Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.109273
Revised: May 26, 2025
Accepted: August 1, 2025
Published online: October 19, 2025
Processing time: 143 Days and 4.7 Hours
This letter critically evaluates the study by Yue et al investigating the association between gray matter volume (GMV) and cognitive/motor function in amnestic mild cognitive impairment (aMCI). Yue et al utilized voxel-based morphometry (VBM) and comprehensive functional assessments, finding significant GMV reductions in aMCI patients compared to controls, notably in temporal, parietal, occipital, and frontal regions. These structural changes correlated significantly with lower cognitive scores (mini-metal state examination, cambridge cognitive examination-Chinese version, activities of daily living) and impaired gait pa
Core Tip: Yue et al's study links reduced gray matter volume (GMV) in specific brain regions (temporal, frontal, occipital, parietal) to both cognitive decline and impaired motor function (gait disturbances) in patients with amnestic mild cognitive impairment (aMCI). Utilizing voxel-based morphometry, the study found correlations between regional atrophy and performance on tests like mini-metal state examination, Cambridge cognitive examination-Chinese version, timed up and go test, and walking speed. This reinforces GMV's potential as an aMCI biomarker and supports the idea of shared brain networks for cognition and motor control. However, the cross-sectional design prevents establishing causality. Future longitudinal research is essential to clarify the temporal relationship between brain atrophy and functional decline in aMCI.
- Citation: Byeon H. Structural brain correlates of neuropsychomotor performance in older adults with early cognitive decline. World J Psychiatry 2025; 15(10): 109273
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/109273.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.109273
Mild cognitive impairment (MCI) represents a critical transitional stage between the cognitive changes of normal aging and the more severe decline characteristic of dementia, particularly Alzheimer's disease (AD)[1]. Among MCI subtypes, amnestic MCI (aMCI), primarily characterized by episodic memory deficits, is considered a significant precursor to AD, with a substantial proportion of individuals progressing to dementia within years[1]. Understanding the neurobiological underpinnings of aMCI is paramount for early detection, prognostic prediction, and the development of effective therapeutic interventions aimed at delaying or preventing the onset of AD[2,3].
While cognitive decline, especially memory impairment, is the hallmark of aMCI, there is growing recognition that subtle motor dysfunction, particularly changes in gait and balance, often co-occurs and may even precede overt cognitive symptoms[4-6]. Gait disturbances in older adults are associated with increased risks of falls, functional dependence, reduced quality of life, and increased mortality[7,8]. Accumulating evidence suggests that cognitive and motor functions are not entirely separate domains but rely on overlapping neural networks, including prefrontal cortex, parietal regions, and subcortical structures involved in executive functions, attention, and sensorimotor integration[9,10]. This concept of a shared underlying pathophysiology implies that investigating structural brain changes in relation to both cognitive and motor deficits in aMCI can provide a more holistic understanding of the neurodegenerative process.
Neuroimaging techniques, particularly magnetic resonance imaging (MRI), offer powerful tools to investigate brain structure alterations associated with MCI. Numerous studies have consistently demonstrated the utility of MRI in detecting structural abnormalities and predicting the progression of cognitive impairment[4,11]. Voxel-based morphometry (VBM) allows for an objective, automated, whole-brain analysis of gray matter volume (GMV) differences between groups and correlation with clinical variables. Previous studies have consistently reported GMV reductions in medial temporal lobe structures, such as the hippocampus and entorhinal cortex, in aMCI[11]. However, the extent of GMV changes in other cortical regions and their specific relationship with both cognitive and motor performance in aMCI warrants further investigation.
In this context, the recent study by Yue et al[2] makes a timely and valuable contribution. The authors aimed to investigate the association between regional GMV, assessed using VBM, and performance on a comprehensive battery of cognitive and motor function tests in a well-characterized cohort of aMCI patients compared to normal controls (NC). This letter aims to provide a critical appraisal of the study by Yue et al[2], evaluating its methodology, findings, strengths, and limitations, and discussing its implications for clinical practice and future research directions in the field of MCI and neurodegenerative diseases.
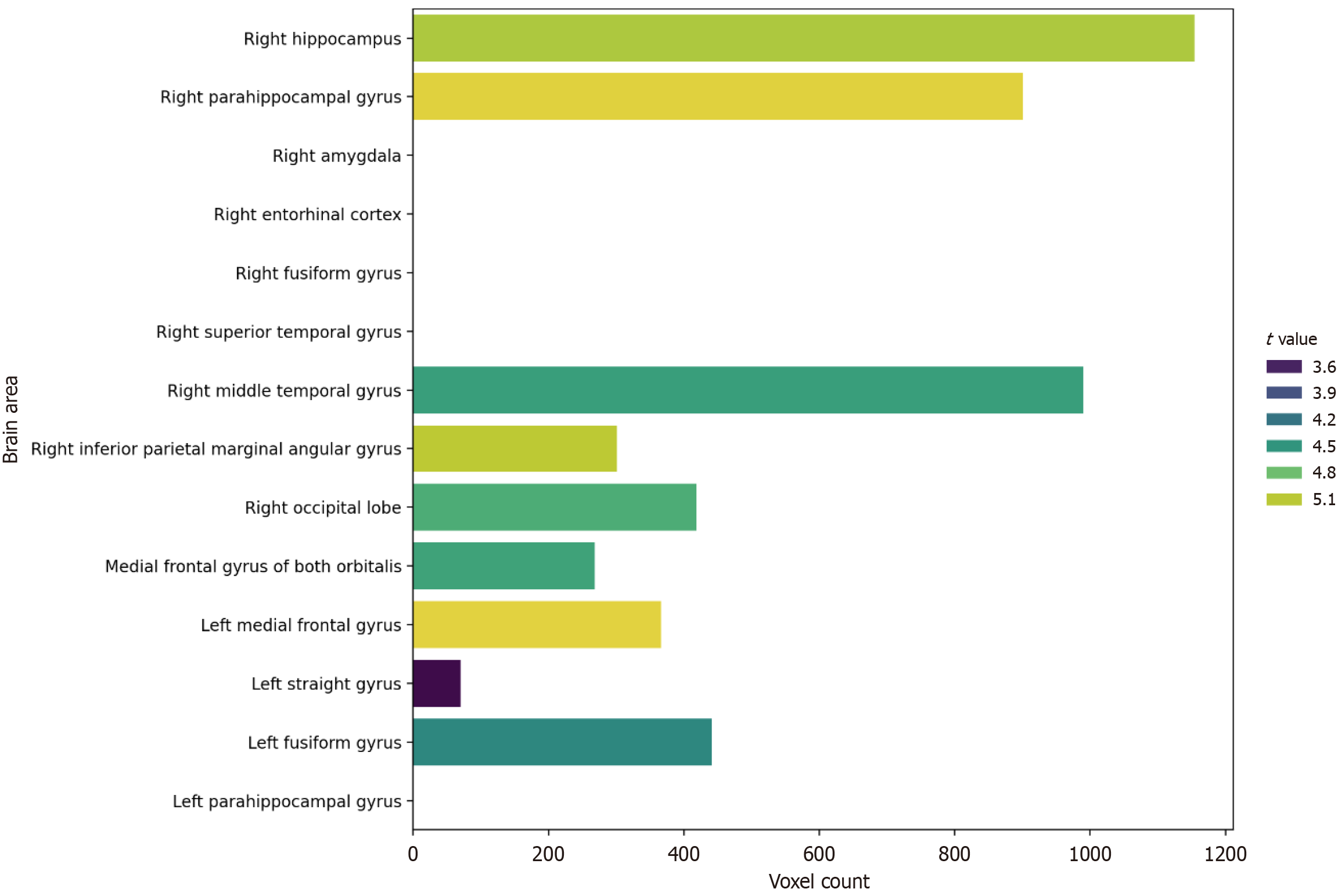
The findings presented by Yue et al[2] align well with and extend the existing body of literature on structural brain changes and functional decline in MCI and AD. The observation of significant GMV reductions in the aMCI group compared to controls, particularly in medial temporal lobe structures like the hippocampus, parahippocampal gyrus, and entorhinal cortex [captured within their regions of interest (ROIs) 1], is a well-established finding and considered a hallmark of early AD pathology[11,12]. These regions are crucial for episodic memory, explaining the characteristic memory deficits in aMCI.
Yue et al's study, however, contributes beyond confirming these known associations[2]. By employing whole-brain VBM, they identified GMV reductions in additional regions, including the middle temporal gyrus (ROI2), inferior parietal angular gyrus (ROI3), occipital lobe (ROI4), bilateral orbital frontal regions (ROI5), and fusiform gyrus (ROI1, ROI7). This pattern of more widespread atrophy, extending beyond the medial temporal lobe, is consistent with the understanding that AD pathology progresses along specific neural networks[11,13]. The involvement of parietal regions is often linked to visuospatial deficits, while frontal lobe changes are associated with executive dysfunction, both of which can manifest in later stages of MCI or early AD. The novel finding of occipital lobe GMV reduction (ROI4) is particularly interesting, as this region is primarily involved in visual processing but also connects with parietal and frontal areas involved in visuospatial attention and executive control, potentially contributing to difficulties in navigating complex environments or performing visually guided motor tasks[14]. Potential mechanisms underlying occipital GMV reduction may involve impaired visuospatial integration and deficits in visual-motor coordination, critical for performing cognitive-motor tasks. Moreover, previous evidence indicates that occipital GMV alterations have also been reported in various ophthalmologic conditions and other brain disorders involving visual processing regions[4,14]. Therefore, future studies are needed to clarify whether these occipital GMV reductions are specifically associated with aMCI or represent broader neuroimaging features common to disorders affecting the visual system.
Crucially, the study links these structural changes to functional performance. The correlation between GMV in temporal and frontal regions (ROI1, ROI2, ROI6) and cognitive scores [mini-metal state examination (MMSE), Cambridge cognitive examination-Chinese version (CAMCOG-C), activities of daily living (ADL)] reinforces the structure-function relationship widely reported in the literature[12]. The more novel contribution lies in linking specific GMV reductions to detailed gait parameters. The negative correlation between left superior occipital gyrus GMV and timed up and go test (TUG) duration, and the positive correlation between frontal (straight gyrus, orbital medial frontal gyrus) and occipital (tabloid fissure, supraoccipital gyrus) GMV with walking speed, provide neuroanatomical correlates for gait disturbances in aMCI. This supports the growing body of evidence suggesting that gait is not merely a simple motor task but requires significant cognitive input, particularly from prefrontal (planning, executive control) and parietal/occipital (visuospatial processing, navigation) regions[9,10,15]. The finding that CAMCOG-C scores negatively correlated with dual task timed up and go test (D-TUG) duration further strengthens the concept of cognitive-motor interference, where compromised cognitive resources (assessed by CAMCOG-C) directly impact the ability to perform a complex motor task while concurrently performing a cognitive task (D-TUG)[5,16].
Compared to previous studies[7], Yue et al's work benefits from the simultaneous, detailed assessment of both cognitive function across multiple domains and quantitative gait parameters (beyond simple TUG time), correlated with whole-brain VBM analysis[2]. While some studies have linked gait speed to global brain atrophy or white matter changes[7], this study provides more specific regional GMV associations with various gait components (speed, TUG, D-TUG) within an aMCI cohort, adding valuable granularity to our understanding.
Yue et al[2] employed a cross-sectional case-control study design to investigate the relationship between GMV and both cognitive and motor functions in individuals with aMCI compared to NC. This design is suitable for exploring group differences and identifying associations between variables at a single time point. The study recruited 45 aMCI patients and 45 age and education-matched NCs, adhering to established diagnostic criteria (Petersen criteria) for aMCI, which strengthens the validity of group comparisons.
A key methodological strength is the use of VBM, an objective and automated neuroimaging technique, to assess GMV across the entire brain. This approach allows for the precise quantification of regional gray matter differences without a priori assumptions about specific ROIs, potentially revealing subtle or unexpected structural alterations compared to ROI-based methods. Furthermore, the authors conducted a comprehensive functional assessment, encompassing standardized neuropsychological tests covering multiple cognitive domains (MMSE, CAMCOG-C, ADL) and a detailed motor function evaluation including basic balance, timed mobility (TUG), dual-task performance (D-TUG), and quantitative gait analysis (step length, speed, frequency). This concurrent assessment of both cognitive and motor domains is a significant advantage, enabling the exploration of their potential interplay in aMCI.
The study's main findings largely align with existing literature while adding valuable details. As expected, the aMCI group exhibited significant GMV reductions compared to the NC group in multiple brain regions. These included not only the hallmark medial temporal lobe structures implicated in early AD pathology (hippocampus, parahippocampal gyrus, entorhinal cortex, amygdala ROI1 and parts of ROI7) but also extended to other cortical areas such as the middle temporal gyrus (ROI2), inferior parietal angular gyrus (ROI3), occipital lobe (ROI4), bilateral orbitofrontal cortex (ROI5), and fusiform gyrus (ROI1, ROI7) (Figure 1). This pattern supports the model of AD pathology originating in the medial temporal lobe and gradually spreading to interconnected cortical networks[11,12]. The identification of significant GMV loss in occipital and orbitofrontal regions is particularly noteworthy, implicating areas involved in visual processing, spatial navigation, and executive functions, which are increasingly recognized as relevant to gait control and overall functioning in MCI/AD[9,14].
Crucially, the study established significant correlations between regional GMV and functional measures. GMV in medial temporal (ROI1, ROI2) and frontal (ROI6) regions showed expected negative correlations with ADL scores (lower ADL indicates poorer function) and positive correlations (ROI6) with global cognitive scores (MMSE, CAMCOG-C) (Figure 2A), reinforcing the link between atrophy in these key areas and cognitive/daily living impairment. Regarding motor function, orbitofrontal GMV (ROI5) positively correlated with walking speed (Figure 2B). Whole-brain linear regression further linked GMV in specific areas like the left superior occipital gyrus (negatively with TUG time) and bilateral frontal straight gyri/right orbitofrontal cortex/occipital regions (positively with walking speed). Moreover, the partial correlation analysis revealing a negative association between CAMCOG-C scores and D-TUG duration (Figure 2C) directly supports the concept of cognitive-motor interference, where reduced cognitive resources impact performance on complex motor tasks[5,16].
Collectively, these findings robustly demonstrate that GMV reduction in aMCI is associated not only with cognitive deficits but also closely linked to motor impairments, particularly gait dysfunction. The implicated frontal, occipital, and central regions align with neural circuits known to be involved in gait regulation, supporting theories of shared neural substrates for cognition and motor control[4].
In addition to examining gray matter structural abnormalities, exploring intrinsic brain connectivity networks offers valuable insights into potential mechanisms underlying cognitive and motor impairments in aMCI. Recent studies utilizing resting-state functional connectivity analyses indicate that disruptions in intrinsic connectivity networks significantly correlate with the cognitive and motor deficits observed in MCI and early-stage AD[2,10]. Integrating structural MRI findings with functional connectivity data could thus provide a comprehensive framework for understanding the relationship between anatomical changes and functional impairments. Future research employing multimodal neuroimaging techniques is warranted to validate these connections and elucidate their mechanistic significance in aMCI.
The statistical methodology, involving appropriate group comparison tests and partial correlation analyses controlling for relevant covariates (age, sex, education, height, weight), adheres to standard practices. The use of linear regression for whole-brain analyses with appropriate correction for multiple comparisons adds rigor. However, interpreting the strength and clinical significance of the reported correlations requires caution. While statistically significant, the magnitude of these correlations might be moderate, potentially limiting the predictive value of GMV in a specific region for an individual's functional score. While the univariate analysis approach employed in this study effectively identified significant associations between regional GMV and functional measures, it might not fully capture the complexity of multivariate brain-behavior relationships. Multivariate correlation analyses have emerged as more sophisticated approaches that can potentially reveal complex interactions between multiple brain regions and diverse clinical outcomes. However, it is important to acknowledge that multivariate methods can also inflate reported effect sizes. Future research should consider incorporating multivariate techniques to enhance the robustness and clinical relevance of identified biomarkers, while remaining mindful of methodological challenges[3,7].
The discussion appropriately contextualizes the findings within models of AD progression and highlights the consistency with existing literature. The interpretation linking specific regional atrophy (e.g., occipital, frontal) to functional impairments (e.g., visuospatial, executive functions impacting gait) is neuroanatomically plausible. Acknowledging the overlap in neural networks controlling cognition and motor function is a key interpretive strength. Nevertheless, conclusions regarding the "key roles" of specific brain regions in gait disorders should be tempered by the study's correlational nature. While these regions are clearly implicated, the design cannot establish causality or their relative importance compared to other potential contributing factors (e.g., white matter integrity, subcortical involvement, peripheral factors). The accurate conclusion is that GMV reduction correlates with impaired function; implying a direct causal or strongly predictive relationship solely based on this cross-sectional data would be an overstatement.
This study possesses several notable strengths. First, the use of objective neuroimaging techniques, specifically VBM, allowed for the quantitative assessment of GMV across the entire brain. This approach minimized researcher bias and enabled sensitive detection of localized GMV changes. Second, a comprehensive evaluation of both cognitive function (including various domains) and motor function (including balance, gait speed, and dual-task gait) was conducted. This detailed assessment facilitated a multifaceted analysis of structure-function relationships. Third, by revealing associations not only with cognitive decline but also with motor function deficits, the study highlighted the heterogeneity of aMCI, emphasizing that it may represent a multifaceted neurodegenerative process rather than solely a syndrome of memory impairment. Fourth, the finding that GMV in specific brain regions was significantly associated with cognitive and motor functions suggests that GMV could potentially serve as an imaging biomarker for the diagnosis, prognosis prediction, or monitoring of treatment response in aMCI.
Despite these strengths, the study is subject to several limitations. First and foremost, the cross-sectional design represents a significant limitation as it precludes the establishment of causality between GMV reduction and functional decline. It is not possible to determine whether reduced GMV leads to functional impairment, whether functional impairment contributes to structural brain changes (e.g., disuse atrophy), or whether a third underlying factor influences both. Longitudinal studies are essential to elucidate temporal relationships. Second, the sample size of 45 participants per group, while potentially reasonable for neuroimaging studies, may limit the statistical power and generalizability of the findings. Furthermore, participants were recruited from a single institutional setting (Geriatrics department), which restricts the generalizability of the results to other populations (e.g., community-dwelling older adults, different racial/ethnic groups). Third, although efforts were made to control for variables such as age, sex, and education level, other potential confounding factors that could influence both GMV and function, such as vascular risk factors, microvascular disease (e.g., white matter hyperintensities), AD and apolipoprotein E (APOE) genotype, undiagnosed psychiatric conditions, and medication use, may not have been fully accounted for. Fourth, VBM, while a powerful technique, has inherent technical limitations. Results can vary depending on image preprocessing steps, brain normalization, and the choice of smoothing kernel size, and registration errors can occur, particularly in brains with significant atrophy. Fifth, the assessment of motor function was conducted in a laboratory setting, lacking more ecologically valid measures of real-world gait patterns or fall history in daily life.
Despite its limitations, the study by Yue et al[2] offers several important clinical implications and guides future research. Clinically, the findings reinforce the need for a comprehensive assessment of aMCI patients that includes not only cognitive testing but also evaluation of motor function, particularly gait and balance. Gait disturbances may serve as an early indicator of underlying neurodegeneration and increased fall risk, warranting proactive management strategies. The association between specific regional GMV atrophy (potentially detectable on clinical MRI scans, although VBM is a research tool) and functional deficits suggests that structural imaging could potentially contribute to prognostic assessments, although more research is needed to establish its predictive utility at the individual level. Furthermore, the link between cognitive performance (CAMCOG-C) and dual-task gait (D-TUG) highlights the potential benefit of interventions like dual-task training, which simultaneously challenge cognitive and motor systems, potentially improving both domains[17].
Future research should prioritize longitudinal studies to track GMV changes and cognitive/motor decline over time, clarifying the temporal sequence and predictive value of brain atrophy. Incorporating multi-modal neuroimaging, including fMRI (to assess functional connectivity within cognitive-motor networks), diffusion tensor imaging (to evaluate white matter tract integrity connecting relevant regions), and positron emission tomography (to measure amyloid and tau pathology), would provide a more comprehensive understanding of the underlying pathophysiology. Research employing more ecologically valid assessments of motor function, such as wearable sensors monitoring daily life activity and gait patterns, is needed to bridge the gap between laboratory findings and real-world functioning.
Investigating the mechanisms linking regional atrophy to functional decline is crucial. This could involve exploring the role of specific neurotransmitter systems, neuroinflammation, or synaptic dysfunction. Studies examining the influence of genetic factors (e.g., APOE genotype) and modifiable risk factors (e.g., vascular health, physical activity, cognitive engagement) on the relationship between GMV and function are also warranted. Finally, intervention studies are needed to determine whether targeting specific mechanisms (e.g., through exercise, cognitive training, pharmacological agents, or neuromodulation) can mitigate GMV loss or attenuate its impact on cognitive and motor function in individuals with aMCI. Replication of these findings in larger, more diverse populations is essential to confirm their generalizability.
In conclusion, Yue et al[2] provide valuable evidence linking reduced gray matter volume in specific temporal, frontal, occipital, and parietal regions to impairments in both cognitive and motor functions in patients with aMCI. Their study effectively utilizes VBM and comprehensive functional assessments to highlight the interconnectedness of brain structure, cognition, and gait control in this at-risk population. While the findings support the potential of regional GMV as a biomarker and emphasize the importance of integrated cognitive-motor assessment and rehabilitation strategies, the cross-sectional nature of the study precludes definitive conclusions about causality. Future longitudinal, multi-modal, and mechanistic research is essential to further elucidate these relationships and translate these findings into effective clinical tools and interventions for individuals progressing towards AD.
| 1. | Zhan R, Mpofu E, Prybutok G, Ingman S. Social networking older adults with mild cognitive impairment: Systematic review protocol on their use of information and communication technology. PLoS One. 2024;19:e0302138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Yue YB, Xu MF, Xu Z, Xu JX, Lin M, Yang Y. Link of gray matter volume to cognitive and motor function in elderly patients with mild cognitive impairment. World J Psychiatry. 2025;15:99859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Mattsson-Carlgren N, Salvadó G, Ashton NJ, Tideman P, Stomrud E, Zetterberg H, Ossenkoppele R, Betthauser TJ, Cody KA, Jonaitis EM, Langhough R, Palmqvist S, Blennow K, Janelidze S, Johnson SC, Hansson O. Prediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma Biomarkers. JAMA Neurol. 2023;80:360-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 211] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 4. | Wu H, Song Y, Yang X, Chen S, Ge H, Yan Z, Qi W, Yuan Q, Liang X, Lin X, Chen J. Functional and structural alterations of dorsal attention network in preclinical and early-stage Alzheimer's disease. CNS Neurosci Ther. 2023;29:1512-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 5. | Čepukaitytė G, Newton C, Chan D. Early detection of diseases causing dementia using digital navigation and gait measures: A systematic review of evidence. Alzheimers Dement. 2024;20:3054-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Fatokun I, Gee M, Nelles K, Ba F, Dadar M, Duchesne S, Sharma B, Masellis M, Black SE, Almeida QJ, Smith EE, Pieruccini-Faria F, Montero-Odasso M, Camicioli R. Dual-task gait and white matter hyperintensities in Lewy body diseases: An exploratory analysis. Front Aging Neurosci. 2023;15:1088050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Nadkarni AN, Mukamal KJ, Zhu X, Siscovick D, Brach JS, Jacob M, Seshadri S, Abe T, Rosano C, Djousse L, Rosso AL. Associations of Neurological Biomarkers in Serum With Gait Measures: The Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2024;79:glae043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Brognara L, Luna OC, Traina F, Cauli O. Inflammatory Biomarkers and Gait Impairment in Older Adults: A Systematic Review. Int J Mol Sci. 2024;25:1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 9. | Koo GK, Gaur A, Tumati S, Kusumo RW, Bawa KK, Herrmann N, Gallagher D, Lanctôt KL. Identifying factors influencing cognitive outcomes after anodal transcranial direct current stimulation in older adults with and without cognitive impairment: A systematic review. Neurosci Biobehav Rev. 2023;146:105047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Wang S, Rao J, Yue Y, Xue C, Hu G, Qi W, Ma W, Ge H, Zhang F, Zhang X, Chen J. Altered Frequency-Dependent Brain Activation and White Matter Integrity Associated With Cognition in Characterizing Preclinical Alzheimer's Disease Stages. Front Hum Neurosci. 2021;15:625232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Luo L, You W, DelBello MP, Gong Q, Li F. Recent advances in psychoradiology. Phys Med Biol. 2022;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | Kim SA, Kim Y, Na DL, Seo SW, Jang H; PREMIER Consortium. Comprehensive Clinical and Behavioral Characteristics of Mild Cognitive Impairment According to Amyloid Positivity: A Large Multi-Center Korean Cohort. Dement Neurocogn Disord. 2025;24:102-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Lau CI, Liu MN, Cheng FY, Wang HC, Walsh V, Liao YY. Can transcranial direct current stimulation combined with interactive computerized cognitive training boost cognition and gait performance in older adults with mild cognitive impairment? a randomized controlled trial. J Neuroeng Rehabil. 2024;21:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 14. | Michele R, Ivana S, Maria DV, Luca B, Domenico L, Maria ZF, Alessandro B, Silvio S, Khalid AO, Valeria M, Pietro A. Tracing in vivo the dorsal loop of the optic radiation: convergent perspectives from tractography and electrophysiology compared to a neuroanatomical ground truth. Brain Struct Funct. 2022;227:1357-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Hwang J, Liu C, Winesett SP, Chatterjee SA, Gruber AD 2nd, Swanson CW, Manini TM, Hass CJ, Seidler RD, Ferris DP, Roy A, Clark DJ. Prefrontal cortical activity during uneven terrain walking in younger and older adults. Front Aging Neurosci. 2024;16:1389488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Knapstad MK, Naterstad I, Bogen B. The association between cognitive impairment, gait speed, and Walk ratio. Front Aging Neurosci. 2023;15:1092990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Gooijers J, Pauwels L, Hehl M, Seer C, Cuypers K, Swinnen SP. Aging, brain plasticity, and motor learning. Ageing Res Rev. 2024;102:102569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/