Published online Feb 19, 2025. doi: 10.5498/wjp.v15.i2.101948
Revised: November 23, 2024
Accepted: December 25, 2024
Published online: February 19, 2025
Processing time: 103 Days and 22.2 Hours
Phelan-McDermid syndrome (PMS) is a rare genetic disorder characterized by in
This report presented an 18-year clinical history of a 36-year-old woman with PMS, marked by intellectual disabilities, social withdrawal, and stereotyped beha
Effective management of PMS requires a thorough clinical history, genetic testing, and long-term supportive care.
Core Tip: Phelan-McDermid syndrome (PMS) is a complex genetic disorder characterized by intellectual disability, delayed language development, autism spectrum disorders, and psychiatric symptoms, such as bipolar disorder. Effective mana
- Citation: Sun YY, Xia Y, Zhi QN, Liu XY. Diagnosis and treatment of bipolar disorder in Phelan-McDermid syndrome: A case report and review of literature. World J Psychiatry 2025; 15(2): 101948
- URL: https://www.wjgnet.com/2220-3206/full/v15/i2/101948.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i2.101948
Phelan-McDermid syndrome (PMS), a rare neurodevelopmental disorder, is caused by haploinsufficiency of the SHANK3 (SH3 and Multiple Ankyrin Repeat Domains 3) gene, located at the terminal region of chromosome 22q13.3 on the long arm of chromosome 22[1]. The genetic variations associated with PMS are heterogeneous and may include terminal or interstitial deletions, translocations, mutations, or ring chromosome abnormalities[2]. This syndrome is characterized by intellectual disabilities, delayed language development, features of autism spectrum disorders, and abnormalities in motor tone[3]. SHANK3 gene abnormalities increase the risk of mental disorders[4]. Studies pointed out that infections, hormonal changes, and life stress can trigger psychiatric symptoms in PMS patients, accompanying by psychiatric disorders typically appearing during adolescence or early adulthood[5]. Approximately 54% of PMS patients may meet the criteria for bipolar disorder, and about half may experience behavioral regression[3]. A 36-year-old woman with PMS and a history of delayed mental development since childhood presented with recurrent manic episodes beginning at the age of 18 years old. Her treatment was complicated by severe reactions, including neuroleptic malignant syndrome (NMS), leading to regression in speech and daily living skills. After one year of treatment, partial improvement was noted; however, the recurrence of manic symptoms required additional interventions.
A 36-year-old woman with PMS presented with developmental delays, mood instability, manic episodes, and impulsive behavior.
The patient was a 36-year-old woman. According to her family’s statement, she could only point and make sounds by age 2 years, said her first word ("mama") at age 3 years, and began walking at the same age. In early childhood, she was often silent, seldom speaking, and had minimal social interaction. Upon starting elementary school, she demonstrated poor academic performance, being unable to perform simple arithmetic (e.g, addition and subtraction within 100) or navigate outside independently. Her daily living activities required family assistance. She was not taken for medical evaluation and dropped out of school after the sixth grade. She continued to live under family care, capable of basic self-care, including dressing, eating, and following simple instructions. At age 18 years, she first exhibited noticeable emotional instability, including excessive talking, grandiosity, hyperactivity, frequent shouting, and reduced sleep (2-3 hours per night) without fatigue. These episodes, which lasted approximately one month, alternated with periods of withdrawal and sparse speech. She was hospitalized during this period and diagnosed with: (1) Intellectual disability with significant behavioral issues requiring attention or treatment; and (2) Bipolar disorder, manic episode without psychotic features. Her IQ, assessed via the Wechsler Intelligence Scale, was 54. Treatment included Clozapine and Oxcarbazepine, although her family could not provide specific dosages. She discontinued medication within 6 months. During remission periods, she displayed stable behavior, used simple speech, and managed limited independence with family support. At 23 years old, she married and gave birth to a daughter at 24 years old. After childbirth, she relapsed, presenting with hype
On January 17, 2022, she was unconscious, disoriented, and displayed generalized rigidity. She was unable to speak, had a tense facial expression, could not eat independently, experienced difficulty swallowing, and persistently vocalized with repetitive "ah, ah" sounds. During her initial hospitalization, the patient was diagnosed with the following: (1) NMS; and (2) Intellectual disability with significant behavioral impairments requiring medical attention. At this stage, diagnosis of bipolar disorder was not made because the patient was unconscious and did not exhibit symptoms of mania. Fo
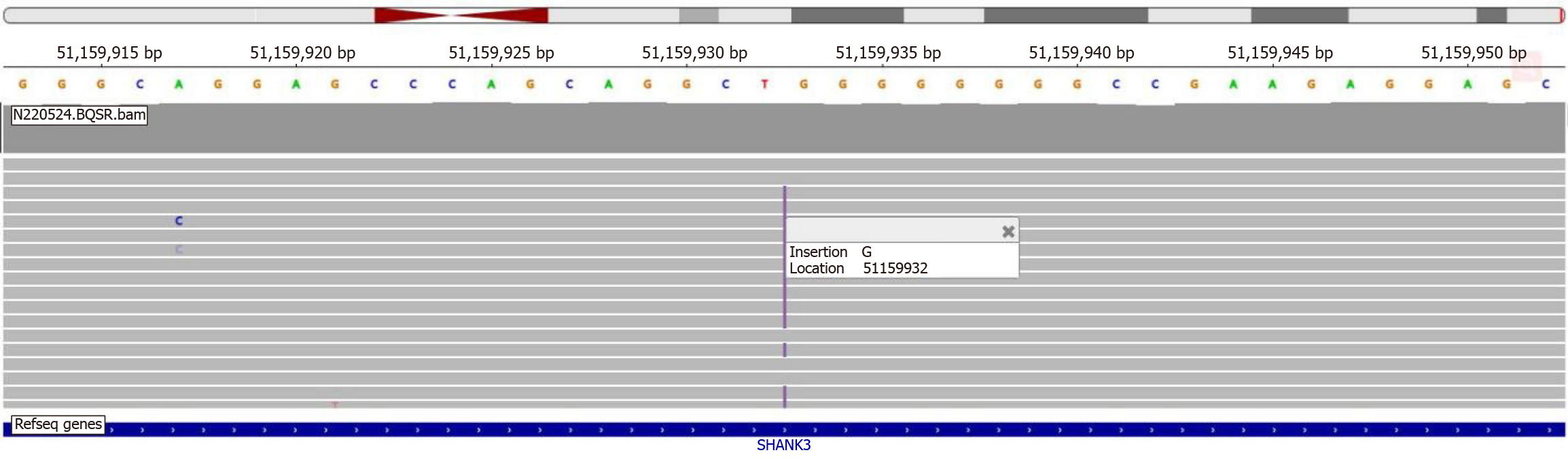
During her hospitalization at the rehabilitation hospital, on the basis of the patient's developmental delay, recurrent manic episodes, decline in daily living skills, and persistent high muscle tone in her limbs, whole-exome sequencing was performed in April 2022. This revealed a mutation in the SHANK3 gene (c.3865dup), aligning with her clinical presen
In January 2024, the patient experienced a relapse. Over the course of a week, she exhibited enhanced excitement, spoke loudly and incessantly, yelled, cursed at family members, and engaged in destructive behaviors, such as smashing objects. She frequently made sexually suggestive remarks, slept only 2-3 hours per night, and remained highly energetic during the day, running around the house continuously. In February 2024, she was admitted to the hospital for the second time. she displayed symptoms consistent with manic syndrome.
The patient had a history of intellectual disability, while without history of cardiac, endocrine, or other systemic diseases.
Findings from the initial admission evaluation were unremarkable.
During the initial examination, the patient’s condition significantly worsened. On physical examination, the patient’s vital signs were as follows: Body temperature, 38.8 °C; blood pressure, 170/110 mmHg; heart rate, 100-130 beats per min; body weight, 45 kg; and height, 158 cm. She experienced urinary and fecal incontinence. Her right hand assumed a claw-like posture, muscle tone was markedly enhanced, and she exhibited rigid flexion spasms. In February 2024, the patient was admitted to the hospital for the second time. On physical examination, no significant abnormalities were detected.
On January 17, 2022, routine blood tests revealed a white blood cell (WBC) count of 4.37 × 109/L, a red blood cell (RBC) count of 3.91 × 1012/L, a hemoglobin level of 106 g/L, and an elevated platelet count of 267 × 109/L. Blood biochemistry on the same day indicated significantly elevated levels of alanine aminotransferase (ALT; 62.5 U/L), aspartate aminotransferase (AST; 136.3 U/L), creatine kinase (CK; 11154 U/L), and CK-MB isoenzyme (CK-MB; 218.21 U/L). On January 18, 2022, blood gas analysis, female tumor markers, and thyroid function tests were all within normal ranges. Electroencephalogram, performed on January 19, 2022, indicated increased fast waves. Routine cerebrospinal fluid (CSF) analysis, CSF biochemistry, and autoimmune encephalitis antibody tests, conducted on February 1, 2022, all returned normal results. Subsequent blood tests indicated progressive improvement. On February 12, 2022, routine blood exami
Magnetic resonance imaging of the head revealed slight white matter hyperintensity of vascular origin in both lateral ventricles, classified as Fazekas grade 1.
Based on her complex medical history, psychiatric evaluation findings, and laboratory test results, the following diag
Given her history of recurrent manic episodes, a NMS episode two years earlier, and the prolonged recovery of speech and motor skills over nearly 11 months of rehabilitation, an extensive literature review and multidisciplinary discussions were undertaken. These discussions highlighted that patients with PMS exhibit elevated sensitivity to antipsychotic medications, necessitating careful use of low-dose regimens[6]. For this hospitalization, she was prescribed Olanzapine at a dose of 1.25-5 mg/day, in combination with Lithium carbonate sustained-release tablets at 0.6-0.9 g/day to stabilize her mood. In cases of severe agitation, intramuscular Estazolam (2-4 mg) was administered as a temporary measure. In addition to pharmacological treatment, psychological counseling and behavioral interventions were implemented. These included 15-minutes sessions 2-3 times daily, reducing aggressive and disruptive behaviors through consistent commu
| Age | Event/observation | Treatment/intervention | Outcome/remarks |
| 2-3 years old | Delayed speech and walking development, lack of social engagement | ||
| 8-13 years old | Learning difficulties, poor academic performance, requires family assistance for personal tasks | Sixth grade suspension due to learning difficulties | |
| 18 years old | Hyperactivity, increased speech, reduced sleep, impulsive behaviors, energy surge | Merriam-Webster Intelligence score: 54 | Inpatient diagnosis: (1) Intellectual disability; and (2) Bipolar disorder (manic episode without psychotic symptoms). Treatment: Clozapine, oxcarbazepine |
| 23 years old | Marriage | Emotional stability | |
| 24 years old | Gave birth to daughter, postpartum mood changes (hyper speech, excessive spending) | Treated with clozapine | Mood stabilized after 2 months, maintained on clozapine 25-75 mg/day |
| 33 years old | Recurrent episodes: Hyperactivity, excessive speech, screaming, insomnia, high energy | Lurasidone 40-80 mg/day, clozapine 25-100 mg/day, lorazepam 2 mg/day | No significant improvement, continued emotional agitation and high-pitched screaming |
| 33 years old (December 2021) | Sluggish movements and rigidity in posture | Discontinued clozapine and lurasidone, switched to olanzapine 5-20 mg/day | Worsened sleep, increased rigidity, difficulty swallowing |
| 34 years old (January 2022) | Increased rigidity, fever (38.8 °C), sweating, elevated BP and HR, urinary/bowel incontinence | Emergency treatment, olanzapine discontinued, Diazepam (10 mg/day) intravenous injection, MECT | Fever and sweating subsided within 1 week, rigidity eased after 2 weeks, continued frequent screaming |
| 34 years old (February-April 2023) | Decreased screaming, but inability to speak, swallow, walk, or perform basic self-care | Rehabilitation, nasal feeding tube, lithium carbonate sustained-release tablets 0.3-0.6 g/day | Improved swallowing by May 2023, slow return to autonomous eating, bowel movements, and basic communication by September 2023. Still unstable gait and prone to falling. Speech and life skills largely restored by April 2023 |
| 36 years old (January 2024) | Disease recurrence: Hyperactivity, excessive speech, reduced sleep, irritability, impulsivity | Olanzapine 125-5 mg/day, lithium carbonate sustained-release tablets 0.6-0.9 g/day, dextrose-potassium-chlorine-insulin drip, physical/psychological therapy | Significant mood improvement, better sleep, stable behavior within 2 weeks |
| 36 years old (March 2024) | Stable mood, no disordered behavior, simple speech, good sleep | Outpatient treatment: Olanzapine 5 mg/day, lithium carbonate sustained-release tablet 0.9 g/day | Mood stable, no disordered behavior or speech issues, good sleep |
| 36 years old (April 2024) | Stable mood, no disordered behavior, simple speech, good sleep | Outpatient treatment: Olanzapine 5 mg/day, lithium carbonate sustained-release tablet 0.9 g/day | Serum lithium concentration: 0.8 mmol/L, patient discharged |
Follow-up conducted 2 months after discharge revealed that the patient’s mood remained stable. The patient and her family actively participated in the treatment and expressed satisfaction with the outcomes. The combination therapy of Olanzapine (5 mg/day) and Lithium carbonate sustained-release tablets (0.9 g/day) proved effective in maintaining her mental stability. During the maintenance phase, her blood lithium concentration was regularly monitored and consis
This 36-year-old woman had a history of delayed mental development since childhood and multiple manic episodes beginning in early adulthood. In 2022, she experienced a regression in life skills over a period of more than a year, followed by another manic episode. To better understand her diagnosis and treatment, three essential questions need to be addressed: What is her diagnosis? What treatment is appropriate for her? How can similar patients be identified for early intervention?
Intellectual disability is a prevalent feature in patients with generalized developmental disorders, such as PMS, mainly manifesting as severe or profound[7,8]. Additionally, several patients experience episodes of decompensation, including mood instability, irritability, and erratic behaviors. The patient’s symptoms are consistent with the psychiatric manifestations commonly associated with PMS[9]. Research indicated that approximately 54% of PMS patients with psychiatric symptoms may meet the diagnostic criteria for bipolar disorder. Notably, bipolar disorder could be more prevalent in individuals with large deletions of the SHANK3 gene (3%), compared with those with small deletions or SHANK3 se
PMS can lead to neurological and psychiatric deterioration, affecting cognition, speech, motor skills, and daily living abilities, with speech regression often occurring first[15,16]. A previous study on PMS patients indicated that 43% experienced regression[15], which is a recognized characteristic of the PMS. These regressions can be triggered by biolo
The diagnosis of PMS is primarily confirmed through molecular genetic testing, such as chromosomal microarray analysis and next-generation sequencing for genome copy number variation. Additionally, for detected deletions, further testing may be needed to assess the extent of the deletions[21]. In this case, the patient underwent whole-exome sequencing in April 2022, which identified a SHANK3 gene mutation (c.3865dup), a loss-of-function frameshift mutation, ultimately leading to a definitive diagnosis of PMS.
PMS management guidelines recommend the utilization of mood stabilizers and atypical antipsychotics for mood disorders. Mood stabilizers, either alone or in combination with antipsychotics, can stabilize mood and behavior in bipolar disorder, while antipsychotic monotherapy is less effective and poorly tolerated[6]. There is a case report that lithium salt reversed the degenerative symptoms and stabilized the patient's behavior. Two patients were administered lithium at doses of 1.5 and 1.0 g/day, and after two years, one patient even recovered to the level of functioning before the occurrence of degeneration[22]. Other PMS patients with emotional instability, hyperactivity, and sleep disturbances have exhibited improvement with lithium doses of 0.4 and 0.7 g/day, with reports indicating that lithium can reverse regression symptoms[23,24]. In 2022, the patient experienced NMS following changes in her antipsychotic medication. Treatment involved discontinuing Olanzapine, initiating hydration, and administering modified electroconvulsive therapy, which helped stabilize her mood and restore her consciousness. Lithium sustained-release tablets were later introduced as part of her rehabilitation. During her 2024 hospitalization, the patient received olanzapine (1.25-5 mg/day) plus lithium sustained-release tablets (0.3-0.9 g/day) using a cautious incremental dosing strategy to minimize extrapyramidal side effects. Second-generation antipsychotic medications can aid in acute stabilization in such cases; however, they should be administered at very low doses due to the risk of triggering catatonia and the generally high incidence of side effects[25]. This patient had mental retardation since childhood, accompanied by poor cognitive function. Magnetic resonance imaging of the skull revealed mild white matter hyperintensities near both lateral ventricles, likely of vascular origin. Structural brain abnormalities are frequently found in patients with PMS, including cortical atrophy, white matter changes, ventricular enlargement, reduced volume of the striatum and left pallidum, and mild cerebellar ectopia. Jesse et al[26] demonstrated that the brain white matter changes were particularly significant in PMS patients, mainly concentrated in the long-range fiber bundles, especially the uncinate fasciculus and fronto-suboccipital fasciculus. Insulin is an important neuropeptide in the central nervous system, influencing the balance of the hypothalamic-pituitary-adrenal axis and regulating the secretion of neurotrophic factors and neurotransmitters[27]. Scholars found that insulin can regulate the synaptic plasticity by upregulating the expression levels of receptors related to synaptic plasticity and post-translational modification[28]. According to this evidence, peripheral intravenous insulin was administered to this patient to enhance cognitive function, recognizing as a strategy referenced in PMS management. Preventing infections is crucial for PMS patients, as infections can trigger regression. Behavioral interventions are vital for managing aggression, repetitive actions, and self-injurious behaviors, mainly guided by the Applied Behavior Analysis principles and reinforced behavioral plans[29,30]. Regression in PMS frequently leads to significant deterioration in life skills, necessitating long-term rehabilitation. In some cases, nasogastric tube care provided by community healthcare centers. Comprehensive management, including behavioral therapies, infection prevention, and ongoing medical and rehabilitative support, is vital to maintain the quality of life and address the complex needs of PMS patients.
PMS is a rare neurodevelopmental disorder, and bipolar disorder may be a component of its psychopathological profile. Clinicians should consider PMS in individuals with intellectual disability and autism-like behaviors during childhood, who later exhibit hyperexcited screaming, irritability, destructive behaviors, reduced sleep, and high energy during adolescence or early adulthood. Regression in speech and daily functioning accompanying these symptoms may further indicate atypical bipolar disorder. Accurate diagnosis requires a comprehensive clinical history and genetic testing to avoid misdiagnosis and inappropriate treatment. Long-term management typically involves structured rehabilitation programs, and in more severe cases, ongoing care, such as nasogastric feeding tube support from community healthcare services. These interventions are crucial to maintain quality of life and ensure consistent medical care for patients with PMS.
We would like to express our sincere gratitude to all the other medical staff involved in providing care for the patient.
| 1. | Cammarata-Scalisi F, Callea M, Martinelli D, Willoughby CE, Tadich AC, Araya Castillo M, Lacruz-Rengel MA, Medina M, Grimaldi P, Bertini E, Nevado J. Clinical and Genetic Aspects of Phelan-McDermid Syndrome: An Interdisciplinary Approach to Management. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Bonaglia MC, Giorda R, Beri S, De Agostini C, Novara F, Fichera M, Grillo L, Galesi O, Vetro A, Ciccone R, Bonati MT, Giglio S, Guerrini R, Osimani S, Marelli S, Zucca C, Grasso R, Borgatti R, Mani E, Motta C, Molteni M, Romano C, Greco D, Reitano S, Baroncini A, Lapi E, Cecconi A, Arrigo G, Patricelli MG, Pantaleoni C, D'Arrigo S, Riva D, Sciacca F, Dalla Bernardina B, Zoccante L, Darra F, Termine C, Maserati E, Bigoni S, Priolo E, Bottani A, Gimelli S, Bena F, Brusco A, di Gregorio E, Bagnasco I, Giussani U, Nitsch L, Politi P, Martinez-Frias ML, Martínez-Fernández ML, Martínez Guardia N, Bremer A, Anderlid BM, Zuffardi O. Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet. 2011;7:e1002173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Kolevzon A, Delaby E, Berry-Kravis E, Buxbaum JD, Betancur C. Neuropsychiatric decompensation in adolescents and adults with Phelan-McDermid syndrome: a systematic review of the literature. Mol Autism. 2019;10:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Kohlenberg TM, Trelles MP, McLarney B, Betancur C, Thurm A, Kolevzon A. Psychiatric illness and regression in individuals with Phelan-McDermid syndrome. J Neurodev Disord. 2020;12:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Frank Y. The Neurological Manifestations of Phelan-McDermid Syndrome. Pediatr Neurol. 2021;122:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Kozol RA, Dallman JE. Drugs prescribed for Phelan-McDermid syndrome differentially impact sensory behaviors in shank3 zebrafish models. F1000Res. 2023;12:84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Zwanenburg RJ, Ruiter SA, van den Heuvel ER, Flapper BC, Van Ravenswaaij-Arts CM. Developmental phenotype in Phelan-McDermid (22q13.3 deletion) syndrome: a systematic and prospective study in 34 children. J Neurodev Disord. 2016;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Ponson L, Gomot M, Blanc R, Barthelemy C, Roux S, Munnich A, Romana S, Aguillon-Hernandez N, Malan V, Bonnet-Brilhault F. 22q13 deletion syndrome: communication disorder or autism? Evidence from a specific clinical and neurophysiological phenotype. Transl Psychiatry. 2018;8:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Verhoeven WMA, Egger JIM, de Leeuw N. A longitudinal perspective on the pharmacotherapy of 24 adult patients with Phelan McDermid syndrome. Eur J Med Genet. 2020;63:103751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Levy T, Foss-Feig JH, Betancur C, Siper PM, Trelles-Thorne MDP, Halpern D, Frank Y, Lozano R, Layton C, Britvan B, Bernstein JA, Buxbaum JD, Berry-Kravis E, Powell CM, Srivastava S, Sahin M, Soorya L, Thurm A, Kolevzon A; Developmental Synaptopathies Consortium. Strong evidence for genotype-phenotype correlations in Phelan-McDermid syndrome: results from the developmental synaptopathies consortium. Hum Mol Genet. 2022;31:625-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Denayer A, Van Esch H, de Ravel T, Frijns JP, Van Buggenhout G, Vogels A, Devriendt K, Geutjens J, Thiry P, Swillen A. Neuropsychopathology in 7 Patients with the 22q13 Deletion Syndrome: Presence of Bipolar Disorder and Progressive Loss of Skills. Mol Syndromol. 2012;3:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Serrada-Tejeda S, Cuadrado ML, Martínez-Piédrola RM, Máximo-Bocanegra N, Sánchez-Herrera-Baeza P, Camacho-Montaño LR, Pérez-de-Heredia-Torres M. Sensory processing and adaptive behavior in Phelan-McDermid syndrome: a cross-sectional study. Eur J Pediatr. 2022;181:3141-3152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Jungová P, Čumová A, Kramarová V, Lisyová J, Ďurina P, Chandoga J, Bӧhmer D. Phelan-McDermid syndrome in adult patient with atypical bipolar psychosis repeatedly triggered by febrility. Neurocase. 2018;24:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Messias E, Kaley SN, McKelvey KD. Adult-onset psychosis and clinical genetics: a case of Phelan-McDermid syndrome. J Neuropsychiatry Clin Neurosci. 2013;25:E27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Reierson G, Bernstein J, Froehlich-Santino W, Urban A, Purmann C, Berquist S, Jordan J, O'Hara R, Hallmayer J. Characterizing regression in Phelan McDermid Syndrome (22q13 deletion syndrome). J Psychiatr Res. 2017;91:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | De Rubeis S, Siper PM, Durkin A, Weissman J, Muratet F, Halpern D, Trelles MDP, Frank Y, Lozano R, Wang AT, Holder JL Jr, Betancur C, Buxbaum JD, Kolevzon A. Delineation of the genetic and clinical spectrum of Phelan-McDermid syndrome caused by SHANK3 point mutations. Mol Autism. 2018;9:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 17. | Egger JI, Zwanenburg RJ, van Ravenswaaij-Arts CM, Kleefstra T, Verhoeven WM. Neuropsychological phenotype and psychopathology in seven adult patients with Phelan-McDermid syndrome: implications for treatment strategy. Genes Brain Behav. 2016;15:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Ware MR, Feller DB, Hall KL. Neuroleptic Malignant Syndrome: Diagnosis and Management. Prim Care Companion CNS Disord. 2018;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Brignell A, Gu C, Holm A, Carrigg B, Sheppard DA, Amor DJ, Morgan AT. Speech and language phenotype in Phelan-McDermid (22q13.3) syndrome. Eur J Hum Genet. 2021;29:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Burdeus-Olavarrieta M, San José-Cáceres A, García-Alcón A, González-Peñas J, Hernández-Jusdado P, Parellada-Redondo M. Characterisation of the clinical phenotype in Phelan-McDermid syndrome. J Neurodev Disord. 2021;13:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Srivastava S, Sahin M, Buxbaum JD, Berry-Kravis E, Soorya LV, Thurm A, Bernstein JA, Asante-Otoo A, Bennett WE Jr, Betancur C, Brickhouse TH, Passos Bueno MR, Chopra M, Christensen CK, Cully JL, Dies K, Friedman K, Gummere B, Holder JL Jr, Jimenez-Gomez A, Kerins CA, Khan O, Kohlenberg T, Lacro RV, Levi LA, Levy T, Linnehan D, Eva L, Moshiree B, Neumeyer A, Paul SM, Phelan K, Persico A, Rapaport R, Rogers C, Saland J, Sethuram S, Shapiro J, Tarr PI, White KM, Wickstrom J, Williams KM, Winrow D, Wishart B, Kolevzon A. Updated consensus guidelines on the management of Phelan-McDermid syndrome. Am J Med Genet A. 2023;191:2015-2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Serret S, Thümmler S, Dor E, Vesperini S, Santos A, Askenazy F. Lithium as a rescue therapy for regression and catatonia features in two SHANK3 patients with autism spectrum disorder: case reports. BMC Psychiatry. 2015;15:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Egger JIM, Verhoeven WMA, Groenendijk-Reijenga R, Kant SG. Phelan-McDermid syndrome due to SHANK3 mutation in an intellectually disabled adult male: successful treatment with lithium. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | van Balkom IDC, Burdeus-Olavarrieta M, Cooke J, de Cuba AG, Turner A; European Phelan-McDermid Syndrome consortium, Vogels A, Maruani A. Consensus recommendations on mental health issues in Phelan-McDermid syndrome. Eur J Med Genet. 2023;66:104770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Pasini A, D'Agati E, Casarelli L, Curatolo P. Dose-dependent effect of risperidone treatment in a case of 22q13.3 deletion syndrome. Brain Dev. 2010;32:425-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Jesse S, Müller HP, Huppertz HJ, Andres S, Ludolph AC, Schön M, Boeckers TM, Kassubek J. Neurodegeneration or dysfunction in Phelan-McDermid syndrome? A multimodal approach with CSF and computational MRI. Orphanet J Rare Dis. 2023;18:274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Schmidt H, Kern W, Giese R, Hallschmid M, Enders A. Intranasal insulin to improve developmental delay in children with 22q13 deletion syndrome: an exploratory clinical trial. J Med Genet. 2009;46:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Zwanenburg RJ, Bocca G, Ruiter SA, Dillingh JH, Flapper BC, van den Heuvel ER, van Ravenswaaij-Arts CM. Is there an effect of intranasal insulin on development and behaviour in Phelan-McDermid syndrome? A randomized, double-blind, placebo-controlled trial. Eur J Hum Genet. 2016;24:1696-1701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Landlust AM, Visser L, Flapper BCT, Ruiter SAJ, Zwanenburg RJ, van Ravenswaaij-Arts CMA, van Balkom IDC. Understanding Behavior in Phelan-McDermid Syndrome. Front Psychiatry. 2022;13:836807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Schroeder KA, Witts BN, Traub MR. Opportunities for ABA intervention in Phelan-McDermid syndrome. Int J Dev Disabil. 2022;68:984-989. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/