Published online Sep 19, 2024. doi: 10.5498/wjp.v14.i9.1308
Revised: August 9, 2024
Accepted: August 20, 2024
Published online: September 19, 2024
Processing time: 112 Days and 23.9 Hours
Generalized anxiety disorder (GAD) is a relatively common mental disorder. Recently, inflammation, an important factor for the development of depression, has attracted increasing attention. Several studies have shown that inflammatory cytokines can affect the pathophysiological processes of several nervous system diseases. We hypothesized that there is a correlation between the levels of lipopolysaccharide (LPS)-stimulated inflammatory cytokines and the clinical symptoms of GAD.
To investigate the predictive effect of LPS-stimulated inflammatory cytokines on symptoms of GAD.
This was a cross-sectional study in which 89 patients with GAD diagnosed at The First Hospital of Hebei Medical University from January 2022 to December 2022 and 70 individuals without anxiety and depression (controls) during the same period were included. Fasting venous blood was collected from all the subjects in heparin tubes, and another 3 ml of blood was supplemented with LPS (10 ng/ml). The plasma levels of 12 cytokines [Interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, IL-17A, IL-12p70, and IFN-α] were detected.
Post-LPS stimulation, the levels of IL-1β, IL-6, IL-8, IL-10, and TNF-α in both the control and GAD groups were significantly elevated above those in the nonstimulated groups, with IL-6 and IL-8 showing marked increases. Increases in IL-8 and TNF-α were statistically significant in the GAD group (P < 0.05). IL-1β, IL-6, IL-8, IL-10, and TNF-α were found to be significantly correlated with Hamilton Anxiety Rating Scale (HAMA) scores (P < 0.05). A negative correlation was observed between IL-10 levels and HAMA scores. Further analysis revealed that TNF-α was associated with mental anxiety, whereas IL-1β, IL-8, and IL-10 were associated with physical anxiety symptoms, with IL-10 showing a negative correlation with physical anxiety. IL-6 was associated with both mental and physical aspects of anxiety.
The physical symptoms of GAD are related to inflammatory factors. IL-1β, IL-8, IL-10, and TNF-a can be used as predictors of physical or mental anxiety in patients with GAD.
Core Tip: In this study, the physical symptoms of generalized anxiety disorder (GAD) were found to be related to inflammatory factors and the innate ability of the body to produce inflammation in vivo. Interleukin (IL)-1β, IL-8, IL-10, and tumor necrosis factor-α can be used as predictors of physical or mental anxiety in patients with GAD. This study provides evidence that lipopolysaccharide-induced inflammatory cytokines can be used to monitor the mental state of GAD patients. Strategies for generalized anxiety disorder that can benefit from personalized treatment with anti-inflammatory drugs may be promising.
- Citation: Wang WY, Liu N, Qi XX, Han B, Sun JN, Chen ZL, Wang MW, Wang YY. Predictive effect of lipopolysaccharide-stimulated inflammatory cytokines on symptoms of generalized anxiety disorder. World J Psychiatry 2024; 14(9): 1308-1318
- URL: https://www.wjgnet.com/2220-3206/full/v14/i9/1308.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i9.1308
Generalized anxiety disorder (GAD) is a relatively common mental disorder characterized by persistent and widespread anxiety and stress, with an unclear pathogenesis. Health facilities at all levels frequently encounter patients with a variety of symptoms. These patients often experience pain in different parts of the body, fatigue, and dysfunction of the head, heart, and gastrointestinal or other organs. Many patients even experience multiple symptoms at the same time. Pain is not limited to physical pain; it also includes psychological and behavioral aspects, especially for some patients. Physical disease is mild or even nonexistent, but patients are highly sensitive to health anxiety and examination behavior. In most patients, most pain is caused by physical pain, but in others, the pain in their own bodies is caused by mental illness, in which anxiety is at the heart of their suffering[1]. However, most patients have physical distress problems or types of illness that are not properly identified and acknowledged at the time of visit. Consequently, prolonged and ultimately unsuccessful treatments often lead to depression. As a result, doctors frequently find such disorders or patients more challenging to treat and requiring higher costs and more time compared to those without anxiety disorders. These health problems significantly contribute to the increasing global burden of disease. At present, it is generally believed that functional somatic symptoms can cause more depression and anxiety disorders. Studies have shown that people with anxiety and depression have different neurobiological correlations compared to people without, such as a stronger thalamic-pituitary-adrenal axis and increased neural activation and activation deficits in working memory due to hypervigilance during magnetic resonance imaging cognitive control tasks[2]. In recent years, inflammation, an im
While the hypothesis linking immune dysregulation to anxiety disorders is compelling, the evidence supporting it is not uniform. A crucial consideration in earlier research was the measurement of basal inflammatory markers in circulation, which are significantly impacted by lifestyle choices and health conditions[4,5]. A comprehensive meta-analysis revealed that the correlation between anxiety and inflammation becomes less pronounced when body mass index (BMI) is taken into account[3]. It appears that baseline inflammation is more closely related to various lifestyle and health factors, including alcohol consumption, BMI, chronic illnesses, and the use of certain medications. At present, few studies have investigated the relationships between various anxiety disorders, especially GAD, and inflammatory responses. It has been reported that ILs and inflammatory chemokines play important roles in the immune regulatory response in GAD[6,7]. On the other hand, studies have shown that inflammatory cytokines can affect cognitive function[8]. In most patients, the association between underlying inflammation and anxiety largely disappeared after adjusting for lifestyle and health factors.
Investigating the response of inflammatory markers to in vitro lipopolysaccharide (LPS) stimulation in blood samples may offer deeper insights into immune regulatory mechanisms. This approach closely mirrors the natural physiological environment, is influenced by strong genetic factors, and elicits an inflammatory reaction that mirrors the body's inherent capacity for inflammatory marker production[9,10,11]. Recent research has indicated that the variability observed in baseline inflammatory level assessments could be mitigated by evaluating markers of inflammation in blood samples stimulated with LPS[12].
Therefore, this study has three main objectives: (1) To observe the basal levels of inflammatory factors in GAD patients and analyze whether there is a correlation between basal inflammatory factors and the clinical symptoms of GAD; (2) To determine whether there is a correlation between the levels of LPS-stimulated cytokines and the clinical symptoms of GAD, given that basic inflammatory factors are influenced by multiple factors; and (3) To examine the relationship between the levels of LPS-stimulated inflammatory factors and mental or physical anxiety as measured by the Hamilton Anxiety Rating Scale (HAMA).
This investigation employed a cross-sectional approach. The subject pool comprised 89 individuals diagnosed with GAD, recruited from the outpatient and inpatient units of the neurology and psychiatry departments at The First Hospital of Hebei Medical University between January 2022 and December 2022. Seventy individuals who did not exhibit anxiety or depression symptoms during the same timeframe were included as controls. The study protocol received ethical approval from the hospital's ethics committee (approval No. 20220581).
The inclusion criteria were as follows: (1) Patients aged 20–70 years; (2) Patients met the International Classification of Disease-10 (ICD-10) version of the diagnostic criteria for GAD; and (3) Patients signed an informed consent form.
The exclusion criteria were as follows: (1) Patients with physical symptoms caused by physical diseases; (2) Patients with language, writing, or reading difficulties; (3) Patients with cognitive dysfunction; (4) Patients diagnosed with other mental illnesses (including bipolar disorder, schizophrenia, and mania); (5) Patients with a strong suicidal tendency; (6) Patients who currently suffer from acute or chronic inflammatory diseases; (7) Patients who have taken antidepressant or antianxiety drugs within 1 mo; and (8) Patients who reported any inflammatory events or ingesting any drugs known to have immunomodulatory effects (such as glucocorticoids) within 2 wk before testing.
The inclusion criteria were as follows: (1) Patients aged 20–70 years; (2) Patients diagnosed according to the ICD-10 version and used the GAD 7-item (GAD-7) scale and Patient Health Questionnaire-9 (PHQ-9) to rule out anxiety and depression; (3) Patients matched the age and sex of the experimental group; and (4) Patients signed an informed consent form.
The exclusion criteria were as follows: (1) Patients had language, writing, or reading difficulties; (2) Patients had cognitive dysfunction; (3) Patients had suffered from mental illness in the past; (4) Patients had acute or chronic inflammatory diseases; and (5) Patients reported any inflammatory events or ingestion of any drugs known to have immunomodulatory effects (such as glucocorticoids) within 2 wk before testing.
The demographic questionnaire included questions about gender, age, race, place of residence and living conditions, marital status, education level, and occupation. Lifestyle questionnaire data included height, weight, smoking history, and drinking history. The presence of chronic diseases such as diabetes, hypertension, cardiovascular and cere
The study utilized three critical scales for mental health evaluation: The PHQ-9, the GAD-7, and the HAMA, with a specific focus on the HAMA. In accordance with the criteria set by the Local Scale Collaboration Group, HAMA scores are interpreted as follows: A total score exceeding 29 points signifies severe anxiety, a score above 21 points indicates notable anxiety, a score over 14 points suggests moderate anxiety, a score greater than 7 points denotes mild anxiety, and a score less than 7 points indicates no apparent anxiety symptoms. HAMA's comprehensive analysis, which includes both physical and psychological elements, serves to depict a patient's emotional state and identify any physical symptoms requiring medical attention.
Additionally, the study employed the GAD-7, which was formulated by Kroenke et al[13]. This scale, a segment of the Patient Health Questionnaire, is pivotal for detecting generalized anxiety and measuring the intensity of symptoms. The Chinese version of the GAD-7 has a sensitivity of 86.2%, specificity of 95.5%, and kappa value of 0.825, highlighting its high reliability and validity. This meticulous approach in scale selection and interpretation aligns with the rigorous standards of Science Citation Index journal publications, ensuring a thorough and credible evaluation of psychological health.
Venous blood from all the subjects was collected in heparin tubes, left at room temperature for 30 min, and centrifuged at 4000 r/min for 10 min to obtain the plasma. Another 3 mL of blood was collected, and LPS (10 ng/mL) was added for stimulation[9,11,14]. The mixture was incubated with slow rotation at 37 °C for 5-6 h and centrifuged at 4000 r/min for 10 min. The plasma was then frozen at -80 °C. Plasma levels of cytokines [IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, TNF-α, interferon (IFN)-γ, IL-17A, IL-12p70, and IFN-α] were detected[11].
After meticulous validation and editing, the data were organized into a database compatible with Statistical Package for the Social Sciences (SPSS) software, and the analyses were performed via SPSS version 21.0. Proportional data are expressed as percentages. Numerical data are presented as the mean with standard deviation (mean ± SD). To compare average values between two groups, the t test was applied. Categorical variables were tested using the χ² test. Pearson correlation was used to analyze the relationship between two continuous variables, and Spearman correlation was used to evaluate associations between dichotomous and continuous variables. Statistical significance was indicated by a P value less than 0.05.
The demographic and clinical data of participants in the GAD group vs those in the control group are shown in Table 1. The GAD group included 89 individuals, whereas the control group included 70 individuals. In terms of sex distribution, age, BMI, smoking habits, alcohol consumption, and the prevalence of diabetes, hypertension, hyperlipidemia, coronary artery disease, or cerebrovascular accidents, no significant differences were observed between the two cohorts (P > 0.05).
| Index | Control group | Generalized anxiety disorder group | t/χ2 | P value |
| Age (yr) | 53.79 ± 16.23 | 58.30 ± 13.70 | 1.863 | 0.065 |
| Sex (male/female) | 25/45 | 39/50 | 1.812 | 0.178 |
| Body mass index (kg/m2) | 24.55 ± 2.06 | 24.46 ± 3.24 | 0.291 | 0.760 |
| Smoking history | 19 (27.14) | 18 (20.22) | 1.050 | 0.305 |
| Drinking history | 9 (12.85) | 8 (8.99) | 0.001 | 0.529 |
| Diabetes | 10 (14.29) | 13 (14.60) | 0.030 | 0.954 |
| Hypertension | 33 (47.14) | 46 (51.69) | 0.323 | 0.570 |
| Coronary heart disease | 8 (11.42) | 18 (20.22) | 2.216 | 0.137 |
| Hyperlipidemia | 7 (10.00) | 10 (11.23) | 0.063 | 0.802 |
| Cerebral infarction | 10 (14.28) | 15 (16.85) | 0.195 | 0.659 |
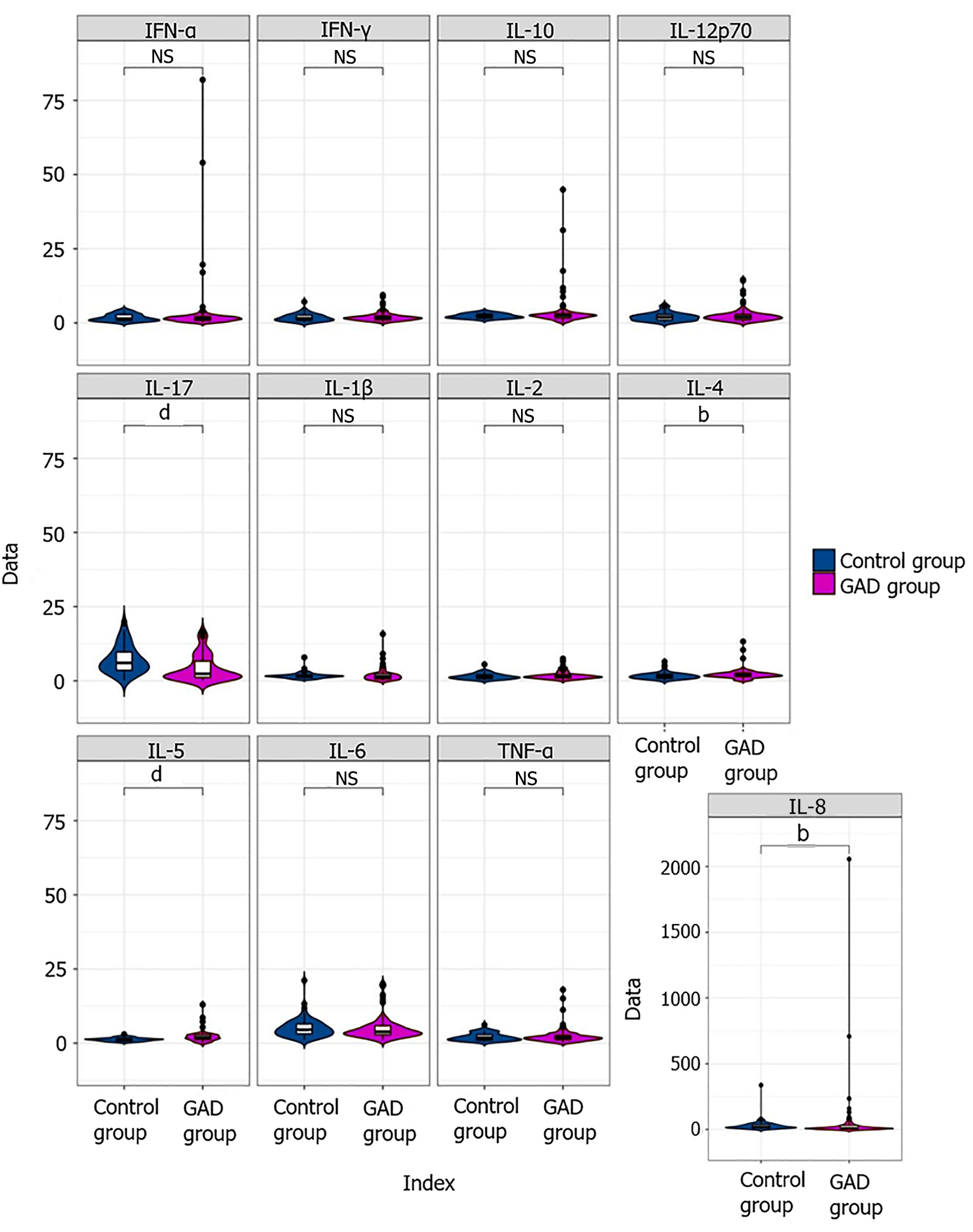
Flow cytometry was used to measure the levels of inflammatory cytokines in the blood. The comparative analysis of these cytokine levels in the peripheral blood of the GAD and control groups is illustrated in Table 2 and Figure 1. Notably, the levels of IL-4 and IL-5 were significantly elevated in the GAD group compared with those in the control group (P < 0.05), whereas the IL-17A levels were significantly lower in the GAD group (P < 0.05).
| Inflammatory factor (normal range) | Control group (mean ± SD) | Generalized anxiety disorder group (mean ± SD) | t value | P value |
| IL-1β (0-12.4 pg/mL) | 1.69 ± 1.04 | 1.96 ± 2.21 | 1.024 | 0.308 |
| IL-2 (0-5.71 pg/mL) | 1.45 ± 0.98 | 1.78 ± 1.40 | 1.684 | 0.094 |
| IL-4 (0-3 pg/mL) | 1.68 ± 1.14 | 2.19 ± 1.82 | 2.040 | 0.043 |
| IL-5 (0-3.10 pg/mL) | 1.27 ± 0.58 | 2.18 ± 1.80 | 4.044 | 0.001 |
| IL-6 (0-7 pg/mL) | 5.03 ± 3.11 | 5.06 ± 3.98 | -0.40 | 0.968 |
| IL-8 (0-20.60 pg/mL) | 30.59 ± 41.33 | 57.53 ± 230.29 | 1.081 | 0.336 |
| IL-10 (0-4.91 pg/mL) | 2.30 ± 0.82 | 3.66 ± 5.86 | 2.163 | 0.056 |
| TNF-α (0-4.60 pg/mL) | 2.05 ± 1.25 | 2.42 ± 2.67 | 1.069 | 0.287 |
| IFN-γ (0-7.42 pg/mL) | 1.77 ± 1.29 | 2.02 ± 1.59 | 1.056 | 0.292 |
| IL-17A (0-20.6 pg/mL) | 7.23 ± 4.84 | 4.27 ± 4.42 | -3.749 | 0.001 |
| IL-12p70 (0-3.4 pg/mL) | 2.07 ± 1.40 | 2.55 ± 2.54 | 1.443 | 0.151 |
| IFN-α (0-8.5 pg/mL) | 1.80 ± 1.20 | 3.42 ± 10.42 | 1.460 | 0.148 |
LPS-stimulated inflammatory factors represent a patient's innate ability to produce inflammatory factors. Inflammatory factors are affected by various factors, such as diabetes, BMI, and other factors. When we compared the levels of inflammatory factors between the two groups, we assessed whether there was a difference in the ability to produce inflammatory factors. In our analysis, we excluded the effects of BMI, diabetes, hypertension, hyperlipidemia, coronary heart disease, and cerebral infarction on inflammatory factors.
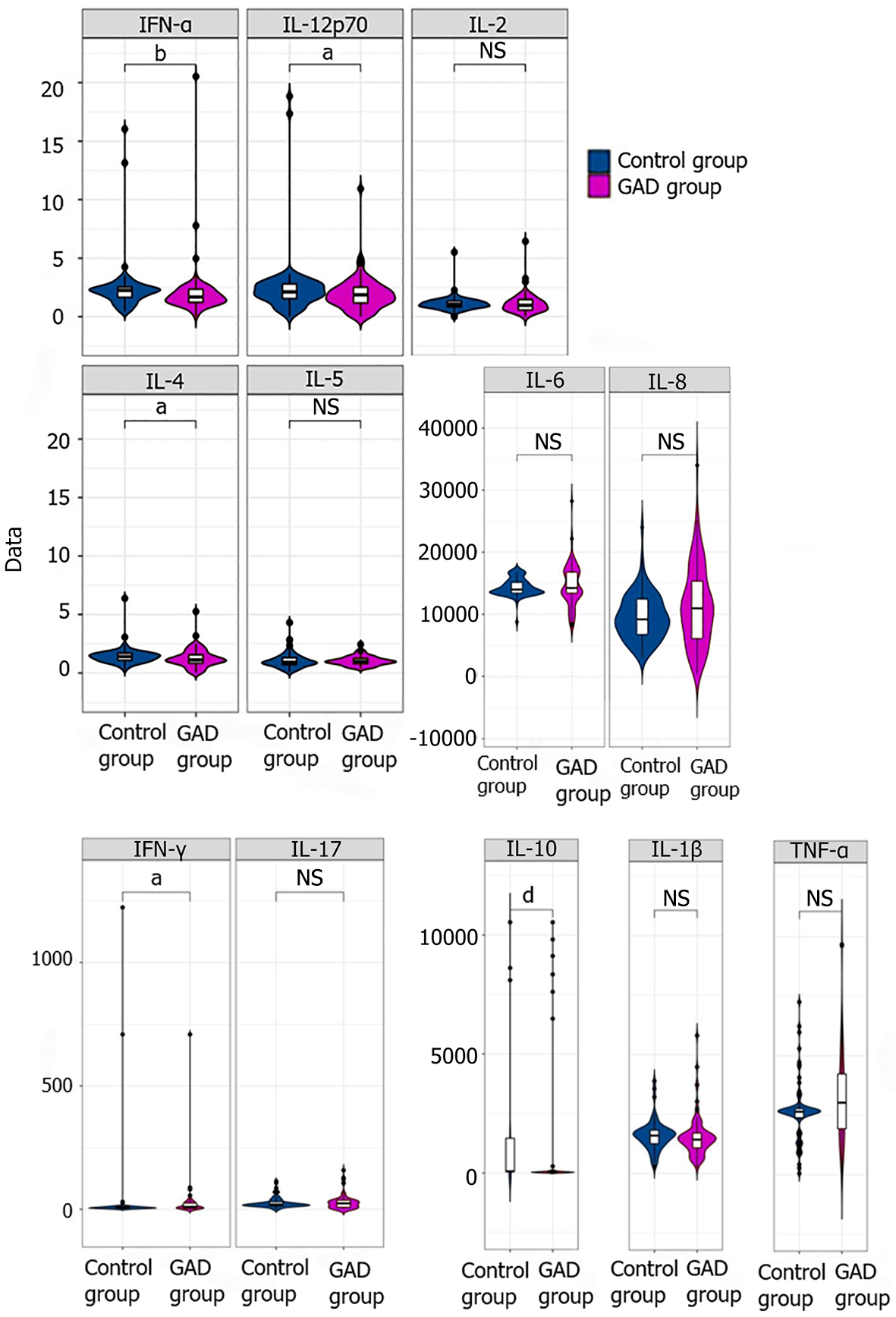
Since LPS is involved in nonspecific immunity and different individuals have varying immune capabilities, we further selected inflammatory factors stimulated by LPS and observed differences between the two groups. After LPS stimulation, flow cytometry was used to compare the levels of inflammatory factors (Table 3 and Figure 2). Compared to the baseline levels (Table 1), the levels of the inflammatory factors IL-1β, IL-6, IL-8, IL-10, TNF-α, and IFN-γ significantly increased in both the control group and the GAD group after stimulation (P < 0.05). Among these, the expression of IL-6 and IL-8 showed the most pronounced increase. The levels of IL-8 and TNF-α in the GAD group after stimulation were significantly higher than those in the control group after stimulation (P < 0.05). However, there were no significant differences in the basic inflammatory factors IL-4, IL-5, and IL-17A which were previously distinct, suggests that GAD is related to the innate production ability of IL-8 and TNF-α in the body.
| Inflammatory factor (normal range) | Control group (mean ± SD) | Generalized anxiety disorder group (mean ± SD) | t value | P value |
| IL-1β (0-12.4 pg/mL) | 1592.06 ± 622.43 | 1538.99 ± 844.21 | -0.440 | 0.660 |
| IL-2 (0-5.71 pg/mL) | 1.12 ± 0.67 | 1.13 ± 0.85 | 0.082 | 0.935 |
| IL-4 (0-3 pg/mL) | 1.44 ± 0.75 | 1.25 ± 0.77 | -1.568 | 0.119 |
| IL-5 (0-3.10 pg/mL) | 1.09 ± 0.68 | 1.04 ± 0.39 | -0.625 | 0.534 |
| IL-6 (0-7 pg/mL) | 14354.85 ± 1420.62 | 14657.66 ± 3347.42 | 0.173 | 0.443 |
| IL-8 (0-20.60 pg/mL) | 9515.79 ± 3803.61 | 11351.12 ± 6349.79 | 2.306 | 0.025 |
| IL-10 (0-4.91 pg/mL) | 1244.80 ± 2727.53 | 1049.45 ± 2877.97 | -0.433 | 0.665 |
| TNF-α (0-4.60 pg/mL) | 2666.29 ± 1272.87 | 3197.81 ± 1887.78 | 1.676 | 0.045 |
| IFN-γ (0-7.42 pg/mL) | 34.72 ± 166.81 | 23.77 ± 74.81 | -0.553 | 0.581 |
| IL-17 (0-20.6 pg/mL) | 26.91 ± 22.767 | 28.78 ± 30.53 | 0.426 | 0.671 |
| IL-12p70 (0-3.4 pg/mL) | 2.50 ± 2.84 | 1.94 ± 1.44 | -1.633 | 0.104 |
| IFN-α (0-8.5 pg/mL) | 2.42 ± 2.24 | 2.01 ± 2.21 | -1.181 | 0.240 |
Some previous studies[11,15] indicated that the correlation of anxiety with inflammation is driven mainly by anxiety symptoms. Herein, we investigated whether these inflammatory factors are related to certain clinical symptoms of GAD. First, we analyzed the correlations between the levels of inflammatory factors and HAMA scores before and after LPS stimulation (Table 4). The results showed that before LPS stimulation, HAMA scores were related to the level of IL-17A; after LPS stimulation, the levels of IL-17A and HAMA scores were not related. However, the levels of five inflammatory factors (IL-1β, IL-6, IL-8, IL-8, IL-8, and TNF-α) and HAMA scores were significantly increased (P < 0.05) after LPS stimulation. Among them, IL-1β, IL-6, IL-8, and TNF-α were positively related to HAMA scores, and IL-10 was negatively related to HAMA scores. This may be because only IL-10 is an anti-inflammatory factor (P < 0.05).
| Inflammatory factor | Correlation with HAMA scores before LPS stimulation | Correlation with HAMA scores after LPS- stimulation | ||
| r value | P value | r value | P value | |
| IL-1β | 0.118 | 0.271 | 0.219 | 0.039 |
| IL-2 | 0.131 | 0.220 | -0.20 | 0.852 |
| IL-4 | 0.106 | 0.322 | 0.663 | 0.558 |
| IL-5 | -0.087 | 0.420 | -0.042 | 0.698 |
| IL-6 | 0.058 | 0.590 | 0.379 | < 0.001 |
| IL-8 | 0.149 | 0.164 | 0.640 | < 0.001 |
| IL-10 | 0.050 | 0.640 | -2.92 | 0.005 |
| TNF-α | 0.108 | 0.313 | 0.621 | < 0.001 |
| IFN-γ | 0.049 | 0.647 | 0.153 | 0.151 |
| IL-17A | 0.245 | 0.021 | 0.138 | 0.197 |
| IL-12p70 | 0.118 | 0.270 | 0.091 | 0.399 |
| IFN-α | 0.036 | 0.737 | 0.144 | 0.178 |
The above results indicate that the correlations between IL-1β, IL-6, IL-8, IL-10, and TNF-α and HAMA score were greater than those between other factors and HAMA score. Therefore, we further analyzed the correlations between IL-1β, IL-6, IL-8, IL-10, and TNF-α and HAMA score. The HAMA was also used to assess both mental anxiety and physical anxiety. These five factors were more stronger related to the mental anxiety and physical anxiety. Therefore, we compared the correlations between the five inflammatory factors and the HAMA score (Table 5). The results revealed that TNF-α was more strongly correlated with mental anxiety, whereas IL-1β, IL-8, and IL-10 were more strongly correlated with physical anxiety (P < 0.05). IL-10 was negatively correlated with physical anxiety (r = -0.292, P < 0.05).
| Inflammatory factor | Correlation with mental anxiety | Correlation with somatic anxiety | ||
| r value | P value | r value | P value | |
| IL-1β | 0.148 | 0.166 | 0.236 | 0.026 |
| IL-6 | 0.374 | < 0.0001 | 0.304 | 0.004 |
| IL-8 | 0.495 | < 0.0001 | 0.587 | < 0.0001 |
| IL-10 | -0.224 | < 0.035 | -0.307 | 0.003 |
| TNF-α | 0.644 | < 0.0001 | 0.503 | < 0.0001 |
The above results revealed that there were four inflammatory factors related to physical anxiety. Therefore, we used these four inflammatory factors and a more detailed factor division in the HAMA to perform a more specific correlation analysis (Table 6). The results showed that IL-1β was obviously correlated with symptoms of the cardiovascular system. Interestingly, IL-8 was related not only to urogenital system symptoms but also to symptoms of the autonomous nervous system. IL-10 was correlated with gastrointestinal symptoms (Table 6).
| Somatic anxiety factor (symptoms) | Correlation with inflammatory factors | |||||
| IL-1β | IL-8 | IL- 10 | ||||
| r value | P value | r value | P value | r value | P value | |
| Muscular system | 0.26 | 0.808 | 0.175 | 0.101 | -0.20 | 0.853 |
| Sensory system | 0.143 | 0.182 | 0.114 | 0.288 | -1.69 | 0.114 |
| Cardiovascular system | 0.30 | 0.004 | 0.119 | 0.265 | 0.029 | 0.788 |
| Respiratory system | 0.012 | 0.908 | 0.118 | 0.273 | -0.092 | 0.392 |
| Gastrointestinal system | -0.052 | 0.630 | 0.027 | 0.801 | -0.503 | < 0.0001 |
| Genitourinary system | 0.102 | 0.341 | 0.322 | 0.002 | -0.124 | 0.245 |
| Autonomic nervous system | 0.077 | 0.471 | 0.517 | < 0.0001 | -0.040 | 0.707 |
The results of this study suggest that the physical symptoms of GAD are related to inflammatory factors and the innate ability of the body to generate inflammation. IL-1β, IL-8, IL-10, and TNF-α may serve as predictors of physical or mental anxiety in GAD. In the future, monitoring these inflammatory factors could improve the diagnosis of patients' mental status and facilitate symptomatic treatment.
However, doctors in general hospitals should be vigilant about potential psychological issues such as depression or anxiety in patients presenting with multiple symptoms or when emotional symptoms co-occur. The high prevalence of depression and anxiety disorders in general hospital patients underscores the importance of these institutions in providing mental health services. Yet, this role is often mismatched with their current service levels: The recognition rates for mental health problems, especially anxiety and depression, are low. One study revealed that only 8.5% of patients with depression and anxiety in general hospitals received advice from psychiatrists, and only 6.4% were prescribed psychiatric medications[13]. Misdiagnosis surveys have shown that common diseases like digestive, cardiovascular, endocrine, and neurological conditions are often mistaken for anxiety and depression, leading to repeated consultations and examinations, delayed diagnosis, and increased economic and psychological burdens[16].
A study by the National Institutes of Health found that physicians correctly diagnosed anxiety or depression in 77% of patients with psychological symptoms as the main complaint, but only 22% when physical symptoms were predominant[17]. This suggests that focusing on somatic symptoms may reduce the recognition rate of depression and anxiety disorders. In China, the recognition rate of these disorders by non-psychiatrists is particularly low[13].
In 1998, Maes et al[18] first reported a significant relationship between the inflammatory response and anxiety induced by stress. Individuals with anxiety had higher levels of IFN-γ and lower levels of IL-10 compared to non-anxious individuals. Low levels of IL-4 and IL-10 in patients with chronic pain and anxiety are linked to increased pain[19], suggesting a strong association between pain and anxiety[20,21]. Recently, the relationship between inflammatory cytokines and cognitive functions has gained attention. Studies have shown that newly diagnosed GAD patients have abnormal inflammatory cytokine levels and execution function, and there is a correlation between the two factors[22]. Another analysis revealed a bidirectional relationship between depression and inflammation[3]. These studies suggest that inflammatory cytokines and anxiety symptoms are correlated.
To evaluate the mental and physical symptoms of patients, this study utilized the scales PHQ-9, GAD-7, and HAMA. The GAD-7 and PHQ-9 are used for screening, while the HAMA was used to analyze the correlation with inflammatory cytokines. Studies have shown that these scales have strong reliability and high detection rates in diagnosing anxiety or depression[23,24].
In this study, significant differences were noted in the baseline inflammatory markers between the GAD group and the control group. Specifically, the GAD group had higher levels of IL-4 and IL-5 and lower levels of IL-17A (P < 0.05), contrasting with the typically reported increases in IL-6, IL-8, IL-1β, TNF-α, and IFN-γ in prior studies. Research has shown increased IL-6 levels in GAD patients compared to controls[25-28]. TNF-α's role in GAD has yielded mixed results, with some studies indicating elevated levels among GAD patients[27,29,30], whereas the most extensive TNF-α study (n = 1010) revealed no differences between GAD patients and controls or any link between TNF-α levels and anxiety symptoms[25]. This aligns with a study of 93 GAD patients with ischemic heart disease, where no significant differences were found using a combined inflammatory index[31]. A study on IL-4[30] found no significant differences between GAD patients and controls when adjusting for demographic and lifestyle factors. IL-10, known for its anti-inflammatory properties, was significantly decreased in GAD patients (odds ratio = 0.35, P = 0.003). Three studies assessed IFN-γ levels in GAD patients (n = 330)[25,29,30]. A domestic study with the largest number (n = 118) indicating elevated levels of IFN-γ even after adjustments for various factors[30]. Research on newly diagnosed GAD outpatients revealed elevated levels of IL-1α, IL-8, and IL-12p70, with IL-1α and IL-8 levels correlating with GAD severity[25].
Our investigation analyzed baseline inflammation and cytokine production in response to GAD in the same participants. Post-LPS stimulation, significant increases in IL-1β, IL-6, IL-8, IL-10, TNF-α, and IFN-γ were observed in both the control and GAD groups, with IL-6 and IL-8 showing particularly marked increases. The GAD group had significant increases in IL-8 and TNF-α levels, suggesting a link between GAD and the body's inherent ability to produce these cytokines.
Additionally, correlations were found between the levels of IL-1β, IL-6, IL-8, IL-10, and TNF-α and HAMA scores (P < 0.05). IL-1β, IL-6, IL-8, and TNF-α were positively correlated with HAMA scores, with IL-8 and TNF-α showing particularly strong correlations. Conversely, IL-10 levels were negatively correlated with HAMA scores. Baseline inflammation was only associated with IL-17A. Research has indicated that the levels of LPS-stimulated inflammatory markers, including IL-6, IL-8, IL-10, IL-18, MCP-1, MMP2, and TNF-β, are linked to the severity of anxiety symptoms[28].
This study highlights the distinct nature of basal inflammation levels compared to those post-LPS stimulation, representing different aspects of the immune system response. It also underscores the importance of cytokine production capacity in understanding anxiety disorders.
The limitations of this study, such as the cross-sectional design and insufficient sample size, should be acknowledged. Further research is needed to explore the potential causal effects of cytokine production capacity on anxiety symptom risk. Understanding the complex interplay between peripheral inflammation, neuroinflammatory responses, and anxiety symptoms could inform the development of targeted treatments for patients with high inflammation.
In general, this study has monitored the mental state of patients with GAD using LPS-induced inflammatory cytokines. Strategies for GAD that can benefit from (personalized) treatment with anti-inflammatory drugs may be promising.
| 1. | Hettema JM. The nosologic relationship between generalized anxiety disorder and major depression. Depress Anxiety. 2008;25:300-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Patriquin MA, Mathew SJ. The Neurobiological Mechanisms of Generalized Anxiety Disorder and Chronic Stress. Chronic Stress (Thousand Oaks). 2017;1:2470547017703993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 3. | Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1916] [Cited by in RCA: 2164] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 4. | Bosma-den Boer MM, van Wetten ML, Pruimboom L. Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutr Metab (Lond). 2012;9:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Enroth S, Johansson A, Enroth SB, Gyllensten U. Strong effects of genetic and lifestyle factors on biomarker variation and use of personalized cutoffs. Nat Commun. 2014;5:4684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Gądek-Michalska A, Bugajski J. Interleukin-1 (IL-1) in stress-induced activation of limbic-hypothalamic-pituitary adrenal axis. Pharmacol Rep. 2010;62:969-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 486] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 8. | Pollmächer T, Haack M, Schuld A, Reichenberg A, Yirmiya R. Low levels of circulating inflammatory cytokines--do they affect human brain functions? Brain Behav Immun. 2002;16:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, Dehart I. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 388] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | de Craen AJ, Posthuma D, Remarque EJ, van den Biggelaar AH, Westendorp RG, Boomsma DI. Heritability estimates of innate immunity: an extended twin study. Genes Immun. 2005;6:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | van Eeden WA, El Filali E, van Hemert AM, Carlier IVE, Penninx BWJH, Lamers F, Schoevers R, Giltay EJ. Basal and LPS-stimulated inflammatory markers and the course of anxiety symptoms. Brain Behav Immun. 2021;98:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | van Eeden WA, van Hemert AM, Carlier IVE, Penninx BWJH, Lamers F, Fried EI, Schoevers R, Giltay EJ. Basal and LPS-stimulated inflammatory markers and the course of individual symptoms of depression. Transl Psychiatry. 2020;10:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Kroenke K, Spitzer RL, Williams JB, Linzer M, Hahn SR, deGruy FV 3rd, Brody D. Physical symptoms in primary care. Predictors of psychiatric disorders and functional impairment. Arch Fam Med. 1994;3:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 473] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | van der Linden MW, Huizinga TW, Stoeken DJ, Sturk A, Westendorp RG. Determination of tumour necrosis factor-alpha and interleukin-10 production in a whole blood stimulation system: assessment of laboratory error and individual variation. J Immunol Methods. 1998;218:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Vogelzangs N, de Jonge P, Smit JH, Bahn S, Penninx BW. Cytokine production capacity in depression and anxiety. Transl Psychiatry. 2016;6:e825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 16. | Zhou MJ, Yao LQ. [Misdiagnosis resource waste of mental illness in general hospitals and its influencing factors]. Zhonghua Xingwei Yixue Yu Naokexue Zazhi. 2006;15:174-175. [DOI] [Full Text] |
| 17. | Kirmayer LJ, Robbins JM, Dworkind M, Yaffe MJ. Somatization and the recognition of depression and anxiety in primary care. Am J Psychiatry. 1993;150:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 359] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpé S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 550] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 19. | Uçeyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum. 2006;54:2656-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, Angermeyer MC, Bernert S, de Girolamo G, Morosini P, Polidori G, Kikkawa T, Kawakami N, Ono Y, Takeshima T, Uda H, Karam EG, Fayyad JA, Karam AN, Mneimneh ZN, Medina-Mora ME, Borges G, Lara C, de Graaf R, Ormel J, Gureje O, Shen Y, Huang Y, Zhang M, Alonso J, Haro JM, Vilagut G, Bromet EJ, Gluzman S, Webb C, Kessler RC, Merikangas KR, Anthony JC, Von Korff MR, Wang PS, Brugha TS, Aguilar-Gaxiola S, Lee S, Heeringa S, Pennell BE, Zaslavsky AM, Ustun TB, Chatterji S; WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291:2581-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2082] [Cited by in RCA: 2069] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 21. | Scott K, McGee MA, Schaaf D, Baxter J. Mental-physical comorbidity in an ethnically diverse population. Soc Sci Med. 2008;66:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Ye G, Tang Z, Qian ZK, Du XD, Pan MZ, Zhu F, Fu JL, Fu T, Liu QC. [Correlation between peripheral blood inflammatory cytokines and executive function in patients with first-episode generalized anxiety disorder]. Jingshen Yixue Zazhi. 2016;29:161-163. [DOI] [Full Text] |
| 23. | Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3056] [Cited by in RCA: 2888] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 24. | Lucchesi C, Baldacci F, Cafalli M, Dini E, Giampietri L, Siciliano G, Gori S. Fatigue, sleep-wake pattern, depressive and anxiety symptoms and body-mass index: analysis in a sample of episodic and chronic migraine patients. Neurol Sci. 2016;37:987-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Tang Z, Ye G, Chen X, Pan M, Fu J, Fu T, Liu Q, Gao Z, Baldwin DS, Hou R. Peripheral proinflammatory cytokines in Chinese patients with generalised anxiety disorder. J Affect Disord. 2018;225:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Hoge EA, Bui E, Palitz SA, Schwarz NR, Owens ME, Johnston JM, Pollack MH, Simon NM. The effect of mindfulness meditation training on biological acute stress responses in generalized anxiety disorder. Psychiatry Res. 2018;262:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 27. | Yang CJ, Liu D, Xu ZS, Shi SX, Du YJ. The pro-inflammatory cytokines, salivary cortisol and alpha-amylase are associated with generalized anxiety disorder (GAD) in patients with asthma. Neurosci Lett. 2017;656:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 353] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 29. | Korkeila J, Runsten S, Ollikainen S, Korkeila K. PW01-161 - Generalized Anxiety Disorder And Immunity Markers In A Stratified Population Sample. Eur Psychiatr. 2010;25:25-E1560. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Hou R, Garner M, Holmes C, Osmond C, Teeling J, Lau L, Baldwin DS. Peripheral inflammatory cytokines and immune balance in Generalised Anxiety Disorder: Case-controlled study. Brain Behav Immun. 2017;62:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 31. | Zahm JL. Generalized anxiety disorder and inflammatory biomarkers in coronary heart disease: Sex-specific effects. Diss Abstr Int Sect B Sci Eng. 2018;78. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/