Published online Feb 19, 2025. doi: 10.5498/wjp.v15.i2.98484
Revised: November 26, 2024
Accepted: December 23, 2024
Published online: February 19, 2025
Processing time: 199 Days and 3.9 Hours
Suicide is defined as the act of a person attempting to take their own life by causing death. Suicide is a complex phenomenon that is influenced by a multitude of factors, including psychosocial, cultural, and religious aspects, as well as genetic, biochemical, and environmental factors. From a biochemical perspective, it is crucial to consider the communication between the endocrine, immune, and nervous systems when studying the etiology of suicide. Several pathologies involve the bidirectional communication between the peripheral activity and the central nervous system by the action of molecules such as cytokines, hormones, and neurotransmitters. These humoral signals, when present in optimal quantities, are responsible for maintaining physiological homeostasis, including mood states. Stress elevates the cortisol and proinflammatory cytokines levels and alter neurotransmitters balance, thereby increasing the risk of developing a psychiatric disorder and subsequently the risk of suicidal behavior. This review provides an integrative perspective about the neurochemical, immunological, and endocrinological disturbances associated with suicidal behavior, with a particular focus on those alterations that may serve as potential risk markers and/or indicators of the state preceding such a tragic act.
Core Tip: The World Health Organization defines suicide as 'any act by which an individual causes injury to himself or herself, regardless of the degree of lethal intent and knowledge of the true mobile'. The etiology of suicide is multifactorial and involves complex interactions among biological, genetic, psychological, social, and environmental factors. The purpose of this manuscript is to describe the complex interactions of neuroimmunoendocrine and genetic factors associated with suicide and their relationships with environmental factors such as exposure to early life adversity, with a focus on alterations in the hypothalamic-pituitary adrenal axis and immune system.
- Citation: Ponce-Regalado MD, Becerril-Villanueva E, Maldonado-García JL, Moreno-Lafont MC, Martínez-Ramírez G, Jacinto-Gutiérrez S, Arreola R, Sánchez-Huerta K, Contis-Montes de Oca A, López-Martínez KM, Bautista-Rodríguez E, Chin-Chan JM, Pavón L, Pérez-Sánchez G. Comprehensive view of suicide: A neuro-immune-endocrine approach. World J Psychiatry 2025; 15(2): 98484
- URL: https://www.wjgnet.com/2220-3206/full/v15/i2/98484.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i2.98484
The term "suicide" has its etymological roots in the Latin words "sui" (oneself) and "caedere" (to kill)[1]. Suicidal ideation refers to persistent thoughts about the desire to end one's own life. In adolescents, ideation is often considered a high-risk factor. An attempt is defined as any act carried out with the intention of harming oneself or taking one's life[2].
The term "suicide" was first used in 1737 by Desfontaines. However, Émile Durkheim, a Frenchman, classified and defined suicidal behavior in 1897. In this case, the author concluded that individuals with greater social integration and whose desires and aspirations are more aligned with social norms are less likely to engage in suicidal behavior[3,4].
The concept of a 'suicidal act' was introduced in 1969 by the World Health Organization (WHO) as 'any act by which an individual causes injury to himself or herself, regardless of the degree of lethal intent and knowledge of the true mobile'. This last WHO definition includes aspects such as voluntariness, the purpose of the act, and conscious or unconscious motivations[5]. The WHO estimates that approximately 20% of global suicides are due to pesticide self-poisoning, with the majority occurring in rural agricultural areas in low- and middle-income countries. Other common methods of suicide include hanging and firearms (WHO, 2023). Due to the complex social, cultural, religious, and psychopathological implications of suicide, it is challenging to propose a universal definition that encompasses all the factors that contribute to it[6].
A suicidal crisis represents the stage at which the individual develops a structured plan for suicide and initiates its implementation. Suicidal ideation is the act of contemplating or devising a plan for suicide[7]. The term "parasuicide" is defined as any behavior that results in self-injury without the intention of ending one's life[8]. A suicide attempt is defined as any action that is intended to result in self-harm but that is ultimately unsuccessful due to the inappropriate methods employed[5]. Frustrated suicide is the term used to describe any suicidal act that is intended to result in death but is ultimately prevented by external factors beyond the individual's control. In such an act, the individual's intention is clearly to cause their own death[9]. The act of suicide is considered to be complete once the individual has taken the final steps to end his or her own life. This can involve a range of different levels of elaboration and planning, with the aim of fulfilling the purpose of the act[5].
Suicide is a significant public health concern worldwide, with the WHO estimating that more than 700000 individuals die by suicide annually[10]. Recently, it has been observed that men over the age of 80 are more likely to commit suicide than younger people are. This is particularly evident when people are confronted with loneliness, chronic pain, and a loss of meaning in their lives[11]. Suicide is the 18th leading cause of death overall and the second leading cause of death among young people between the ages of 15 and 29[12].
The prevalence of suicide among adults in low- and middle-income countries varies by region and individual income level but is, on average, lower than that in high-income countries. Notably, young people in low- and middle-income countries have much greater rates of suicide attempts than young people in high-income countries. Additionally, women, as well as individuals with psychiatric disorders, people living with human immunodeficiency virus (HIV), and people with poor socioeconomic status, are highly vulnerable populations[10].
As reported by Zhang[13], suicide represents the seventh leading cause of lost years of potential life, surpassing liver disease, diabetes, and HIV. In the United States, one in seven young adults have presented some type of suicidal ideation during their lives, and at least five percent have attempted suicide. The financial impact on the United States healthcare system is estimated to exceed $70 billion, with significant unquantified costs borne by families affected in terms of loss of earnings.
It is estimated that for each completed suicide, there are between 100 and 200 suicide attempts by adolescents. The prevalence of suicide attempts among adolescents aged 15 to 16 years old is estimated to range from 4.1% to 23.5%[14].
The primary factors associated with a neurobiological response to suicide include anatomical and functional alterations of the central nervous system (CNS), fluctuations in neurotransmitter concentrations, genetic influences, and psychiatric disorders such as depression, schizophrenia, anxiety, personality disorders, and substance use disorders[15]. In addition to the aforementioned variables, certain mediators of inflammation, including cytokines, chemokines, hormones, and growth factors, are also implicated in the etiology of suicidal behavior[16].
Given the intricate network of factors and interactions underlying suicide, it is crucial to elucidate the endocrinological, immunological, and neurochemical alterations associated with suicidal behavior. This will facilitate a more comprehensive understanding of this challenging clinical condition. Additionally, other pertinent factors, such as genetic and anatomic factors, will be addressed. In recent years, a plethora of evidence demonstrating that the nervous, endocrine, and immune systems establish bidirectional communication, known as neuroimmunoendocrine interactions (NIEs), has been published[17]. This communication is carried out through the controlled release of soluble mediators, including hormones, cytokines, and neurotransmitters. The variation in the levels of these soluble mediators exerts a regulatory influence on physiological processes, which in turn modulates the body's response to various factors, including stress[18,19]. This neuroendocrine-immunological interaction facilitates the development of behavioral alterations.
Recently, microglia have gained significant attention in the field of suicidal behavior. It has been hypothesized that alterations in microglial function may be associated with the development of risk factors for suicidal behavior, including early life adversity (ELA), stressful events, and psychiatric disorders. As discussed below, risk factors associated with suicide are linked to inflammatory, oxidative stress, and neuroendocrine alterations. These alterations have been hypothesized to initiate microglial synaptic remodeling, leading to altered susceptibility or resistance to suicide[20].
It has been evidenced in postmortem studies that suicidal depressed subjects have increased microglial priming in the cingulum area[21]. Microglial priming is characterized by epigenetic changes that favor the overexpression of proinflammatory cytokine mRNA such as interleukin (IL)-1 in microglia, which favors an increase in the production of proinflammatory cytokines in response to stressful stimuli[22,23]. Additionally, it has been proposed that these inflammatory changes promote a disruption of the blood-brain barrier that favors leukocyte migration in the periphery. The impact of the inflammatory response on the pathophysiology of suicide will be discussed later[24]. Interestingly, it has been proposed that hormonal changes during adolescence could enhance microglial priming depending on the amount of testosterone or estrogen present during adolescence, it has been hypothesized that the higher the concentration of these hormones, the higher the risk of developing psychiatric illnesses due to microglial priming[24].
Postmortem studies have reported alterations in glial cell populations of suicidal psychiatric patients and have identified alterations in glial fibrillary acidic protein proteins or in astrocytes immunoreactive for vimentin. In addition, a higher density of glial cells was found in the dentate gyrus of the hippocampus[25]. Inflammatory changes in the morphology of astrocytes in the anterior cingulate cortex have been observed. These changes include significantly larger cell bodies, a higher number of nodes, and a reduced number of branches. Additionally, the total length of branches and the total number of spines are decreased. Furthermore, depressed suicidals exhibit more branching processes in the auditory association cortex[26]. Recently it has been described in postmortem studies that oligodendrocytes from depressed suicides have a lower expression of antioxidant molecules such as superoxide dismutase-1, superoxide dismutase-2, glutathione peroxidase-1, catalase, and alkylglycerone phosphate synthase. It has been proposed that a decrease in these antioxidant molecules favors inflammation and changes in neurotransmitter synthesis, which will be discussed below[27].
It is well established that the psychosocial environment plays a pivotal role in suicidal behavior. Poverty, unemployment, and social isolation are key contributors to this phenomenon[28]. In addition, other potential risk factors for an increased likelihood of suicidal acts include psychiatric disorders, psychosocial adversity, and stressful life events[29]. Regarding pychiatric disorders, there is a strong correlation between suicidal behavior and mood states[12].
It has been hypothesized that psychosocial difficulties and neurobiological anomalies may contribute to suicidal behavior. Notably, over 90% of individuals who commit suicide have a psychiatric illness, with major depressive disorder (MDD) being the most prevalent major psychiatric condition[6]. A significant proportion of suicides are associated with mood disorders, with 60% of all suicides having a mood disorder at the time of death[12]. This can be associated with a set of characteristics or symptoms that are consistently present in mood disorders, including anhedonia and hopelessness, anxiety, agitation and panic, and impulsivity and aggression. Additionally, other factors such as excessive alcohol and drug use may contribute to this phenomenon. Other psychiatric diagnoses, including anxiety disorders, schizophrenia, and bipolar disorder, have been strongly linked to suicidal behavior. In fact, approximately 20% of individuals who have attempted or completed suicide have been diagnosed with bipolar disorder, while 15% have been diagnosed with unipolar depression[6,12,30].
In terms of gender, completed suicide is more prevalent among men, whereas suicide attempts are more common among women[6,31,32]. However, individuals who are single, divorced, or widowed are more at risk of attempting suicide in the United States than those who are married. In this context, widowed individuals are at the highest risk of suicide attempts or completed suicide, followed by those who are divorced or single. The lowest prevalence of suicide attempts or completed suicide is observed among individuals who are married with children[33]. This evidence provides support for the hypothesis that family and social support can act as protective factors in reducing the incidence of suicide.
The neurobiology of suicide is complex, so it is not yet possible to determine exactly why some patients decide to commit suicide and others do not, nor can we identify a specific triggering factor. As we have seen, there are many psychosocial factors associated with suicide, so this is an area that needs much more research. Given the high prevalence of suicidal behavior and completed suicides in patients with psychiatric disorders, it is prudent in these cases to consider suicide as the most severe phase of a psychiatric disorder, especially when completed events are considered[34,35].
Adverse events in early life strongly influence suicide rates and suicide attempts. A family history of suicide, having suffered sexual harassment, and having suffered an infection have also been found to be factors associated with suicide[36,37]. Precipitating factors in vulnerable individuals have been suggested to lead to psychological changes such as feelings of hopelessness and overwhelm, which in turn may lead to social isolation. Exposure to violence is one of the most studied risk factors for suicide. All of these biological and psychological risk factors lead to various adverse outcomes in adulthood, including an increased risk of suicide[38,39].
ELA is defined as exposure to adverse experiences during the first years of life, including adverse childhood expe
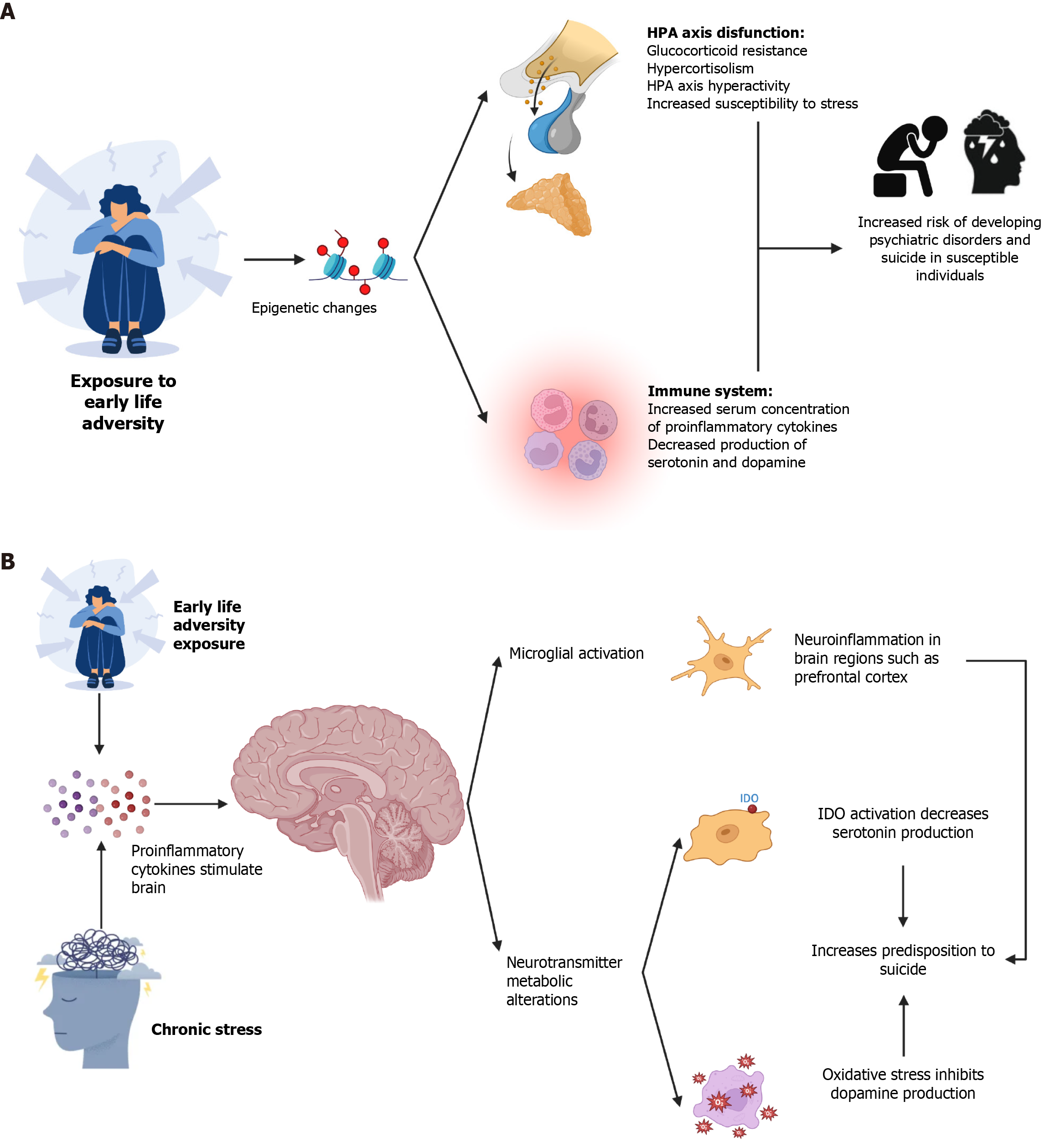
Several studies have shown that individuals exposed to ACE have increased levels of inflammatory markers[43-46]. There is strong evidence that ACE is associated with an increased risk of disease in adulthood, characterized by chronic inflammation. Some diseases that may be triggered involve metabolic, autoimmune, or psychiatric disorders[47]. There is evidence that ELA exposure is associated with epigenetic changes in the immune system and the functioning of the hypothalamic-pituitary adrenal (HPA) axis, which modify and determine the stress response[47]. The results of some studies suggest that ELA is associated with increased hyperactivity of the HPA axis due to epigenetic changes that cause decreased glucocorticoid receptor (GR) signaling[48-53]. As described below, the stress response is regulated by the HPA axis and is accompanied by increased circulating cortisol levels, catecholamines, and proinflammatory cytokines. HPA axis hyperactivity and chronic inflammation are associated with the development of glucocorticoid resistance, which is a feature observed in individuals with MDD, a condition associated with suicide (Figure 1A)[43,54].
On the other hand, exposure to adverse life events (ALSs) has been demonstrated to induce epigenetic changes in the immune system, resulting in the development of a chronic proinflammatory state. Long-term studies have shown that people exposed to ALSs have increased inflammatory markers such as C-reactive protein (CRP) or IL-6 during adolescence or adulthood[48,55-57]. As described below, chronic inflammation has been associated with changes in the metabolism of neurotransmitters such as serotonin or dopamine, so it has been proposed that changes in the immune system and the HPA axis increase susceptibility to developing psychopathology[58]. Finally, hyperactivity of the HPA axis and chronic inflammation modify microglial functioning, causing increased microglial activation[59,60]. These changes trigger increased proinflammatory cytokine production and decreased serotonin production. It has been proposed that microglial activation caused by chronic stress causes epigenetic changes in microglia, generating a senescent phenotype characterized by increased production of proinflammatory cytokines and increased expression of major histocompatibility complex-II, which has been proposed as a possible mechanism to explain the development of psychopathology, mood disorders, and suicide[23,61,62].
The anatomy of NIE interactions involves a complex network of molecules, cells, tissues, and organs that comprise specialized systems, all of which work together to maintain a homeostatic state[63]. The nervous system includes two large arms. The central and peripheral nervous systems primarily coordinate cognitive functions and facilitate com
The HPA axis is of enormous relevance because it is the backbone of NIE interactions, and one of its primary roles is activating effector mechanisms to fight against any stimulus capable of generating tension in the body and reestablishing a normal state as soon as possible. When an organism experiences a stressful stimulus, whether due to the environment that surrounds it, a cognitive perception of vulnerability or a potentially pathogenic agent, the organism's physiological response is to activate the HPA axis. First, the paraventricular nucleus of the hypothalamus releases corticotropin-releasing hormone (CRH). This hormone aims to stimulate the pituitary gland to release adrenocorticotropin, which in turn travels to the adrenal glands to produce cortisol. This escalation of synthesis of the HPA axis must be regulated since its constant activation triggers alterations at the cellular, molecular, and structural level in multiple systems. For example, in the particular case of those mood disorders that have been related to a high incidence of suicidal acts, there is major depression, in which a significant increase in cortisol levels has been established due to hyperactivity of the HPA axis, which correlates with cognitive impairment, decision making, and emotional processing in patients with major depression and suicide attempts[70,71]. Another critical function of cortisol is the release of energy, increasing the mobilization of proteins and fats, as well as the regulation of inflammatory processes[72]. Recent studies evidence of alterations in the HPA axis and its association with the risk of suicide[73]. In this sense, and the search for biological markers for suicide attempt and completed suicide, the literature shows that plasma cortisol levels are elevated in patients with suicide attempts compared to healthy individuals[74]. It is also crucial to consider the clinical characteristics of patients when examining the relationship between plasma cortisol levels and suicidal behavior, as this can help to prevent misinterpretation[75]. This is because cortisol levels increase as part of the normal aging process, possibly due to a diminished activation of negative feedback mechanisms[75,76].
A review of the literature revealed that most studies exploring the potential role of the HPA axis as a risk factor in suicidal behavior have focused on individuals with major depression, physical or sexual abuse, and impulsive behavior. However, the findings of these studies are not always conclusive. For instance, while Westrin and colleagues reported elevated cortisol levels in suicidal patients compared to healthy volunteers, Lindqvist and colleagues observed the opposite result. These discrepancies may be attributed to the variability between studies, including the heterogeneity of the samples and the type of analytical method employed[77,78].
A recent meta-analysis of 2269 determinations revealed that the activity of the HPA axis varies with age. In patients under 40 years of age, there was a positive correlation between the HPA axis and suicidal behavior. The effect was reversed in samples of patients over 40 years of age, demonstrating that the role of the HPA axis is more complex than previously recognized based on the association with mood disorders: (1) In samples of patients under 40 years of age, there was a positive correlation between the increase in the HPA axis and the occurrence of suicidal acts; and (2) In samples of patients over 40 years of age, the effect was reversed, thus demonstrating that the role of the HPA axis is more complex than previously recognized based on the association with mood disorders[78]. It has been proposed that elevated plasma cortisol concentrations are associated with insensitivity of the GR and binding protein 5 (FKBP5)[77]. One proposed mechanism to increase cortisol production is based on the overexpression of FKBP5. The FKBP5 gene encodes a GR chaperone that plays an important role in intracellular signaling and is crucial for homeostatic regulation of the stress response[77,79]. Consequently, in patients who have attempted suicide, it is postulated that overexpression of FKBP5 reduces the affinity of cortisol for the GR complex, thereby increasing GR resistance and plasma cortisol levels[80]. Notably, several studies have demonstrated the presence of FKBP5 polymorphisms in various populations, which have been associated with an increased risk of suicide[81].
It is crucial to acknowledge that HPA axis hyperactivity has been linked to an elevated risk of suicide, irrespective of the presence or absence of a psychiatric condition. This suggests that the role of the HPA axis extends beyond glucocorticoid resistance, which is commonly observed in patients with MDD[82]. Exposure to ELA causes inflammation and HPA axis alterations that increase the risk of developing stress, which in turn increases the risk of suicidal behavior[82-84]. It has been postulated that alterations in the genes encoding the GR and the CRH receptor type 1 and 2 are associated with suicidal behaviors[84-86]. In postmortem studies conducted on teenagers who died by suicide, a decreased density of GR-α receptors was observed in the medial prefrontal cortex and amygdala in comparison with control subjects[87].
Furthermore, individuals who were exposed to ELA and who died by suicide exhibited reduced GR expression in the hippocampus, in addition to the expression of GR variants. This evidence suggests that ELA causes epigenetic changes in GR expression[88]. In other postmortem studies, a higher concentration of CRH and vasopressin was found in brain regions such as the forebrain, the raphe nucleus, and the locus coeruleus of suicide victims[89,90]. In addition, it has been reported that individuals who have died by suicide have a greater volume and weight of the adrenal glands, which may be due to hypertrophy resulting from chronic activation of the HPA axis[91-94].
In general, the findings align with the hypothesis of a loss or increase in allostatic load put forth by McEwen. This hypothesis posits that uncontrolled activity of the HPA axis typically results in the deregulation of various systems, including NIE interactions[95]. In conclusion, the HPA axis plays a role in the diathesis of suicidal behavior. Therefore, glucocorticoids and cortisol may be a trait biomarker for this behavior[74,76,96].
In general, the immune system is responsible for protecting and preserving the integrity of the body against a wide range of potential pathogens, including bacteria, viruses, parasites, fungi, and toxins. The immune response can be divided into two major stages: The innate immune response and the adaptive immune response. In each case, characteristic cell lineages are involved, which, upon activation, secrete soluble mediators called cytokines and/or ILs. These proteins play fundamental roles as activation and signaling proteins. The role of cytokines in the pathophysiology of psychiatric disorders was postulated when observing a greater incidence of depressive symptoms in patients with an inflammatory condition, as well as in those patients therapeutically treated with cytokines[97,98]. In recent years, a considerable body of literature has been published on the role of cytokines, growth factors, and chemokines in peripheral blood and brain in the pathophysiology of behavior and suicidal acts associated with behavioral disorders (Figure 1B)[18,99,100].
Studies in individuals with suicidal ideation that evaluated the concentration of circulating proinflammatory cytokines have demonstrated alterations in the concentration of proinflammatory cytokines and CRP[18,101-105]. CRP has been demonstrated to be a dependable prognostic biomarker for predicting the risk of patients who have a history of suicide attempts[106].
IL-1: This IL family comprises 11 members. It is a cytokine considered proinflammatory. IL-1α and IL-1β are members of this family. Its synthesis is primarily by macrophages[107]. The role of IL-1α as an active proinflammatory cytokine has been evaluated in a population of patients with various psychiatric disorders who have attempted suicide. However, the results did not show a significant difference[108].
IL-2: IL-2 is a growth factor for T, B, and natural killer lymphocytes and is also involved in interferon synthesis and inflammatory reactions[109]. In patients who have attempted suicide, there is a reduction in IL-2 levels compared to those who have not attempted suicide but are depressed[101]. One hypothesis associated with this decrease in IL-2 Levels is related to the increase in sIL-2R concentrations in these patients. The sIL-2R/IL-2 complex is internalized and undergoes degradation, which explains the decrease in its levels. Furthermore, Isung et al[110] reported in a cohort with over 13 years of follow-up that a reduction in IL-2 was associated with patients who completed a suicide attempt.
IL-4: IL-4 plays a role in the differentiation of Th2 Lymphocytes and the maturation of plasma cells and is strongly associated with allergic processes[111]. The peripheral levels of this cytokine are lower in suicidal patients with major depression than in healthy volunteers[112]. These observations are preferentially associated with the depressive state because changes in levels are observed between patients with depression and those with suicidal attempts[110,113].
IL-6: The IL-6 family of cytokines consists of seven members (IL-6, IL-11, leukemia inhibitor factor, oncostatin M, ciliary neurotrophic factor, cardiotrophin 1, and cardiotrophin-like cytokine) with proinflammatory and anti-inflammatory properties and are significant players in hematopoiesis; however, they have also been implicated in inflammatory and autoimmune diseases[114,115]. According to a recent meta-analysis, IL-6 is the cytokine with the most significant relevance in suicidal behavior; its role in behavioral disorders such as depression has been reported[116]. MDD is a primary risk factor for suicide, and increased release of IL-6 has been detected in this condition; moreover, increased IL-6levels are associated with prognosis and therapeutic response in MDD patients. Drugs, herbal medications, exercise, and other interventions have been explored as potential regulators of IL-6 in inflammation-dependent depression[117]. In concordance, transgenic mice overexpressing IL-6 in the CNS display increased stress-induced glucocorticoid levels, likely inducing vulnerability to depressive-like behaviors[118].
IL-10: IL-10 is a cytokine with high activity in inhibiting inflammation and plays a crucial role in pathological processes such as cancer and autoimmune diseases[119]. The relevance of IL-10 in depression has been demonstrated in animal models. For example, in mice with learned helplessness, which results in object recognition and spatial memory imp
Interferons: Interferons (IFNs) are employed as therapeutic agents in the treatment of viral infections. Additionally, they are utilized in the management of various cancers and the treatment of autoimmune disorders, such as multiple sclerosis. Most of the literature concerning interferon treatments concerns IFN-α, IFN-β, and IFN-γ[123]. Interferons are pivotal in numerous cellular processes through transcriptional regulation of immunologically relevant genes. Its functions include upregulation of pathogen recognition, antigen processing and presentation, antiviral state, inhibition of cellular proliferation and apoptosis, activation of microbicidal effector functions, immunomodulation, and leukocyte trafficking[124]. An association between alterations in mood and the administration of IFN-α was observed in patients who developed depressive symptoms[98]. This relationship occurs because IFN-α is a potent inducer of indolamine 2,3-dioxygenase (IDO-1) and tryptophan 2,3-dioxygenase, two important enzymes in the kynurenine pathway. The activation of this pathway results in the blockade of tryptophan synthesis into serotonin, thereby promoting the formation of neurotoxic metabolites[58,125]. Notably, an elevated kynurenine pathway has been observed in patients with MDD who have attempted suicide compared to those with MDD without suicidal ideation and healthy volunteers[126]. Although the number of reports is limited, it is worth noting a case series of 11 patients with multiple sclerosis who were treated with interferon beta and who presented with major depression and suicidal ideation or attempted suicide. These findings highlight the importance of being especially vigilant in interferon-treated patients[127].
Tumor necrosis factor-alpha: Tumor necrosis factor-alpha (TNF-a) is a classic proinflammatory cytokine produced by macrophages; its role in suicide is not entirely clear, although, in these patients, there is a tendency toward increased circulation; however, TNF-a polymorphisms have been reported to be associated with the development of suicidal ideation in patients with acute coronary syndrome[128,129]. Recently, it was described an increased in plasma TNF-a levels[101]. Additionally, in a study of patients with MDD and suicide attempts that there is a correlation between higher TNF-a concentrations, cognitive impairment and increased suicidal behavior[105].
Transforming growth factor-beta: This cytokine is associated with the control of cell growth, proliferation, differentiation and apoptosis; its role in the immune response is the regulation of inflammation[130]. It has been reported that transforming growth factor-beta (TGF-β) levels are elevated in suicidal patients with MDD compared to healthy volunteers, so this cytokine has been related to stressful events to counteract the deleterious effects of stress in those patients with suicidal behavior[131]. Additionally, TGF-β polymorphisms have been found to be associated with an increased predisposition to suicide[132].
Vascular endothelial growth factor: This growth factor is involved in the correct functioning of the CNS, participating in processes such as neurogenesis, neuronal patterning, neuroprotection and glial growth[133], so subtle changes in its secretion or expression become relevant in psychiatric and neurological conditions, such as decreased cognition in Alzheimer’s disease[134]. In the case of patients who have attempted suicide, a decrease in peripheral glucose levels has been observed compared to those in healthy volunteers, and these results also correlate with the severity of the depressive condition and the decreased CSF and serum glucose levels; this fact could explain the downregulation of hippocampal neurogenesis and the inadequate response to pharmacological treatment[110,135].
Cytokine and chemokine alterations have been linked to the development of psychiatric disorders such as MDD, which are associated with an increased risk of suicide. Postmortem studies have shown elevated concentrations of proinflammatory cytokines in the orbitofrontal cortex and the prefrontal cortex of suicide victims[99,136-138]. In contrast, the levels of some chemokines have been shown to be reduced in the prefrontal cortex of individuals who die by suicide[138]. Furthermore, postmortem studies have revealed elevated expression of the Toll-like receptors (TLRs) TLR2, TLR3, TLR4, TLR6, and TLR10 in the mPFC. TLRs, which recognize molecular patterns associated with damage (damage-associated molecular patterns, or DAMPs) or pathogens (pathogen-associated molecular patterns, or PAMPs), are essential components of the inflammatory response. After activation, TLRs promote the synthesis of proinflammatory cytokines. Although the participation of TLRs in suicide is not completely understood, it has been suggested that TLRs contribute to neuroinflammation[136].
Among the altered cytokines reported in the brain, an increase in IL-1 has been reported in postmortem brain samples of suicide victims, which correlates with the increase in mRNA levels in the brains of suicide victims[136]. The potential role of IL-1β is related to its biological activity at the CNS level, as it has been demonstrated to exert local effects on neuronal and glial functioning, as well as on neurodevelopment and in response to injuries[139]. In the same way, TNF-a were greater in the postmortem brains of teenager suicide victims than in those of healthy controls[136,140]. Another cytokine implicated in the pathophysiology of suicide is IL-2. It has been proposed that reduced cerebral IL-2 Levels may be linked to the loss of cholinergic neurons in the medial septum of the hippocampus, which could impair com
IL-5 promotes the proliferation, activation, and differentiation of eosinophils. It is primarily produced by Th2 lymphocytes[142]. This cytokine has been explored in postmortem samples from suicidal patients in the Brodmann 11 region. However, its expression levels have not been determined. It appears that there is a significant difference in this region of the orbitofrontal cortex, which is associated with decision-making, reward processing, and long-term memory[137]. Moreover, it was reported that the plasma and CSF levels of IL-6 are greater in patients who attempted suicide than in those in the control group; in particular, the levels of IL-6 are highest in patients who attempted violent suicide[140]. Increased levels of IL-6 seem to be related to neurotransmitters, as a positive correlation was found between IL-6 Levels and 5-HIAA (5-hydroxyindoleacetic acid, a metabolite of serotonin) and HVA (homovanillic acid, a metabolite of dopamine) CSF levels in violent suicide attempts[140].
IL-13 exerts an inhibitory effect on macrophages and is an inducer of IgE, regulating numerous aspects of allergic inflammation[143]. The role of this cytokine in suicidal behavior has been observed with greater frequency in suicidal men, as indicated by a study that revealed increased mRNA levels of this cytokine in the orbitofrontal cortex in postmortem male patients. This has led to the suggestion that it may serve as a marker in suicidal behavior[137,144].
Additionally, the mRNA levels of this cytokine were found to be elevated in the prefrontal cortex region of adolescent suicide victims[136,137]. Additionally, increased plasma levels were noted in suicide attempters[101].
Furthermore, alterations in chemokines have been observed in individuals who have committed suicide. A significant decrease in the levels of CCL1, CCL8, CCL13, CCL15, CCL17, CCL19, CCL20, and CXCL11 was observed in suicide completers[138]. The serum levels of monocyte chemoattractant protein 1 (MCP-1/CCL2) and RANTES/CCL5 were lower in patients with depression and suicidal ideation than in healthy controls, whereas the eotaxin/CCL11 ratio was elevated[145].
Finally, as previously mentioned, there is evidence that ELA exposure is associated with an increased risk of suicide due to changes in the HPA axis and inflammation in the long term. Systemic or chronic inflammation is associated with several adverse effects, including anxiety, anhedonia, fatigue, sleep disturbances, and sickness behavior. The presence of a febrile response, anorexia, lack of motivation, social deprivation, and reduced movement characterizes this latter phenomenon. These changes result from proinflammatory cytokines circulating in the bloodstream, which stimulate the brain and trigger metabolic changes in the production of neurotransmitters such as serotonin and dopamine[58]. The introduction of IFN-α for the treatment of hepatitis C led to an increase in depressive symptoms and suicidal ideation in patients receiving IFN-α treatment. This observation provided one of the earliest indications that cytokines may be linked to suicide[146,147]. The relationship between inflammation and suicide can be understood through two main pathways. The first is through the activation of microglia, previously reviewed. The second pathway is associated with an imbalance in the production of serotonin and dopamine, both of which have been linked to an increased risk of developing psychiatric disorders such as MDD in susceptible individuals which is associated with an increased risk of suicide[18,43]. This results from an increase in IDO-1 activity, which is caused by chronic inflammation. This increase in activity leads to increased kynurenine production and a decrease in serotonin production from tryptophan. On the other hand, oxidative stress induced by inflammation impairs the synthesis of tetrahydrobiopterin, a vital cofactor in dopamine production, thereby reducing dopamine synthesis (Figure 1A)[58].
Biogenic amines play a fundamental role in adequately maintaining metal health and homeostasis[148]. Among them are serotonin (5-HT) and dopamine. These two neurotransmitters have been shown to alter psychiatric disorders sig
This system is critical in individuals since alterations in this system are associated with anxiety, depression, aggressiveness, and impulsivity disorders. The neurotransmission of 5-HT is mediated by at least 14 types of receptors and a transporter (SERT)[154]. Among the types of 5-HT receptors that have been associated with suicidal behaviors are 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, 5-HT4, and 5-HTT; among these, 5-HT1A has generated significant interest in the investigation of suicidal acts due to its role in memory, recognition, learning memory and neurogenesis of the hip
Serotonin is associated with suicide risk, with low concentrations of 5-hydroxyindoleacetic acid, a metabolite of 5-HT, in cerebrospinal fluid, potentially indicating a greater risk of suicide[6,12]. This has been corroborated in postmortem brain samples, and it is therefore proposed that a deficit in serotonin transporter binding has a significant impact on the pathophysiology of suicide[158].
Another system that has garnered significant interest in elucidating the underlying mechanisms of behavior and, in particular, emotions is dopamine. This neurotransmitter plays a key role in regulating many brain functions, including mood, aggression, and attention[159]. Dopamine is produced by dopaminergic neurons in the ventral tegmental area of the midbrain and the substantia nigra[159]. Individuals with high levels of this neurotransmitter exhibit hyperactivity and impulsive behavior. Conversely, individuals with low levels of this neurotransmitter demonstrate anhedonia. This is because nonadrenergic activity predisposes individuals to depressive events, which are associated with a decrease in the density of dopamine transporters[160,161].
The close relationship between thyroid hormones and psychiatric symptoms has long been explored. Recently, novel evidence has emerged showing an intriguing association between thyroid status and suicidal behavior, especially in patients with MDD or thyroid illness. With some exceptions[162], recent investigations have demonstrated that distinct alterations in thyroid status are present in MDD patients who have attempted suicide. Some authors have shown that, in comparison with nonattempters, MDD patients with suicidal attempts exhibit elevated scores in tests for depression, anxiety, or psychotic symptoms, which are accompanied by increased levels of thyroid-stimulating hormone (TSH) and anti-thyroid antibodies such as anti-thyroglobulin and anti-thyroid peroxidase (TPOAb)[163-165]. Interestingly, high TSH levels were positively correlated with elevated scores for anxiety and psychotic symptoms, and TSH and TPOAb levels were independently associated with suicide attempts[163]. Moreover, these investigations demonstrated that higher TSH and anti-thyroid antibody levels are risk factors for suicide attempts in MDD patients[163,164].
In contrast with the consistent evidence about TSH, the data on other hormones from the hypothalamus hypophysis thyroid (HHT) axis are inconclusive. Liu et al[164] and Peng et al[163] showed no significant changes in the levels of triiodothyronine (T3) and thyroxine (T4) in MMD patients who attempted suicide compared with those who did not attempt suicide[163,164]. In contrast, other authors reported lower or normal free T4 levels in MDD patients with suicidal behavior[165-168], and Wang et al[167] reported that free T4 levels are predictors of suicidal attempts in depressive patients. Regarding the participation of thyrotropin release hormone (TRH), a unique study reported reduced central TRH function in depressed patients with current suicidal behavior[166].
The high risk of suicide behavior in MDD patients with thyroid disorders is still more evident in depressive individuals who develop subclinical hypothyroidism (SCH), an endocrine disorder characterized by high TSH and normal T3 and T4 serum levels[169]. SCH is highly common in MDD patients, reaching a prevalence of 60%[170,171]. Interestingly, MDD patients with SCH had significantly greater suicide attempts (25.4% vs 12.2%) and a 67% greater risk of suicide than did depressive patients without SCH. Moreover, these patients exhibited higher scores for anxiety, depression, and psychotic symptoms, as well as elevated levels of TSH, than did those without suicide attempts[170].
A relationship between thyroid alterations and suicidal behavior has also been documented in patients with hyper or hypothyroidism. Three different case reports have shown that patients with hyperthyroidism (Graves’ disease) and no previous history of mental disorders present with suicidal ideation or attempts accompanied by undetectable TSH levels[172-174]. Similarly, patients with Graves’ disease have a greater risk of suicide than their controls, although this difference did not reach statistical significance[175].
Concerning hypothyroidism, Todorov et al[176] presented a case report of suicide attempts in an adult patient without a past psychiatric history but with severe hypothyroidism characterized by high TSH and anti-thyroid antibody serum levels and reduced free T3 and T4 serum concentrations. Similarly, patients with hypothyroidism caused by Hashimoto’s thyroiditis showed a significantly increased risk of death by suicide. Interestingly, the median time to death by unnatural causes (including suicide) was 3 years for patients with Hashimoto syndrome and 4 years for controls[177].
Most of the evidence indicates that TSH and anti-thyroid serum levels may contribute to the onset of suicidal behavior in patients with MDD or hypothyroidism. In contrast, the role of other hormones in the HHT axis is not completely clear since there are controversial results about thyroid hormone levels, and the participation of TRH has been minimally explored. Finally, we would like to emphasize the critical relationship between TSH levels and suicide, since its increased concentration is a common denominator in patients with suicide attempts, and the prevalence and risk of suicide attempts are strongly associated with the levels of TSH. Lang et al[171] demonstrated that the risk of committing suicide was 4.5 times greater in MDD patients with a TSH > 10 mIU/L than in those with a TSH < 10 mIU/L. In addition, the prevalence of suicide attempts in MDD patients with higher TSH levels was significantly greater (67.20%) that in depressive patients with TSH < 10 mIU/L (18.40%)[164]. Finally, network analysis by Peng et al[163] revealed that TSH is a key point in the onset of suicide, exhibiting substantial associations with metabolic disturbances, anxiety, and psychotic symptoms in patients with suicidal behavior.
The molecular genetic basis of suicide remains incompletely understood. However, the evolution of genetic and biochemical processes is believed to be a potential avenue for further investigation. Additionally, epigenetic factors, such as negative social and environmental events (e.g., posttraumatic stress), may also play a role. The monitoring of various genetic patterns may prove valuable in identifying those responsible for alterations that could lead to a fatal outcome. With appropriate clinical follow-up and intervention, it may be possible to prevent such outcomes. Although there is evidence that diverse pathways associated with specific single-nucleotide variants could affect the same related brain regions to trigger the suicidal process, analogous to the malignant evolution of the physiological process, it is necessary to determine how to address alterations in physiological and biochemical processes. The interactome of suicide (protein-protein interactions and genetic interactions) is an important goal in this regard. Genetic studies have demonstrated the pivotal role of the serotonergic system in the genesis of suicidal ideation, suicide attempts, and suicidal behaviors, as well as impulsivity and aggressiveness[178-180]. A number of studies have indicated a correlation between specific polymorphic variants of the tryptophan hydroxylase (TPH) gene (a limiting enzyme in serotonin synthesis), the serotonin transporter (5-HTT, SERT or SLC6A4), and the serotonin receptor 2A (5-HT2A) and suicidal behaviors[181-183].
First, the gene encoding THP, an enzyme crucial for the synthesis of 5-HT, exists in two distinct isoforms: THP-1 and THP-2. A meta-analysis was conducted to investigate the correlation between the gene variants TPH-1 (A218C and A779C) and TPH2 (G-703T, A-473T and G19918A) and suicidal behavior. The findings of this meta-analysis indicate that the TPH-1 variant is associated with an increased risk of suicidal behavior, whereas no such association was observed for the TPH-2 variants[181]. In a population of patients with schizophrenia, no association was found between the 473T/A and hCV245410 variants of the TPH-2 gene and suicidal behavior[184].
The serotonin transporter (SERT) has also been investigated in patients with suicidal behavior. The results of studies on the association between the SLC6A4 (serotonin transporter) and 5-HTTLPR (serotonin-transporter-linked promoter region) and suicidal behavior have been controversial. However, a meta-analysis identified an association between the gene variant and violent suicidal behavior[185].
An association between the 5-HT2A receptor and suicidal ideation in patients with depression has been previously described. Compared with individuals with the T/C or T/7 genotype, individuals with the 102C allele exhibited a positive correlation between the item score for suicidal ideation and the C/C genotype. Additionally, the SERT gene S/L polymorphism has been linked to completed suicide. In contrast, the L/L genotype was twice as prevalent in suicide victims diagnosed with depression as in the control group[186].
Another topic worthy of further investigation is the diathesis of suicide, which is defined as a genetic predisposition toward suicidal acts. Brent and colleagues proposed the hypothesis that this phenomenon is highly familiar and may be heritable. The clinical phenotype of completed and attempted suicide is transmitted within families. Furthermore, the rates of suicide attempts are elevated in the family members of suicide completers, and completion rates are elevated in the family members of attempters[187]. In a separate study, the precursors of early-onset suicidal behavior included mood disorders, impulsive aggression, a history of suicide attempts in one's parents, and sexual abuse. The authors posit that the transmission of suicidal behavior from one generation to the next can be prevented or, at the very least, reduced among at-risk youth by attending to specific domains or factors[188].
The results of studies conducted on twins indicate that heritability estimates for suicidal behavior range from 21% to 50%, with a broader phenotype that includes suicidal ideation or planning reaching up to 55%[189]. Regarding monozygotic twins, a higher concordance rate for suicide is observed than for dizygotic twins[187]. A second group of studies that strongly reinforce the role of genetics are those in which a greater risk of suicide attempts has been observed in individuals with a family history of suicide[190].
A recent study identified potential genetic risk loci associated with suicidal ideation and behaviors. This genome-wide association study included 633778 United States military veterans with diverse ancestry and identified ESR1 (encoding an estrogen receptor), DRD2 (encoding a D2 dopamine receptor), TRAF3 (encoding TNF receptor-associated factor 3), and DCC (encoding the netrin receptor DCC) as cross-ancestry candidate risk genes. This meta-analysis identified more than 200 genome-wide significant single nucleotide variants associated with suicidal thoughts and behaviors in individuals of diverse ancestry. These variants were localized to specific regions on chromosomes 2, 6, 9, 11, 14, 16, and 18[191]. Recently, ESR1 and TRAF3 have been identified as risk loci for posttraumatic stress disorder and other disorders, including depression and anxiety, which are considered risk factors for suicidal ideation and suicidal behaviors[192]. TRAF3 is a regulator of type-1 interferon production and has been linked to MDD, antisocial behavior, and attention-deficit/hyperactivity disorder. Notably, lithium, a medication used in bipolar disorder treatment, modifies the expression of TRAF3 and several other inflammatory genes. DCC is expressed in the brain, where it regulates a number of different processes. It has been associated with a range of psychiatric phenotypes, and elevated levels have been observed in the prefrontal cortex of individuals who have died by suicide[193,194]. DRD2 is highly expressed in specific regions of the brain, has been associated with numerous psychiatric phenotypes (including suicide), and is involved as a regulator of other risk factors for suicide, including impulsive behavior, schizophrenia, and mood disorders[195-198].
Imaging methodology has proven to be a valuable tool for studying the anatomy and function of the brain, particularly in the context of psychiatric disorders and neurological diseases. The use of brain images from suicidal patients has facilitated the identification of structural alterations in multiple regions of the brain. These include the orbitofrontal cortex, the amygdala, the temporal-parietal-limbic networks of the hippocampus, and the putamen and cerebellum[198-200]. In addition to these findings, a meta-analysis revealed that suicidal behavior is associated with structural deficits in several brain regions, including the gyrus rectus, superior temporal gyrus, and caudate nucleus, as well as functional overactivation in the anterior and posterior cingulate cortex of patients with suicidal ideation[201].
As previously stated, the amygdala exhibits structural alterations. The significance of this region lies in its subcortical location within the medial temporal lobe and its extensive connections with the majority of the brain, including nuclei involved in emotional and anxiety-related processes[202]. Furthermore, the amygdala is connected to the prefrontal and inferotemporal cortices, which, in turn, are linked to the medial temporal memory systems, including the hippocampus and the entorhinal cortex. Another crucial connection is with the cortex. The ventromedial prefrontal cortex is linked to a range of processes, including the assessment of sensory, cognitive, and affective information, as well as decision-making. Based on this, the role of morphological alterations of the amygdala and its participation in ideation can be explained. Furthermore, the suicidal act can be understood in the context of these findings[203,204]. The prefrontal cortex and hippocampus are functionally related to emotion, stress, cognitive functions, and aspects involved in suicidal behavior.
From an inflammatory perspective, the use of non-steroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, naproxen, celecoxib, and aspirin, has been demonstrated to reduce suicidal ideation[205]. Furthermore, the use of aspirin without concomitant administration of other NSAIDs has been associated with reduced levels of depression, anxiety, and stress in cancer patients[206].
On the other hand, the use of TNF-α antagonists has been demonstrated to reduce suicidal behaviors in patients with hidradenitis suppurativa[207]. In the case of immunotherapies such as tocilizumab (a humanized monoclonal antibody against the IL-6 receptor), a reduction in the risk of depression has been observed in patients with rheumatoid arthritis[208]. It is important to note, however, that certain treatments, such as infliximab (a chimeric monoclonal antibody that blocks TNF-α), which is commonly used for chronic inflammatory diseases[209], have been associated with an increase in suicide attempts in rheumatoid arthritis patients[210] and suicidal ideation in bipolar disorder patients[211]. Another drug of interest is minocycline, which has demonstrated efficacy in reducing depressive symptoms due to its anti-inflammatory effects. Nevertheless, further clinical studies are necessary to ascertain its efficacy in suicide prevention[212].
From a neuroendocrine perspective, levothyroxine is a commonly utilized therapeutic agent for hypothyroidism, and its use is associated with a reduction in depressive symptoms. Nevertheless, only a limited number of studies have investigated the influence of levothyroxine dosage on suicidal behavior[213,214]. Some case reports have indicated a potential correlation between levothyroxine overdose and suicidal ideation, underscoring the necessity for cautious administration and comprehensive clinical investigations to elucidate the benefits and risks associated with its use.
In this context, selenium also merits attention as a substance known to improve thyroid function and reduce inflammation in diseases such as Hashimoto's thyroiditis. Nevertheless, elevated selenium intake has been linked to an elevated risk of postpartum depression. However, adequate selenium levels have been demonstrated to mitigate depressive symptoms[215]. This highlights the necessity for precise, evidence-based dosing to prevent adverse effects.
The presence of hypercortisolemia, a condition frequently observed in individuals with a history of suicide attempts and MDD[216], suggests the potential efficacy of mifepristone as a therapeutic agent. This antagonist of progesterone and GR has been shown to have antidepressant and anxiolytic effects in preclinical studies by counteracting the effects of allopregnanolone[217]. Nevertheless, further preclinical and clinical studies are required to define its efficacy and safety in humans.
It has been established that estrogen (E2) and progesterone (P4) levels are closely associated with mood. A rise in suicidal behaviors has been documented during the premenstrual phase[218], when estrogen and progesterone levels are at their lowest[219]. Moreover, women in menopause are at an elevated risk for suicidal ideation, indicating that both hormone replacement therapy and selective estrogen receptor modulators could have a substantial impact on suicide prevention. Nevertheless, further research is essential to gain a deeper understanding of their role in this context.
Suicide prevention has been addressed through the implementation of psychosocial prevention programs and the utilization of pharmacological and clinical interventions. The implementation of prevention programs has resulted in a notable decline in the suicide rate in countries such as Japan, which has historically grappled with a high prevalence of suicide. In this country, a series of strategic government policies have been introduced gradually since 2000, including legislation aimed at reducing alcohol-related harm, promoting independent living, and supporting mental health. Given the variability of risk factors at the local level, suicide-specific prevention programs have been implemented at the municipal level[220]. Nevertheless, the efficacy of suicide prevention programs is not always evident, and enhanced collaboration between policymakers, researchers, and practitioners, along with augmented funding for suicide research, is imperative[221].
At the clinical level, there is evidence to suggest that clozapine and lithium may be effective in the prophylaxis of suicide attempts in select populations. Nevertheless, the efficacy of antidepressants, antipsychotics, electroconvulsive therapy, and transcranial magnetic stimulation remains constrained. Novel pharmacological approaches, such as the use of ketamine, are demonstrating potential efficacy[222]. Clinical studies have demonstrated that the intravenous administration of a single low dose of ketamine, an N-methyl-D-aspartate receptor antagonist, has a rapid antidepressant effect and can reduce suicidal ideation[223,224].
The antidepressant effects of ketamine have been demonstrated in preclinical studies, and new ketamine-like compounds with reduced potential for addiction are currently being investigated as treatments for depression and suicidal ideation[225,226]. Moreover, stereoisomers of ketamine have been subjected to experimental scrutiny, demonstrating that R-ketamine exhibits more efficacious antidepressant effects and a reduced incidence of adverse effects relative to S-ketamine[227]. Other novel drugs, including selective GluN2B antagonists such as CP101, 606 (Traxoprodil), CERC-301 (Rislenemdaz), and Ro 25-6981, have demonstrated promising results in both experimental and clinical studies, offering potential strategies for addressing depression and suicide risk[226].
Suicide is a significant global public health concern that is frequently overlooked and stigmatized. The intricate neurobiology of suicide encompasses a multitude of factors, including anatomical and functional alterations of the CNS, as well as abnormalities in the concentrations of neurotransmitters, cytokines, chemokines, HPA axis hyperactivity, and thyroid hormones. This intricate network of interactions between the nervous, endocrine, and immune systems, also known as NIEs, plays a pivotal role in the neurobiology of suicide. In addition, genetic factors and psychiatric disorders such as depression, schizophrenia, and anxiety are also considered potential risk factors involved in suicidal behavior.
We thank Julieta Mendoza Torreblanca for her help in preparing the figures.
| 1. | Mitra S, Kodancha PG. Defining Suicide in Clinical Trials-How Do We Fare? Indian J Psychol Med. 2022;44:85-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Sokero TP, Melartin TK, Rytsälä HJ, Leskelä US, Lestelä-Mielonen PS, Isometsä ET. Suicidal ideation and attempts among psychiatric patients with major depressive disorder. J Clin Psychiatry. 2003;64:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Stack S. Emile Durkheim and altruistic suicide. Arch Suicide Res. 2004;8:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Na PJ, Bommersbach T, Pietrzak RH, Rhee TG. Durkheim's Theory of Social Integration and Suicide Revisited: Is It Diversity of Social Networks or Perceived Strength of Social Support That Matters? J Clin Psychiatry. 2022;84:22m14477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Klonsky ED, May AM, Saffer BY. Suicide, Suicide Attempts, and Suicidal Ideation. Annu Rev Clin Psychol. 2016;12:307-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 1010] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 6. | Turecki G, Brent DA, Gunnell D, O'Connor RC, Oquendo MA, Pirkis J, Stanley BH. Suicide and suicide risk. Nat Rev Dis Primers. 2019;5:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 593] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 7. | Harmer B, Lee S, Rizvi A, Saadabadi A. Suicidal Ideation. 2024 Apr 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 8. | Welch SS. A review of the literature on the epidemiology of parasuicide in the general population. Psychiatr Serv. 2001;52:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 136] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Giegling I, Olgiati P, Hartmann AM, Calati R, Möller HJ, Rujescu D, Serretti A. Personality and attempted suicide. Analysis of anger, aggression and impulsivity. J Psychiatr Res. 2009;43:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Lovero KL, Dos Santos PF, Come AX, Wainberg ML, Oquendo MA. Suicide in Global Mental Health. Curr Psychiatry Rep. 2023;25:255-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | De Leo D. Late-life suicide in an aging world. Nat Aging. 2022;2:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Sher L, Oquendo MA. Suicide: An Overview for Clinicians. Med Clin North Am. 2023;107:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 13. | Zhang RW. Evidence-Based Suicide Screening and Prevention Protocol for Licensed Nursing Staff: A Systematic Literature Review and Recommendations. J Psychosoc Nurs Ment Health Serv. 2022;60:21-27. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Beautrais AL. Subsequent mortality in medically serious suicide attempts: a 5 year follow-up. Aust N Z J Psychiatry. 2003;37:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Hawton K, Saunders KE, O'Connor RC. Self-harm and suicide in adolescents. Lancet. 2012;379:2373-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 1322] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 16. | Ganança L, Oquendo MA, Tyrka AR, Cisneros-Trujillo S, Mann JJ, Sublette ME. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology. 2016;63:296-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Fernández-Sevillano J, González-Ortega I, MacDowell K, Zorrilla I, López MP, Courtet P, Gabilondo A, Martínez-Cengotitabengoa M, Leza JC, Sáiz P, González-Pinto A. Inflammation biomarkers in suicide attempts and their relation to abuse, global functioning and cognition. World J Biol Psychiatry. 2022;23:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Brundin L, Bryleva EY, Thirtamara Rajamani K. Role of Inflammation in Suicide: From Mechanisms to Treatment. Neuropsychopharmacology. 2017;42:271-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 19. | O'Connor DB, Gartland N, O'Connor RC. Stress, cortisol and suicide risk. Int Rev Neurobiol. 2020;152:101-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Gonçalves de Andrade E, González Ibáñez F, Tremblay MÈ. Microglia as a Hub for Suicide Neuropathology: Future Investigation and Prevention Targets. Front Cell Neurosci. 2022;16:839396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014;42:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 412] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 22. | Norden DM, Muccigrosso MM, Godbout JP. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology. 2015;96:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 23. | Niraula A, Sheridan JF, Godbout JP. Microglia Priming with Aging and Stress. Neuropsychopharmacology. 2017;42:318-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 309] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 24. | Brenhouse HC, Schwarz JM. Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci Biobehav Rev. 2016;70:288-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Mahar I, Labonte B, Yogendran S, Isingrini E, Perret L, Davoli MA, Rachalski A, Giros B, Turecki G, Mechawar N. Disrupted hippocampal neuregulin-1/ErbB3 signaling and dentate gyrus granule cell alterations in suicide. Transl Psychiatry. 2017;7:e1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonté B, Turecki G, Mechawar N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36:2650-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 27. | Chandley MJ, Szebeni A, Szebeni K, Wang-Heaton H, Garst J, Stockmeier CA, Lewis NH, Ordway GA. Markers of elevated oxidative stress in oligodendrocytes captured from the brainstem and occipital cortex in major depressive disorder and suicide. Prog Neuropsychopharmacol Biol Psychiatry. 2022;117:110559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Beautrais AL, Joyce PR, Mulder RT. Access to firearms and the risk of suicide: a case control study. Aust N Z J Psychiatry. 1996;30:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Batty GD, Kivimäki M, Bell S, Gale CR, Shipley M, Whitley E, Gunnell D. Psychosocial characteristics as potential predictors of suicide in adults: an overview of the evidence with new results from prospective cohort studies. Transl Psychiatry. 2018;8:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 30. | Dome P, Rihmer Z, Gonda X. Suicide Risk in Bipolar Disorder: A Brief Review. Medicina (Kaunas). 2019;55:403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 31. | Barrigon ML, Cegla-Schvartzman F. Sex, Gender, and Suicidal Behavior. Curr Top Behav Neurosci. 2020;46:89-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Lange S, Rehm J, Tran A, L Bagge C, Jasilionis D, Kaplan MS, Meščeriakova-Veliulienė O, Štelemėkas M, Probst C. Comparing gender-specific suicide mortality rate trends in the United States and Lithuania, 1990-2019: putting one of the "deaths of despair" into perspective. BMC Psychiatry. 2022;22:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Serrano CC, Dolci GF. Suicide prevention and suicidal behavior. Gac Med Mex. 2021;157:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Nugent AC, Ballard ED, Park LT, Zarate CA Jr. Research on the pathophysiology, treatment, and prevention of suicide: practical and ethical issues. BMC Psychiatry. 2019;19:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Hawton K, Pirkis J. Suicide is a complex problem that requires a range of prevention initiatives and methods of evaluation. Br J Psychiatry. 2017;210:381-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Roy A. Combination of family history of suicidal behavior and childhood trauma may represent correlate of increased suicide risk. J Affect Disord. 2011;130:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Rajalin M, Hirvikoski T, Renberg ES, Åsberg M, Jokinen J. Exposure to Early Life Adversity and Interpersonal Functioning in Attempted Suicide. Front Psychiatry. 2020;11:552514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Johnson JG, Cohen P, Gould MS, Kasen S, Brown J, Brook JS. Childhood adversities, interpersonal difficulties, and risk for suicide attempts during late adolescence and early adulthood. Arch Gen Psychiatry. 2002;59:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 342] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 39. | Duffy KA, McLaughlin KA, Green PA. Early life adversity and health-risk behaviors: proposed psychological and neural mechanisms. Ann N Y Acad Sci. 2018;1428:151-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 40. | Malave L, van Dijk MT, Anacker C. Early life adversity shapes neural circuit function during sensitive postnatal developmental periods. Transl Psychiatry. 2022;12:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 41. | Ashworth E, Jarman I, McCabe P, McCarthy M, Provazza S, Crosbie V, Quigg Z, Saini P. Suicidal Crisis among Children and Young People: Associations with Adverse Childhood Experiences and Socio-Demographic Factors. Int J Environ Res Public Health. 2023;20:1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 42. | Ports KA, Merrick MT, Stone DM, Wilkins NJ, Reed J, Ebin J, Ford DC. Adverse Childhood Experiences and Suicide Risk: Toward Comprehensive Prevention. Am J Prev Med. 2017;53:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Danese A, J Lewis S. Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology. 2017;42:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 44. | Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 882] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 45. | Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1371] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 46. | Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 475] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 47. | Elwenspoek MMC, Kuehn A, Muller CP, Turner JD. The effects of early life adversity on the immune system. Psychoneuroendocrinology. 2017;82:140-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 48. | Kuhlman KR, Cole SW, Irwin MR, Craske MG, Fuligni AJ, Bower JE. The role of early life adversity and inflammation in stress-induced change in reward and risk processes among adolescents. Brain Behav Immun. 2023;109:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Lussier AA, Zhu Y, Smith BJ, Cerutti J, Fisher J, Melton PE, Wood NM, Cohen-Woods S, Huang RC, Mitchell C, Schneper L, Notterman DA, Simpkin AJ, Smith ADAC, Suderman MJ, Walton E, Relton CL, Ressler KJ, Dunn EC. Association between the timing of childhood adversity and epigenetic patterns across childhood and adolescence: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC) prospective cohort. Lancet Child Adolesc Health. 2023;7:532-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 50. | Mposhi A, Turner JD. How can early life adversity still exert an effect decades later? A question of timing, tissues and mechanisms. Front Immunol. 2023;14:1215544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 51. | Ochi S, Dwivedi Y. Dissecting early life stress-induced adolescent depression through epigenomic approach. Mol Psychiatry. 2023;28:141-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 52. | Murgatroyd C, Spengler D. Epigenetic programming of the HPA axis: early life decides. Stress. 2011;14:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Anacker C, O'Donnell KJ, Meaney MJ. Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues Clin Neurosci. 2014;16:321-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 54. | Brådvik L. Suicide Risk and Mental Disorders. Int J Environ Res Public Health. 2018;15:2028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 256] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 55. | Kuhlman KR, Horn SR, Chiang JJ, Bower JE. Early life adversity exposure and circulating markers of inflammation in children and adolescents: A systematic review and meta-analysis. Brain Behav Immun. 2020;86:30-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 56. | Reid BM, Doom JR, Argote RB, Correa-Burrows P, Lozoff B, Blanco E, Gahagan S. Pathways to inflammation in adolescence through early adversity, childhood depressive symptoms, and body mass index: A prospective longitudinal study of Chilean infants. Brain Behav Immun. 2020;86:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | David J, Measelle J, Ostlund B, Ablow J. Association between early life adversity and inflammation during infancy. Dev Psychobiol. 2017;59:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Ferat-Osorio E, Maldonado-García JL, Pavón L. How inflammation influences psychiatric disease. World J Psychiatry. 2024;14:342-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 59. | Jeon SW, Kim YK. Neuron-Microglia Crosstalk in Neuropsychiatric Disorders. Adv Exp Med Biol. 2023;1411:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 60. | Shin SH, Kim YK. Early Life Stress, Neuroinflammation, and Psychiatric Illness of Adulthood. Adv Exp Med Biol. 2023;1411:105-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 61. | Zhu X, Chen Z, Shen W, Huang G, Sedivy JM, Wang H, Ju Z. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther. 2021;6:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 62. | Baharikhoob P, Kolla NJ. Microglial Dysregulation and Suicidality: A Stress-Diathesis Perspective. Front Psychiatry. 2020;11:781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Del Rey A, Besedovsky HO. Immune-Neuro-Endocrine Reflexes, Circuits, and Networks: Physiologic and Evolutionary Implications. Front Horm Res. 2017;48:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 64. | Pavlov VA, Chavan SS, Tracey KJ. Molecular and Functional Neuroscience in Immunity. Annu Rev Immunol. 2018;36:783-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 328] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 65. | Daëron M. The immune system as a system of relations. Front Immunol. 2022;13:984678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 66. | Görgens SW, Eckardt K, Jensen J, Drevon CA, Eckel J. Exercise and Regulation of Adipokine and Myokine Production. Prog Mol Biol Transl Sci. 2015;135:313-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 67. | Licinio J, Frost P. The neuroimmune-endocrine axis: pathophysiological implications for the central nervous system cytokines and hypothalamus-pituitary-adrenal hormone dynamics. Braz J Med Biol Res. 2000;33:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | França K, Lotti TM. Psycho-Neuro-Endocrine-Immunology: A Psychobiological Concept. Adv Exp Med Biol. 2017;996:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Pérez AR, Maya-Monteiro CM, Carvalho VF. Editorial: Neuroendocrine-Immunological Interactions in Health and Disease. Front Endocrinol (Lausanne). 2021;12:718893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Giletta M, Calhoun CD, Hastings PD, Rudolph KD, Nock MK, Prinstein MJ. Multi-Level Risk Factors for Suicidal Ideation Among at-Risk Adolescent Females: The Role of Hypothalamic-Pituitary-Adrenal Axis Responses to Stress. J Abnorm Child Psychol. 2015;43:807-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 71. | Andela CD, van Haalen FM, Ragnarsson O, Papakokkinou E, Johannsson G, Santos A, Webb SM, Biermasz NR, van der Wee NJ, Pereira AM. MECHANISMS IN ENDOCRINOLOGY: Cushing's syndrome causes irreversible effects on the human brain: a systematic review of structural and functional magnetic resonance imaging studies. Eur J Endocrinol. 2015;173:R1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 72. | Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 1697] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 73. | Turecki G, Ernst C, Jollant F, Labonté B, Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012;35:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 74. | Westrin A, Ekman R, Träskman-Bendz L. Alterations of corticotropin releasing hormone (CRH) and neuropeptide Y (NPY) plasma levels in mood disorder patients with a recent suicide attempt. Eur Neuropsychopharmacol. 1999;9:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 75. | O'Connor DB, Ferguson E, Green JA, O'Carroll RE, O'Connor RC. Cortisol levels and suicidal behavior: A meta-analysis. Psychoneuroendocrinology. 2016;63:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 76. | Lindqvist D, Isaksson A, Träskman-Bendz L, Brundin L. Salivary cortisol and suicidal behavior--a follow-up study. Psychoneuroendocrinology. 2008;33:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology (Berl). 2013;227:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 78. | Melhem NM, Keilp JG, Porta G, Oquendo MA, Burke A, Stanley B, Cooper TB, Mann JJ, Brent DA. Blunted HPA Axis Activity in Suicide Attempters Compared to those at High Risk for Suicidal Behavior. Neuropsychopharmacology. 2016;41:1447-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 79. | Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1544] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 80. | Hernández-Díaz Y, González-Castro TB, Tovilla-Zárate CA, Juárez-Rojop IE, López-Narváez ML, Pérez-Hernández N, Rodríguez-Pérez JM, Genis-Mendoza AD. Association between polymorphisms of FKBP5 gene and suicide attempt in a Mexican population: A case-control study. Brain Res Bull. 2021;166:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Hernández-Díaz Y, Genis-Mendoza AD, González-Castro TB, Tovilla-Zárate CA, Juárez-Rojop IE, López-Narváez ML, Nicolini H. Association and Genetic Expression between Genes Involved in HPA Axis and Suicide Behavior: A Systematic Review. Genes (Basel). 2021;12:1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Berardelli I, Serafini G, Cortese N, Fiaschè F, O'Connor RC, Pompili M. The Involvement of Hypothalamus-Pituitary-Adrenal (HPA) Axis in Suicide Risk. Brain Sci. 2020;10:653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 83. | O'Connor DB, Branley-Bell D, Green JA, Ferguson E, O'Carroll RE, O'Connor RC. Effects of childhood trauma, daily stress, and emotions on daily cortisol levels in individuals vulnerable to suicide. J Abnorm Psychol. 2020;129:92-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 84. | McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2696] [Cited by in RCA: 2160] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 85. | Roy A, Hodgkinson CA, Deluca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J Psychiatr Res. 2012;46:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 86. | Sanabrais-Jiménez MA, Sotelo-Ramirez CE, Ordoñez-Martinez B, Jiménez-Pavón J, Ahumada-Curiel G, Piana-Diaz S, Flores-Flores G, Flores-Ramos M, Jiménez-Anguiano A, Camarena B. Effect of CRHR1 and CRHR2 gene polymorphisms and childhood trauma in suicide attempt. J Neural Transm (Vienna). 2019;126:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Pandey GN, Rizavi HS, Ren X, Dwivedi Y, Palkovits M. Region-specific alterations in glucocorticoid receptor expression in the postmortem brain of teenage suicide victims. Psychoneuroendocrinology. 2013;38:2628-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 88. | Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, Turecki G. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biol Psychiatry. 2012;72:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 89. | Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 297] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 90. | Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 91. | Chaurasia S, Ganvir R, Pandey RK, Singh S, Yadav J, Malik R, Choubal S, Arora A. Gross Changes in Adrenal Glands in Suicidal and Sudden Death Cases: A Postmortem Study. Cureus. 2023;15:e51175. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 92. | Stein E, McCrank E, Schaefer B, Goyer R. Adrenal gland weight and suicide. Can J Psychiatry. 1993;38:563-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | Szigethy E, Conwell Y, Forbes NT, Cox C, Caine ED. Adrenal weight and morphology in victims of completed suicide. Biol Psychiatry. 1994;36:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Dumser T, Barocka A, Schubert E. Weight of adrenal glands may be increased in persons who commit suicide. Am J Forensic Med Pathol. 1998;19:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 95. | McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1043] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 96. | van Heeringen K, Audenaert K, Van de Wiele L, Verstraete A. Cortisol in violent suicidal behaviour: association with personality and monoaminergic activity. J Affect Disord. 2000;60:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Ducasse D, Olié E, Guillaume S, Artéro S, Courtet P. A meta-analysis of cytokines in suicidal behavior. Brain Behav Immun. 2015;46:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 98. | Lotrich FE. Major depression during interferon-alpha treatment: vulnerability and prevention. Dialogues Clin Neurosci. 2009;11:417-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 99. | Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 600] [Article Influence: 33.3] [Reference Citation Analysis (3)] |
| 100. | Pandey GN. Inflammatory and Innate Immune Markers of Neuroprogression in Depressed and Teenage Suicide Brain. Mod Trends Pharmacopsychiatry. 2017;31:79-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Janelidze S, Mattei D, Westrin Å, Träskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav Immun. 2011;25:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 102. | Mendlovic S, Mozes E, Eilat E, Doron A, Lereya J, Zakuth V, Spirer Z. Immune activation in non-treated suicidal major depression. Immunol Lett. 1999;67:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Karlović D, Serretti A, Vrkić N, Martinac M, Marčinko D. Serum concentrations of CRP, IL-6, TNF-α and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res. 2012;198:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 104. | O'Donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, O'Farrelly C, Malone KM. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety. 2013;30:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 105. | Chen MH, Bai YM, Hsu JW, Huang KL, Tsai SJ. Proinflammatory cytokine levels, cognitive function, and suicidal symptoms of adolescents and young adults with major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2024;274:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 106. | Gibbs HM, Davis L, Han X, Clothier J, Eads LA, Cáceda R. Association between C-reactive protein and suicidal behavior in an adult inpatient population. J Psychiatr Res. 2016;79:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1380] [Article Influence: 172.5] [Reference Citation Analysis (0)] |
| 108. | Sowa-Kućma M, Styczeń K, Siwek M, Misztak P, Nowak RJ, Dudek D, Rybakowski JK, Nowak G, Maes M. Are there differences in lipid peroxidation and immune biomarkers between major depression and bipolar disorder: Effects of melancholia, atypical depression, severity of illness, episode number, suicidal ideation and prior suicide attempts. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:372-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 109. | Abbas AK, Trotta E, R Simeonov D, Marson A, Bluestone JA. Revisiting IL-2: Biology and therapeutic prospects. Sci Immunol. 2018;3:eaat1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 110. | Isung J, Mobarrez F, Nordström P, Asberg M, Jokinen J. Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J Biol Psychiatry. 2012;13:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 111. | Lin YJ, Goretzki A, Schülke S. Immune Metabolism of IL-4-Activated B Cells and Th2 Cells in the Context of Allergic Diseases. Front Immunol. 2021;12:790658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 112. | Kim YK, Lee SW, Kim SH, Shim SH, Han SW, Choi SH, Lee BH. Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 113. | Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, Nishawala M, Katz Y, Gaite MR, Gonzalez CJ. A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J Child Adolesc Psychopharmacol. 2009;19:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 114. | Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. 2014;2:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 115. | Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2296] [Cited by in RCA: 2446] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 116. | Neupane SP, Daray FM, Ballard ED, Galfalvy H, Itzhaky L, Segev A, Shelef A, Tene O, Rizk MM, Mann JJ, Zalsman G. Immune-related biomarkers and suicidal behaviors: A meta-analysis. Eur Neuropsychopharmacol. 2023;75:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 117. | Ting EY, Yang AC, Tsai SJ. Role of Interleukin-6 in Depressive Disorder. Int J Mol Sci. 2020;21:2194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 118. | Raber J, O'Shea RD, Bloom FE, Campbell IL. Modulation of hypothalamic-pituitary-adrenal function by transgenic expression of interleukin-6 in the CNS of mice. J Neurosci. 1997;17:9473-9480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Saraiva M, Vieira P, O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020;217:e20190418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 741] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 120. | Worthen RJ, Garzon Zighelboim SS, Torres Jaramillo CS, Beurel E. Anti-inflammatory IL-10 administration rescues depression-associated learning and memory deficits in mice. J Neuroinflammation. 2020;17:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 121. | Anjum S, Qusar MMAS, Shahriar M, Islam SMA, Bhuiyan MA, Islam MR. Altered serum interleukin-7 and interleukin-10 are associated with drug-free major depressive disorder. Ther Adv Psychopharmacol. 2020;10:2045125320916655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 122. | Wiener CD, Moreira FP, Portela LV, Strogulski NR, Lara DR, da Silva RA, Souza LDM, Jansen K, Oses JP. Interleukin-6 and Interleukin-10 in mood disorders: A population-based study. Psychiatry Res. 2019;273:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 123. | Walter MR. The Role of Structure in the Biology of Interferon Signaling. Front Immunol. 2020;11:606489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 124. | Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2690] [Cited by in RCA: 3116] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 125. | Bradley KA, Case JA, Khan O, Ricart T, Hanna A, Alonso CM, Gabbay V. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res. 2015;227:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 126. | Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, Mann JJ, Postolache TT. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun. 2011;25:1272-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 127. | Fragoso YD, Frota ER, Lopes JS, Noal JS, Giacomo MC, Gomes S, Gonçalves MV, da Gama PD, Finkelsztejn A. Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin Neuropharmacol. 2010;33:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 128. | van Loo G, Bertrand MJM. Death by TNF: a road to inflammation. Nat Rev Immunol. 2023;23:289-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 569] [Reference Citation Analysis (0)] |
| 129. | Kang HJ, Kim JW, Lee JY, Kim SW, Shin IS, Hong YJ, Ahn Y, Jeong MH, Kim JM. Time-Specific Associations of Tumor Necrosis Factor-α Levels and Polymorphisms (-850 C/T or -308 G/A) With Suicidal Ideation in Acute Coronary Syndrome Patients. Front Psychiatry. 2021;12:739823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Massagué J, Sheppard D. TGF-β signaling in health and disease. Cell. 2023;186:4007-4037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 531] [Article Influence: 177.0] [Reference Citation Analysis (0)] |
| 131. | Tombácz D, Maróti Z, Kalmár T, Csabai Z, Balázs Z, Takahashi S, Palkovits M, Snyder M, Boldogkői Z. High-Coverage Whole-Exome Sequencing Identifies Candidate Genes for Suicide in Victims with Major Depressive Disorder. Sci Rep. 2017;7:7106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 132. | Omrani MD, Bagheri M, Bushehri B, Azizi F, Anoshae MR. The association of TGF-β1 codon 10 polymorphism with suicide behavior. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:772-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 133. | Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1148] [Cited by in RCA: 1609] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 134. | Mahoney ER, Dumitrescu L, Moore AM, Cambronero FE, De Jager PL, Koran MEI, Petyuk VA, Robinson RAS, Goyal S, Schneider JA, Bennett DA, Jefferson AL, Hohman TJ. Brain expression of the vascular endothelial growth factor gene family in cognitive aging and alzheimer's disease. Mol Psychiatry. 2021;26:888-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 135. | Isung J, Aeinehband S, Mobarrez F, Mårtensson B, Nordström P, Asberg M, Piehl F, Jokinen J. Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry. 2012;2:e196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 136. | Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res. 2012;46:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 137. | Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Möller HJ, Chen HH, Postolache TT. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117:198-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 138. | Shinko Y, Otsuka I, Okazaki S, Horai T, Boku S, Takahashi M, Ueno Y, Sora I, Hishimoto A. Chemokine alterations in the postmortem brains of suicide completers. J Psychiatr Res. 2020;120:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 139. | Srinivasan D, Yen JH, Joseph DJ, Friedman W. Cell type-specific interleukin-1beta signaling in the CNS. J Neurosci. 2004;24:6482-6488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 140. | Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Björkqvist M, Träskman-Bendz L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 411] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 141. | Meola D, Huang Z, Ha GK, Petitto JM. Loss of Neuronal Phenotype and Neurodegeneration: Effects of T Lymphocytes and Brain Interleukin-2. J Alzheimers Dis Parkinsonism. 2013;10:003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 142. | Takatsu K. Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:463-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 143. | Junttila IS. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front Immunol. 2018;9:888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 496] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 144. | Wunderlich U, Bronisch T, Wittchen HU, Carter R. Gender differences in adolescents and young adults with suicidal behaviour. Acta Psychiatr Scand. 2001;104:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 145. | Grassi-Oliveira R, Brieztke E, Teixeira A, Pezzi JC, Zanini M, Lopes RP, Bauer ME. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Braz J Psychiatry. 2012;34:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 146. | Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J Hepatol. 1994;21:241-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 147. | Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 148. | Pendyam S, Mohan A, Kalivas PW, Nair SS. Role of perisynaptic parameters in neurotransmitter homeostasis--computational study of a general synapse. Synapse. 2012;66:608-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 149. | Ryding E, Lindström M, Träskman-Bendz L. The role of dopamine and serotonin in suicidal behaviour and aggression. Prog Brain Res. 2008;172:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 150. | Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velázquez MA, Garcés-Alvarez ME, Hurtado-Alvarado G, Quintero-Fabian S, Pavón L. Immunomodulatory effects mediated by serotonin. J Immunol Res. 2015;2015:354957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 151. | Arreola R, Alvarez-Herrera S, Pérez-Sánchez G, Becerril-Villanueva E, Cruz-Fuentes C, Flores-Gutierrez EO, Garcés-Alvarez ME, de la Cruz-Aguilera DL, Medina-Rivero E, Hurtado-Alvarado G, Quintero-Fabián S, Pavón L. Immunomodulatory Effects Mediated by Dopamine. J Immunol Res. 2016;2016:3160486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 152. | Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 185] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 153. | Miller JM, Hesselgrave N, Ogden RT, Sullivan GM, Oquendo MA, Mann JJ, Parsey RV. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biol Psychiatry. 2013;74:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 154. | Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1817] [Cited by in RCA: 1884] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 155. | Ogren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekström JC, Svenningsson P, Meister B, Kehr J, Stiedl O. The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res. 2008;195:54-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 156. | Stefulj J, Büttner A, Kubat M, Zill P, Balija M, Eisenmenger W, Bondy B, Jernej B. 5HT-2C receptor polymorphism in suicide victims. Association studies in German and Slavic populations. Eur Arch Psychiatry Clin Neurosci. 2004;254:224-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 157. | Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Faludi G, Sarosi A, Palkovits M. Regional distribution and relative abundance of serotonin(2c) receptors in human brain: effect of suicide. Neurochem Res. 2006;31:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 158. | Jokinen J, Nordström AL, Nordström P. CSF 5-HIAA and DST non-suppression--orthogonal biologic risk factors for suicide in male mood disorder inpatients. Psychiatry Res. 2009;165:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 159. | Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharmacology. 1996;35:1503-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 386] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 160. | Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 161. | Felger JC, Treadway MT. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology. 2017;42:216-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 327] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 162. | Su M, Li E, Tang C, Zhao Y, Liu R, Gao K. Comparison of blood lipid profile/thyroid function markers between unipolar and bipolar depressed patients and in depressed patients with anhedonia or suicidal thoughts. Mol Med. 2019;25:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 163. | Peng P, Wang Q, Lang X, Liu T, Zhang XY. Clinical symptoms, thyroid dysfunction, and metabolic disturbances in first-episode drug-naïve major depressive disorder patients with suicide attempts: A network perspective. Front Endocrinol (Lausanne). 2023;14:1136806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 164. | Liu W, Wu Z, Sun M, Zhang S, Yuan J, Zhu D, Yan G, Hou K. Association between fasting blood glucose and thyroid stimulating hormones and suicidal tendency and disease severity in patients with major depressive disorder. Bosn J Basic Med Sci. 2022;22:635-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 165. | Toloza FJK, Mao Y, Menon L, George G, Borikar M, Thumma S, Motahari H, Erwin P, Owen R, Maraka S. Association of Thyroid Function with Suicidal Behavior: A Systematic Review and Meta-Analysis. Medicina (Kaunas). 2021;57:714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 166. | Duval F, Mokrani MC, Erb A, Gonzalez Opera F, Calleja C, Paris V. Relationship between chronobiological thyrotropin and prolactin responses to protirelin (TRH) and suicidal behavior in depressed patients. Psychoneuroendocrinology. 2017;85:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 167. | Wang ST, He XY, Le J, Sun T, Peng R. Associations Between Vitamin K and Suicide Attempts in Patients with Depression: A Case-Control Study. J Inflamm Res. 2024;17:3423-3431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 168. | Peng R, Dai W, Li Y. Low serum free thyroxine level is correlated with lipid profile in depressive patients with suicide attempt. Psychiatry Res. 2018;266:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 169. | Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, Wemeau JL. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur Thyroid J. 2013;2:215-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 513] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 170. | Tian X, Liu XE, Bai F, Li M, Qiu Y, Jiao Q, Li J, Zhang XY. Sex differences in correlates of suicide attempts in Chinese Han first-episode and drug-naïve major depressive disorder with comorbid subclinical hypothyroidism: A cross-sectional study. Brain Behav. 2024;14:e3578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 171. | Lang X, Hou X, Shangguan F, Zhang XY. Prevalence and clinical correlates of subclinical hypothyroidism in first-episode drug-naive patients with major depressive disorder in a large sample of Chinese. J Affect Disord. 2020;263:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 172. | Mizutani K, Nishimura K, Ichihara A, Ishigooka J. Dissociative disorder due to Graves' hyperthyroidism: a case report. Gen Hosp Psychiatry. 2014;36:450.e1-450.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 173. | Fijtman A, Downie S. Is it all in my neck? - Bipolar disorder with mixed features and suicidal ideation related to thyrotoxicosis: A case report. Bipolar Disord. 2021;23:104-107. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 174. | Petrich CE, Bui MP, Farrell HM. A case of a suicide attempt associated with hyperthyroidism. Gen Hosp Psychiatry. 2013;35:576.e9-576.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 175. | Ferløv-Schwensen C, Brix TH, Hegedüs L. Death by Suicide in Graves' Disease and Graves' Orbitopathy: A Nationwide Danish Register Study. Thyroid. 2017;27:1475-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 176. | Todorov L, Ait Boudaoud A, Pascal de Raykeer R, Radu A, Lahlou-Laforêt K, Limosin F, Lemogne C, Czernichow S. A Case of Violent Suicide Attempt in a Context of Myxedema Psychosis following Radioiodine Treatment in a Patient with Graves' Disease. Case Rep Psychiatry. 2019;2019:4972760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 177. | Heiberg Brix T, Ferløv-Schwensen C, Thvilum M, Hegedüs L. Death by unnatural causes, mainly suicide, is increased in patients with Hashimoto's thyroiditis. A nationwide Danish register study. Endocrine. 2019;65:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 178. | Du L, Faludi G, Palkovits M, Bakish D, Hrdina PD. Serotonergic genes and suicidality. Crisis. 2001;22:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 179. | Courtet P, Jollant F, Castelnau D, Buresi C, Malafosse A. Suicidal behavior: relationship between phenotype and serotonergic genotype. Am J Med Genet C Semin Med Genet. 2005;133C:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 180. | Arango V, Huang YY, Underwood MD, Mann JJ. Genetics of the serotonergic system in suicidal behavior. J Psychiatr Res. 2003;37:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 181. | González-Castro TB, Juárez-Rojop I, López-Narváez ML, Tovilla-Zárate CA. Association of TPH-1 and TPH-2 gene polymorphisms with suicidal behavior: a systematic review and meta-analysis. BMC Psychiatry. 2014;14:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 182. | Rujescu D, Thalmeier A, Möller HJ, Bronisch T, Giegling I. Molecular genetic findings in suicidal behavior: what is beyond the serotonergic system? Arch Suicide Res. 2007;11:17-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 183. | Bondy B, Buettner A, Zill P. Genetics of suicide. Mol Psychiatry. 2006;11:336-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 184. | De Luca V, Voineskos D, Wong GW, Shinkai T, Rothe C, Strauss J, Kennedy JL. Promoter polymorphism of second tryptophan hydroxylase isoform (TPH2) in schizophrenia and suicidality. Psychiatry Res. 2005;134:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 185. | Fanelli G, Serretti A. The influence of the serotonin transporter gene 5-HTTLPR polymorphism on suicidal behaviors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:375-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 186. | Du L, Bakish D, Lapierre YD, Ravindran AV, Hrdina PD. Association of polymorphism of serotonin 2A receptor gene with suicidal ideation in major depressive disorder. Am J Med Genet. 2000;96:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 187. | Brent DA, Mann JJ. Family genetic studies, suicide, and suicidal behavior. Am J Med Genet C Semin Med Genet. 2005;133C:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 395] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 188. | Melhem NM, Brent DA, Ziegler M, Iyengar S, Kolko D, Oquendo M, Birmaher B, Burke A, Zelazny J, Stanley B, Mann JJ. Familial pathways to early-onset suicidal behavior: familial and individual antecedents of suicidal behavior. Am J Psychiatry. 2007;164:1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 189. | Antypa N, Serretti A, Rujescu D. Serotonergic genes and suicide: a systematic review. Eur Neuropsychopharmacol. 2013;23:1125-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 190. | Roy A, Rylander G, Sarchiapone M. Genetic studies of suicidal behavior. Psychiatr Clin North Am. 1997;20:595-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 191. | Kimbrel NA, Ashley-Koch AE, Qin XJ, Lindquist JH, Garrett ME, Dennis MF, Hair LP, Huffman JE, Jacobson DA, Madduri RK, Trafton JA, Coon H, Docherty AR, Mullins N, Ruderfer DM, Harvey PD, McMahon BH, Oslin DW, Beckham JC, Hauser ER, Hauser MA; Million Veteran Program Suicide Exemplar Workgroup, the International Suicide Genetics Consortium, the Veterans Affairs Mid-Atlantic Mental Illness Research, Education, and Clinical Center Workgroup, and the Veterans Affairs Million Veteran Program. Identification of Novel, Replicable Genetic Risk Loci for Suicidal Thoughts and Behaviors Among US Military Veterans. JAMA Psychiatry. 2023;80:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 192. | Nievergelt CM, Maihofer AX, Atkinson EG, Chen CY, Choi KW, Coleman JRI, Daskalakis NP, Duncan LE, Polimanti R, Aaronson C, Amstadter AB, Andersen SB, Andreassen OA, Arbisi PA, Ashley-Koch AE, Austin SB, Avdibegoviç E, Babić D, Bacanu SA, Baker DG, Batzler A, Beckham JC, Belangero S, Benjet C, Bergner C, Bierer LM, Biernacka JM, Bierut LJ, Bisson JI, Boks MP, Bolger EA, Brandolino A, Breen G, Bressan RA, Bryant RA, Bustamante AC, Bybjerg-Grauholm J, Bækvad-Hansen M, Børglum AD, Børte S, Cahn L, Calabrese JR, Caldas-de-Almeida JM, Chatzinakos C, Cheema S, Clouston SAP, Colodro-Conde L, Coombes BJ, Cruz-Fuentes CS, Dale AM, Dalvie S, Davis LK, Deckert J, Delahanty DL, Dennis MF, Desarnaud F, DiPietro CP, Disner SG, Docherty AR, Domschke K, Dyb G, Kulenović AD, Edenberg HJ, Evans A, Fabbri C, Fani N, Farrer LA, Feder A, Feeny NC, Flory JD, Forbes D, Franz CE, Galea S, Garrett ME, Gelaye B, Gelernter J, Geuze E, Gillespie CF, Goleva SB, Gordon SD, Goçi A, Grasser LR, Guindalini C, Haas M, Hagenaars S, Hauser MA, Heath AC, Hemmings SMJ, Hesselbrock V, Hickie IB, Hogan K, Hougaard DM, Huang H, Huckins LM, Hveem K, Jakovljević M, Javanbakht A, Jenkins GD, Johnson J, Jones I, Jovanovic T, Karstoft KI, Kaufman ML, Kennedy JL, Kessler RC, Khan A, Kimbrel NA, King AP, Koen N, Kotov R, Kranzler HR, Krebs K, Kremen WS, Kuan PF, Lawford BR, Lebois LAM, Lehto K, Levey DF, Lewis C, Liberzon I, Linnstaedt SD, Logue MW, Lori A, Lu Y, Luft BJ, Lupton MK, Luykx JJ, Makotkine I, Maples-Keller JL, Marchese S, Marmar C, Martin NG, Martínez-Levy GA, McAloney K, McFarlane A, McLaughlin KA, McLean SA, Medland SE, Mehta D, Meyers J, Michopoulos V, Mikita EA, Milani L, Milberg W, Miller MW, Morey RA, Morris CP, Mors O, Mortensen PB, Mufford MS, Nelson EC, Nordentoft M, Norman SB, Nugent NR, O'Donnell M, Orcutt HK, Pan PM, Panizzon MS, Pathak GA, Peters ES, Peterson AL, Peverill M, Pietrzak RH, Polusny MA, Porjesz B, Powers A, Qin XJ, Ratanatharathorn A, Risbrough VB, Roberts AL, Rothbaum AO, Rothbaum BO, Roy-Byrne P, Ruggiero KJ, Rung A, Runz H, Rutten BPF, de Viteri SS, Salum GA, Sampson L, Sanchez SE, Santoro M, Seah C, Seedat S, Seng JS, Shabalin A, Sheerin CM, Silove D, Smith AK, Smoller JW, Sponheim SR, Stein DJ, Stensland S, Stevens JS, Sumner JA, Teicher MH, Thompson WK, Tiwari AK, Trapido E, Uddin M, Ursano RJ, Valdimarsdóttir U, Van Hooff M, Vermetten E, Vinkers CH, Voisey J, Wang Y, Wang Z, Waszczuk M, Weber H, Wendt FR, Werge T, Williams MA, Williamson DE, Winsvold BS, Winternitz S, Wolf C, Wolf EJ, Xia Y, Xiong Y, Yehuda R, Young KA, Young RM, Zai CC, Zai GC, Zervas M, Zhao H, Zoellner LA, Zwart JA, deRoon-Cassini T, van Rooij SJH, van den Heuvel LL; AURORA Study; Estonian Biobank Research Team; FinnGen Investigators; HUNT All-In Psychiatry, Stein MB, Ressler KJ, Koenen KC. Genome-wide association analyses identify 95 risk loci and provide insights into the neurobiology of post-traumatic stress disorder. Nat Genet. 2024;56:792-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 99] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 193. | Li HJ, Qu N, Hui L, Cai X, Zhang CY, Zhong BL, Zhang SF, Chen J, Xia B, Wang L, Jia QF, Li W, Chang H, Xiao X, Li M, Li Y. Further confirmation of netrin 1 receptor (DCC) as a depression risk gene via integrations of multi-omics data. Transl Psychiatry. 2020;10:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 194. | Dieperink E, Ho SB, Tetrick L, Thuras P, Dua K, Willenbring ML. Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. Gen Hosp Psychiatry. 2004;26:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 195. | Wang Y, Liu L, Xin L, Fan D, Ding N, Hu Y, Cai G, Wang L, Xia Q, Li X, Yang X, Zou Y, Pan F. The -141C Ins/Del and Taq1A polymorphism in the dopamine D2 receptor gene may confer susceptibility to schizophrenia in Asian populations. J Clin Neurosci. 2016;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 196. | Abdolmaleky HM, Thiagalingam S, Wilcox M. Genetics and epigenetics in major psychiatric disorders: dilemmas, achievements, applications, and future scope. Am J Pharmacogenomics. 2005;5:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 197. | Docherty AR, Mullins N, Ashley-Koch AE, Qin X, Coleman JRI, Shabalin A, Kang J, Murnyak B, Wendt F, Adams M, Campos AI, DiBlasi E, Fullerton JM, Kranzler HR, Bakian AV, Monson ET, Rentería ME, Walss-Bass C, Andreassen OA, Behera C, Bulik CM, Edenberg HJ, Kessler RC, Mann JJ, Nurnberger JI Jr, Pistis G, Streit F, Ursano RJ, Polimanti R, Dennis M, Garrett M, Hair L, Harvey P, Hauser ER, Hauser MA, Huffman J, Jacobson D, Madduri R, McMahon B, Oslin DW, Trafton J, Awasthi S, Berrettini WH, Bohus M, Chang X, Chen HC, Chen WJ, Christensen ED, Crow S, Duriez P, Edwards AC, Fernández-Aranda F, Galfalvy H, Gandal M, Gorwood P, Guo Y, Hafferty JD, Hakonarson H, Halmi KA, Hishimoto A, Jain S, Jamain S, Jiménez-Murcia S, Johnson C, Kaplan AS, Kaye WH, Keel PK, Kennedy JL, Kim M, Klump KL, Levey DF, Li D, Liao SC, Lieb K, Lilenfeld L, Marshall CR, Mitchell JE, Okazaki S, Otsuka I, Pinto D, Powers A, Ramoz N, Ripke S, Roepke S, Rozanov V, Scherer SW, Schmahl C, Sokolowski M, Starnawska A, Strober M, Su MH, Thornton LM, Treasure J, Ware EB, Watson HJ, Witt SH, Woodside DB, Yilmaz Z, Zillich L, Adolfsson R, Agartz I, Alda M, Alfredsson L, Appadurai V, Artigas MS, Van der Auwera S, Azevedo MH, Bass N, Bau CHD, Baune BT, Bellivier F, Berger K, Biernacka JM, Bigdeli TB, Binder EB, Boehnke M, Boks MP, Braff DL, Bryant R, Budde M, Byrne EM, Cahn W, Castelao E, Cervilla JA, Chaumette B, Corvin A, Craddock N, Djurovic S, Foo JC, Forstner AJ, Frye M, Gatt JM, Giegling I, Grabe HJ, Green MJ, Grevet EH, Grigoroiu-Serbanescu M, Gutierrez B, Guzman-Parra J, Hamshere ML, Hartmann AM, Hauser J, Heilmann-Heimbach S, Hoffmann P, Ising M, Jones I, Jones LA, Jonsson L, Kahn RS, Kelsoe JR, Kendler KS, Kloiber S, Koenen KC, Kogevinas M, Krebs MO, Landén M, Leboyer M, Lee PH, Levinson DF, Liao C, Lissowska J, Mayoral F, McElroy SL, McGrath P, McGuffin P, McQuillin A, Mehta D, Melle I, Mitchell PB, Molina E, Morken G, Nievergelt C, Nöthen MM, O'Donovan MC, Ophoff RA, Owen MJ, Pato C, Pato MT, Penninx BWJH, Potash JB, Power RA, Preisig M, Quested D, Ramos-Quiroga JA, Reif A, Ribasés M, Richarte V, Rietschel M, Rivera M, Roberts A, Roberts G, Rouleau GA, Rovaris DL, Sanders AR, Schofield PR, Schulze TG, Scott LJ, Serretti A, Shi J, Sirignano L, Sklar P, Smeland OB, Smoller JW, Sonuga-Barke EJS, Trzaskowski M, Tsuang MT, Turecki G, Vilar-Ribó L, Vincent JB, Völzke H, Walters JTR, Weickert CS, Weickert TW, Weissman MM, Williams LM, Wray NR, Zai CC, Agerbo E, Børglum AD, Breen G, Demontis D, Erlangsen A, Gelernter J, Glatt SJ, Hougaard DM, Hwu HG, Kuo PH, Lewis CM, Li QS, Liu CM, Martin NG, McIntosh AM, Medland SE, Mors O, Nordentoft M, Olsen CM, Porteous D, Smith DJ, Stahl EA, Stein MB, Wasserman D, Werge T, Whiteman DC, Willour V; VA Million Veteran Program (MVP); MVP Suicide Exemplar Workgroup; Suicide Working Group of the Psychiatric Genomics Consortium; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium; Bipolar Disorder Working Group of the Psychiatric Genomics Consortium; Schizophrenia Working Group of the Psychiatric Genomics Consortium; Eating Disorder Working Group of the Psychiatric Genomics Consortium; German Borderline Genomics Consortium, Coon H, Beckham JC, Kimbrel NA, Ruderfer DM. GWAS Meta-Analysis of Suicide Attempt: Identification of 12 Genome-Wide Significant Loci and Implication of Genetic Risks for Specific Health Factors. Am J Psychiatry. 2023;180:723-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 198. | Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychol Med. 2012;42:1203-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 199. | Lee YJ, Kim S, Gwak AR, Kim SJ, Kang SG, Na KS, Son YD, Park J. Decreased regional gray matter volume in suicide attempters compared to suicide non-attempters with major depressive disorders. Compr Psychiatry. 2016;67:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 200. | Peng H, Wu K, Li J, Qi H, Guo S, Chi M, Wu X, Guo Y, Yang Y, Ning Y. Increased suicide attempts in young depressed patients with abnormal temporal-parietal-limbic gray matter volume. J Affect Disord. 2014;165:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 201. | van Heeringen K, Bijttebier S, Desmyter S, Vervaet M, Baeken C. Is there a neuroanatomical basis of the vulnerability to suicidal behavior? A coordinate-based meta-analysis of structural and functional MRI studies. Front Hum Neurosci. 2014;8:824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 202. | Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48:631-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 203. | Smith DV, Hayden BY, Truong TK, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci. 2010;30:2490-2495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 204. | Domínguez-Borràs J, Vuilleumier P. Amygdala function in emotion, cognition, and behavior. Handb Clin Neurol. 2022;187:359-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 205. | Shaikh NF, Sambamoorthi U. Prescription Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Depression among Adults with Inflammatory Chronic Conditions in the United States. Psychiatr Q. 2020;91:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 206. | Hu K, Sjölander A, Lu D, Walker AK, Sloan EK, Fall K, Valdimarsdóttir U, Hall P, Smedby KE, Fang F. Aspirin and other non-steroidal anti-inflammatory drugs and depression, anxiety, and stress-related disorders following a cancer diagnosis: a nationwide register-based cohort study. BMC Med. 2020;18:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 207. | Gupta MA, Vujcic B, Sheridan AD, Gupta AK. Reduced risk of suicidal behaviours associated with the treatment of hidradenitis suppurativa with tumour necrosis factor alpha antagonists: results from the US FDA Adverse Events Reporting System pharmacovigilance database. J Eur Acad Dermatol Venereol. 2020;34:1564-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 208. | Baghdadi LR. Tocilizumab Reduces Depression Risk in Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis. Psychol Res Behav Manag. 2024;17:3419-3441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 209. | Rahmati-Dehkordi F, Birang N, Jalalian MN, Tamtaji Z, Dadgostar E, Aschner M, Shafiee Ardestani M, Jafarpour H, Mirzaei H, Nabavizadeh F, Tamtaji OR. Can infliximab serve as a new therapy for neuropsychiatric symptoms? Naunyn Schmiedebergs Arch Pharmacol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 210. | Thillard EM, Gautier S, Babykina E, Carton L, Amad A, Bouzillé G, Beuscart JB, Ficheur G, Chazard E. Psychiatric Adverse Events Associated With Infliximab: A Cohort Study From the French Nationwide Discharge Abstract Database. Front Pharmacol. 2020;11:513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 211. | Ulual P, Burhan HS, Güçlü O. Infliximab induced severe depression and suicidal thoughts in patient with bipolar disorder. Eur Psychiatr. 2023;66:S762-S763. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 212. | Zazula R, Husain MI, Mohebbi M, Walker AJ, Chaudhry IB, Khoso AB, Ashton MM, Agustini B, Husain N, Deakin J, Young AH, Berk M, Kanchanatawan B, Ng CH, Maes M, Berk L, Singh AB, Malhi GS, Dean OM. Minocycline as adjunctive treatment for major depressive disorder: Pooled data from two randomized controlled trials. Aust N Z J Psychiatry. 2021;55:784-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 213. | Korkmaz HA, Dizdarer C, Hazan F, Karaarslan U. Attempted suicide with levothyroxine in an adolescent girl. J Pediatr Endocrinol Metab. 2013;26:129-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 214. | Gill AS, Rai HK, Karunakaran A, Chaudhuri A. Suicide Attempt With Levothyroxine Overdose. Cureus. 2023;15:e36172. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 215. | Sajjadi SS, Foshati S, Haddadian-Khouzani S, Rouhani MH. The role of selenium in depression: a systematic review and meta-analysis of human observational and interventional studies. Sci Rep. 2022;12:1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 216. | Genis-Mendoza AD, Dionisio-García DM, Gonzalez-Castro TB, Tovilla-Zaráte CA, Juárez-Rojop IE, López-Narváez ML, Castillo-Avila RG, Nicolini H. Increased Levels of Cortisol in Individuals With Suicide Attempt and Its Relation With the Number of Suicide Attempts and Depression. Front Psychiatry. 2022;13:912021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 217. | Choudhary D, Sasibhushana RB, Shankaranarayana Rao BS, Srikumar BN. Mifepristone blocks the anxiolytic- and antidepressant-like effects of allopregnanolone in male rats. Int J Neurosci. 2024;134:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 218. | Saunders KE, Hawton K. Suicidal behaviour and the menstrual cycle. Psychol Med. 2006;36:901-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 219. | Eisenlohr-Moul TA, Bowers SM, Prinstein MJ, Schmalenberger KM, Walsh EC, Young SL, Rubinow DR, Girdler SS. Effects of acute estradiol and progesterone on perimenstrual exacerbation of suicidal ideation and related symptoms: a crossover randomized controlled trial. Transl Psychiatry. 2022;12:528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 220. | Okamura K, Ikeshita K, Kimoto S, Makinodan M, Kishimoto T. Suicide prevention in Japan: Government and community measures, and high-risk interventions. Asia Pac Psychiatry. 2021;13:e12471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 221. | Platt S, Niederkrotenthaler T. Suicide Prevention Programs. Crisis. 2020;41:S99-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 222. | Griffiths JJ, Zarate CA Jr, Rasimas JJ. Existing and Novel Biological Therapeutics in Suicide Prevention. Focus (Am Psychiatr Publ). 2023;21:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 223. | Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 598] [Article Influence: 42.7] [Reference Citation Analysis (14)] |
| 224. | Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2275] [Cited by in RCA: 2670] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 225. | Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 226. | Duman RS. Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Res. 2018;7:F1000 Faculty Rev-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 227. | Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 491] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/