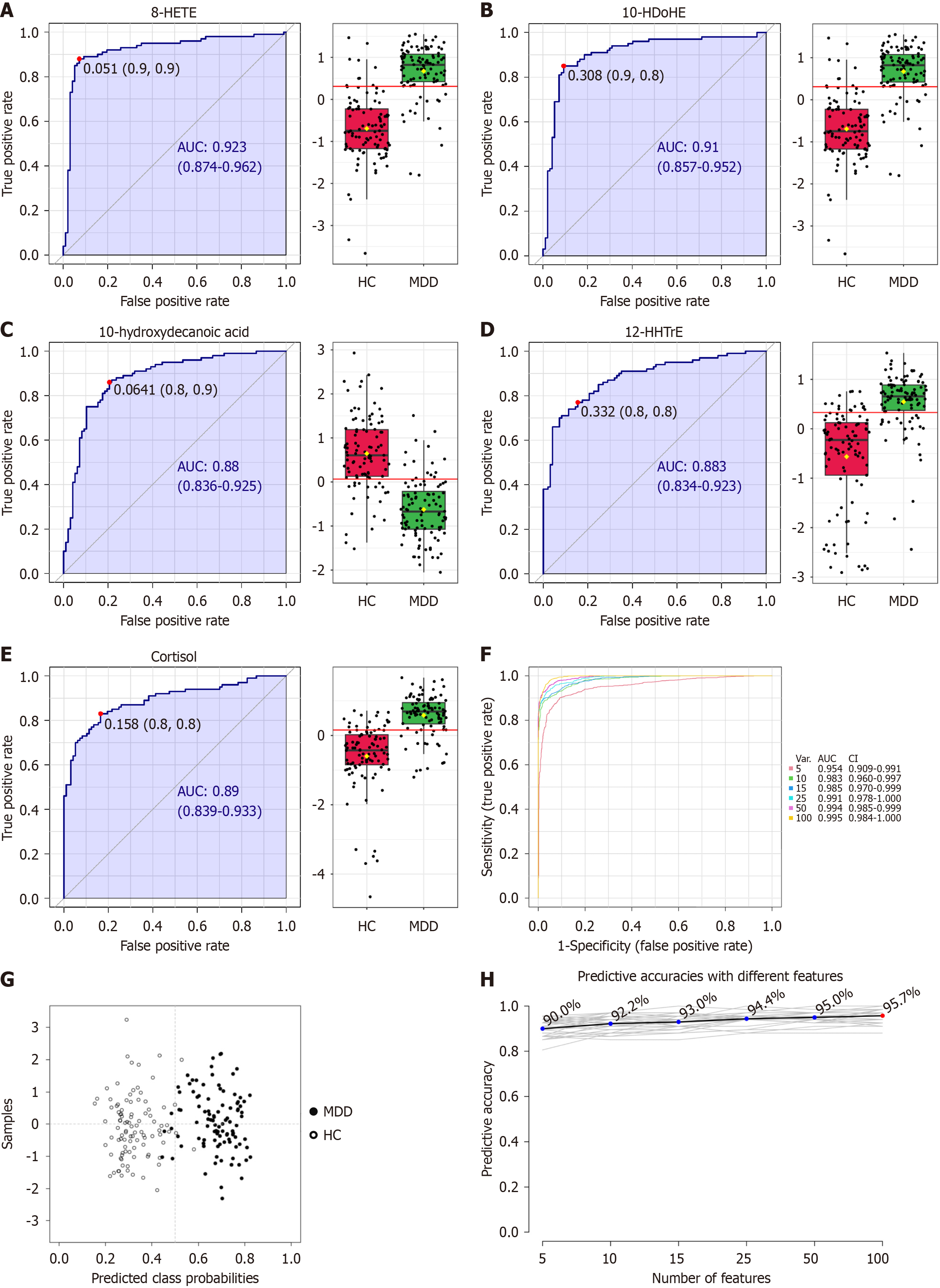
ROC analyses were performed on each plasma metabolite to evaluate the diagnostic significance of the dysregulated metabolites. 8-HETE, 10-HDoHE, 10-hydroxydecanoic acid, 12-HHTrE, and cortisol were identified as the top 5 efficient diagnostic biomarkers for MDD from HCs, with an AUC of 0.923, 0.910, 0.880, 0.883, and 0.890, respectively. ROC analysis demonstrated the following performance metrics: For 8-HETE, the sensitivity, specificity, positive predictive value, and negative predictive value were 90%, 10%, 50.76%, and 49.24%, respectively; for 10-HDoHE, these values were 90%, 20%, 53.7%, and 65.9%; for 10-hydroxydecanoic acid, they were 80%, 10%, 47.8%, and 32.7%; and for both 12-HHTrE and cortisol, the corresponding values were 80%, 20%, 50.76%, and 49.24%. Figure 3A-E displays the relative concentrations of these metabolite biomarkers for depression. Multiple ROC curves constructed with 5, 15, 25, 50, and 100 lipids are shown in Figure 3F. The top five curves attained an AUC of 0.954, and the 100 lipids achieved an AUC of 0.995. Figure 3G and H illustrates the predictive accuracy of each PLS-DA model developed with different numbers of features.
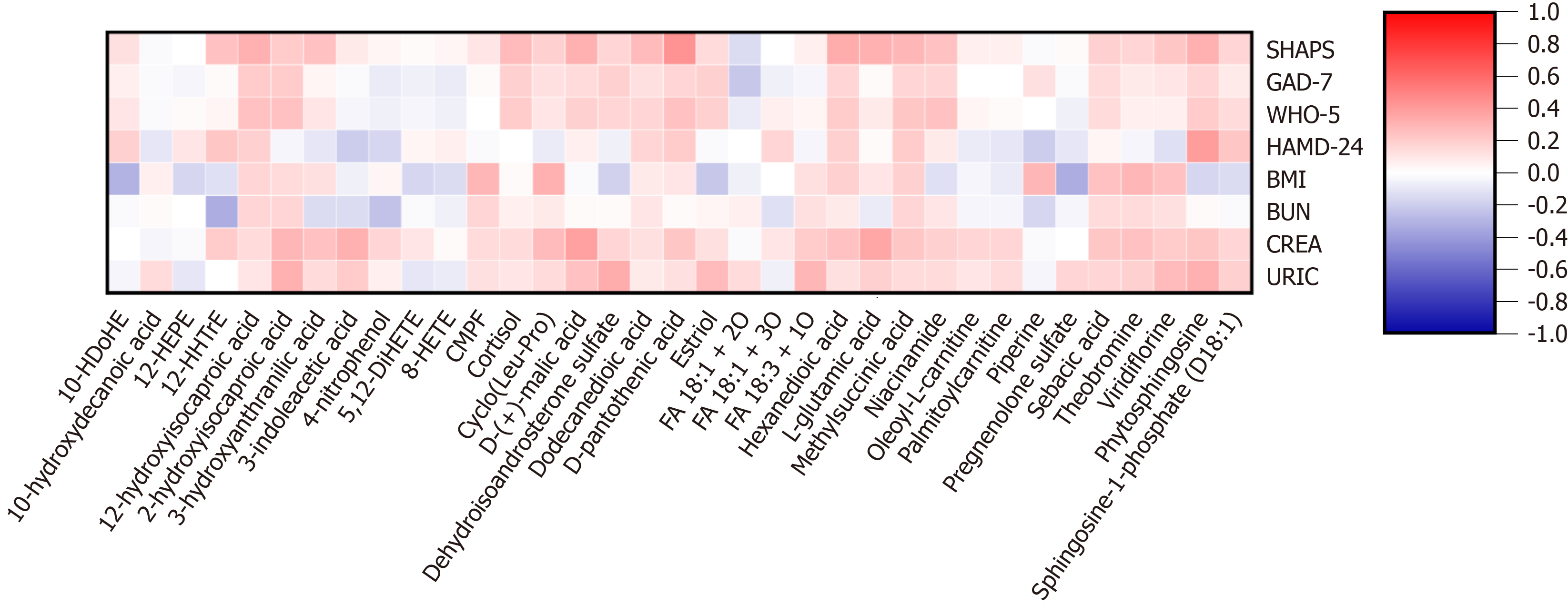
Lipids (e.g., 12-hydroxydodecanoic acid and hexanedioic acid), steroids (e.g., cortisol and dehydroisoandrosterone sulfate), and some other metabolites (e.g., phytosphingosine and 2-hydroxyisocaproic acid) were found to be positively correlated with psychological variables (e.g., SHAPS, WHO-5, HAMD-24, and GAD-7). Lipids (e.g., sebacic acid and methylsuccinic acid), amino acids [e.g., cyclo(Leu-Pro) and L-glutamic acid], and some other metabolites [e.g., d-(+)-malic acid, viridiflorine, and 2-hydroxyisocaproic acid] were found to be positively correlated with clinical variables (e.g., BMI, BUN, CREA, and URIC). Spearman correlations of metabolites and variables are shown in Figure 4. The results of supplementary regression analysis are shown in the supplementary table[23,24].
The present study employed GC-MS-based metabolomics to investigate the metabolomic profile of patients with MDD and identify potential diagnostic biomarkers. The primary findings are as follows: (1) 35 differential metabolites responsible for discriminating MDD patients and HCs were identified. We found that MDD patients showed a decrease in 13 types of differential metabolites (mainly belongs to lipids and alkaloids) in patients with depression and an increase in 22 types (mainly belongs to lipids, steroids, carnitines, and amino acids); (2) The AUC values of 14 metabolites are above 0.8. Additionally, a combination of five metabolites for the diagnosis of MDD patients was established, demonstrating the ability to distinguish MDD patients from HCs with an AUC of 0.954; and (3) The concentrations of some lipids and steroids (e.g., 12-hydroxydodecanoic acid and cortisol) were positively correlated with a number of metabolic markers reflecting BMI and renal functions (i.e., CREA, URIC, and BUN). Some lipids and amino acids [e.g., sebacic acid and cyclo(Leu-Pro)] were positively correlated with psychological variables (e.g., SHAPS, WHO-5, HAMD-24, and GAD-7).
Lipids: Fatty acids (FAs), constituting the majority of lipids in human physiology and dietary sources, are essential for numerous physiological and pathological processes[25,26]. Dietary FAs can be classified into saturated FAs (SFAs), monounsaturated FAs (MUFAs), and polyunsaturated FAs (PUFAs) based on the amount and type of double bonds, as well as chain length[27]. In the present study, the levels of most SFAs (hexanedioic acid, 12-hydroxydodecanoic acid, dodecanedioic acid, and methylsuccinic acid) increased while some (sebacic acid and 10-hydroxydecanoic acid) decreased in patients with MDD compared with HCs. A human cross-sectional investigation indicated a significant positive con
The levels of ω-6 PUFAs (8-HETE, 12-HHTrE, and 5,12-DiHETE) increased while those of ω-3 PUFAS (10-HDoHE and 12-HEPE) decreased in patients with MDD compared with the HC group. Previous studies mainly focused on ω-3 and ω-6 PUFAs in samples of patients with MDD. Several small clinical studies noted that ω-6 levels were elevated in the tissues of depressed patients relative to controls, and the severity of depression correlated with ω-6 levels[40]. A study presents compelling evidence that a diet high in ω-6 may increase the risk of depression in the general population. ω-6 FAs are believed to have a possibly positive correlation with depression, particularly due to their pro-inflammatory effects[34]. Notably, some investigations have identified a reduction in ω-3 levels in patients with MDD. For instance, ω-3 levels were significantly diminished in patients with depression[41], and reduced total ω-3 levels and elevated ω-6/ω-3 ratio were also observed in individuals with prenatal depression[42]. Conversely, ω-3 dietary supplementation during pregnancy or postpartum alleviates depressive symptoms, and ω-3 adjunctive treatment offers a viable option for managing depression and anxiety symptoms in individuals with recent onset psychosis[43,44]. The antidepressant effects of ω-3 PUFAs are associated with their capacity to reduce inflammatory responses[45]. A further study demonstrated that ω-3 PUFAs can mitigate hippocampal neuroinflammation in mice by modulating TLR4 expression, hence enhancing depression-like behavior[46]. Moreover, hippocampus atrophy is a significant indicator in individuals with depression, and ω-3 PUFAs facilitate hippocampal neurogenesis[47]. In summary, PUFAs are crucial for brain function and neurological disorders, influencing neurotransmission, neuroinflammation, mood, and cognition[48]. PUFAs constitute the fundamental components of the brain and are essential for the optimal functioning of neurons, synapses, and neural transmission. The lack of a crucial element, ω-3 FAs, in preference to ω-6 FAs, might worsen mental disease and serve as a potential catalyst for neurodegenerative alterations[47]. One study confirms that major depression is associated with a high ω-6/ω-3 ratio and elevation in the cytokine IL-6[49]. Research indicates that an imbalanced diet of PUFAs, characterized by elevated ω-6 FAs at the expense of ω-3, resulted in atherosclerosis and diminished cognitive performance[50].
Steroids: Steroids are present in all eukaryotic species and exhibit a wide range of biological functions[51]. The current study found elevated levels of cortisol, estriol, dehydroisoandrosterone sulphate, and pregnenolone sulphate in patients with MDD compared to HCs. Cortisol plays a multifaceted role in MDD. A review suggests that higher levels of cortisol are a risk for subsequent depression. Diurnal rhythms are disrupted, there is heightened resistance to the feedback mechanism of glucocorticoids, excessive cortisol may precipitate MDD, baseline cortisol levels may be elevated, and the post-awakening cortisol surge is intensified in individuals predisposed to MDD[52]. Evidence has amassed in favour of a contemporary paradigm suggesting that cortisol exhibits immune-enhancing capabilities, potentially leading to the heightened inflammation observed in depression[53]. For example, Munhoz et al[54] indicated that, in contrast to the anti-inflammatory effects of glucocorticoids through NF-kB inhibition, rats subjected to chronic unpredictable stress exhibited heightened glucocorticoid levels, leading to increased NF-kB activation and proinflammatory gene expression triggered by acute stress exposure. Furthermore, pre-treatment with a GR antagonist weakened this effect, thus suggesting the putative immune potentiating properties of glucocorticoids. Estrogen, an important steroid hormone secreted by the ovaries in the female body, exists in three forms, including estrone (E1), estradiol (E2), and estriol (E3)[55]. Some re
Research has demonstrated a negative correlation between plasma pregnenolone concentrations and HAMD scores after electroconvulsive therapy[71]. Previous studies indicated that pregnenolone levels were decreased in the cere
Amino acids: The current study found increased levels of L-glutamic acid and 2-hydroxyisocaproic acid, while cyclo(Leu-Pro) and 3-hydroxyanthranilic acid (3-HAA) were decreased in patients with MDD compared to HCs. Increasing evidence suggests that glutamatergic signaling may be involved in the pathophysiology of MDD. A meta-analysis indicated that blood glutamate concentrations were markedly elevated in patients with MDD compared to controls, exhibiting considerable heterogeneity[74]. L-glutamic acid (glutamate) is recognized as the principal excitatory neurotransmitter in the neurological system. It significantly influences brain development by impacting neuronal migration, differentiation, axon formation, and survival[75]. 3-HAA is involved in the kynurenine-tryptophan metabolic pathway. A study demonstrated the novel neuroprotective activity of the tryptophan metabolite 3-HAA[76]. The kynurenine derivative 3-HAA is recognized for its role in modulating the immune system and demonstrating anti-inflammatory properties by suppressing T-cell cytokine release and affecting macrophage activity[77]. Our results suggest that cyclo(Leu-Pro) and 2-hydroxyisocaproic acid may be related to the metabolic disorders in the early metabolic stage or subsequent metabolic steps, and further targeted exploration is needed.
Others: Regarding carnitines, in the present study, the levels of palmitoylcarnitine and oleoyl-L-carnitine increased in patients with MDD compared with HCs. Acylcarnitine is an acylated derivative of carnitine that efficiently facilitates FA oxidation to produce energy for essential functions. It has been documented to mitigate the symptoms of depression. A study revealed that palmitoylcarnitine and carnitine levels were markedly decreased in the serum of individuals with depression[78]. However, Moaddel et al[79] found a reduction in acylcarnitine concentrations after ketamine treatment compared to placebo. Additional comprehensive research is required to clarify the relationship between carnitines and depression. The observed differences in carnitine levels across studies may reflect varying degrees of mitochondrial impairment or compensatory mechanisms in different patient populations or treatment contexts. Concerning alkaloids, in the present study, the levels of piperine and viridiflorine decreased in patients with MDD compared with HCs. Piperine is the primary chemical component in pepper, exhibiting antioxidant effects and immunological modulation, while also facilitating the reversal of HPA axis dysfunction caused by chronic stress[80]. Research indicates that the co-treatment approach employing piperine may serve as an effective alternative therapy for mitigating chronic stress[81]. Trans-resveratrol and piperine may partially function through the 5-HT-cAMP-PKA-CREB-BDNF signaling pathway. This study also confirmed the above views. There are few studies on the direct relationship between viridiflorine and MDD. However, alkaloids often have a variety of biological activities, including anti-inflammatory, antioxidant, and neuroprotective effects, and these properties may have potential effects on MDD.
There are also other metabolites involved in MDD. For example, theobromine, 3-indoleacetic acid, and 4-nitrophenol decreased, and phytosphingosine, D-pantothenic acid, D-(+)-malic acid, and niacinamide increased in patients with MDD compared with HCs. However, due to the limited number of studies on the relationship between these metabolites and MDD, further research is required.
In this study, the concentration of lipids, amino acids, and some other metabolites is associated with multiple indicators of kidney function and BMI. A cross-sectional study of a Chinese population revealed a significant correlation between the severity of depression and depressive symptoms and renal function levels, which remained after adjusting for confounding variables, including chronic kidney disease, hypertension, and diabetes. This implies that minor alterations in early renal function may be associated with depression[82]. Microvascular illness in the kidney is thought to mirror conditions in the brain, and there may be a connection between the two organs[83]. However, few studies have explored the association between MDD and renal function from the perspective of metabolomics. This study also examined the reciprocal nature of the relationship between metabolites and BMI. A review explicitly examines shared biological pathways that may mechanistically elucidate the connection between depression and obesity, encompassing genetics and modifications in systems responsible for homeostatic regulation[84]. Nonetheless, not all patients demonstrate comparable dysregulations in associating biological mechanisms. Consequently, our research encompassed metabolomics to deliver a comprehensive characterization of the biological pathways linking depression.
Regarding psychological variables, the concentration of lipids and steroids is correlated with psychological factors (SHAPS, WHO-5, HAMD-24, and GAD-7). Anhedonia is regarded as a fundamental characteristic of MDD. The neu
In summary, our study provides new evidence for the biomarkers of MDD. Nevertheless, our results need careful interpretation owing to the following limitations: First, there was a considerable percentage of female volunteers in both the MDD group and the HC group. This was primarily due to the challenges in recruiting male participants, particularly in obtaining fecal biosamples from healthy male controls. To ensure a matched comparison between patients and controls, we intentionally included a higher number of healthy female participants. While this approach allowed us to maintain sample consistency and comparability, it may introduce a potential bias related to gender differences in metabolic profiles. Future studies should aim to achieve a more balanced gender distribution to further validate our findings and explore potential sex-specific metabolic signatures in MDD. Moreover, similar to other cross-sectional studies, this study did not conduct a longitudinal analysis, so it is difficult to explain causal relationships. A key limitation of this study is the recruitment of all subjects from a single clinical site, which may introduce site-specific biases and limit the generalizability of the findings. To address these limitations, future studies should recruit heterogeneous subjects from multiple clinical sites, ensuring diversity in demographics, lifestyle, and baseline health conditions.
Our study reported metabolites (some lipids, steroids, amino acids, carnitines, and alkaloids) responsible for discriminating MDD patients and HCs. This metabolite profile may facilitate the development of a laboratory-based diagnostic test for MDD. The mechanisms underlying the association between psychological or clinical variables and differential metabolites also deserve further exploration. These findings indicate that metabolomic profiling offers valuable insights into the biological mechanisms underlying psychological symptoms in MDD. The observed associations between specific metabolites and psychological variables hold promise for advancing personalized treatment strategies, enabling more targeted interventions based on individual metabolic profiles. Furthermore, metabolomic profiling could serve as a tool to monitor treatment response and refine psychotherapy interventions, potentially enhancing therapeutic outcomes.
| 1. | Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1434] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 2. | Zheng P, Wang Y, Chen L, Yang D, Meng H, Zhou D, Zhong J, Lei Y, Melgiri ND, Xie P. Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol Cell Proteomics. 2013;12:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Fekadu A, Demissie M, Birhane R, Medhin G, Bitew T, Hailemariam M, Minaye A, Habtamu K, Milkias B, Petersen I, Patel V, Cleare AJ, Mayston R, Thornicroft G, Alem A, Hanlon C, Prince M. Under detection of depression in primary care settings in low and middle-income countries: a systematic review and meta-analysis. Syst Rev. 2022;11:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Zhang X, Zhang Z, Diao W, Zhou C, Song Y, Wang R, Luo X, Liu G. Early-diagnosis of major depressive disorder: From biomarkers to point-of-care testing. TrAC Trend Anal Chem. 2023;159:116904. [RCA] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 5. | Li Z, Ruan M, Chen J, Fang Y. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci Bull. 2021;37:863-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 6. | Jesulola E, Micalos P, Baguley IJ. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet? Behav Brain Res. 2018;341:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 7. | Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 614] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 8. | Pu J, Liu Y, Zhang H, Tian L, Gui S, Yu Y, Chen X, Chen Y, Yang L, Ran Y, Zhong X, Xu S, Song X, Liu L, Zheng P, Wang H, Xie P. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol Psychiatry. 2021;26:4265-4276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 9. | Paige LA, Mitchell MW, Krishnan KR, Kaddurah-Daouk R, Steffens DC. A preliminary metabolomic analysis of older adults with and without depression. Int J Geriatr Psychiatry. 2007;22:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Zheng P, Fang Z, Xu XJ, Liu ML, Du X, Zhang X, Wang H, Zhou J, Xie P. Metabolite signature for diagnosing major depressive disorder in peripheral blood mononuclear cells. J Affect Disord. 2016;195:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Ali-Sisto T, Tolmunen T, Toffol E, Viinamäki H, Mäntyselkä P, Valkonen-Korhonen M, Honkalampi K, Ruusunen A, Velagapudi V, Lehto SM. Purine metabolism is dysregulated in patients with major depressive disorder. Psychoneuroendocrinology. 2016;70:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Szczypiński JJ, Gola M. Dopamine dysregulation hypothesis: the common basis for motivational anhedonia in major depressive disorder and schizophrenia? Rev Neurosci. 2018;29:727-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Taylor GT, Cabrera O, Hoffman J. The Neuroendocrinology of Anhedonia. In: Ritsner M, editor. Anhedonia: A Comprehensive Handbook Volume I. Dordrecht: Springer, 2014. [DOI] [Full Text] |
| 14. | Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom. 2015;84:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1669] [Cited by in RCA: 2665] [Article Influence: 242.3] [Reference Citation Analysis (0)] |
| 15. | de Vries LP, van de Weijer MP, Bartels M. The human physiology of well-being: A systematic review on the association between neurotransmitters, hormones, inflammatory markers, the microbiome and well-being. Neurosci Biobehav Rev. 2022;139:104733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Bot M, Milaneschi Y, Al-Shehri T, Amin N, Garmaeva S, Onderwater GLJ, Pool R, Thesing CS, Vijfhuizen LS, Vogelzangs N, Arts ICW, Demirkan A, van Duijn C, van Greevenbroek M, van der Kallen CJH, Köhler S, Ligthart L, van den Maagdenberg AMJM, Mook-Kanamori DO, de Mutsert R, Tiemeier H, Schram MT, Stehouwer CDA, Terwindt GM, Willems van Dijk K, Fu J, Zhernakova A, Beekman M, Slagboom PE, Boomsma DI, Penninx BWJH; BBMRI-NL Metabolomics Consortium. Metabolomics Profile in Depression: A Pooled Analysis of 230 Metabolic Markers in 5283 Cases With Depression and 10,145 Controls. Biol Psychiatry. 2020;87:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 17. | Altmaier E, Emeny RT. Use of Metabolomics and Proteomics to Reveal Pathophysiological Pathways in Anxiety Disorders. Adv Biol Psychiatry. 2014;29. [DOI] [Full Text] |
| 18. | Hall DM, Cole TJ. What use is the BMI? Arch Dis Child. 2006;91:283-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Hryhorczuk C, Sharma S, Fulton SE. Metabolic disturbances connecting obesity and depression. Front Neurosci. 2013;7:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 20. | Liang H, Wang JM, Wei XQ, Su XQ, Zhang BX. Thyroid function, renal function, and depression: an association study. Front Psychiatry. 2023;14:1182657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 21. | Gan X, Li X, Cai Y, Yin B, Pan Q, Teng T, He Y, Tang H, Wang T, Li J, Zhu Z, Zhou X, Li J. Metabolic features of adolescent major depressive disorder: A comparative study between treatment-resistant depression and first-episode drug-naive depression. Psychoneuroendocrinology. 2024;167:107086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251-W257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2063] [Cited by in RCA: 2155] [Article Influence: 195.9] [Reference Citation Analysis (0)] |
| 23. | Leclercq S, Ahmed H, Amadieu C, Petit G, Koistinen V, Leyrolle Q, Poncin M, Stärkel P, Kok E, Karhunen PJ, de Timary P, Laye S, Neyrinck AM, Kärkkäinen OK, Hanhineva K, Delzenne N. Blood metabolomic profiling reveals new targets in the management of psychological symptoms associated with severe alcohol use disorder. Elife. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Sotelo-Orozco J, Abbeduto L, Hertz-Picciotto I, Slupsky CM. Association Between Plasma Metabolites and Psychometric Scores Among Children With Developmental Disabilities: Investigating Sex-Differences. Front Psychiatry. 2020;11:579538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother. 2002;56:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 366] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Chilton FH, Dutta R, Reynolds LM, Sergeant S, Mathias RA, Seeds MC. Precision Nutrition and Omega-3 Polyunsaturated Fatty Acids: A Case for Personalized Supplementation Approaches for the Prevention and Management of Human Diseases. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | de Carvalho CCCR, Caramujo MJ. The Various Roles of Fatty Acids. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 531] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 28. | Tsuboi H, Watanabe M, Kobayashi F, Kimura K, Kinae N. Associations of depressive symptoms with serum proportions of palmitic and arachidonic acids, and α-tocopherol effects among male population--a preliminary study. Clin Nutr. 2013;32:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Li D, Liang H, Tong Y, Zheng H, Li Y. Association between saturated fatty acid intake and depressive symptoms in midlife women: A prospective study. J Affect Disord. 2020;267:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes (Lond). 2013;37:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 312] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 31. | Hryhorczuk C, Florea M, Rodaros D, Poirier I, Daneault C, Des Rosiers C, Arvanitogiannis A, Alquier T, Fulton S. Dampened Mesolimbic Dopamine Function and Signaling by Saturated but not Monounsaturated Dietary Lipids. Neuropsychopharmacology. 2016;41:811-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Gupta S, Knight AG, Gupta S, Keller JN, Bruce-Keller AJ. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem. 2012;120:1060-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 33. | Zheng X, Chen Y, Lin SQ, Liu T, Liu CA, Ruan GT, Ge YZ, Xie HL, Song MM, Shi JY, Wang ZW, Yang M, Liu XY, Zhang HY, Zhang Q, Deng L, Shi HP. The relationship between different fatty acids intake and the depressive symptoms: A population-based study. J Affect Disord. 2024;357:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Wolfe AR, Ogbonna EM, Lim S, Li Y, Zhang J. Dietary linoleic and oleic fatty acids in relation to severe depressed mood: 10 years follow-up of a national cohort. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Sartorius T, Ketterer C, Kullmann S, Balzer M, Rotermund C, Binder S, Hallschmid M, Machann J, Schick F, Somoza V, Preissl H, Fritsche A, Häring HU, Hennige AM. Monounsaturated fatty acids prevent the aversive effects of obesity on locomotion, brain activity, and sleep behavior. Diabetes. 2012;61:1669-1679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Alemany R, Navarro MA, Vögler O, Perona JS, Osada J, Ruiz-Gutiérrez V. Olive oils modulate fatty acid content and signaling protein expression in apolipoprotein E knockout mice brain. Lipids. 2010;45:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Montserrat-de la Paz S, Del Carmen Naranjo M, Lopez S, Del Carmen Millan-Linares M, Rivas-Dominguez A, Jaramillo-Carmona SM, Abia R, Muriana FJG, Bermudez B. Immediate-release niacin and a monounsaturated fatty acid-rich meal on postprandial inflammation and monocyte characteristics in men with metabolic syndrome. Clin Nutr. 2023;42:2138-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 38. | Ruan GT, Xie HL, Yuan KT, Lin SQ, Zhang HY, Liu CA, Shi JY, Ge YZ, Song MM, Hu CL, Zhang XW, Liu XY, Yang M, Wang KH, Zheng X, Chen Y, Hu W, Cong MH, Zhu LC, Deng L, Shi HP. Prognostic value of systemic inflammation and for patients with colorectal cancer cachexia. J Cachexia Sarcopenia Muscle. 2023;14:2813-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Bahadorpour S, Hajhashemy Z, Mohammadi S, Mokhtari E, Heidari Z, Saneei P. Total fat and omega-3 fatty acids intake in relation to serum brain-derived neurotrophic factor (BDNF) levels and psychological disorders in Iranian adults. Sci Rep. 2023;13:5392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31 Suppl:S157-S161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 270] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 396] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 42. | Lin PY, Chang CH, Chong MF, Chen H, Su KP. Polyunsaturated Fatty Acids in Perinatal Depression: A Systematic Review and Meta-analysis. Biol Psychiatry. 2017;82:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 43. | Robinson DG, Gallego JA, John M, Hanna LA, Zhang JP, Birnbaum ML, Greenberg J, Naraine M, Peters BD, McNamara RK, Malhotra AK, Szeszko PR. A potential role for adjunctive omega-3 polyunsaturated fatty acids for depression and anxiety symptoms in recent onset psychosis: Results from a 16 week randomized placebo-controlled trial for participants concurrently treated with risperidone. Schizophr Res. 2019;204:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | Hsu MC, Tung CY, Chen HE. Omega-3 polyunsaturated fatty acid supplementation in prevention and treatment of maternal depression: Putative mechanism and recommendation. J Affect Disord. 2018;238:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 45. | Freeman MP. Omega-3 fatty acids in major depressive disorder. J Clin Psychiatry. 2009;70 Suppl 5:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Hu L, Zeng X, Yang K, Peng H, Chen J. n-3 polyunsaturated fatty acids improve depression-like behavior by inhibiting hippocampal neuroinflammation in mice via reducing TLR4 expression. Immun Inflamm Dis. 2022;10:e707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Stachowicz K. The role of polyunsaturated fatty acids in neuronal signaling in depression and cognitive processes. Arch Biochem Biophys. 2023;737:109555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 48. | Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 1140] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 49. | Dinan T, Siggins L, Scully P, O'Brien S, Ross P, Stanton C. Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. J Psychiatr Res. 2009;43:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Wani AL, Bhat SA, Ara A. Omega-3 fatty acids and the treatment of depression: a review of scientific evidence. Integr Med Res. 2015;4:132-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Wallimann P, Marti T, Fürer A, Diederich F. Steroids in Molecular Recognition. Chem Rev. 1997;97:1567-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 187] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 52. | Herbert J. Cortisol and depression: three questions for psychiatry. Psychol Med. 2013;43:449-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 53. | Amasi-Hartoonian N, Sforzini L, Cattaneo A, Pariante CM. Cause or consequence? Understanding the role of cortisol in the increased inflammation observed in depression. Curr Opin Endocr Metab Res. 2022;24:100356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 54. | Munhoz CD, Sorrells SF, Caso JR, Scavone C, Sapolsky RM. Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci. 2010;30:13690-13698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 55. | Wu YC, Hill RA, Gogos A, van den Buuse M. Sex differences and the role of estrogen in animal models of schizophrenia: interaction with BDNF. Neuroscience. 2013;239:67-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 56. | Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1337] [Article Influence: 70.4] [Reference Citation Analysis (6)] |
| 57. | Zsido RG, Villringer A, Sacher J. Using positron emission tomography to investigate hormone-mediated neurochemical changes across the female lifespan: implications for depression. Int Rev Psychiatry. 2017;29:580-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830-6838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 283] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 59. | Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med. 2004;71 Suppl 2:S4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 345] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 61. | Di Paolo T, Falardeau P, Morissette M. Striatal D-2 dopamine agonist binding sites fluctuate during the rat estrous cycle. Life Sci. 1988;43:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Henderson JA, Bethea CL. Differential effects of ovarian steroids and raloxifene on serotonin 1A and 2C receptor protein expression in macaques. Endocrine. 2008;33:285-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 430] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 65. | Paslakis G, Luppa P, Gilles M, Kopf D, Hamann-Weber B, Lederbogen F, Deuschle M. Venlafaxine and mirtazapine treatment lowers serum concentrations of dehydroepiandrosterone-sulfate in depressed patients remitting during the course of treatment. J Psychiatr Res. 2010;44:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Morita T, Senzaki K, Ishihara R, Umeda K, Iwata N, Nagai T, Hida H, Nabeshima T, Yukawa K, Ozaki N, Noda Y. Plasma dehydroepiandrosterone sulfate levels in patients with major depressive disorder correlate with remission during treatment with antidepressants. Hum Psychopharmacol. 2014;29:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 68. | Dhir A, Kulkarni S. Involvement of sigma (sigma1) receptors in modulating the anti-depressant effect of neurosteroids (dehydroepiandrosterone or pregnenolone) in mouse tail-suspension test. J Psychopharmacol. 2008;22:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Bermack JE, Debonnel G. The role of sigma receptors in depression. J Pharmacol Sci. 2005;97:317-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Markopoulou K, Papadopoulos A, Juruena MF, Poon L, Pariante CM, Cleare AJ. The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology. 2009;34:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Turan Ş, Yıldırım A, Aksoy-Poyraz C, Bolayırlı M, Savrun M. Effects of electroconvulsive therapy on plasma levels of neuroactive steroids in inpatients with major depression. Int J Psychiatry Clin Pract. 2014;18:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 72. | Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 363] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 73. | Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239-3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 542] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 74. | Inoshita M, Umehara H, Watanabe SY, Nakataki M, Kinoshita M, Tomioka Y, Tajima A, Numata S, Ohmori T. Elevated peripheral blood glutamate levels in major depressive disorder. Neuropsychiatr Dis Treat. 2018;14:945-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 300] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 76. | Krause D, Suh HS, Tarassishin L, Cui QL, Durafourt BA, Choi N, Bauman A, Cosenza-Nashat M, Antel JP, Zhao ML, Lee SC. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: role of hemeoxygenase-1. Am J Pathol. 2011;179:1360-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 77. | Xue C, Gu X, Zheng Q, Shi Q, Yuan X, Chu Q, Jia J, Su Y, Bao Z, Lu J, Li L. Effects of 3-HAA on HCC by Regulating the Heterogeneous Macrophages-A scRNA-Seq Analysis. Adv Sci (Weinh). 2023;10:e2207074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 78. | Zu X, Xin J, Xie H, Xu X, Shen Y, Wang J, Tian S, Wen Y, Li H, Yang J, Fang Y. Characteristics of gut microbiota and metabolic phenotype in patients with major depressive disorder based on multi-omics analysis. J Affect Disord. 2024;344:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 79. | Moaddel R, Zanos P, Farmer CA, Kadriu B, Morris PJ, Lovett J, Acevedo-Diaz EE, Cavanaugh GW, Yuan P, Yavi M, Thomas CJ, Park LT, Ferrucci L, Gould TD, Zarate CA Jr. Comparative metabolomic analysis in plasma and cerebrospinal fluid of humans and in plasma and brain of mice following antidepressant-dose ketamine administration. Transl Psychiatry. 2022;12:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 80. | Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. 2010;10:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 81. | Xu Y, Zhang C, Wu F, Xu X, Wang G, Lin M, Yu Y, An Y, Pan J. Piperine potentiates the effects of trans-resveratrol on stress-induced depressive-like behavior: involvement of monoaminergic system and cAMP-dependent pathway. Metab Brain Dis. 2016;31:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 82. | Lin M, Huang H, Yao J, Liang J, Li L, Lin W, Lin L, Hong F, Lu J, Bi Y, Wang W, Wen J, Chen G. Association between Depression and Renal Hyperfiltration in a General Chinese Population. Kidney Blood Press Res. 2019;44:1441-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Simões E Silva AC, Miranda AS, Rocha NP, Teixeira AL. Neuropsychiatric Disorders in Chronic Kidney Disease. Front Pharmacol. 2019;10:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 84. | de Wit LM, van Straten A, van Herten M, Penninx BW, Cuijpers P. Depression and body mass index, a u-shaped association. BMC Public Health. 2009;9:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 285] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 85. | Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008;10:291-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 86. | Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 966] [Cited by in RCA: 985] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 87. | Bush DE, DeSousa NJ, Vaccarino FJ. Self-administration of intravenous amphetamine: effect of nucleus accumbens CCKB receptor activation on fixed-ratio responding. Psychopharmacology (Berl). 1999;147:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Donati RJ, Rasenick MM. G protein signaling and the molecular basis of antidepressant action. Life Sci. 2003;73:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 89. | Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front Mol Neurosci. 2018;11:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 325] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 90. | Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol. 2009;65:467-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1507] [Cited by in RCA: 1112] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 91. | Edmondson OJ, MacLeod AK. Psychological Well-Being and Anticipated Positive Personal Events: Their Relationship to Depression. Clin Psychol Psychother. 2015;22:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Liu X, Zheng P, Zhao X, Zhang Y, Hu C, Li J, Zhao J, Zhou J, Xie P, Xu G. Discovery and validation of plasma biomarkers for major depressive disorder classification based on liquid chromatography-mass spectrometry. J Proteome Res. 2015;14:2322-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/