Published online Mar 19, 2025. doi: 10.5498/wjp.v15.i3.101178
Revised: November 26, 2024
Accepted: December 27, 2024
Published online: March 19, 2025
Processing time: 153 Days and 21.5 Hours
Diabetes is associated with increased cognitive decline and dementia due to the loss of myelinated nerve fiber function, which is linked to oligodendrocyte dysfunction. The voltage-gated proton channel 1 (Hv1) is important for the cellular proton extrusion machinery. However, its role in regulating diabetes-induced cognitive dysfunction is unclear.
To investigate the role of Hv1 in cognitive impairment induced by diabetes and its potential mechanisms, focusing on neuroinflammation, oligodendrocyte apop
A diabetes model was established by administering a high-fat diet and streptozotocin injections in mice. Hv1 knockout (KO) and wild-type mice were used to evaluate cognitive function via behavioral tests and neuroinflammation using immunofluorescence. Oligodendrocyte apoptosis was assessed with the terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling assay, and axonal demyelination was analyzed using electron microscopy.
Hv1 expression was significantly increased in the corpus callosum of diabetic mice. Hv1 KO alleviated cognitive impairment, reduced oligodendrocyte apoptosis, and decreased the expression of inflammatory factors, including interleukin-1 and tumor necrosis factor-α, in diabetic mice. Electron microscopy revealed a reduction in myelin thickness and an increased g-ratio in diabetic mice, which were reversed by Hv1 KO.
Hv1 plays a role in diabetes-induced cognitive dysfunction by modulating neuroinflammation and myelin integrity. Hv1 KO demonstrates therapeutic potential in mitigating diabetes-related cognitive decline and associated complications.
Core Tip: This study demonstrates that voltage-gated proton channel 1 (HV-1) knockout reduces neuroinflammation and alleviates axonal demyelination in diabetic mice, suggesting that HV-1 is a potential therapeutic target for treating diabetes-associated cognitive decline. The findings highlight the role of HV-1 in modulating microglial activity and promoting oligodendrocyte survival, which contributes to improved cognitive function in diabetic models. These insights offer new avenues for developing strategies to mitigate cognitive dysfunction related to diabetes by targeting HV-1.
- Citation: Li CY, Zhang SJ, Xu JL, Yang Y, Zeng ZX, Ma DL. Inhibition of the microglial voltage-gated proton channel 1 channel ameliorates diabetes-associated cognitive dysfunction by regulating axon demyelination. World J Psychiatry 2025; 15(3): 101178
- URL: https://www.wjgnet.com/2220-3206/full/v15/i3/101178.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i3.101178
Cognitive impairment is a serious complication of diabetes, which occurs globally[1-3]. Increased oxidative stress in the brain causes chronic neuroinflammation, which has been implicated in the pathogenesis of Alzheimer’s disease[4,5]. Although microglia are crucial for maintaining brain homeostasis, they also contribute to various central nervous system (CNS) disorders[3,6,7]. Studies have shown that the mechanical changes in the CNS microenvironment influence glial cell behavior. However, the specific regulatory mechanisms governing microglial functions remain unclear.
The voltage-gated proton channel 1 (Hv1) is a unique ion channel in mammalian cells, characterized by a conserved voltage-sensor domain commonly seen in the voltage-gated channel family. However, its distinct feature is the lack of a pore domain[8,9]. It is mainly responsible for extruding protons from the cell under acidic potential of hydrogen (pH) conditions and in response to membrane depolarization[10]. Hv1 also maintains cytoplasmic pH homeostasis, which requires the activity of nicotinamide adenine dinucleotide phosphate oxidase (NOX)[10]. Several studies have indicated that in addition to immune cells, Hv1 is expressed in other cellular populations, such as those within the nervous system.
Microglia act as intrinsic macrophages in the CNS and are essential for host defense and tissue repair. Previous studies on Hv1 in the CNS have focused on microglia. Using electrophysiology, quantitative real-time polymerase chain reaction, Western blot, and immunohistochemistry techniques, Hv1 expression has been detected in the microglia in rodent models and human brain tissues. Notably, the microglia expression levels of Hv1 have been shown to vary across different species[11,12]. Wu et al[12] observed significantly higher Hv1 currents in the microglia in mice than in rats, as the hydrogen ion currents in rats were only at 8% of that in mice. While studies have shown significant Hv1 levels and hydrogen ion currents in cultured human microglia, it is unclear if substantial Hv1 currents exist in native human microglia. Wu[13] showed that Hv1 activation can lead to NOX-dependent reactive oxygen species (ROS) generation in brain microglia. ROS production was notably decreased in the activated microglia of mice lacking Hv1, which conferred a neuroprotective effect against NOX-induced neuronal death and brain injury following a stroke[12,13].
Recent studies have demonstrated that microglia regulate myelin maintenance and regeneration. Microglia activity is increased in pathological conditions, and these activated microglia proliferate at lesion sites. This results in the increased production of proinflammatory cytokines and inflammatory mediators, such as ROS, which can directly damage neuronal myelin[13]. Interleukin (IL)-1 produced by activated microglia inhibits oligodendrocyte proliferation. These microglia can also remodel synapses, leading to cognitive decline. Furthermore, microglia can affect myelin integrity by influencing lipid metabolism in oligodendrocytes. Therefore, microglial cells are potential therapeutic targets for preventing myelin destruction in various diseases.
Studies suggest that the myelin sheath is protected in Hv1-deficient mice. Liu et al[14] showed protective effects against demyelination and motor deficits were ameliorated in a cuprizone-induced model of multiple sclerosis in Hv1-deficient mice due to. decreased ROS levels, improved oligodendrocyte precursor cells (OPC) proliferation, increased mature olses, and enhanced remyelination[14]. Building on this, Yu et al[15] discovered that OPC apoptosis was reduced in a model with Hv1-deficient microglia, while their proliferation and differentiation were increased by regulating ROS and inflammatory cytokine production.
However, the exact contribution of Hv1 to early cognitive impairments associated with diabetes is still unclear. Herein, we explored the role of Hv1 in corpus callosum injury and cognitive impairment in diabetic mice. We also investigated its potential links to the dysregulation of myelin sheath formation. Collectively, our results indicate that Hv1 plays a neuroprotective role against cognitive decline associated with diabetes, suggesting its potential as a novel therapeutic target for treating diabetes-related cognitive disorders.
Hv1 mice were generated in the Clapham laboratory and were backcrossed with C57BL/6 mice for more than 10 generations, as previously reported[12]. All animal procedures were approved by the Animal Care and Use Committee at the Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China, and were performed according to the Public Health Service Policy on Human Care and Use of Laboratory Animals.
To create the diabetic mouse model, five-week-old male C57BL/6J mice were acclimatized for a week. Then, they were fed a high-fat diet (containing 60% of calories from fat) for eight weeks and then intraperitoneally injected with streptozotocin (STZ) (40 mg/kg body weight) (Sigma-Aldrich, S0130 dissolved in 50 mmol/L citrate buffer pH 4.5). The mice in the control group were given the same volume of citrate buffer and fed a standard normal chow (10% of calories from fat). A week after the STZ injection, blood was drawn from the tail vein to determine the blood glucose levels. Mice with a fasting blood glucose concentration above 11.1 mmol/L were classified as diabetic and were used for subsequent experiments. The control group was injected with an equivalent volume of the citrate buffer without STZ. The STZ-treated mice were given 10% sucrose water for seven days to avoid hypoglycemic shock.
The terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL) assay was performed using the one-step TUNEL kit (Wuhan, Hubei Province, China) according to the manufacturer’s instructions[16,17]. Mice brains were sliced into 4 μm- thick sections and embedded in paraffin. After deparaffinization using xylene and subsequent rehydration using an ethanol gradient, the sections were treated with the TUNEL reagent mixture for 60 minutes and then counterstained with 4’,6-diamidino-2-phenylindole for 5 minutes. The stained samples were examined using a fluorescence microscope (SV120, OLYMPUS, Japan).
The mice were anesthetized using intraperitoneal injections of 1% pentobarbital sodium. The hearts of the mice were rinsed with physiological saline and then perfused with 4% formaldehyde for 10 minutes until their limbs convulsed and their spinal column stiffened. The brain tissues were extracted and fixed in 4% formaldehyde at 4 °C overnight. Then, 20 μm-thick frozen sections of the brain were sliced at the coronal plane. The sections were first blocked using bovine serum albumin, membrane-permeabilized, and incubated with primary antibodies [rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba1), 1:500; mouse anti-tumor necrosis factor (TNF)-α, 1:100; mouse anti-neuro-glia antigen 2 (NG2), 1:100; mouse anti-IL-1β, 1: 100] at 4 °C overnight. After washing thrice with phosphate buffer saline (PBS), the sections were incubated with the respective fluorescent secondary antibodies at 20 °C for two hours. After washing the sections thrice with PBS, the sections were mounted on slides[18,19] and observed under a Recordbio NE610 microscope. Inflammation factors and microglial proliferation were detected at two weeks post-modeling.
Neurological function was examined using the eight-arm radial maze test based on a previously described method[20]. We assessed the working and reference memory four weeks after the diagnosis of diabetes. This test involves a radial maze consisting of eight arms with bits of food hidden in each arm. The mice are placed in the center and allowed to find the food by themselves. The trial is considered complete when the mice either eat all the food or the 25 minutes are over.
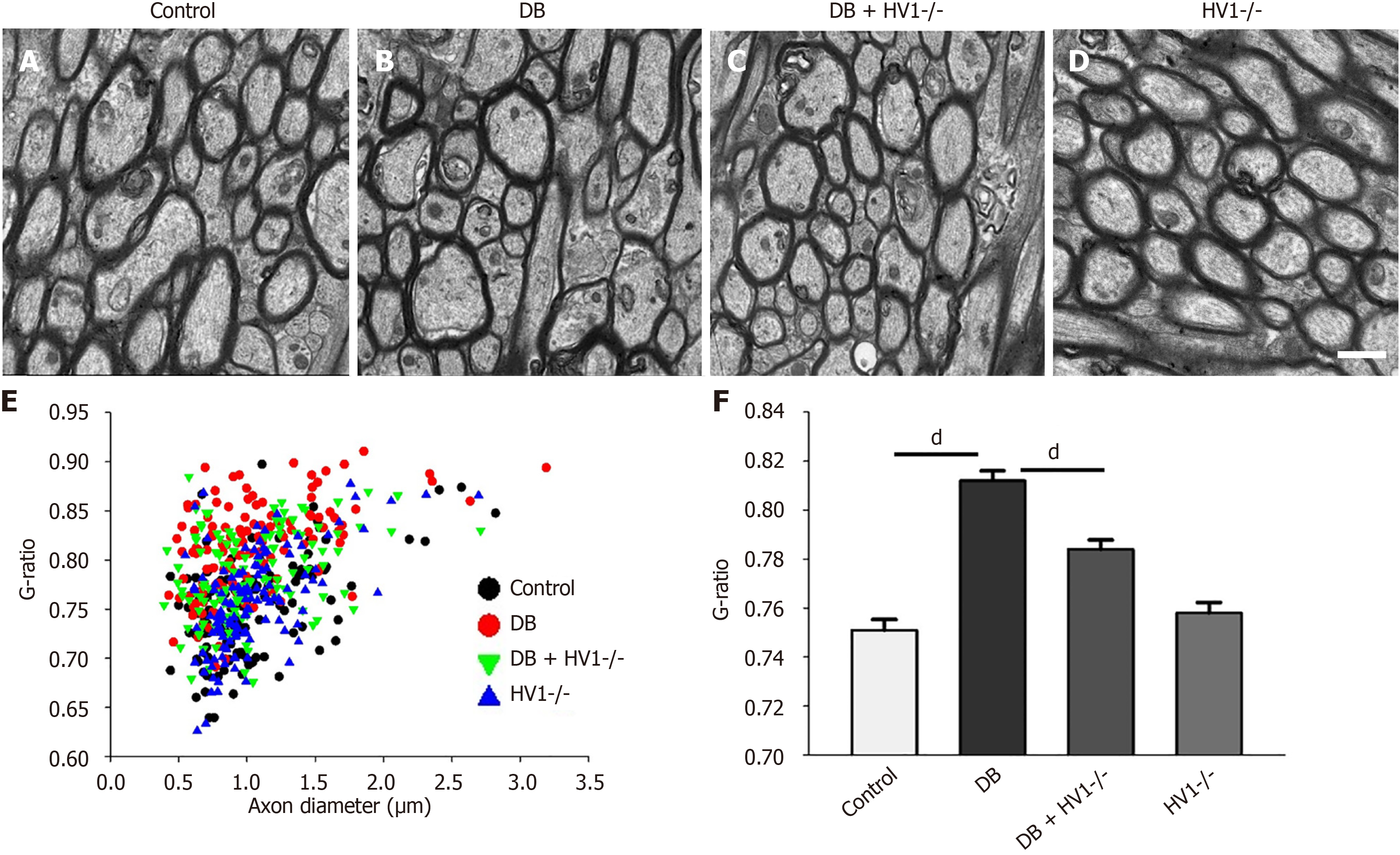
Transmission electron microscopy images were captured from four different regions of the corpus callosum of each animal. The diameters of individual axons and their myelin sheaths were measured using the ImageJ software. The g-ratio of each axon was determined as the ratio of the axon diameter to that of the total axon fiber[21].
After carefully removing the meninges, the microglia were purified using the primary cell extraction technique. The tissue segments were trimmed to 1 cm-long pieces and rinsed with PBS. After soaking the segments in fetal bovine serum (50 × concentration), 1 mmol dithiothreitol, and 30 mmol ethylenediaminetetraacetic acid, they were incubated at 37 °C with a rotation speed of 250 rpm for 20 minutes. This process was repeated. After filtering the suspensions, the leftover tissues were minced into 0.5 cm pieces and subjected to enzymatic digestion at 37 °C and 250 rpm for 50 minutes in Roswell park memorial institute 1640 medium. The samples were randomly divided into four groups: PBS-treated control, high glucose, high glucose + siRNA-Hv1, and scrambled RNA-treated groups (n = 4/group).
To identify the proliferating cells in mouse tissues, the mice were intraperitoneally injected with a thymidine analog, 5-ethynyl-2’-deoxyuridine (EdU) (50 mg/kg, E10415), for three consecutive days before tissue collection. Tissue samples were collected 24 hours after the last injection, and EdU was detected as described previously[22,23].
The student’s unpaired t-test was used to compare two different groups. Variations within groups were evaluated using one-way analysis of variance, supplemented with Dunnett’s post hoc test for multiple comparisons. Statistical sig
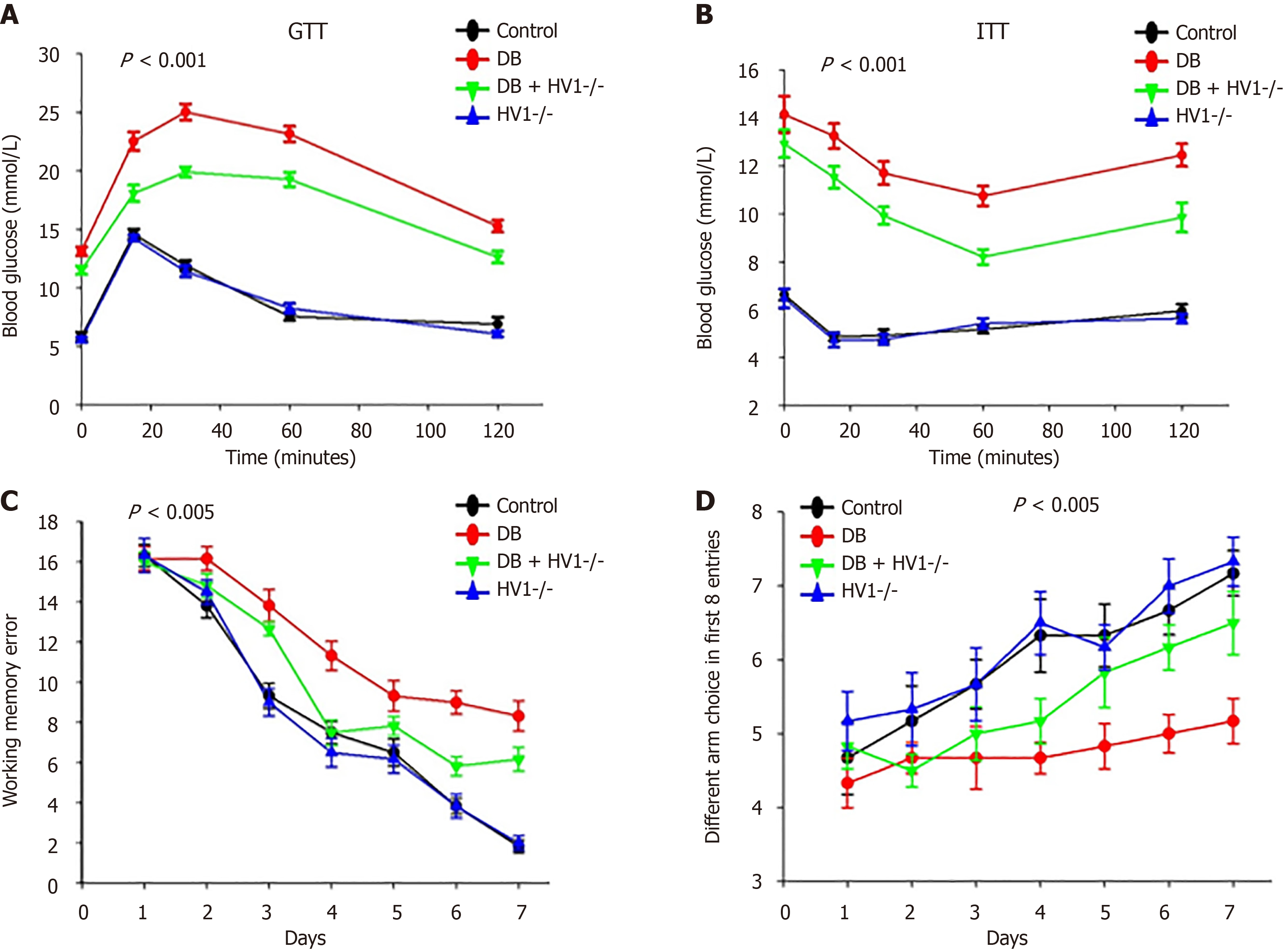
We conducted glucose tolerance tests (1 g glucose/kg body weight) and insulin tolerance tests insulin tolerance tests, 1 unit human insulin/kg, respectively) to assess whether Hv1 knockout (KO) affected glucose tolerance and insulin resistance. The results showed that Hv1 KO improved glucose tolerance and insulin resistance in diabetic mice. Behavioral tests were conducted four weeks after modeling to assess the motor function and cognitive ability. The results showed that the diabetic group exhibited more working memory errors and fewer different arm choices in the first eight entries than the control group (Figure 1). These results indicate that Hv1 gene deletion can rescue cognitive impairment in diabetic mice.
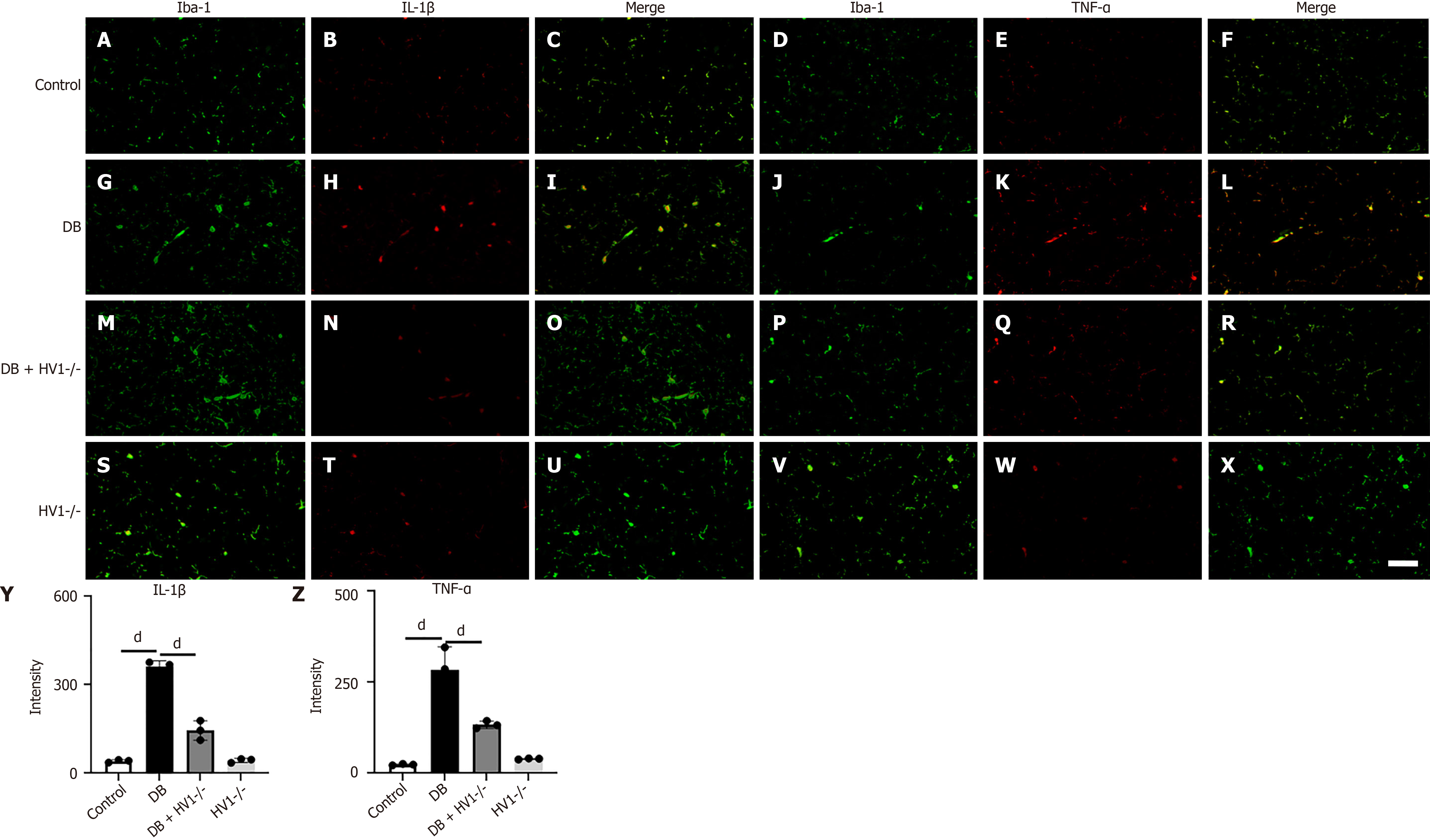
In the corpus callosum, we compared the expression levels of IL-1β and TNF-α with the control group at P2 (two weeks post-modeling) (Figure 2A-F). The average grayscale values of IL-1β (Figure 2G-I) and TNF-α (Figure 2J-L) in microglial cells in the corpus callosum were significantly elevated in the diabetic group. This response was effectively reversed by Hv1 gene KO treatment (Figure 2M-R). Conversely, there was no significant difference in the IL-1β and TNF-α levels in the Hv1 gene KO group (Figure 2S-X).
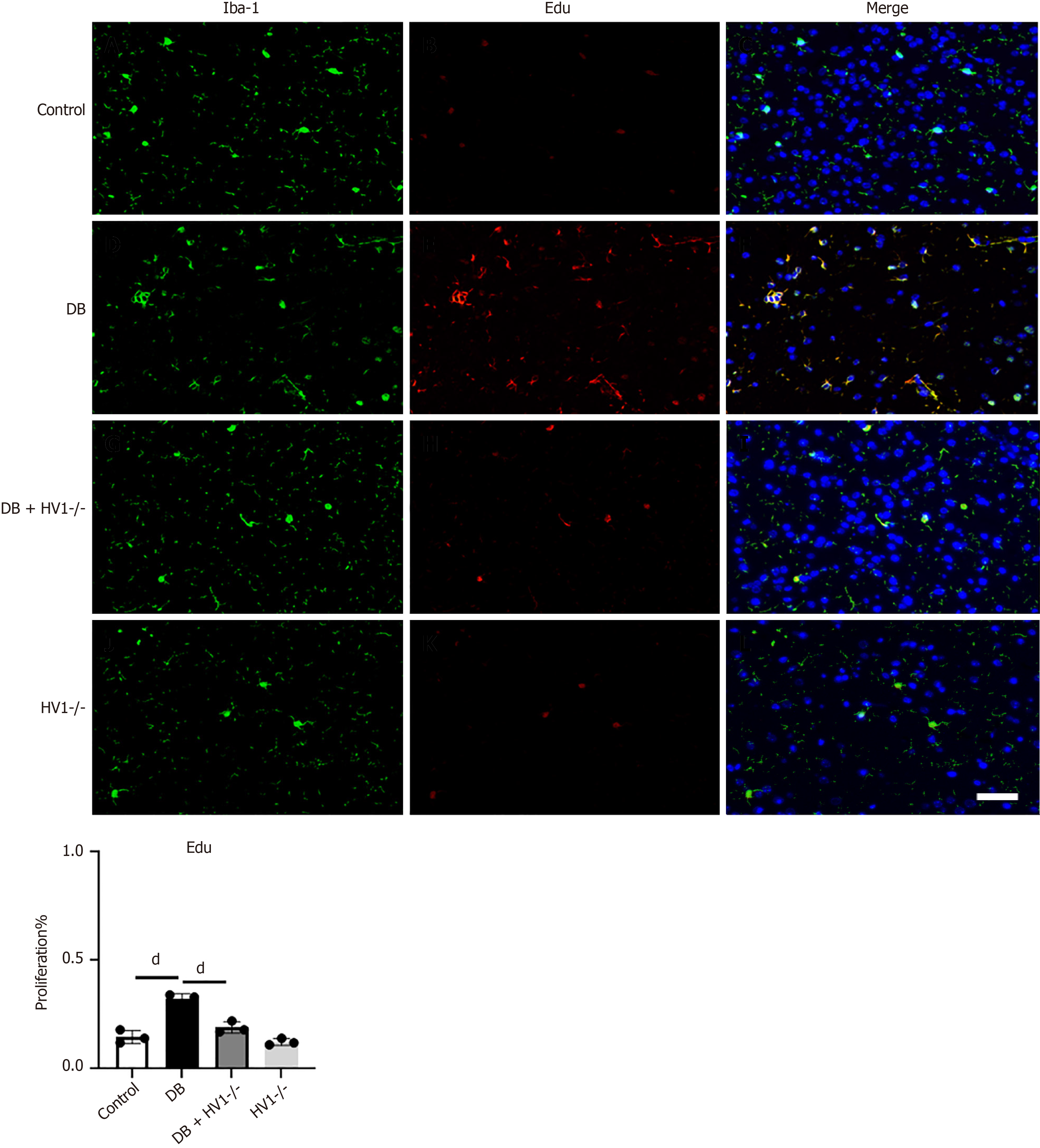
We investigated the association between Hv1 gene deletion and the proliferation of microglia in the corpus callosum. To address this question, we injected EdU into the corpus callosum of the mice at P2 (two weeks post-modeling). Three days later, the mice brains were collected and subjected to immunofluorescence staining to identify Iba 1+ microglia, which represent activated microglia, in the corpus callosum at P2 (Figure 3). We found that the EdU expression at P2 was higher in the diabetic group than in the control group, which was restored by Hv1 gene deletion.
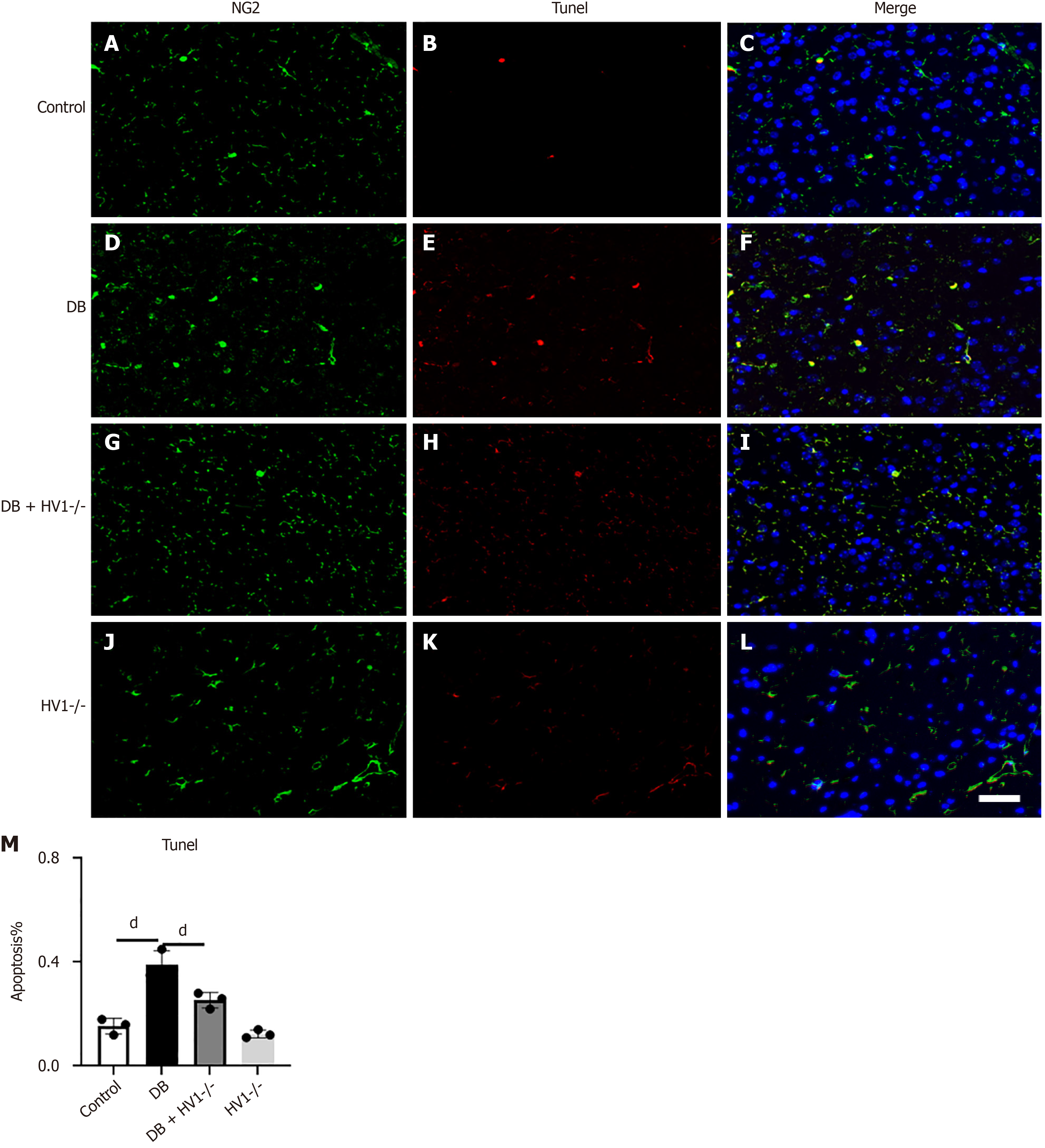
Five weeks after establishing the diabetes model, the NG2-positive OPCs exhibited increased apoptosis than the control group. The number of OPCs significantly decreased after five weeks. However, the Hv1 gene knockdown reduced OPC apoptosis, and the number of OPCs recovered was higher than that of the diabetic group (Figure 4).
Electron microscopy observations showed that the density of myelinated axons significantly decreased in the corpus callosum five weeks after diabetes modeling, exhibiting a loose fibrous tissue morphology (Figure 5A and B) compared with the control group. Notably, the myelin sheath was also significantly thinner in this group than in the control group. Hv1 KO improved these conditions (Figure 5C and D). The average elliptical ratio of the control group mice reached 0.75, while that of the diabetic mice significantly increased to 0.81. After the Hv1 KO intervention, the average elliptical ratio was 0.78, indicating that Hv1 KO can reverse the decrease in myelin thickness in diabetic mice (Figure 5E and F).
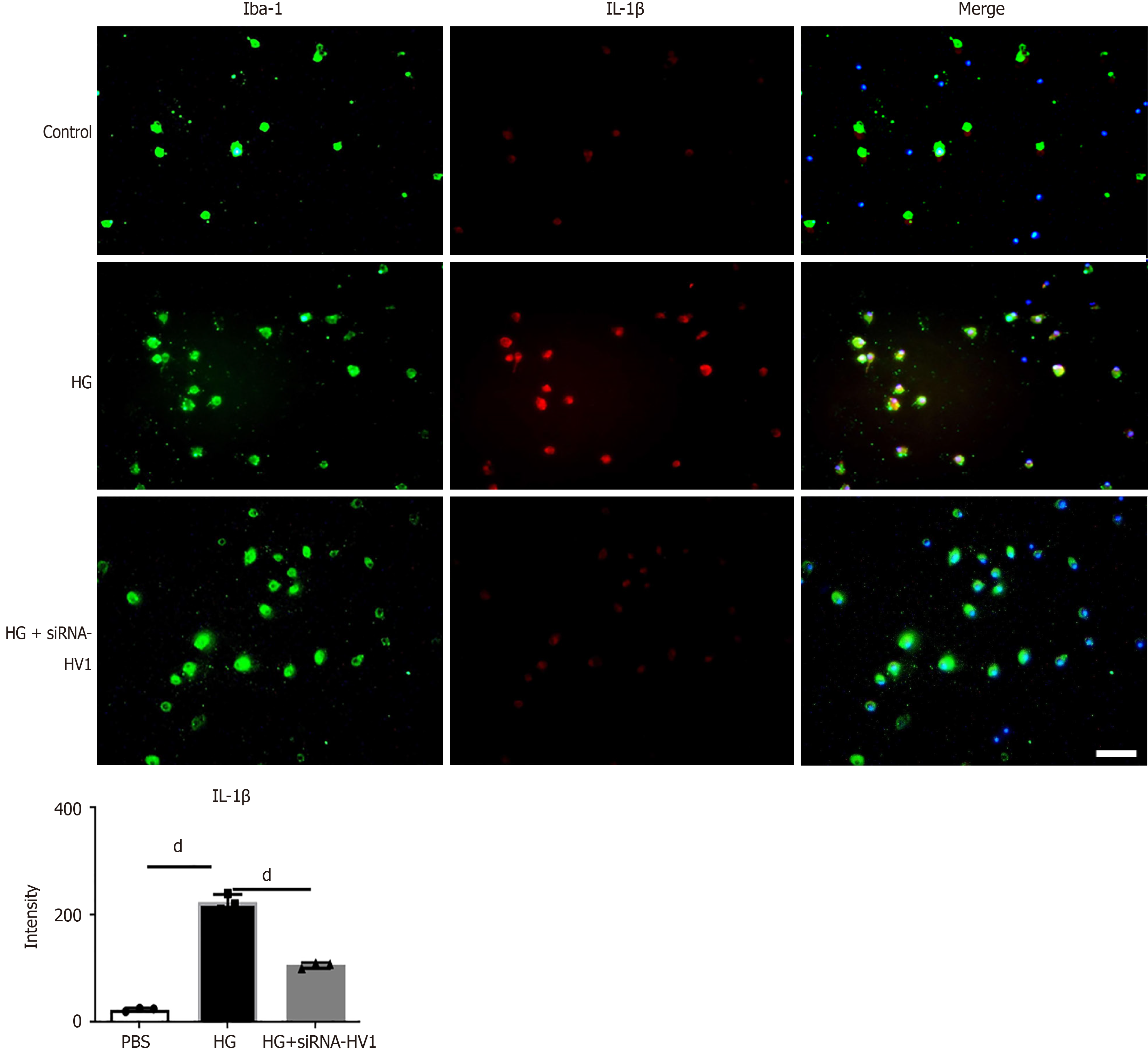
The IL-1β levels were higher in the microglial cells in the high glucose and high osmolarity group than the control group. The IL-1β levels were higher in the microglial cells in the high glucose and high osmolarity group than in the control group. After treatment with siRNA-Hv1, the IL-1β levels in the microglial cells decreased (Figure 6).
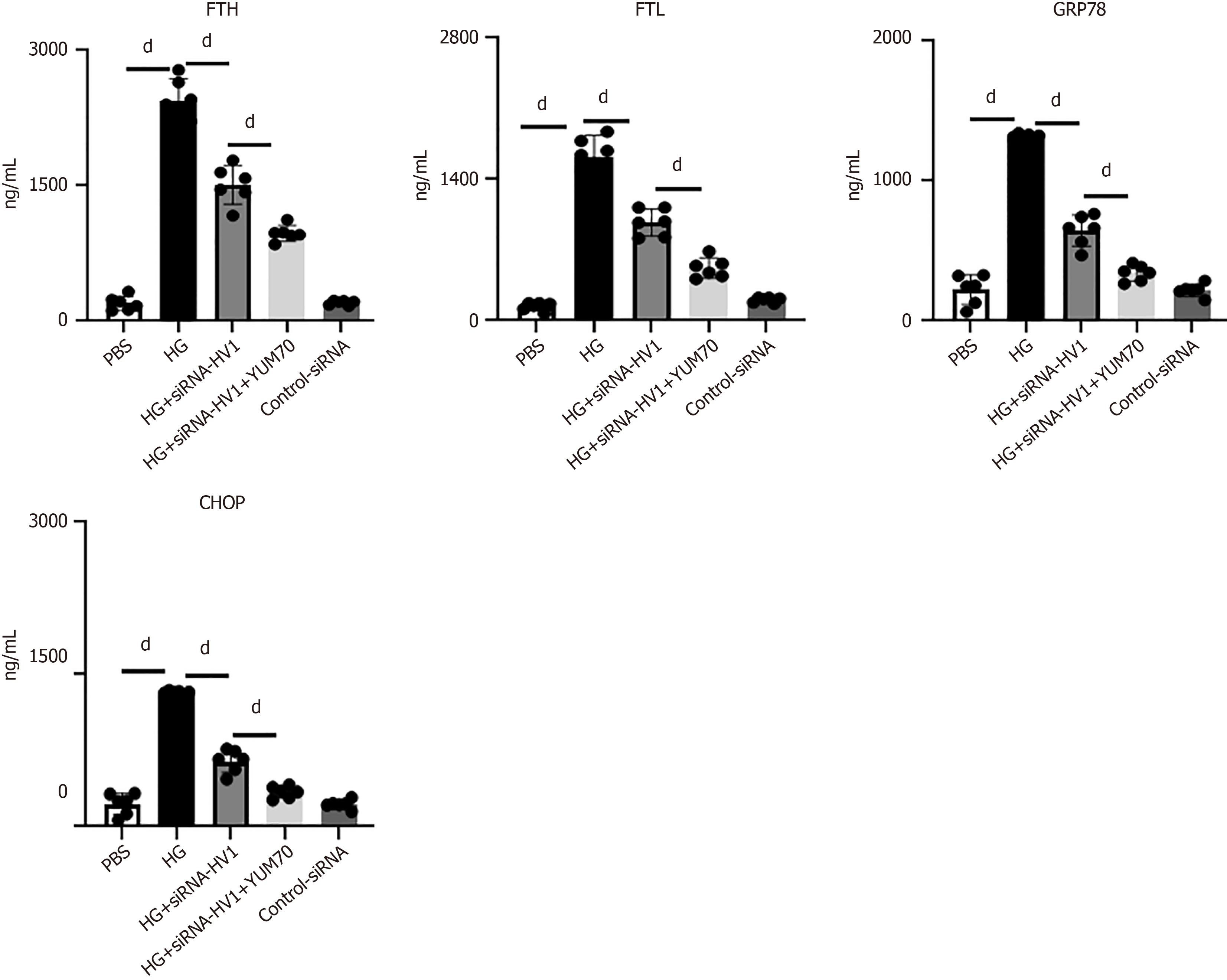
We observed a significant increase in the ferroptosis markers, including ferritin heavy chain/light-chain (FTH/FTL), CCAAT/enhancer-binding protein homologous protein (CHOP), and glucose-regulated protein 78 (GRP78), in the cultured microglia, indicating the occurrence of ferroptosis. Surprisingly, when cells were pretreated with siRNA-Hv1 before high glucose treatment, the expression levels of all four ferroptosis markers were inhibited. Further, when the YUM70 was added, the effect of siRNA-Hv1 was enhanced, while the control RNA did not affect the expression (Figure 7).
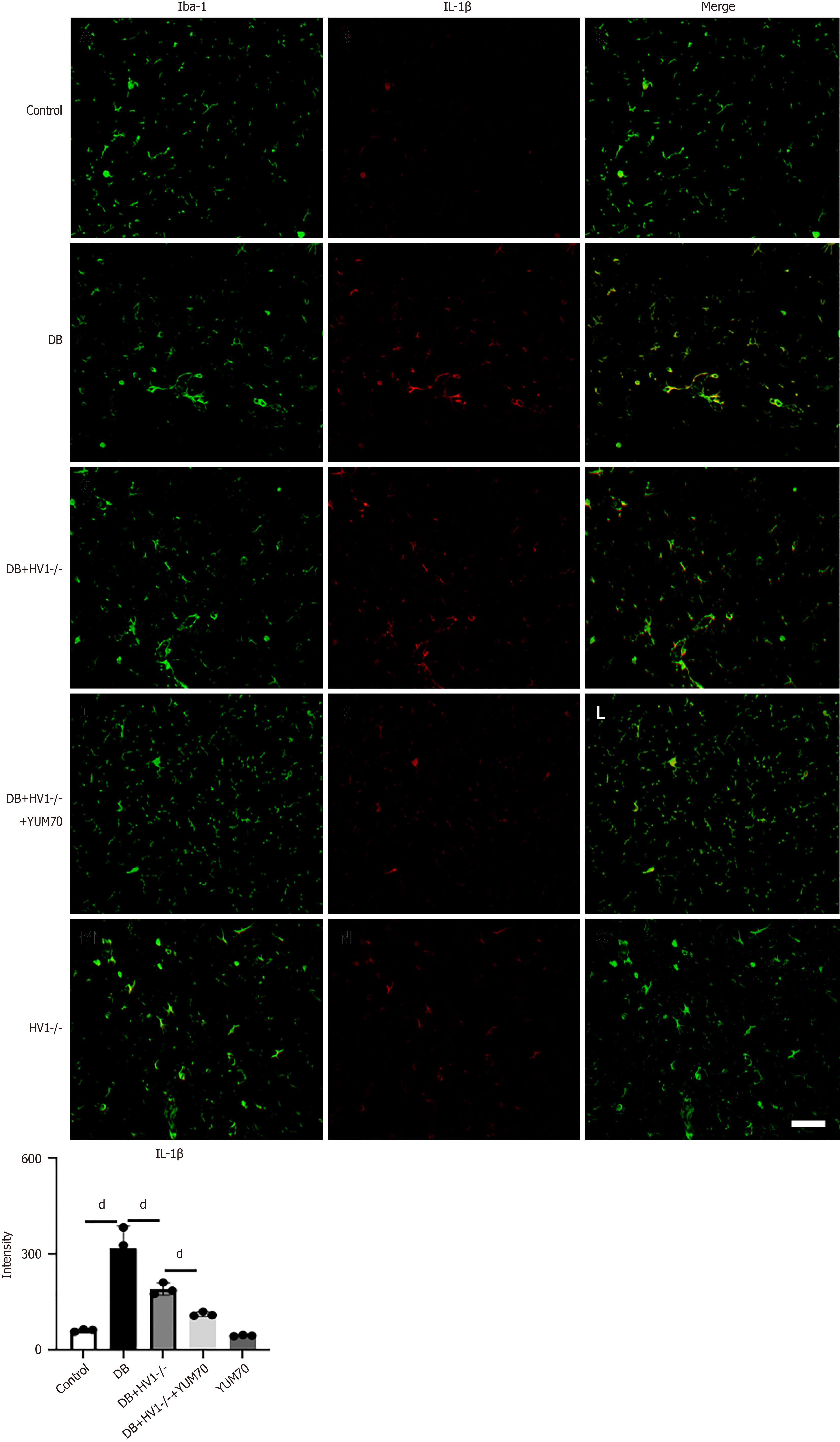
The IL-1β levels were higher in the microglial cells of the diabetic group than in the control group. The IL-1β levels were higher in the microglial cells of the diabetic group than in the control group. After Hv1 KO, the IL-1β levels in the microglial cells decreased. When the YUM70 was added, the effect of Hv1 was enhanced, indicating that Hv1 gene KO inhibits IL-1β by suppressing the GRP78 pathway (Figure 8).
Diabetes-related cognitive dysfunction is a burgeoning clinical complication that affects the quality of life of patients, overburdening their families and society. This study provides in-depth insights into the potential mechanisms and therapeutic potential of Hv1 in diabetes. Upregulated Hv1 levels might serve as markers of neural damage in the pathogenesis of diabetes. Under diabetic conditions, the excessive activation of Hv1 may lead to abnormal proton flow, affecting the intracellular pH balance and redox state. This might lead to diabetes-related neural cell dysfunction. Behavioral testing showed that Hv1 gene KO improved working memory and spatial learning abilities in diabetic mice. These improvements might alleviate neuroinflammation and axonal demyelination by Hv1 gene KO, indicating the regulatory role of Hv1 in the behavioral manifestations during diabetic cognitive impairment.
Several studies have shown the involvement of myelin, a fatty sheath around the axon of a nerve, in learning and memory functions. Oligodendrocytes are the only myelin-forming cells that produce a cell membrane that repeatedly wraps around the axon to form a myelin sheath. If the survival and maturation of OPCs are disturbed, axonal demyelination occurs. Therefore, maintaining the survival and differentiation of OPCs is important to reduce demyelination[24-27]. Studies have shown that oxidative stress and inflammation are key triggers of OPC damage. Activated microglia release large amounts of ROS, which induce demyelination, axonal degeneration, and OPC damage or even apoptosis. Therefore, microglia are promising therapeutic candidates for preventing myelin damage and enhancing the recovery of OPC function.
Neuroinflammation, an inflammatory response occurring in CNS, can be caused by various factors, including infection, autoimmune diseases, metabolic disorders, neurodegenerative diseases, and injuries[28-30]. The cells involved in neuroinflammation primarily include microglia, astrocytes, and, in some cases, infiltrating peripheral immune cells. Microglia are the main immune cells of the CNS that are activated upon sensing tissue damage or pathogens. Activated microglia and astrocytes release proinflammatory cytokines (such as IL-1β and TNF-α) and anti-inflammatory cytokines, influencing neuronal function and survival. In certain cases, neuroinflammation can damage the blood-brain barrier, allowing peripheral immune cells to enter the CNS, further intensifying the inflammatory response[31-33]. Neuroinflammation is associated with various neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. Modulating the activity or expression levels of molecules, such as Hv1, can be considered a potential therapeutic approach for neuroinflammation as it can control the inflammatory process and improve neuronal function. The neuroprotective effects of Hv1 gene KO in diabetic mice may involve multiple mechanisms. Apart from decreasing the expression of inflammatory factors, the Hv1 gene KO in diabetic mice may also protect the nervous system against oxidative stress, thus preserving mitochondrial function and promoting the release of neurotrophic factors. In addition to improving diabetic cognitive impairment, Hv1 can alleviate other complications such as diabetic retinopathy, diabetic nephropathy, and diabetic foot. Modulating Hv1 might offer a novel strategy for preventing and treating these complications.
Although it is well known that Hv1 knockdown reduces ROS production, how this reduction affects M1 activation in microglia is still unclear. Our findings showed that Hv1 knockdown reduced ferroptosis-dependent proinflammatory microglial activation by reducing ROS. Ferroptosis is a distinct type of cell death characterized by ferrous iron overload and lipid peroxidation[34,35]. Multiple studies have found that attenuating the intracellular accumulation of ferrous iron and lipid peroxidation associated with iron toxicity restores mitochondrial function in neuronal cells[36,37]. Regulation of oxidative stress can attenuate ferroptosis and promote the ability of microglia/macrophages to shift from M1 to M2 polarization[38,39]. Our study found that Hv1 knockdown significantly reduced the markers of ferroptosis, including FTH/FTL, CHOP, and GRP78, suggesting that Hv1 may regulate ferroptosis and promote microglia to M1 polarization through the ROS/GRP78 pathway, which was further validated by subsequent in vivo experiments.
Although our study identified that Hv1 affects OPC survival, how it impairs the survival and differentiation of OPCs and the specific molecular mechanisms in the OPCs have not been addressed. Moreover, although we verified that Hv1 knockdown facilitates myelin formation in mice and mitigates OPC apoptosis, further research should be conducted on Hv1-specific blocking drugs to ensure their safety and feasibility for clinical applications.
This study emphasizes the multiple roles of Hv1 in diabetes-related cognitive dysfunction and reveals its potential as a therapeutic target. Novel treatment strategies targeting the regulation of the activity or expression of Hv1 might help improve the cognitive function and overall health of diabetic patients.
| 1. | Kotecha P, Chen W, Donahoo WT, Jaffee M, Bian J, Guo J. Continuous glucose monitoring and all-cause mortality in insulin-using population with diabetes and cognitive impairment. Diabetes Obes Metab. 2024;26:4795-4798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Rovner BW, Casten RJ. Medication beliefs and depression in Black individuals with diabetes and mild cognitive impairment. J Am Geriatr Soc. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Satpathy B, Sa N, Behera A, Sahu PK. Dose-Dependent Attenuation of the Efficacy of Clitoria ternatea by Cobalt Oxide Nanoparticles Against Diabetes-Induced Cognitive Impairment. Mol Neurobiol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Paul S, Bhardwaj J, Binukumar BK. Cdk5-mediated oligodendrocyte myelin breakdown and neuroinflammation: Implications for the link between Type 2 Diabetes and Alzheimer's disease. Biochim Biophys Acta Mol Basis Dis. 2024;1870:166986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 5. | Zhu W, Zhang H, Niu T, Liu K, Fareeduddin Mohammed Farooqui H, Sun R, Chen X, Yuan Y, Wang S. Microglial SCAP deficiency protects against diabetes-associated cognitive impairment through inhibiting NLRP3 inflammasome-mediated neuroinflammation. Brain Behav Immun. 2024;119:154-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Thomas SD, Abdalla S, Eissa N, Akour A, Jha NK, Ojha S, Sadek B. Targeting Microglia in Neuroinflammation: H3 Receptor Antagonists as a Novel Therapeutic Approach for Alzheimer's Disease, Parkinson's Disease, and Autism Spectrum Disorder. Pharmaceuticals (Basel). 2024;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 7. | Sales ISL, de Souza AG, Chaves Filho AJM, Sampaio TL, da Silva DMA, Valentim JT, Chaves RC, Soares MVR, Costa Júnior DC, Barbosa Filho JM, Macêdo DS, de Sousa FCF. Antidepressant-like effect of riparin I and riparin II against CUMS-induced neuroinflammation via astrocytes and microglia modulation in mice. Behav Pharmacol. 2024;35:314-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Fernández M, Alvear-Arias JJ, Carmona EM, Carrillo C, Pena-Pichicoi A, Hernandez-Ochoa EO, Neely A, Alvarez O, Latorre R, Garate JA, Gonzalez C. Trapping Charge Mechanism in Hv1 Channels (CiHv1). Int J Mol Sci. 2023;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Szanto TG, Feher A, Korpos E, Gyöngyösi A, Kállai J, Mészáros B, Ovari K, Lányi Á, Panyi G, Varga Z. 5-Chloro-2-Guanidinobenzimidazole (ClGBI) Is a Non-Selective Inhibitor of the Human H(V)1 Channel. Pharmaceuticals (Basel). 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci U S A. 2009;106:7642-7647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Murugan M, Zheng J, Wu G, Mogilevsky R, Zheng X, Hu P, Wu J, Wu LJ. The voltage-gated proton channel Hv1 contributes to neuronal injury and motor deficits in a mouse model of spinal cord injury. Mol Brain. 2020;13:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Wu LJ, Wu G, Akhavan Sharif MR, Baker A, Jia Y, Fahey FH, Luo HR, Feener EP, Clapham DE. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci. 2012;15:565-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Wu LJ. Microglial voltage-gated proton channel Hv1 in ischemic stroke. Transl Stroke Res. 2014;5:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Liu J, Tian D, Murugan M, Eyo UB, Dreyfus CF, Wang W, Wu LJ. Microglial Hv1 proton channel promotes cuprizone-induced demyelination through oxidative damage. J Neurochem. 2015;135:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Yu Y, Yu Z, Xie M, Wang W, Luo X. Hv1 proton channel facilitates production of ROS and pro-inflammatory cytokines in microglia and enhances oligodendrocyte progenitor cells damage from oxygen-glucose deprivation in vitro. Biochem Biophys Res Commun. 2018;498:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Fu YB, Ahmed Z, Yang H, Horbach C. TUNEL Assay and DAPI Staining Revealed Few Alterations of Cellular Morphology in Naturally and Artificially Aged Seeds of Cultivated Flax. Plants (Basel). 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Wang H, Yang LL, Ji YL, Chen YH, Hu J, Zhang C, Zhang J, Xu DX. Different fixative methods influence histological morphology and TUNEL staining in mouse testes. Reprod Toxicol. 2016;60:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Baetas-da-Cruz W, Macedo-Silva RM, Santos-Silva A, Henriques-Pons A, Madeira MF, Corte-Real S, Cavalcante LA. Destiny and intracellular survival of Leishmania amazonensis in control and dexamethasone-treated glial cultures: protozoa-specific glycoconjugate tagging and TUNEL staining. J Histochem Cytochem. 2004;52:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Bahmani P, Schellenberger E, Klohs J, Steinbrink J, Cordell R, Zille M, Müller J, Harhausen D, Hofstra L, Reutelingsperger C, Farr TD, Dirnagl U, Wunder A. Visualization of cell death in mice with focal cerebral ischemia using fluorescent annexin A5, propidium iodide, and TUNEL staining. J Cereb Blood Flow Metab. 2011;31:1311-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Shibata M, Yamasaki N, Miyakawa T, Kalaria RN, Fujita Y, Ohtani R, Ihara M, Takahashi R, Tomimoto H. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke. 2007;38:2826-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Pirolli NH, Jay SM. Analysis of Bacterial Extracellular Vesicles by Immunogold Transmission Electron Microscopy. Methods Mol Biol. 2024;2843:15-23. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Oshchepkov MS, Kovalenko LV, Kalistratova AV, Tkachenko SV, Gorunova ON, Bystrova NA, Kochetkov KA. New Hybrid Ethylenediurea (EDU) Derivatives and Their Phytoactivity. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Pessina F, Romussi A, Piccini D, Mazzucco G, Varasi M, Doksani Y. Enrichment of DNA replication intermediates by EdU pull down. Methods Cell Biol. 2024;182:83-94. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Dehestani M, Kozareva V, Blauwendraat C, Fraenkel E, Gasser T, Bansal V. Transcriptomic changes in oligodendrocytes and precursor cells associate with clinical outcomes of Parkinson's disease. Mol Brain. 2024;17:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Nguyen PT, Makowiecki K, Lewis TS, Fortune AJ, Clutterbuck M, Reale LA, Taylor BV, Rodger J, Cullen CL, Young KM. Low intensity repetitive transcranial magnetic stimulation enhances remyelination by newborn and surviving oligodendrocytes in the cuprizone model of toxic demyelination. Cell Mol Life Sci. 2024;81:346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 26. | Sasmita AO, Depp C, Nazarenko T, Sun T, Siems SB, Ong EC, Nkeh YB, Böhler C, Yu X, Bues B, Evangelista L, Mao S, Morgado B, Wu Z, Ruhwedel T, Subramanian S, Börensen F, Overhoff K, Spieth L, Berghoff SA, Sadleir KR, Vassar R, Eggert S, Goebbels S, Saito T, Saido T, Saher G, Möbius W, Castelo-Branco G, Klafki HW, Wirths O, Wiltfang J, Jäkel S, Yan R, Nave KA. Oligodendrocytes produce amyloid-β and contribute to plaque formation alongside neurons in Alzheimer's disease model mice. Nat Neurosci. 2024;27:1668-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 27. | Lopes FB, Fernandes JPS, Uliassi E. Tackling Neuroinflammation in Cognitive Disorders with Single-targeted and Multi-targeted Histamine H3 Receptor Modulators. Curr Top Med Chem. 2024;24:2421-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | He L, Ye J, Zhuang X, Shi J, Wu W. Omega-3 polyunsaturated fatty acids alleviate endoplasmic reticulum stress-induced neuroinflammation by protecting against traumatic spinal cord injury through the histone deacetylase 3/ peroxisome proliferator-activated receptor-γ coactivator pathway. J Neuropathol Exp Neurol. 2024;83:939-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Hoang TA, Jin L, Nicolazzo JA, Trevaskis NL. Acute Neuroinflammation Alters the Transport of a Model Therapeutic Protein from the Brain into Lymph and Blood. Mol Pharm. 2024;21:5138-5149. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Monda A, La Torre ME, Messina A, Di Maio G, Monda V, Moscatelli F, De Stefano M, La Marra M, Padova MD, Dipace A, Limone P, Casillo M, Monda M, Messina G, Polito R. Exploring the ketogenic diet's potential in reducing neuroinflammation and modulating immune responses. Front Immunol. 2024;15:1425816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 31. | Liu Y, Meng X, Tang C, Zheng L, Tao K, Guo W. Aerobic exercise modulates RIPK1-mediated MAP3K5/JNK and NF-κB pathways to suppress microglia activation and neuroinflammation in the hippocampus of D-gal-induced accelerated aging mice. Physiol Behav. 2024;286:114676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 32. | Pranclova V, Nedvedova L, Kotounova E, Hönig V, Dvorakova M, Davidkova M, Bily T, Vancova M, Ruzek D, Palus M. Unraveling the role of human microglia in tick-borne encephalitis virus infection: insights into neuroinflammation and viral pathogenesis. Microbes Infect. 2024;26:105383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (3)] |
| 33. | Shen Y, Liu F, Zhang M. Therapeutic potential of plant-derived natural compounds in Alzheimer's disease: Targeting microglia-mediated neuroinflammation. Biomed Pharmacother. 2024;178:117235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 34. | Lei G, Zhuang L, Gan B. The roles of ferroptosis in cancer: Tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell. 2024;42:513-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 306] [Reference Citation Analysis (0)] |
| 35. | Liu G, Xie X, Liao W, Chen S, Zhong R, Qin J, He P, Xie J. Ferroptosis in cardiovascular disease. Biomed Pharmacother. 2024;170:116057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 36. | Zhou Q, Meng Y, Li D, Yao L, Le J, Liu Y, Sun Y, Zeng F, Chen X, Deng G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct Target Ther. 2024;9:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 431] [Article Influence: 215.5] [Reference Citation Analysis (0)] |
| 37. | Wang X, Zhou Y, Wang D, Wang Y, Zhou Z, Ma X, Liu X, Dong Y. Cisplatin-induced ototoxicity: From signaling network to therapeutic targets. Biomed Pharmacother. 2023;157:114045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 94] [Reference Citation Analysis (0)] |
| 38. | Qiu Z, Zhang H, Xia M, Gu J, Guo K, Wang H, Miao C. Programmed Death of Microglia in Alzheimer's Disease: Autophagy, Ferroptosis, and Pyroptosis. J Prev Alzheimers Dis. 2023;10:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 39. | Yu H, Chang Q, Sun T, He X, Wen L, An J, Feng J, Zhao Y. Metabolic reprogramming and polarization of microglia in Parkinson's disease: Role of inflammasome and iron. Ageing Res Rev. 2023;90:102032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |