Published online Nov 19, 2025. doi: 10.5498/wjp.v15.i11.107604
Revised: May 21, 2025
Accepted: August 18, 2025
Published online: November 19, 2025
Processing time: 221 Days and 18.9 Hours
Schizophrenia is a multifaceted neurodevelopmental disorder characterized by hallucinations, delusions, cognitive deficits, and emotional dysregulation. The prefrontal cortex (PFC), essential for executive functions, working memory, and emotional regulation, is notably impaired in this condition. This review consolidates current insights into the role of PFC dysfunction in schizophrenia, with a focus on its implications for therapeutic strategies. The neuroanatomical and neurobiological foundations of PFC dysfunction are explored, emphasizing structural abnormalities, functional dysconnectivity, and microcircuit disruptions that contribute to cognitive deficits and impaired decision-making. Clinical implications are discussed, particularly the correlation between PFC dysfunction and the severity and progression of schizophrenia symptoms. Additionally, pharmacological and non-pharmacological approaches aimed at modulating PFC activity are reviewed as potential therapeutic options. In conclusion, a deeper understanding of PFC dysfunction is pivotal for developing targeted treatments, and ongoing research offers promising avenues for enhancing outcomes for individuals affected by this debilitating disorder.
Core Tip: Schizophrenia is characterized by cognitive deficits, emotional dysregulation, and executive dysfunction. This review highlights the central role of prefrontal cortex (PFC) dysfunction in the manifestation of these symptoms. Structural abnormalities, functional dysconnectivity, and microcircuit dysregulation within the PFC contribute significantly to the disorder’s pathology. A comprehensive understanding of these neurobiological mechanisms is vital for the development of targeted therapeutic approaches. Both pharmacological and non-pharmacological treatments aimed at modulating PFC activity show considerable promise in alleviating the core symptoms of schizophrenia. Ongoing research into PFC dysfunction remains essential for devising effective therapies and improving outcomes for individuals affected by this complex disorder.
- Citation: Cheng BF, Liang Y, Wu Q. Unraveling the mysteries of schizophrenia: Insights into prefrontal cortex dysfunction and therapeutic implications. World J Psychiatry 2025; 15(11): 107604
- URL: https://www.wjgnet.com/2220-3206/full/v15/i11/107604.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i11.107604
Schizophrenia, a debilitating neurodevelopmental disorder, affects approximately 1% of the global population, with a lifetime prevalence[1]. This complex condition severely impacts those affected, often disrupting their ability to maintain relationships, employment, and independent living, while also placing significant strain on healthcare systems and economies worldwide. The disorder is diagnostically characterized by three core symptom domains: (1) Positive symptoms, such as hallucinations and delusions; (2) Negative symptoms, including social withdrawal, anhedonia, and blunted affect; and (3) Cognitive impairments, including working memory deficits, executive dysfunction, and attention problems[2]. Despite decades of research, the underlying pathophysiological mechanisms of schizophrenia remain incompletely understood, with a combination of genetic, environmental, and neurodevelopmental factors likely contributing to its onset, adding complexity to this multifaceted disorder.
The prefrontal cortex (PFC) plays a central role in understanding this enigma, as it is responsible for higher-order cognitive functions such as decision-making, working memory, and social behavior. Alterations in PFC structure and function have long been implicated in schizophrenia, with these changes thought to underlie many of the cognitive and negative symptoms observed in patients. Neuroimaging studies, including those by Weinberger et al[3], provide compelling evidence of PFC dysfunction in schizophrenia, underscoring its critical role in the disorder’s pathology and highlighting the urgent need for a deeper understanding of this vital brain region. Notably, studies utilizing paired transcranial magnetic stimulation (TMS) combined with electroencephalography have offered insights into the pathophysiology of schizophrenia, particularly implicating prefrontal GABAergic and glutamatergic dysfunctions in the manifestation of symptoms[4]. Recent research employing interleaved TMS and functional magnetic resonance imaging (fMRI) has provided causal evidence of interhemispheric dysconnectivity within the PFC in schizophrenia[5]. These cognitive neuroimaging findings collectively emphasize the critical role of prefrontal cortical abnormalities in the pathophysiology of schizophrenia.
Understanding the nature of PFC dysfunction in schizophrenia is essential for the development of targeted therapeutic interventions to address the core symptoms of the disorder, particularly the negative symptoms and cognitive impair
Schizophrenia’s neurobiological mechanisms are complex, involving a dynamic interplay of neurotransmitter imbalances, developmental disruptions, and circuit-level abnormalities, with the PFC consistently implicated as a central hub of dysfunction.
One of the most enduring frameworks is the dopamine hypothesis, which has evolved significantly since its introduction. Initially conceptualized as a model of generalized dopamine hyperactivity, the hypothesis has been refined in light of accumulating evidence, now emphasizing regional specificity. Elevated dopamine transmission in subcortical regions, such as the striatum, is associated with positive symptoms like hallucinations and delusions, while reduced dopamine signaling in the PFC correlates with negative symptoms (e.g., anhedonia, social withdrawal) and cognitive deficits (e.g., working memory impairments), as outlined by Howes and Kapur[6]. This revised model proposes a potential relationship between hypo- and hyperdopaminergic states. For example, animal studies have shown that lesions in dopamine neurons in the PFC result in elevated dopamine levels, increased metabolites, and increased dopamine D2 receptor density in the striatum[7]. Similarly, excessive dopaminergic signaling in the striatum may reduce cortical dopamine levels, contributing to cognitive deficits[8], emphasizing the complex interaction between these regions. Advances in positron emission tomography studies confirm that dopaminergic dysregulation in schizophrenia primarily occurs at the presynaptic level, with limited evidence supporting significant alterations in dopamine D2/3 receptor levels[9]. Nevertheless, the substantial role of striatal dopamine D2/D3 receptors in the negative symptoms of schizophrenia should not be overlooked, as a recent fMRI finding suggest that their antagonism may exacerbate these symptoms in patients treated with antipsychotic medications[10].
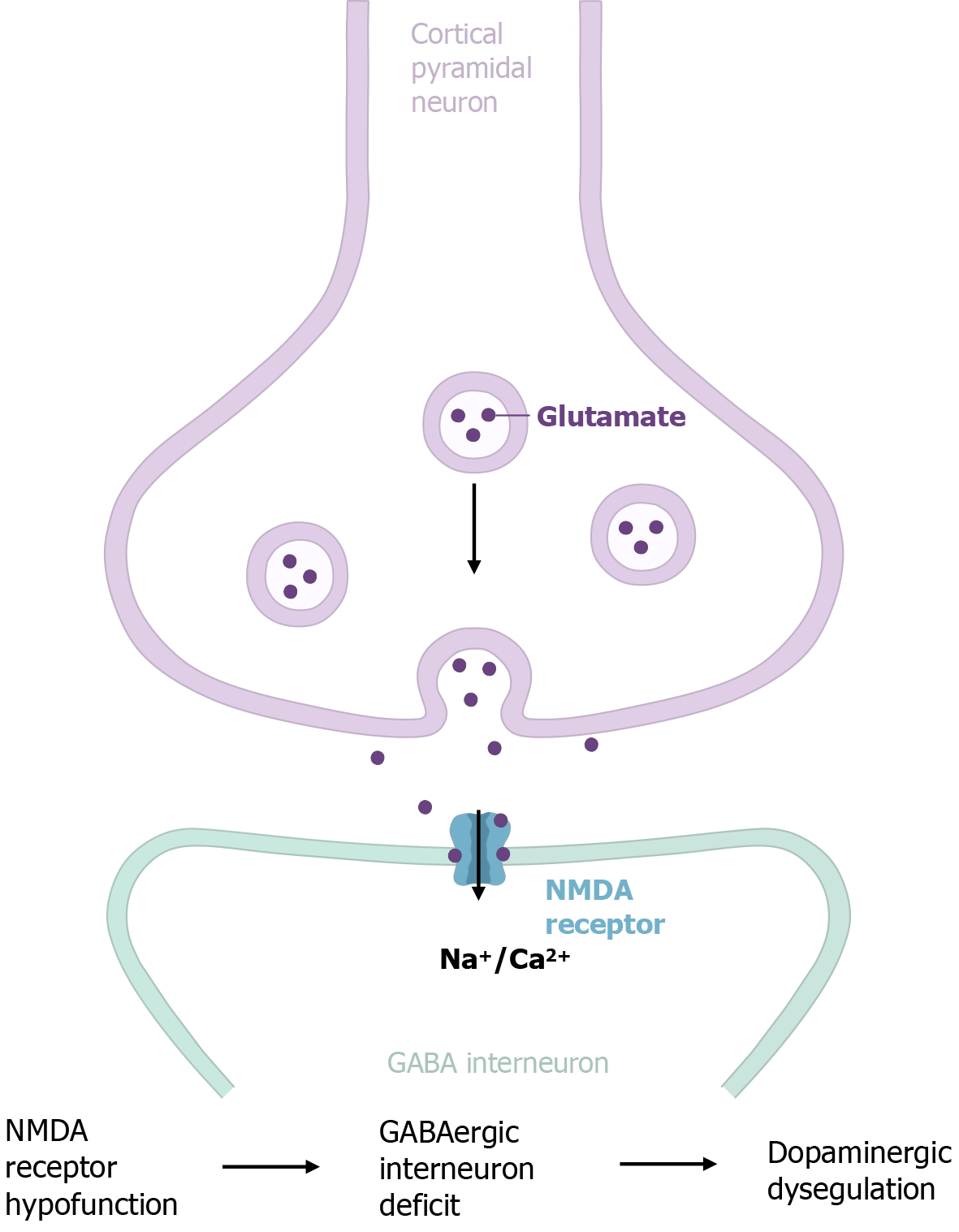
In parallel with the dopamine hypothesis, the glutamate hypothesis posits that deficits in glutamate transmission play a pivotal role in the pathophysiology of schizophrenia. Evidence strongly supports the idea that alterations in prefrontal connectivity, especially involving glutamate signaling at N-methyl-D-aspartate (NMDA) receptors, are central to the disorder[11,12]. According to the glutamate hypothesis, the hypofunction of NMDA receptors on GABAergic inter
The causal relationship between the glutamate and dopamine hypotheses in schizophrenia remains a subject of ongoing debate. Carlsson et al[17] proposed a model in which glutamatergic projections from the frontal cortex exert bimodal modulation of dopamine activity in the ventral tegmental area. Previous evidence indicates that dopaminergic dysregulation in schizophrenia may stem from impaired NMDA receptor function[9,18]. Conversely, alternative hypotheses suggest that altered dopamine function in schizophrenia could disrupt NMDA receptor-mediated transmission. Despite these competing views, increasing evidence suggests that NMDA receptor hypofunction typically precedes dopaminergic dysregulation[19,20], with the former emerging earlier in development[21-23]. NMDA receptor dysfunction may initiate deficits in GABAergic interneurons, leading to aberrant local circuit dynamics and long-range disconnections between brain regions, including the dopamine system[24-26] (Figure 1). These disruptions are thought to underlie the core clinical features of schizophrenia. Collectively, these insights point to a bidirectional relationship between the dopamine and glutamate systems, offering a more integrated perspective on the neurobiological basis of schizophrenia.
The neurodevelopmental hypothesis further enhances this neurobiological framework, proposing that schizophrenia arises from early disruptions in brain maturation, with the PFC’s prolonged development rendering it particularly vulnerable. Recent studies analyzing gene expression in brain tissue, comparing fetal to postnatal stages, have consistently shown that genes associated with schizophrenia exhibit higher expression during fetal development than in postnatal life[27-29]. Moreover, schizophrenia-specific differentially expressed genes converge on pathways related to neurodevelopment and synaptic function[30], with several key regulatory factors genetically linked to schizophrenia and other neurodevelopmental disorders[31,32].
Environmental factors further shape schizophrenia’s neurodevelopmental trajectory. Pregnancy complications, such as preeclampsia, intrauterine infections, and immune incompatibility, have been linked to an increased risk of schizophrenia. Insults during critical developmental periods, such as prenatal exposure to infection or malnutrition, may disrupt PFC synaptic pruning and myelination, processes that continue into adolescence and early adulthood. Preclinical models, such as maternal immune activation paradigms, have reproduced PFC-dependent cognitive deficits in offspring, supporting the link between early immune challenges and lasting alterations in prefrontal circuitry. Elevated microglial and astroglial activity, along with increased pro-inflammatory cytokines such as interleukin-6 and interleukin-8, have been observed in the PFC of patients with schizophrenia. This neuroinflammatory response amplifies oxidative stress, disrupts synaptic activity mediated by neurotrophic factors such as BDNF, and leads to the loss of parvalbumin (PV) interneurons. The interplay between glial hyperactivity, reduced neurotrophic support, and impaired GABAergic neurotransmission likely contributes to dysregulated microcircuitry within the PFC, which may underlie the cognitive deficits observed in schizophrenia.
Environmental factors further shape neurodevelopmental trajectory of schizophrenia. Substantial evidence links early developmental adversities, such as pregnancy complications, including premature birth, preeclampsia, intrauterine infections, and immune incompatibility, with an increased risk of schizophrenia[33]. Insults during critical developmental periods, such as prenatal exposure to infection or malnutrition, could alter PFC synaptic pruning and myelination, processes that continue into adolescence and early adulthood, coinciding with the typical onset of schizophrenia[34]. Preclinical models, such as maternal immune activation paradigms, have reproduced PFC-dependent cognitive deficits in offspring, supporting this hypothesis by demonstrating lasting alterations in prefrontal circuitry[35]. Increasing evidence suggests that activation of microglia and astroglia, along with elevated pro-inflammatory cytokines like interleukin-6 and interleukin-8, are observed in the PFC of patients with schizophrenia[36,37]. This neuroinflammatory response amplifies oxidative stress, which, in turn, disrupts synaptic activity mediated by BDNF and leads to the loss of PV interneurons[38]. The interplay between glial hyperactivity, reduced neurotrophic support, and impaired GABAergic neurotransmission likely contributes to dysregulated microcircuitry within the PFC, which may underlie the cognitive deficits observed in schizophrenia[38].
It is important to note that, gene-environment interactions are particularly relevant in this context. For example, prenatal stress has been shown to exacerbate PFC dysfunction in genetically predisposed individuals[39]. Furthermore, this inflammatory state may interact with genetic vulnerabilities, such as polymorphisms in immune-related genes, amplifying PFC dysfunction and contributing to the disorder’s heterogeneity. Ursini et al[40] demonstrated a significant interaction between obstetric complications, adverse intrauterine and perinatal events that may affect fetal health, and schizophrenia polygenic risk. Their study suggests that certain schizophrenia risk genes may sensitize the placenta to environmental stress, increasing the likelihood of impaired fetal development and pregnancy complications. These findings support the idea that schizophrenia’s neurobiological roots lie in a cascade of events that begin well before symptom onset, with the PFC acting as a critical nexus where developmental disturbances manifest as clinical symptoms. Together, these neurobiological mechanisms, including the dopamine hypothesis, glutamate hypothesis, and neurodevelopmental processes shaped by genetic and environmental factors, illustrate schizophrenia as a disorder characterized by disrupted PFC networks. These insights not only enhance our understanding of its etiology but also provide a foundation for developing targeted interventions.
Lesion studies have demonstrated that distinct subdivisions of the PFC regulate different aspects of executive function, each contributing uniquely to goal-directed behavior[41]. The lateral PFC is primarily involved in the selection, monitoring, and manipulation of cognitive task sets, while the medial PFC plays a pivotal role in updating and adapting these task sets. The orbitofrontal cortex is essential for assigning social and emotional significance to task sets, thus optimizing goal-directed behavior[41]. Extensive research over the past several decades has consistently identified dysfunction in the dorsolateral PFC (DLPFC) as central to the pathophysiology of schizophrenia[42-44]. This region is strongly associated with the hallmark cognitive impairments of schizophrenia, particularly deficits in working memory and cognitive control. Notably, while specific PFC subregions are linked to distinct functions, there is considerable overlap and extensive functional interaction among these regions, as emphasized by Duncan[45].
Schizophrenia is conceptualized as a brain dysconnectivity disorder[46], characterized by a failure in the functional integration of distributed but specific neuronal systems that depend on neuromodulatory inputs[47]. For instance, frontal regions receiving dopaminergic projections from the mesocorticolimbic pathway are particularly implicated[46]. Longitudinal studies suggest that neuroanatomical changes may precede symptom onset and progress throughout the course of schizophrenia, supporting a neurodevelopmental model[48]. As a result, there is an increasing focus on understanding structural changes, as they may offer valuable insights and improve the potential for early intervention. Meta-analyses of volumetric imaging studies in schizophrenia have consistently identified progressive gray matter loss in the PFC[49-51], characterized by reduced total PFC gray matter volume, altered neurodevelopmental trajectories of PFC thickness (including age-related thinning), and diminished hemispheric asymmetry in cortical complexity[52,53]. These structural abnormalities correlate with cognitive deficits and may reflect aberrant synaptic pruning during adolescence[54], a proposed mechanism underlying PFC dysfunction in schizophrenia. Post-mortem analyses have further supported these findings, revealing decreased neuronal density and reduced dendritic spine density in the superficial layer pyramidal neurons of the DLPFC[55,56]. These alterations in synaptic morphology are thought to contribute to the cognitive impairments observed in schizophrenia. Additionally, meta-analytic evidence from individuals with first-episode schizophrenia suggests that cognitive deficits may not only manifest early in the disease but could even precede its onset[57,58]. Advances in diffusion tensor imaging (DTI), which measures white matter fiber tract integrity using markers such as fractional anisotropy (FA), axial diffusivity, and radial diffusivity (RD), have also provided critical insights. A large-scale ENIGMA study found lower FA across the whole brain in patients with schizophrenia, with the most pronounced reductions observed in fronto-thalamic tracts[59]. Similarly, Yao et al[60] reported reduced FA in the white matter of the right deep frontal and left deep temporal lobes in patients with first-episode schizophrenia. These findings collectively highlight the presence of disrupted anatomical connections in the PFC, even during the early stages of the disorder.
Impaired structural connectivity has been identified as a key mechanism contributing to altered functional dynamics and disrupted global brain function in schizophrenia[61]. Non-invasive imaging studies in schizophrenia further support this notion, converging on the consensus that functional disconnection involves the PFC, along with critical subcortical (e.g., thalamic) and associative cortical (e.g., temporal) regions[62,63]. Resting-state fMRI studies have revealed disrupted communication between the PFC and regions such as the hippocampus, thalamus, and striatum, which contributes to the breakdown of cognitive and emotional processes, as demonstrated by Sutherland et al[64]. Recent investigations have also examined the relationship between schizotypy dimensions and brain structural connectivity, offering further insights into the connection between structural and functional variations in schizophrenia. For example, a DTI-based study found a positive correlation between negative schizotypy and FA in the right uncinate fasciculus. In contrast, positive schizotypy showed a negative correlation with FA in the right cingulum, and disorganized schizotypy was negatively associated with FA in the left cingulum[64]. These findings suggest distinct structural connectivity patterns that underlie different schizotypy dimensions.
Emerging evidence has clarified the synaptic mechanisms underlying these dysconnections. A recent study using dynamic causal modeling suggests that the observed functional disconnection in schizophrenia may be driven by modulatory neurotransmitters influencing NMDA receptor-mediated changes in synaptic efficacy (i.e., synchronous gain) within the PFC[65]. This dysregulation of synaptic efficacy subsequently leads to secondary deficits in GABAergic neurotransmission, disrupting the excitation-inhibition balance or cortical gain control, as demonstrated in both human and rodent models[64]. Specifically, NMDA receptor hypofunction on GABAergic interneurons may result in the disinhibition of glutamatergic pyramidal neurons, causing a cortical excitation-inhibition imbalance, as reviewed by Javitt[14]. Post-mortem studies consistently show reduced expression of glutamic acid decarboxylase 67, the enzyme responsible for gamma aminobutyric acid synthesis, and PV, a marker for fast-spiking interneurons, in the PFC of patients with schizophrenia[66,67]. Additionally, optogenetic studies in rodent models have demonstrated that perturbations in excitation-inhibition balance within the PFC can replicate cognitive deficits characteristic of schizophrenia[16]. These deficits impair inhibitory control, leading to dysregulated gamma oscillations, high-frequency brain waves crucial for cognitive functions such as working memory and attention[68,69]. The loss of inhibitory tone disrupts the PFC’s ability to coordinate with other brain regions, including the hippocampus and thalamus, contributing to the fragmented cognition and perception characteristic of the disorder[70]. It is hypothesized that NMDA receptor hypofunction occurs first, initiating a cascade of dysfunction within local GABAergic circuits and disrupting input-output connectivity in the PFC[71]. These alterations ultimately result in the cognitive and social deficits that define schizophrenia. Overall, the cumulative evidence supports a model in which structural and functional dysconnectivity in the PFC, mediated by abnormalities in synaptic modulation and gain control, plays a central role in the pathophysiology of the disorder.
As previously noted, cognitive deficits, a core symptom of schizophrenia, are observable even before the onset of the illness[57,58]. Among these impairments, working memory dysfunction, characterized by the inability to store and process task-relevant information for executive control during brief sensory input intervals, is especially prominent[72]. Converging evidence highlights structural alterations in the DLPFC as a central contributor to these cognitive deficits[42]. Specifically, dysregulated microcircuitry within the DLPFC, where layer 3 pyramidal neurons exhibit reduced somal size, spine density, total dendritic length, and disrupted connectivity, impairs delay-period activity critical for working memory maintenance[73,74]. At the cellular level, GABAergic interneurons, classified by molecular markers such as PV and somatostatin (SST), are essential for regulating DLPFC microcircuit dynamics[73]. PV basket cells demonstrate activity-dependent reductions in glutamic acid decarboxylase 67 expression[75], impairing gamma oscillation generation during working memory delays[76], while SST interneuron depletion hampers distractor resistance through inadequate suppression of task-irrelevant inputs[40,70,71]. Conversely, enhanced PV chandelier cell-mediated inhibition may reflect an insufficient compensatory response to pyramidal hypoactivity[42,55]. These multilevel disruptions in DLPFC microcircuitry position neural circuit biomarkers as critical targets for addressing cognitive deficits in schizophrenia.
In summary, the PFC in schizophrenia is characterized by structural (gray/white matter loss), functional (network dysconnectivity), and synaptic (NMDA/gamma aminobutyric acid dysfunction) abnormalities. Disruption of DLPFC microcircuitry, particularly involving pyramidal neurons and PV/SST interneurons, underpins working memory deficits and excitation/inhibition imbalance. These multilevel pathologies converge to drive cognitive and perceptual fragmentation in the disorder.
Schizophrenia’s complex symptomatology requires a multifaceted therapeutic approach. However, current treatments often fail to comprehensively address the full range of symptoms, particularly those associated with PFC dysfunction. Therapeutic strategies increasingly target PFC dysfunction, encompassing pharmacotherapy, neuromodulation, and behavioral interventions. Emerging approaches, including trace amine-associated receptor 1 (TAAR1) agonists, stem cells, and psychedelics, focus on addressing synaptic and circuit-level pathologies. However, challenges such as metabolic side effects, inconsistent efficacy [e.g., transcranial direct current stimulation (tDCS)], and ethical concerns [e.g., deep brain stimulation (DBS)] persist. Precision medicine, integrating multiple modalities, offers the potential to optimize treatment outcomes.
First-generation antipsychotics, such as haloperidol, primarily target subcortical dopamine D2 receptors, effectively reducing positive symptoms like hallucinations and delusions through dopamine blockade in the mesolimbic pathway. However, their efficacy in addressing PFC-mediated cognitive deficits and negative symptoms is limited, as these symptoms are more closely associated with cortical hyperdopaminergic activity rather than subcortical hyperactivity[77]. This dissociation reveals a critical therapeutic gap, as cognitive and negative symptoms are strong predictors of long-term functional outcomes, including employment and social integration[78].
Second-generation antipsychotics (SGAs), such as risperidone and clozapine, exhibit a broader receptor profile, antagonizing serotonin serotonin 2A receptors in addition to modulating D2 receptors. This dual action may enhance dopaminergic transmission in the PFC, potentially alleviating cognitive and negative symptoms[79]. Clozapine’s unique efficacy in treatment-resistant schizophrenia is partly attributed to its effects on PFC circuitry, though the evidence remains mixed due to variability in patient response and side-effect profiles[80]. Notably, SGAs are associated with significant metabolic adverse effects, including weight gain, dyslipidemia, and type 2 diabetes mellitus, which increase cardiovascular mortality risk, one of the leading contributors to reduced life expectancy in this population[81]. Importantly, even the modest cognitive improvements attributed to SGAs in PFC-dependent functions are overshadowed by their metabolic liabilities, highlighting the need for therapies that can decouple therapeutic efficacy from systemic toxicity[82].
Emerging pharmacological strategies aim to directly target PFC dysfunction by modulating neurotransmitter systems beyond dopamine. A promising approach involves NMDA receptor modulation via glycine transport inhibitors, which enhance glutamatergic signaling by increasing synaptic glycine levels, a co-agonist of NMDA receptors. Preclinical and early clinical studies suggest these agents may improve cognitive deficits in schizophrenia by restoring the excitatory-inhibitory balance in the PFC[83]. Similarly, α7 nicotinic acetylcholine receptor agonists, such as varenicline, target cholinergic pathways to enhance PFC-dependent cognitive processes, including attention and working memory. Phase II trials have shown modest cognitive benefits, though challenges such as receptor desensitization and off-target effects persist[84].
Non-pharmacological interventions have gained prominence as complementary neuromodulation strategies to enhance PFC function, including non-invasive methods such as TMS, tDCS, and magnetic seizure therapy, as well as invasive approaches like electroconvulsive therapy (ECT) and DBS. High-frequency repetitive TMS over the DLPFC improves cognitive and negative symptoms by enhancing cortical excitability and strengthening PFC-subcortical connectivity[85], with meta-analyses confirming sustained improvements in working memory, language function[86], and moderate reductions in treatment-resistant auditory hallucinations[87,88]. These therapeutic benefits are accompanied by favorable safety considerations, with a recent systematic review confirming that repetitive TMS has a positive risk-benefit profile in schizophrenia and noting minimal adverse events[89]. In contrast, tDCS has shown mixed efficacy, with preliminary evidence supporting improvement in negative symptoms but inconsistent cognitive effects[90], highlighting the need for larger trials and standardized protocols[91]. ECT remains a widely recognized intervention for treatment-resistant schizophrenia, rapidly improving both positive and negative symptoms[92,93], and potentially modulating neuroinflammation via microglial regulation[94]. However, cognitive risks limit its widespread use[95,96]. Magnetic seizure therapy, a novel non-invasive brain stimulation technique inducing therapeutic seizures, has shown promise as an alternative to ECT due to reduced neurocognitive side effects[97,98] and efficacy in alleviating positive symptoms in schizophrenia[99]. While transcutaneous noninvasive vagus nerve stimulation, being safer than invasive vagus nerve stimulation, has shown limited efficacy in stable schizophrenia, adherence challenges may contribute to these results[100]. DBS, an invasive technique targeting PFC-subcortical circuits (e.g., thalamo-prefrontal pathways) in treatment-resistant cases, has shown preliminary therapeutic potential. Recent exploratory trials in ultra-treatment-resistant schizophrenia cohorts suggest that DBS may alleviate severe negative symptoms by modulating dysfunctional networks[101], although ethical and safety considerations currently restrict its use[102]. Collectively, these interventions are thought to enhance GABAergic inhibitory neurotransmission[103,104], though further clinical validation is essential to optimize their implementation and establish a therapeutic hierarchy in schizophrenia.
Cognitive remediation therapy (CRT) offers a behavioral approach to enhance PFC function through targeted training exercises. By engaging neuroplasticity mechanisms, CRT aims to improve areas such as working memory and executive function, with neuroimaging studies showing increased PFC activation post-intervention[105]. Meta-analytic evidence supports the efficacy of CRT in ameliorating positive symptom domains in schizophrenia[106], though its impact may be diminished in patients experiencing acute symptom-related distress or attentional disruptions during training sessions[105]. Computer-based programs targeting attention and problem-solving have shown potential for enhancing functional outcomes, particularly vocational success, when paired with psychosocial support[107]. However, individual variability in response and the need for sustained engagement present challenges to its broader adoption.
Additional potential therapeutic strategies targeting synaptic dysfunction and molecular pathologies within the PFC have emerged in the context of schizophrenia. Among these, TAAR1 agonism presents a promising approach, leveraging its role in modulating PFC circuitry. TAAR1 is highly expressed in layer 5 PFC pyramidal neurons and is genetically linked to schizophrenia susceptibility through mutations identified in clinical cohorts[108-110]. Preclinical studies show that TAAR1 agonists normalize dysregulated dopaminergic signaling in midbrain pathways and enhance PFC glutamatergic transmission by restoring NMDA receptor function[108,111,112]. Notably, murine studies reveal that TAAR1 deletion induces PFC dysfunction, manifesting as aberrant perseverative behaviors (e.g., compulsive repetition) and heightened impulsivity, phenotypes that mirror core cognitive and behavioral deficits seen in schizophrenia[108]. These impairments are mechanistically tied to PFC glutamatergic hypofunction. TAAR1 agonists have demonstrated broad efficacy in animal models, improving positive, negative, and cognitive symptom domains[113]. Early clinical trials support their translational potential, showing symptom remission without the adverse effects typically associated with D2 blockade[114]. These findings suggest that TAAR1 agonism may provide a viable therapeutic strategy to address the complex PFC dysfunction underlying schizophrenia.
Emerging evidence further highlights synaptic dysfunction as a central mechanism in schizophrenia pathogenesis. Single-cell transcriptomic analyses of the human PFC reveal altered expression of synaptic genes associated with one-carbon metabolism and neuronal depolarization[30,115]. These transcriptional signatures, enriched in enzymes critical to one-carbon pathways and chromatin remodeling, suggest interactions between genetic and environmental risk factors[30]. For example, folate supplementation during neurodevelopment reduces psychosis risk, while adult administration alleviates schizophrenia symptoms, highlighting the therapeutic potential of metabolic modulation[116,117]. Concurrently, stem cell-based strategies targeting GABAergic dysfunction, a consistent pathological hallmark tied to PFC microcircuit dysregulation and cognitive deficits, have shown promise[118]. Preclinical studies indicate that transplantation of PV and SST interneurons into the medial PFC rescues behavioral deficits and restores neural circuitry integrity in rodent models, suggesting that GABAergic interneuron transplantation could potentially rewire aberrant cortical networks[119,120]. Additionally, psychedelic-assisted therapies, such as psilocybin, are being explored for their ability to reset PFC connectivity and enhance neuroplasticity, with preliminary findings indicating potential benefits for mood and cognition in psychiatric disorders[121]. These advances collectively emphasize novel therapeutic avenues that bridge metabolic regulation, synaptic repair, and circuit-level restoration to address the molecular and phenotypic heterogeneity of schizophrenia. Taken together, these developments signal a shift toward precision medicine, where treatments are tailored to individual profiles of PFC dysfunction. The combination of pharmacological agents (e.g., NMDA modulators) with neuromodulation (e.g., TMS) or behavioral interventions (e.g., CRT) holds promise for synergistically addressing the heterogeneous nature of schizophrenia, offering hope for enhanced symptom management and improved quality of life (Table 1).
| Category | Interventions | Examples | Target/mechanism | Key considerations |
| Pharmacological | First-generation antipsychotics | Haloperidol | D2 receptor antagonism (mesolimbic pathway) | Reduces positive symptoms (hallucinations/delusions); minimal impact on PFC-mediated cognitive/negative symptoms |
| Second-generation antipsychotics | Risperidone, clozapine | D2 + serotonin 2A receptor modulation | Partial PFC dopaminergic enhancement; metabolic side effects (weight gain, diabetes) | |
| NMDA receptor modulators | Glycine transport inhibitors | Enhances glutamatergic signaling via NMDA co-agonism | Improves PFC excitatory-inhibitory balance; potential cognitive benefits | |
| α7 nicotinic acetylcholine receptor agonists | Varenicline | Boosts cholinergic signaling in PFC | Modest cognitive benefits; receptor desensitization remains a challenge | |
| TAAR1 agonists | Ulotaront, ralmitaront | Modulates PFC dopaminergic/glutamatergic transmission | Effective for positive, negative, and cognitive symptoms without D2-related side effects | |
| Folate supplementation | l-methylfolate | Corrects one-carbon metabolism deficits | Reduces psychosis risk/symptoms via metabolic-epigenetic interactions | |
| Non-pharmacological | Neuromodulation | Repetitive TMS, tDCS, magnetic seizure therapy, ECT, DBS | Targets PFC-subcortical circuits | Enhances PFC connectivity; variable cognitive/negative symptom efficacy in resistant cases |
| Cognitive remediation therapy | Working memory/executive function | Neuroplasticity-driven training | Improves functional outcomes; requires sustained engagement | |
| Emerging/experimental | Stem cell-based strategies | GABAergic interneuron transplantation | Restores PFC microcircuitry via interneuron grafting | Preclinical rescue of behavioral deficits; ethical/technical challenges remain |
| Psychedelic-assisted therapy | Psilocybin | Resets PFC connectivity via neuroplasticity | Potential benefits for mood/cognition; unproven in schizophrenia populations | |
| Combination approaches | Integrated therapies | Pharmacological agents + neuromodulation + behavioral interventions | Synergistic targeting of PFC dysfunction | Addressing heterogeneity may improve functional outcomes and quality of life |
While current treatment paradigms primarily focus on symptom management, characterizing the prognostic value of prefrontal structural and functional alterations in schizophrenia could revolutionize precision interventions. Gray matter reductions, particularly in the DLPFC[122-124], are consistently associated with poorer clinical and functional outcomes, including impaired cognitive control[125,126], increased hospitalization frequency, and diminished social functioning[127]. Reduced white matter integrity in the PFC, as measured by DTI, is also notably pronounced in patients with poor prognosis[128,129]. Additionally, cortical morphological features, such as prefrontal sulcal enlargement, have differential treatment implications, being linked to poor clozapine response[130-132]. Likewise, reduced gyrification in the frontal and temporal cortices correlates with poor antipsychotic response[133]. Functional neuroimaging also provides valuable insights, with a substantial body of evidence from resting-state fMRI studies indicating altered cortical connectivity as biomarkers for predicting antipsychotic efficacy[134-136]. For instance, functional dynamics within the fronto-parietal network may predict clinical improvements in negative symptoms of schizophrenia[137]. Collectively, these converging findings suggest that neuroimaging biomarkers, particularly those related to prefrontal neuroplasticity, show considerable promise in predicting treatment efficacy. However, while these biomarkers demonstrate clinical potential, individual-level validation studies are essential to establish reliable predictive frameworks for personalized treatment strategies.
PFC dysfunction is a core feature of schizophrenia, contributing to cognitive impairments, negative symptoms, and certain positive symptoms. Advances in neuroscience techniques have improved our understanding of PFC alterations, guiding the development of pharmacological, neuromodulation, and cognitive remediation strategies tailored to specific patterns of PFC dysfunction[79,89,138]. Continued research is crucial to enhance outcomes and quality of life for those affected by this debilitating disorder. Translating these insights into effective treatments remains a significant challenge. Future therapeutic approaches will likely combine pharmacological interventions targeting specific neurotransmitter systems with neuromodulation techniques and cognitive remediation, all tailored to individual PFC dysfunction profiles. Further unraveling the complexities of PFC dysfunction in schizophrenia will promote the development of interventions that address the full spectrum of symptoms, ultimately improving outcomes and quality of life for those affected by this devastating disorder.
| 1. | Velligan DI, Rao S. The Epidemiology and Global Burden of Schizophrenia. J Clin Psychiatry. 2023;84:MS21078COM5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 84] [Reference Citation Analysis (0)] |
| 2. | Correll CU, Schooler NR. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr Dis Treat. 2020;16:519-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 547] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 3. | Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 469] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Noda Y, Barr MS, Zomorrodi R, Cash RFH, Farzan F, Rajji TK, Chen R, Daskalakis ZJ, Blumberger DM. Evaluation of short interval cortical inhibition and intracortical facilitation from the dorsolateral prefrontal cortex in patients with schizophrenia. Sci Rep. 2017;7:17106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Webler RD, Hamady C, Molnar C, Johnson K, Bonilha L, Anderson BS, Bruin C, Bohning DE, George MS, Nahas Z. Decreased interhemispheric connectivity and increased cortical excitability in unmedicated schizophrenia: A prefrontal interleaved TMS fMRI study. Brain Stimul. 2020;13:1467-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2033] [Cited by in RCA: 1887] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 7. | Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 382] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Torrisi SA, Laudani S, Contarini G, De Luca A, Geraci F, Managò F, Papaleo F, Salomone S, Drago F, Leggio GM. Dopamine, Cognitive Impairments and Second-Generation Antipsychotics: From Mechanistic Advances to More Personalized Treatments. Pharmaceuticals (Basel). 2020;13:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19:15-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 418] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 10. | Osugo M, Wall MB, Selvaggi P, Zahid U, Finelli V, Chapman GE, Whitehurst T, Onwordi EC, Statton B, McCutcheon RA, Murray RM, Marques TR, Mehta MA, Howes OD. Striatal dopamine D2/D3 receptor regulation of human reward processing and behaviour. Nat Commun. 2025;16:1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Catts VS, Lai YL, Weickert CS, Weickert TW, Catts SV. A quantitative review of the postmortem evidence for decreased cortical N-methyl-D-aspartate receptor expression levels in schizophrenia: How can we link molecular abnormalities to mismatch negativity deficits? Biol Psychol. 2016;116:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Terry-Lorenzo RT, Fan RH, Khin NA, Singh JB. Therapeutic potential of D-amino acid oxidase inhibitors for cognitive impairment associated with schizophrenia: learnings from luvadaxistat. Int J Neuropsychopharmacol. 2024;28:pyae066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 13. | Nakazawa K, Sapkota K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther. 2020;205:107426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 14. | Javitt DC. Glycine transport inhibitors and the treatment of schizophrenia. Biol Psychiatry. 2008;63:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1540] [Cited by in RCA: 1849] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 16. | Tanqueiro SR, Mouro FM, Ferreira CB, Freitas CF, Fonseca-Gomes J, Simões do Couto F, Sebastião AM, Dawson N, Diógenes MJ. Sustained NMDA receptor hypofunction impairs brain-derived neurotropic factor signalling in the PFC, but not in the hippocampus, and disturbs PFC-dependent cognition in mice. J Psychopharmacol. 2021;35:730-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Carlsson A, Waters N, Carlsson ML. Neurotransmitter interactions in schizophrenia-therapeutic implications. Eur Arch Psychiatry Clin Neurosci. 1999;249 Suppl 4:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Gleich T, Deserno L, Lorenz RC, Boehme R, Pankow A, Buchert R, Kühn S, Heinz A, Schlagenhauf F, Gallinat J. Prefrontal and Striatal Glutamate Differently Relate to Striatal Dopamine: Potential Regulatory Mechanisms of Striatal Presynaptic Dopamine Function? J Neurosci. 2015;35:9615-9621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 2013;3:e238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 300] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 20. | Chini M, Hanganu-Opatz IL. Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends Neurosci. 2021;44:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 21. | Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci. 2013;7:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Nakajima M, Halassa MM. Thalamic control of functional cortical connectivity. Curr Opin Neurobiol. 2017;44:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 23. | Gao WJ, Yang SS, Mack NR, Chamberlin LA. Aberrant maturation and connectivity of prefrontal cortex in schizophrenia-contribution of NMDA receptor development and hypofunction. Mol Psychiatry. 2022;27:731-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Nakazawa K, Jeevakumar V, Nakao K. Spatial and temporal boundaries of NMDA receptor hypofunction leading to schizophrenia. NPJ Schizophr. 2017;3:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res. 2015;167:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 26. | Buck SA, Quincy Erickson-Oberg M, Logan RW, Freyberg Z. Relevance of interactions between dopamine and glutamate neurotransmission in schizophrenia. Mol Psychiatry. 2022;27:3583-3591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18:727-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 359] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 28. | Jaffe AE, Shin J, Collado-Torres L, Leek JT, Tao R, Li C, Gao Y, Jia Y, Maher BJ, Hyde TM, Kleinman JE, Weinberger DR. Developmental regulation of human cortex transcription and its clinical relevance at single base resolution. Nat Neurosci. 2015;18:154-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | Birnbaum R, Jaffe AE, Hyde TM, Kleinman JE, Weinberger DR. Prenatal expression patterns of genes associated with neuropsychiatric disorders. Am J Psychiatry. 2014;171:758-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Ruzicka WB, Mohammadi S, Fullard JF, Davila-Velderrain J, Subburaju S, Tso DR, Hourihan M, Jiang S, Lee HC, Bendl J; PsychENCODE Consortium§, Voloudakis G, Haroutunian V, Hoffman GE, Roussos P, Kellis M; PsychENCODE Consortium. Single-cell multi-cohort dissection of the schizophrenia transcriptome. Science. 2024;384:eadg5136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 31. | Li M, Santpere G, Imamura Kawasawa Y, Evgrafov OV, Gulden FO, Pochareddy S, Sunkin SM, Li Z, Shin Y, Zhu Y, Sousa AMM, Werling DM, Kitchen RR, Kang HJ, Pletikos M, Choi J, Muchnik S, Xu X, Wang D, Lorente-Galdos B, Liu S, Giusti-Rodríguez P, Won H, de Leeuw CA, Pardiñas AF; BrainSpan Consortium; PsychENCODE Consortium; PsychENCODE Developmental Subgroup, Hu M, Jin F, Li Y, Owen MJ, O'Donovan MC, Walters JTR, Posthuma D, Reimers MA, Levitt P, Weinberger DR, Hyde TM, Kleinman JE, Geschwind DH, Hawrylycz MJ, State MW, Sanders SJ, Sullivan PF, Gerstein MB, Lein ES, Knowles JA, Sestan N. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science. 2018;362:eaat7615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 546] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 32. | Maussion G, Diallo AB, Gigek CO, Chen ES, Crapper L, Théroux JF, Chen GG, Vasuta C, Ernst C. Investigation of genes important in neurodevelopment disorders in adult human brain. Hum Genet. 2015;134:1037-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Murray RM, Bhavsar V, Tripoli G, Howes O. 30 Years on: How the Neurodevelopmental Hypothesis of Schizophrenia Morphed Into the Developmental Risk Factor Model of Psychosis. Schizophr Bull. 2017;43:1190-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 34. | Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2579] [Cited by in RCA: 2409] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 35. | Raymann S, Schalbetter SM, Schaer R, Bernhardt AC, Mueller FS, Meyer U, Weber-Stadlbauer U. Late prenatal immune activation in mice induces transgenerational effects via the maternal and paternal lineages. Cereb Cortex. 2023;33:2273-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 476] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 37. | Tsai SJ. Role of interleukin 8 in depression and other psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 38. | Tendilla-Beltrán H, Sanchez-Islas NDC, Marina-Ramos M, Leza JC, Flores G. The prefrontal cortex as a target for atypical antipsychotics in schizophrenia, lessons of neurodevelopmental animal models. Prog Neurobiol. 2021;199:101967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Carboni E, Barros VG, Ibba M, Silvagni A, Mura C, Antonelli MC. Prenatal restraint stress: an in vivo microdialysis study on catecholamine release in the rat prefrontal cortex. Neuroscience. 2010;168:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Ursini G, Punzi G, Chen Q, Marenco S, Robinson JF, Porcelli A, Hamilton EG, Mitjans M, Maddalena G, Begemann M, Seidel J, Yanamori H, Jaffe AE, Berman KF, Egan MF, Straub RE, Colantuoni C, Blasi G, Hashimoto R, Rujescu D, Ehrenreich H, Bertolino A, Weinberger DR. Convergence of placenta biology and genetic risk for schizophrenia. Nat Med. 2018;24:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 41. | Rolls ET, Cheng W, Feng J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2020;2:fcaa196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 303] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 42. | Smucny J, Dienel SJ, Lewis DA, Carter CS. Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia. Neuropsychopharmacology. 2022;47:292-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 43. | Arnsten AFT, Woo E, Yang S, Wang M, Datta D. Unusual Molecular Regulation of Dorsolateral Prefrontal Cortex Layer III Synapses Increases Vulnerability to Genetic and Environmental Insults in Schizophrenia. Biol Psychiatry. 2022;92:480-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Chadha R, Meador-Woodruff JH. Downregulated AKT-mTOR signaling pathway proteins in dorsolateral prefrontal cortex in Schizophrenia. Neuropsychopharmacology. 2020;45:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 45. | Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1549] [Cited by in RCA: 1241] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 46. | Harikumar A, Solovyeva KP, Misiura M, Iraji A, Plis SM, Pearlson GD, Turner JA, Calhoun VD. Revisiting Functional Dysconnectivity: a Review of Three Model Frameworks in Schizophrenia. Curr Neurol Neurosci Rep. 2023;23:937-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 47. | Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 522] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 48. | Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 905] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 49. | Liu J, Wen F, Yan J, Yu L, Wang F, Wang D, Zhang J, Yan C, Chu J, Li Y, Li Y, Cui Y. Gray Matter Alterations in Pediatric Schizophrenia and Obsessive-Compulsive Disorder: A Systematic Review and Meta-Analysis of Voxel-Based Morphometry Studies. Front Psychiatry. 2022;13:785547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Howes OD, Cummings C, Chapman GE, Shatalina E. Neuroimaging in schizophrenia: an overview of findings and their implications for synaptic changes. Neuropsychopharmacology. 2023;48:151-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 51. | Dabiri M, Dehghani Firouzabadi F, Yang K, Barker PB, Lee RR, Yousem DM. Neuroimaging in schizophrenia: A review article. Front Neurosci. 2022;16:1042814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 52. | Zhen Y, Zheng H, Zheng Y, Zheng Z, Yang Y, Tang S. Altered Hemispheric Asymmetry of Functional Hierarchy in Schizophrenia. Brain Sci. 2025;15:313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 53. | Gutman BA, van Erp TGM, Alpert K, Ching CRK, Isaev D, Ragothaman A, Jahanshad N, Saremi A, Zavaliangos-Petropulu A, Glahn DC, Shen L, Cong S, Alnaes D, Andreassen OA, Doan NT, Westlye LT, Kochunov P, Satterthwaite TD, Wolf DH, Huang AJ, Kessler C, Weideman A, Nguyen D, Mueller BA, Faziola L, Potkin SG, Preda A, Mathalon DH, Bustillo J, Calhoun V, Ford JM, Walton E, Ehrlich S, Ducci G, Banaj N, Piras F, Piras F, Spalletta G, Canales-Rodríguez EJ, Fuentes-Claramonte P, Pomarol-Clotet E, Radua J, Salvador R, Sarró S, Dickie EW, Voineskos A, Tordesillas-Gutiérrez D, Crespo-Facorro B, Setién-Suero E, van Son JM, Borgwardt S, Schönborn-Harrisberger F, Morris D, Donohoe G, Holleran L, Cannon D, McDonald C, Corvin A, Gill M, Filho GB, Rosa PGP, Serpa MH, Zanetti MV, Lebedeva I, Kaleda V, Tomyshev A, Crow T, James A, Cervenka S, Sellgren CM, Fatouros-Bergman H, Agartz I, Howells F, Stein DJ, Temmingh H, Uhlmann A, de Zubicaray GI, McMahon KL, Wright M, Cobia D, Csernansky JG, Thompson PM, Turner JA, Wang L. A meta-analysis of deep brain structural shape and asymmetry abnormalities in 2,833 individuals with schizophrenia compared with 3,929 healthy volunteers via the ENIGMA Consortium. Hum Brain Mapp. 2022;43:352-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 54. | Mallya AP, Deutch AY. (Micro)Glia as Effectors of Cortical Volume Loss in Schizophrenia. Schizophr Bull. 2018;44:948-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Lewis DA. The chandelier neuron in schizophrenia. Dev Neurobiol. 2011;71:118-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Moyer CE, Shelton MA, Sweet RA. Dendritic spine alterations in schizophrenia. Neurosci Lett. 2015;601:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 57. | Zhang X, Yang M, Du X, Liao W, Chen D, Fan F, Xiu M, Jia Q, Ning Y, Huang X, Wu F, Soares JC, Cao B, Wang L, Chen H. Correction: Glucose disturbances, cognitive deficits and white matter abnormalities in first-episode drug-naive schizophrenia. Mol Psychiatry. 2020;25:3454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull. 2014;40:744-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 366] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 59. | Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, Andreassen OA, Arango C, Banaj N, Bouix S, Bousman CA, Brouwer RM, Bruggemann J, Bustillo J, Cahn W, Calhoun V, Cannon D, Carr V, Catts S, Chen J, Chen JX, Chen X, Chiapponi C, Cho KK, Ciullo V, Corvin AS, Crespo-Facorro B, Cropley V, De Rossi P, Diaz-Caneja CM, Dickie EW, Ehrlich S, Fan FM, Faskowitz J, Fatouros-Bergman H, Flyckt L, Ford JM, Fouche JP, Fukunaga M, Gill M, Glahn DC, Gollub R, Goudzwaard ED, Guo H, Gur RE, Gur RC, Gurholt TP, Hashimoto R, Hatton SN, Henskens FA, Hibar DP, Hickie IB, Hong LE, Horacek J, Howells FM, Hulshoff Pol HE, Hyde CL, Isaev D, Jablensky A, Jansen PR, Janssen J, Jönsson EG, Jung LA, Kahn RS, Kikinis Z, Liu K, Klauser P, Knöchel C, Kubicki M, Lagopoulos J, Langen C, Lawrie S, Lenroot RK, Lim KO, Lopez-Jaramillo C, Lyall A, Magnotta V, Mandl RCW, Mathalon DH, McCarley RW, McCarthy-Jones S, McDonald C, McEwen S, McIntosh A, Melicher T, Mesholam-Gately RI, Michie PT, Mowry B, Mueller BA, Newell DT, O'Donnell P, Oertel-Knöchel V, Oestreich L, Paciga SA, Pantelis C, Pasternak O, Pearlson G, Pellicano GR, Pereira A, Pineda Zapata J, Piras F, Potkin SG, Preda A, Rasser PE, Roalf DR, Roiz R, Roos A, Rotenberg D, Satterthwaite TD, Savadjiev P, Schall U, Scott RJ, Seal ML, Seidman LJ, Shannon Weickert C, Whelan CD, Shenton ME, Kwon JS, Spalletta G, Spaniel F, Sprooten E, Stäblein M, Stein DJ, Sundram S, Tan Y, Tan S, Tang S, Temmingh HS, Westlye LT, Tønnesen S, Tordesillas-Gutierrez D, Doan NT, Vaidya J, van Haren NEM, Vargas CD, Vecchio D, Velakoulis D, Voineskos A, Voyvodic JQ, Wang Z, Wan P, Wei D, Weickert TW, Whalley H, White T, Whitford TJ, Wojcik JD, Xiang H, Xie Z, Yamamori H, Yang F, Yao N, Zhang G, Zhao J, van Erp TGM, Turner J, Thompson PM, Donohoe G. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 532] [Article Influence: 66.5] [Reference Citation Analysis (18)] |
| 60. | Yao L, Lui S, Liao Y, Du MY, Hu N, Thomas JA, Gong QY. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 61. | Naim-Feil J, Rubinson M, Freche D, Grinshpoon A, Peled A, Moses E, Levit-Binnun N. Altered Brain Network Dynamics in Schizophrenia: A Cognitive Electroencephalography Study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Chen P, Ye E, Jin X, Zhu Y, Wang L. Association between Thalamocortical Functional Connectivity Abnormalities and Cognitive Deficits in Schizophrenia. Sci Rep. 2019;9:2952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 63. | Ramirez-Mahaluf JP, Tepper Á, Alliende LM, Mena C, Castañeda CP, Iruretagoyena B, Nachar R, Reyes-Madrigal F, León-Ortiz P, Mora-Durán R, Ossandon T, Gonzalez-Valderrama A, Undurraga J, de la Fuente-Sandoval C, Crossley NA. Dysconnectivity in Schizophrenia Revisited: Abnormal Temporal Organization of Dynamic Functional Connectivity in Patients With a First Episode of Psychosis. Schizophr Bull. 2023;49:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 64. | Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 65. | Hoffmann J, Meller T, Maj C, Hoffmann P, Forstner AJ, Nöthen MM, Nenadić I. Differential Association of Schizotypy Dimensions With Brain Structural Connectivity and Moderation by Schizophrenia Polygenic Risk. Schizophr Bull. 2025;51:S149-S159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 66. | Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 67. | Hashimoto T, Arion D, Unger T, Maldonado-Avilés JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 419] [Cited by in RCA: 403] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 68. | Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 872] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 69. | Liang Y, Li J, Tian Y, Tang P, Liu C, Chen X. The Anterior Cingulate Cortex Promotes Long-Term Auditory Cortical Responses through an Indirect Pathway via the Rhinal Cortex in Mice. J Neurosci. 2023;43:4262-4278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 70. | Anderson MC, Hulbert JC. Active Forgetting: Adaptation of Memory by Prefrontal Control. Annu Rev Psychol. 2021;72:1-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 71. | Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 72. | Ma L, Skoblenick K, Seamans JK, Everling S. Ketamine-Induced Changes in the Signal and Noise of Rule Representation in Working Memory by Lateral Prefrontal Neurons. J Neurosci. 2015;35:11612-11622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Miller EK, Lundqvist M, Bastos AM. Working Memory 2.0. Neuron. 2018;100:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 548] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 74. | Schoonover KE, Dienel SJ, Lewis DA. Prefrontal cortical alterations of glutamate and GABA neurotransmission in schizophrenia: Insights for rational biomarker development. Biomark Neuropsychiatry. 2020;3:100015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 749] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 76. | Volk DW, Sampson AR, Zhang Y, Edelson JR, Lewis DA. Cortical GABA markers identify a molecular subtype of psychotic and bipolar disorders. Psychol Med. 2016;46:2501-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 77. | Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50:873-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 262] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 78. | Takeda T, Umehara H, Matsumoto Y, Yoshida T, Nakataki M, Numata S. Schizophrenia and cognitive dysfunction. J Med Invest. 2024;71:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 79. | Lähteenvuo M, Tiihonen J. Antipsychotic Polypharmacy for the Management of Schizophrenia: Evidence and Recommendations. Drugs. 2021;81:1273-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 80. | Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 307] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 81. | Raedler TJ. Cardiovascular aspects of antipsychotics. Curr Opin Psychiatry. 2010;23:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 82. | Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA; CATIE Investigators; Neurocognitive Working Group. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 776] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 83. | Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, Bear MF, Umbricht D, Hajos M, Potter WZ, Lee CM. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3:102mr2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 84. | Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 328] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 85. | Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipović SR, Hummel FC, Jääskeläinen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schönfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125:2150-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1225] [Cited by in RCA: 1435] [Article Influence: 119.6] [Reference Citation Analysis (0)] |
| 86. | Jiang Y, Guo Z, Xing G, He L, Peng H, Du F, McClure MA, Mu Q. Effects of High-Frequency Transcranial Magnetic Stimulation for Cognitive Deficit in Schizophrenia: A Meta-Analysis. Front Psychiatry. 2019;10:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 87. | Mehta DD, Siddiqui S, Ward HB, Steele VR, Pearlson GD, George TP. Functional and structural effects of repetitive transcranial magnetic stimulation (rTMS) for the treatment of auditory verbal hallucinations in schizophrenia: A systematic review. Schizophr Res. 2024;267:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 88. | Slotema CW, Blom JD, van Lutterveld R, Hoek HW, Sommer IE. Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol Psychiatry. 2014;76:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 89. | Blyth SH, Cruz Bosch C, Raffoul JJ, Chesley J, Johnson B, Borodge D, Sagarwala R, Masters R, Brady RO Jr, Vandekar S, Ward HB. Safety of rTMS for Schizophrenia: A Systematic Review and Meta-analysis. Schizophr Bull. 2025;51:392-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 90. | Palm U, Segmiller FM, Epple AN, Freisleder FJ, Koutsouleris N, Schulte-Körne G, Padberg F. Transcranial direct current stimulation in children and adolescents: a comprehensive review. J Neural Transm (Vienna). 2016;123:1219-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 91. | Cheng PWC, Louie LLC, Wong YL, Wong SMC, Leung WY, Nitsche MA, Chan WC. The effects of transcranial direct current stimulation (tDCS) on clinical symptoms in schizophrenia: A systematic review and meta-analysis. Asian J Psychiatr. 2020;53:102392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 92. | Sanghani SN, Petrides G, Kellner CH. Electroconvulsive therapy (ECT) in schizophrenia: a review of recent literature. Curr Opin Psychiatry. 2018;31:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 93. | Davarinejad O, Hendesi K, Shahi H, Brand S, Khazaie H. A Pilot Study on Daily Intensive ECT over 8 Days Improved Positive and Negative Symptoms and General Psychopathology of Patients with Treatment-Resistant Schizophrenia up to 4 Weeks after Treatment. Neuropsychobiology. 2019;77:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Wang Y, Zhang X. The role of immune inflammation in electroconvulsive therapy for schizophrenia: Treatment mechanism, and relationship with clinical efficacy: Immune-inflammation in ECT for schizophrenia. Psychiatry Res. 2024;332:115708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 95. | Liu Y, Jia LN, Wu H, Jiang W, Wang Q, Wang D, Xiong YB, Ren YP, Ma X, Tang YL. Adjuvant electroconvulsive therapy with antipsychotics is associated with improvement in auditory mismatch negativity in schizophrenia. Psychiatry Res. 2022;311:114484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 96. | Porter RJ, Baune BT, Morris G, Hamilton A, Bassett D, Boyce P, Hopwood MJ, Mulder R, Parker G, Singh AB, Outhred T, Das P, Malhi GS. Cognitive side-effects of electroconvulsive therapy: what are they, how to monitor them and what to tell patients. BJPsych Open. 2020;6:e40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 97. | Tang VM, Blumberger DM, McClintock SM, Kaster TS, Rajji TK, Downar J, Fitzgerald PB, Daskalakis ZJ. Magnetic Seizure Therapy in Treatment-Resistant Schizophrenia: A Pilot Study. Front Psychiatry. 2017;8:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Tang VM, Blumberger DM, Throop A, McClintock SM, Voineskos D, Downar J, Knyahnytska Y, Mulsant BH, Fitzgerald PB, Daskalakis ZJ. Continuation Magnetic Seizure Therapy for Treatment-Resistant Unipolar or Bipolar Depression. J Clin Psychiatry. 2021;82:20m13677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Zhang XY, Chen HD, Liang WN, Yang XH, Cai DB, Huang X, Huang XB, Liu CY, Zheng W. Adjunctive Magnetic Seizure Therapy for Schizophrenia: A Systematic Review. Front Psychiatry. 2021;12:813590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Hasan A, Wolff-Menzler C, Pfeiffer S, Falkai P, Weidinger E, Jobst A, Hoell I, Malchow B, Yeganeh-Doost P, Strube W, Quast S, Müller N, Wobrock T. Transcutaneous noninvasive vagus nerve stimulation (tVNS) in the treatment of schizophrenia: a bicentric randomized controlled pilot study. Eur Arch Psychiatry Clin Neurosci. 2015;265:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 101. | Corripio I, Roldán A, Sarró S, McKenna PJ, Alonso-Solís A, Rabella M, Díaz A, Puigdemont D, Pérez-Solà V, Álvarez E, Arévalo A, Padilla PP, Ruiz-Idiago JM, Rodríguez R, Molet J, Pomarol-Clotet E, Portella MJ. Deep brain stimulation in treatment resistant schizophrenia: A pilot randomized cross-over clinical trial. EBioMedicine. 2020;51:102568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 102. | Mikell CB, Sinha S, Sheth SA. Neurosurgery for schizophrenia: an update on pathophysiology and a novel therapeutic target. J Neurosurg. 2016;124:917-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 103. | Daskalakis ZJ, Möller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 104. | Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol. 2013;124:1309-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 105. | Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1159] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 106. | Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34:523-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 729] [Cited by in RCA: 637] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 107. | McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 769] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 108. | Espinoza S, Lignani G, Caffino L, Maggi S, Sukhanov I, Leo D, Mus L, Emanuele M, Ronzitti G, Harmeier A, Medrihan L, Sotnikova TD, Chieregatti E, Hoener MC, Benfenati F, Tucci V, Fumagalli F, Gainetdinov RR. TAAR1 Modulates Cortical Glutamate NMDA Receptor Function. Neuropsychopharmacology. 2015;40:2217-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 109. | John J, Kukshal P, Bhatia T, Chowdari KV, Nimgaonkar VL, Deshpande SN, Thelma BK. Possible role of rare variants in Trace amine associated receptor 1 in schizophrenia. Schizophr Res. 2017;189:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 110. | Mühlhaus J, Dinter J, Jyrch S, Teumer A, Jacobi SF, Homuth G, Kühnen P, Wiegand S, Grüters A, Völzke H, Raile K, Kleinau G, Krude H, Biebermann H. Investigation of Naturally Occurring Single-Nucleotide Variants in Human TAAR1. Front Pharmacol. 2017;8:807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Espinoza S, Ghisi V, Emanuele M, Leo D, Sukhanov I, Sotnikova TD, Chieregatti E, Gainetdinov RR. Postsynaptic D2 dopamine receptor supersensitivity in the striatum of mice lacking TAAR1. Neuropharmacology. 2015;93:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 112. | Sukhanov I, Caffino L, Efimova EV, Espinoza S, Sotnikova TD, Cervo L, Fumagalli F, Gainetdinov RR. Increased context-dependent conditioning to amphetamine in mice lacking TAAR1. Pharmacol Res. 2016;103:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 113. | Halff EF, Rutigliano G, Garcia-Hidalgo A, Howes OD. Trace amine-associated receptor 1 (TAAR1) agonism as a new treatment strategy for schizophrenia and related disorders. Trends Neurosci. 2023;46:60-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 114. | Heffernan MLR, Herman LW, Brown S, Jones PG, Shao L, Hewitt MC, Campbell JE, Dedic N, Hopkins SC, Koblan KS, Xie L. Ulotaront: A TAAR1 Agonist for the Treatment of Schizophrenia. ACS Med Chem Lett. 2022;13:92-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 115. | Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu Rev Anim Biosci. 2019;7:263-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 116. | Eryilmaz H, Dowling KF, Huntington FC, Rodriguez-Thompson A, Soare TW, Beard LM, Lee H, Blossom JC, Gollub RL, Susser E, Gur RC, Calkins ME, Gur RE, Satterthwaite TD, Roffman JL. Association of Prenatal Exposure to Population-Wide Folic Acid Fortification With Altered Cerebral Cortex Maturation in Youths. JAMA Psychiatry. 2018;75:918-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 117. | Roffman JL, Petruzzi LJ, Tanner AS, Brown HE, Eryilmaz H, Ho NF, Giegold M, Silverstein NJ, Bottiglieri T, Manoach DS, Smoller JW, Henderson DC, Goff DC. Biochemical, physiological and clinical effects of l-methylfolate in schizophrenia: a randomized controlled trial. Mol Psychiatry. 2018;23:316-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 118. | Jahangir M, Zhou JS, Lang B, Wang XP. GABAergic System Dysfunction and Challenges in Schizophrenia Research. Front Cell Dev Biol. 2021;9:663854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 119. | Donegan JJ, Tyson JA, Branch SY, Beckstead MJ, Anderson SA, Lodge DJ. Stem cell-derived interneuron transplants as a treatment for schizophrenia: preclinical validation in a rodent model. Mol Psychiatry. 2017;22:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 120. | Perez SM, Boley A, Lodge DJ. Region specific knockdown of Parvalbumin or Somatostatin produces neuronal and behavioral deficits consistent with those observed in schizophrenia. Transl Psychiatry. 2019;9:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 121. | Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, Bloomfield M, Rickard JA, Forbes B, Feilding A, Taylor D, Pilling S, Curran VH, Nutt DJ. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. 2016;3:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 974] [Article Influence: 97.4] [Reference Citation Analysis (1)] |
| 122. | Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63:759-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 123. | Zhang Y, Catts VS, Sheedy D, McCrossin T, Kril JJ, Shannon Weickert C. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry. 2016;6:e982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 124. | Arango C, Rapado-Castro M, Reig S, Castro-Fornieles J, González-Pinto A, Otero S, Baeza I, Moreno C, Graell M, Janssen J, Parellada M, Moreno D, Bargalló N, Desco M. Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry. 2012;69:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 125. | Chen Z, Deng W, Gong Q, Huang C, Jiang L, Li M, He Z, Wang Q, Ma X, Wang Y, Chua SE, McAlonan GM, Sham PC, Collier DA, McGuire P, Li T. Extensive brain structural network abnormality in first-episode treatment-naive patients with schizophrenia: morphometrical and covariation study. Psychol Med. 2014;44:2489-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 126. | Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 127. | Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 128. | Mitelman SA, Newmark RE, Torosjan Y, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. White matter fractional anisotropy and outcome in schizophrenia. Schizophr Res. 2006;87:138-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 129. | Dazzan P. Neuroimaging biomarkers to predict treatment response in schizophrenia: the end of 30 years of solitude? Dialogues Clin Neurosci. 2014;16:491-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 130. | Pigoni A, Delvecchio G, Dusi N, Schiena G, Andreella A, Finos L, Cecchetto F, Perlini C, Gloria Rossetti M, Ferro A, Bellani M, Lasalvia A, Ruggeri M, Brambilla P; PICOS and First projects. Insula volumes in first-episode and chronic psychosis: A longitudinal MRI study. Schizophr Res. 2022;241:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 131. | Suzuki T, Uchida H, Watanabe K, Kashima H. Factors associated with response to clozapine in schizophrenia: a review. Psychopharmacol Bull. 2011;44:32-60. [PubMed] |
| 132. | Pang TSW, Chun JSW, Wong TY, Chu ST, Ma CF, Honer WG, Chan SKW. A systematic review of neuroimaging studies of clozapine-resistant schizophrenia. Schizophrenia (Heidelb). 2023;9:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 133. | Palaniyappan L, Marques TR, Taylor H, Handley R, Mondelli V, Bonaccorso S, Giordano A, McQueen G, DiForti M, Simmons A, David AS, Pariante CM, Murray RM, Dazzan P. Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013;70:1031-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 134. | Hadley JA, Kraguljac NV, White DM, Ver Hoef L, Tabora J, Lahti AC. Change in brain network topology as a function of treatment response in schizophrenia: a longitudinal resting-state fMRI study using graph theory. NPJ Schizophr. 2016;2:16014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 135. | Kraguljac NV, White DM, Hadley JA, Visscher K, Knight D, ver Hoef L, Falola B, Lahti AC. Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. Neuroimage Clin. 2016;10:146-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 136. | Kraguljac NV, White DM, Hadley N, Hadley JA, Ver Hoef L, Davis E, Lahti AC. Aberrant Hippocampal Connectivity in Unmedicated Patients With Schizophrenia and Effects of Antipsychotic Medication: A Longitudinal Resting State Functional MRI Study. Schizophr Bull. 2016;42:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 137. | Nejad AB, Madsen KH, Ebdrup BH, Siebner HR, Rasmussen H, Aggernæs B, Glenthøj BY, Baaré WF. Neural markers of negative symptom outcomes in distributed working memory brain activity of antipsychotic-naive schizophrenia patients. Int J Neuropsychopharmacol. 2013;16:1195-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 138. | Fitapelli B, Lindenmayer JP. Advances in Cognitive Remediation Training in Schizophrenia: A Review. Brain Sci. 2022;12:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/