Published online Jul 19, 2024. doi: 10.5498/wjp.v14.i7.1106
Revised: May 10, 2024
Accepted: May 27, 2024
Published online: July 19, 2024
Processing time: 104 Days and 0.8 Hours
Major depressive disorder (MDD) in adolescents and young adults contributes significantly to global morbidity, with inconsistent findings on brain structural changes from structural magnetic resonance imaging studies. Activation likeli
To identify consistent brain structural changes in adolescents and young adults with MDD using ALE meta-analysis.
We performed a comprehensive literature search in PubMed, Web of Science, Embase, and Chinese National Knowledge Infrastructure databases for neuroi
Twenty-two studies comprising fourteen diffusion tensor imaging (DTI) studies and eight voxel-based morphome
Structural alterations in the right caudate head, right insula, and right lentiform nucleus putamen in young MDD patients may contribute to its recurrent nature, offering insights for targeted therapies.
Core Tip: This activation likelihood estimation (ALE) meta-analysis illuminates significant structural brain changes in adolescents and young adults with major depressive disorder (MDD), particularly in the right caudate head, right insula, and right lentiform nucleus putamen, highlighting their potential as neural markers. By employing ALE across diffusion tensor imaging and voxel-based morphometry studies, the research reveals consistent patterns of reduced fractional anisotropy, underscoring the recurrent nature of MDD. These insights provide a deeper understanding of its neuropathology and highlights the critical role of specialized neuroimaging in unraveling the complex mechanisms underlying MDD.
- Citation: Shu YP, Zhang Q, Hou YZ, Liang S, Zheng ZL, Li JL, Wu G. Multimodal abnormalities of brain structures in adolescents and young adults with major depressive disorder: An activation likelihood estimation meta-analysis. World J Psychiatry 2024; 14(7): 1106-1117
- URL: https://www.wjgnet.com/2220-3206/full/v14/i7/1106.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i7.1106
Major depressive disorder (MDD), a complex neuropsychiatric condition characterized by pervasive feelings of sadness, pessimism, heightened sensitivity, and cognitive dysregulation, significantly contributes to the global disease burden across diverse age groups, especially among adolescents and young adults[1]. In the global context, the prevalence of self-reported depressive symptoms in adolescents is approximately 34%, with regions such as the Middle East, Africa, and Asia reporting the highest prevalence of elevated depressive symptoms in this demographic group[2]. In addition, the prevalence of MDD among young people has markedly increased in the past decade[3]. This demographic variation emphasizes the critical need for age-specific research that encompasses both adolescents and young adults to effectively address and understand the nuances of MDD within these populations. Depressive symptoms during these crucial developmental periods are often underrecognized, thus potentially leading to rapid disease progression and long-term detrimental effects on educational attainment, social integration, and overall quality of life[4]. The onset of MDD during these stages of significant biological, psychological, and social transformations introduces unique challenges, thus underscoring the importance of specialized research aimed at elucidating the distinct etiology, pathophysiology, and treatment responses of MDD in adolescents and young adults[5,6].
In recent years, the rapid advancement of neuroimaging research has provided a robust foundation for investigating the neurophysiology of MDD patients. Despite decades of fundamental science, clinical neuroscience, and psychiatric research, the pathophysiology of severe MDD remains incompletely understood[7]. Neuroimaging methods offer a powerful, noninvasive avenue to study the neurobiological mechanisms underlying psychiatric disorders[8]. These neuroimaging techniques have yielded invaluable insights into the impact of depression on brain structures in adole
Although researchers have used sMRI techniques to analyze changes in brain structure in adolescents and young adults with MDD, the findings of these studies are inconsistent and remain controversial[14-35]. This inconsistency underscores the complexity of MDD across these age groups and the influence of individual and methodological differences. The high prevalence and distinct nature of adolescent and young adult MDD necessitate a nuanced understanding of its neural underpinnings. sMRI studies, including those utilizing DTI and VBM, have provided valuable insights into brain structural anomalies associated with MDD[36]. However, the variability in research outcomes highlights the complexity of the disease and the impact of individual and methodological differences[37]. This variability calls for a comprehensive and systematic approach to synthesize existing research, such as by utilizing activation likelihood estimation (ALE) meta-analysis. By integrating a wealth of available data, ALE meta-analysis aims to extract coherent and actionable insights, thus bridging the gap in our understanding of the neural basis of MDD among adolescents and young adults. Importantly, the ALE meta-analysis method overcomes the challenges of method diversity and result heterogeneity, thus helping to screen for credible and practically valuable research results[38]. Previously, Yuan et al[39] used the ALE method to conduct a meta-analysis of MDD patients; however, they did not include a young population and may not have identified the consistently vulnerable brain regions in the resting state that may differ between adolescent depression patients and adults.
In this study, we hypothesized that populations with depressive disorders among adolescents and young adults will exhibit distinct brain outcome alteration patterns compared to healthy control (HC) groups, thus potentially revealing neural damage mechanisms associated with depressive disorders. We employed ALE analysis to exclusively focus on multimodal brain structure anomalies by using methodologies from DTI and VBM studies, thus aiming for a more comprehensive understanding of consistent brain structural changes in patients with depressive disorders among adolescents and young adults.
In alignment with PRISMA[40], a comprehensive literature search was systematically executed across four major electronic databases up to November 19, 2023. This review was registered with PROSPERO (ID: CRD42023371521). These databases included PubMed, Web of Science, Embase, and Chinese National Knowledge Infrastructure. The search terms were as follows: (adolescent OR youngster OR young people OR youth OR childhood OR teenage OR teen OR juvenile) AND (depression OR depression neurosis OR depressive disorder OR major depression OR melancholia) AND (white matter OR white brain matter OR cerebellar white matter OR white matter integrity) AND (diffusion tensor* OR DTI OR magnetic resonance imaging OR tractography OR mean diffusivity OR axial diffusivity OR radial diffusivity OR fractional anisotropy OR structural connectivity OR structural changes OR structural MRI OR voxel-based morphometry OR VBM) AND (magnetic resonance OR MRI OR functional MRI OR fMRI OR neuroimaging). In addition, review articles and the reference lists of the included articles were also checked to identify potential omitted studies in the searches.
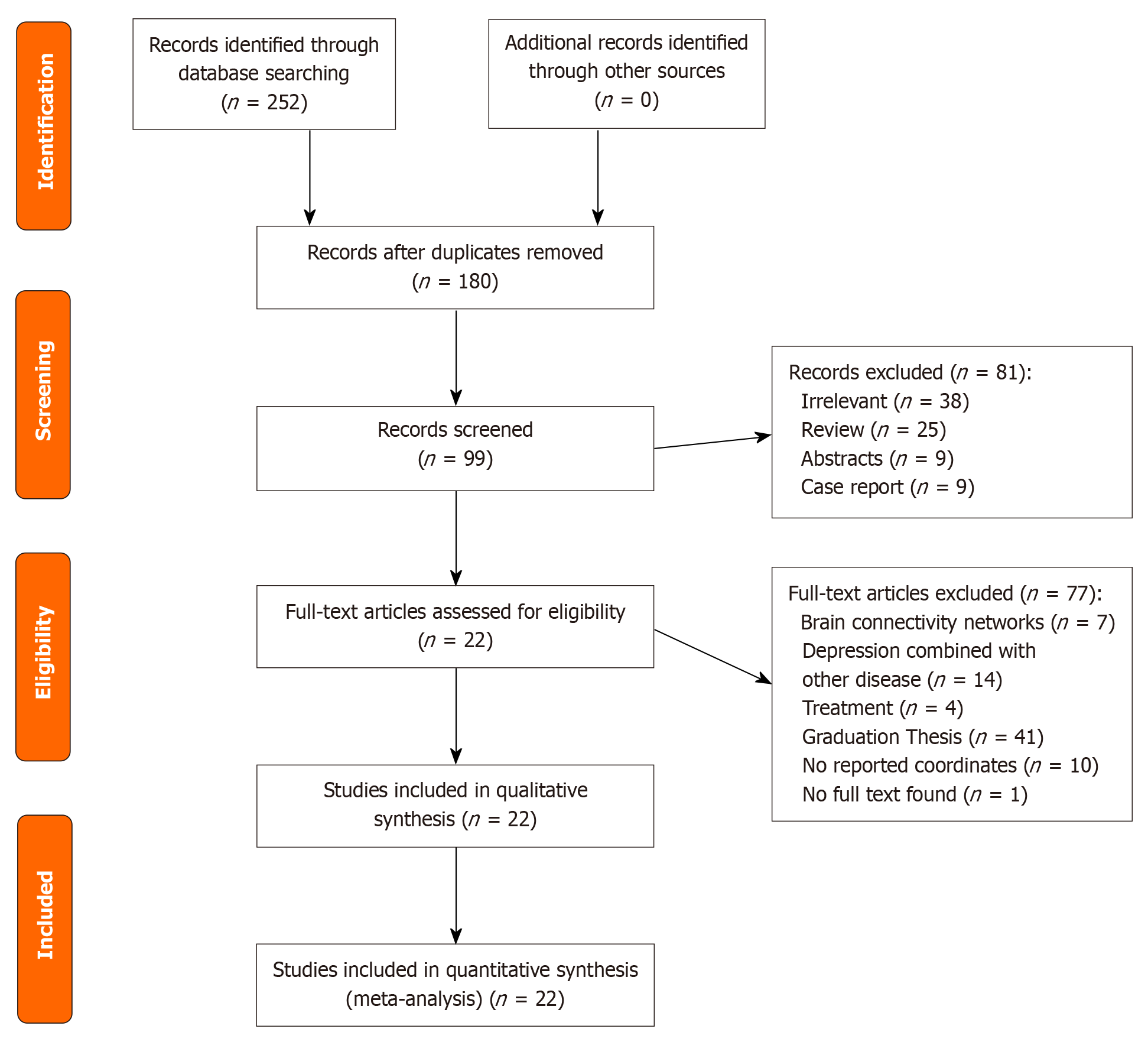
Studies were included in this analysis if they met the following criteria: (1) Had a diagnosis of depression according to the Diagnostic and Statistical Manual of Mental Disorders edition in use at the time of the study’s publication; (2) included adolescent or young adult participants; (3) were right-handed; and (4) had results reported for the whole brain in stereotactic space, either in the Montreal Neurological Institute (MNI) or Talairach coordinates for DTI or VBM studies. Studies were excluded if they met at least one of the following criteria: (1) Were abstracts, case reports, systematic reviews, or meta-analyses; (2) were intervention studies; (3) used regional homogeneity or amplitude of low-frequency fluctuations (ALFF); (4) focused on brain connectivity networks or depression in combination with other diseases; or (5) were studies wherein the full text was not available or did not report of coordinates.
To prevent data duplication, when two or more studies used the same dataset, only the study with the largest sample size and most comprehensive information was selected. For longitudinal or intervention studies, only baseline data were considered.
Two authors (H YZ and ZQ) independently extracted data from each study by using a predefined data extraction form. Any disagreements were resolved through discussion among the authors. Information on the authors, publication year, sample size, characteristics of the study population (age and sex), age range, and MRI technical details (MRI scanner, field strength, processing software, standard stereotactic space, method, differential brain region, corrective methods, and thresholds) was obtained (Table 1). The coordinates in each study were independently extracted following the require
| Ref. | Sample size | Age, mean ± SD | Sex, M/F | MRI equipment & field strength | Processing software | Method | Differential brain region | Corrective methods | Quality | |||
| Patient | HCs | Patient | HCs | Patient | HCs | |||||||
| Cullen et al[14], 2010 | 14 | 14 | 16.79 ± 1.29 | 16.81 ± 1.50 | 4/10 | 6/8 | Siemens Trio Tim 3.0 T | FSL | DTI | 10 | NA | 4/1/1 |
| Liu et al[15], 2010 | 12 | 16 | Approximately 30.38 | Approximately 29.75 | 4/12 | 4/12 | Siemens 1.5 T | SPM12 | DTI | 14 | Puncor < 0.001 | 4/1/1 |
| Henderson et al[16], 2013 | 17 | 16 | 6.80 ± 2.20 | 16.40 ± 1.40 | 9/8 | 6/10 | Siemens Allegra 3.0 T | FSL | TBSS | 4 | Puncor < 0.001 | 4/1/1 |
| Bessette et al[17], 2014 | 31 | 31 | 17.10 ± 1.88 | 17.00 ± 2.40 | 7/24 | 12/19 | Siemens Allegra MRI 3.0 T | FSL | TBSS | 56 | TFCE P < 0.05 | 4/1/1 |
| Jiang et al[18], 2015 | 35 | 34 | 29.54 ± 8.57 | 31.91 ± 8.80 | 17/18 | 17/17 | GE Milwaukee WI 3.0 T | FSL | DTI | 10 | Puncor < 0.001 | 4/1/1 |
| Xiao et al[19], 2015 | 22 | 22 | 20.14 ± 1.64 | 20.77 ± 1.41 | 12/10 | 12/10 | Siemens Magnetom Symphon 1.5 T | FSL | TBSS | 10 | P < 0.01 | 4/1/1 |
| Geng et al[20], 2016 | 26 | 31 | 15.60 ± 1.27 | 15.60 ± 1.38 | 7/19 | 14/17 | GE Signa HDX 3.0 T | PANDA software | DTI | 4 | AlphaSim, P < 0.05 | 4/1/1 |
| Tatham et al[21], 2016 | 55 | 18 | 36.40 ± 10.50 | 33.20 ± 10.20 | NA | NA | GE Signa HDX 3.0 T | FSL | TBSS | 3 | FEW, P < 0.05 | 4/1/1 |
| Chang et al[22], 2018 | 108 | 156 | 20.61 ± 4.91 | 22.25 ± 4.35 | 38/93 | 63/45 | GE Signa HDX 3.0 T | SPM8 | DTI/GMV | 5 & 17 | P < 0.01 | 4/1/1 |
| Wu et al[23], 2018 | 23 | 17 | 19.44 ± 4.61 | 18.07 ± 3.85 | NA | NA | GE Signa HDX 3.0 T | PANDA | DTI | 6 | P < 0.05 | 4/1/1 |
| Wei et al[24], 2020 | 49 | 49 | 30.03 ± 0.91 | 31.12 ± 9.95 | 11/38 | 18/31 | GE Signa HDX 3.0 T | SPM8 | DTI | 6 | GRF, P < 0.001 | 4/1/1 |
| Wang et al[25], 2020 | 18 | 18 | 15.77 ± 1.18 | 16.18 ± 0.95 | 10/8 | 10/8 | Siemens Trio Tim 3.0 T | NIT | FOCA | 3 | FDR, P < 0.05 | 4/1/1 |
| Lee et al[26], 2021 | 19 | 22 | 15.03 ± 1.45 | 15.96 ± 1.02 | NA | NA | Siemens Trio Tim 3.0 T | FSL | TBSS | 4 | FWE, P < 0.05 | 4/1/1 |
| Roelofs et al[27], 2022 | 22 | 21 | 15.93 ± 1.45 | 15.09 ± 1.80 | 2/20 | 4/17 | Philips Achieva 3.0 T | FSL | TBSS | 2 | FWE, P < 0.05 | 4/1/1 |
| Ding et al[28], 2010 | 18 | 18 | 15.78 ± 1.20 | 16.20 ± 0.90 | 10/8 | 10/8 | Siemens Trio Tim 3.0 T | SPM5 | GMV | 11 | P < 0.05 | 4/1/1 |
| Shad et al[29], 2012 | 22 | 22 | 16.00 ± 2.10 | 15.00 ± 2.10 | 10/12 | 11/11 | GE Milwaukee WI 1.5 T | SPM5 | GMV | 25 | FWE, P < 0.05 | 4/1/1 |
| Vulser et al[30], 2015 | 119 | 461 | 14.45 ± 0.36 | 14.40 ± 0.41 | 41/78 | 158/303 | NA | SPM8 | GMV | 8 | FWE, P < 0.05 | 4/1/1 |
| Straub et al[31], 2019 | 60 | 43 | 17.30 ± 3.44 | 17.62 ± 3.85 | 12/48 | 5/38 | Siemens Allegra 3.0 T | SPM 12 | GMV | 4 | FWE, P < 0.05 | 4/1/1 |
| Chen et al[32], 2023 | 95 | 78 | 18.14 ± 4.47 | 17.85 ± 4.30 | 95/0 | 38/40 | GE 750 1.5 T | SPM12 | GMV | 6 | GRF, P < 0.05 | 4/1/1 |
| Vulser et al[33], 2023 | 265 | 128 | 14.41 ± 0.52 | 14.40 ± 0.43 | NA | NA | Siemens Corp 3.0 T | SPM 12 | GMV | 4 | FWE, P < 0.05 | 4/1/1 |
| Deng et al[34], 2023 | 31 | 29 | NA | NA | 8/23 | 11/18 | GE Brivo MR355 1.5 T | SPM8 | GMV | 4 | FWE, P < 0.05 | 4/1/1 |
| Kang et al[35], 2023 | 54 | 167 | 35.69 ± 13.47 | 35.82 ± 12.91 | 21/33 | 67/100 | Siemens Trio Tim 3.0 T | SPM 12 | GMV | 18 | Puncor < 0.001 | 4/1/1 |
The quality of the included studies was assessed by using the Newcastle Ottawa Quality Assessment Scale (NOS). The NOS has three levels and a total of eight items: (1) Four items for subject selection; (2) one item for comparability between groups; and (3) three items for outcome measurement. The total score is 9 points. A result ≥ 5 points was included in the data analysis. Each study was independently reviewed and rated by two authors (H YZ and ZQ). If disagreements occurred, the papers were discussed by the authors’ group to determine a consensus score.
The ALE meta-analysis method was conducted by using GingerALE 3.0.2 software (www.brainmap.org/ale)[41]. ALE models each alteration focus as the center of a spherical Gaussian probability distribution. This approach is used to create spatial probability maps that highlight consistent brain region involvement in certain tasks or conditions[42]. For the ALE meta-analysis, our study was conducted in the MNI standard space. Hence, it was essential to utilize the Lancaster transformation within GingerALE 3.0.2 to convert the three-dimensional coordinates of brain regions in the Talairach space to the MNI space.
Subsequently, Gaussian function smoothing with a full width at half maximum (FWHM) was performed based on the sample size of each test group. Using the FWHM values, Gaussian functions were simulated on the three-dimensional brain mask of coordinates for a set of aberrantly activated brain regions that were reported in the study group. This process yielded three-dimensional modeling activation (MA) maps for each study group.
Afterwards, based on three-dimensional (3D)-MA maps, a 3D ALE map was generated of the Gaussian probability distribution of the activated brain regions between different study groups, and the P value of the activation probability of the brain regions was calculated according to the Gaussian model to construct a 3D-P value distribution map. Moreover, the statistical test threshold was set by a 3D-P value distribution plot. The main parameters were as follows: the cluster-level familywise error (FWE) correction was set at P < 0.05, the threshold permutations were set at P < 0.001 with 1000 permutations, and finally, a threshold map (ALE image) was obtained. Finally, our study used Mango software (http://rii.uthscsa.edu/mango/) to check and analyze the resulting ALE images.
The jackknife sensitivity analysis method was employed to assess the reproducibility of the meta-analysis outcomes. In this approach, a single study was systematically excluded from the dataset, followed by ALE meta-analysis of the remaining study data using GingerALE 3.0.2 software. This procedure was repeated 14 times (each time removing one study) to verify the consistency of the results after the exclusion of a study and to compare these results with the original analysis.
The systematic search generated 252 related articles, 22 of which[14-35] were ultimately selected for inclusion in this meta-analysis (Figure 1). These included 14 studies utilizing DTI and 8 utilizing VBM. Notably, the study by Chang et al[22] encompassed analyses using both DTI and VBM methodologies, thereby contributing to a total of nine VBM-based investigations. Finally, the DTI studies included 451 individuals diagnosed with MDD and 465 HCs, with the identification of alterations in 137 distinct brain areas. The VBM studies included 664 individuals diagnosed with MDD and 946 HCs and identified alterations in 80 distinct brain areas (Table 1).
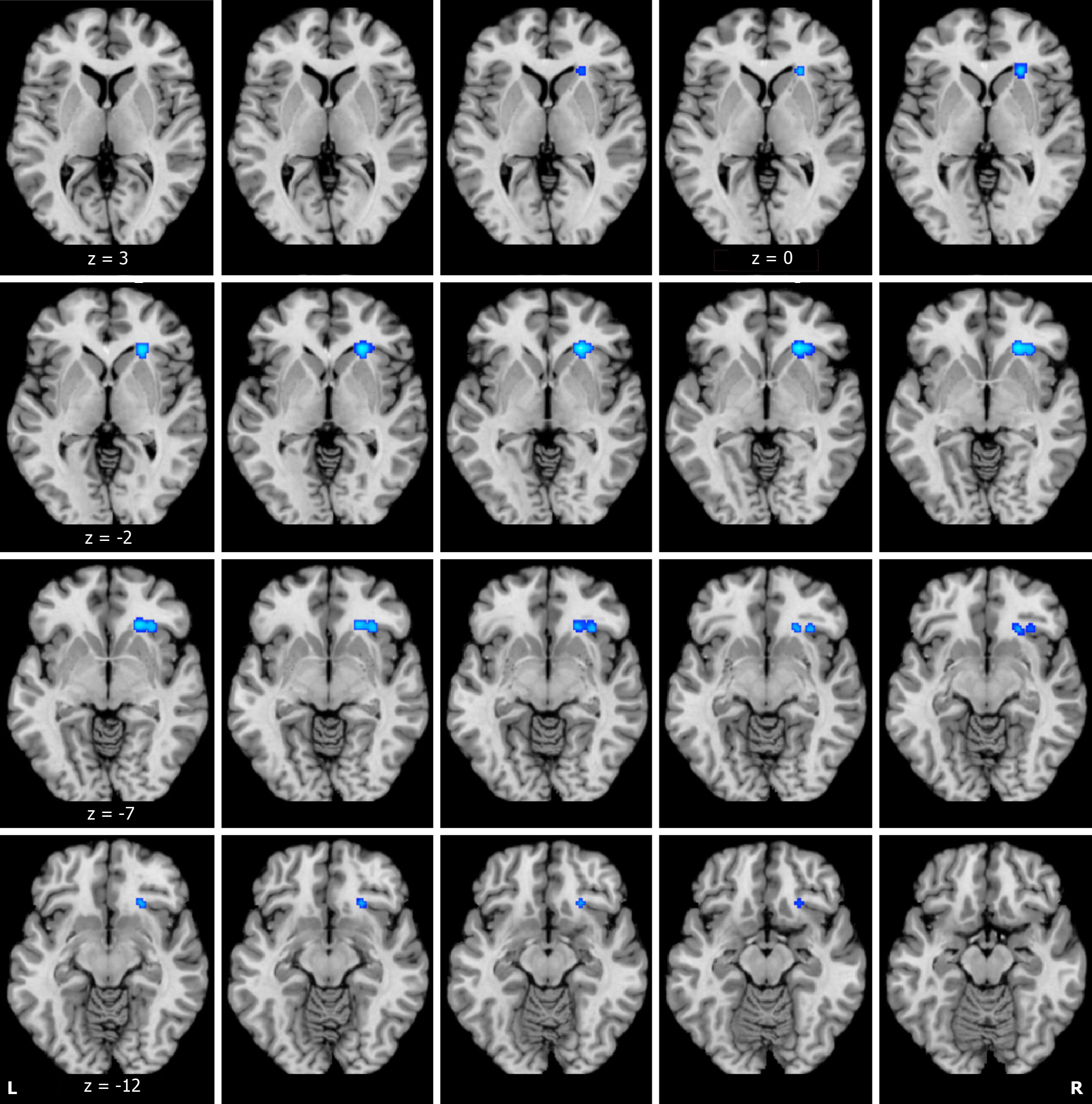
ALE analysis based on DTI structural data demonstrated significant reductions in fractional anisotropy (FA) values in the right caudate head, right insula, and right lentiform nucleus putamen in adolescents and young adults with depression in comparison to HCs (Figure 2 and Table 2). However, no brain regions where FA values increased were identified. In contrast, ALE analysis based on VBM structural data did not reveal any significant changes in gray matter volume.
| Research methods | Anatomical label | Peak MNI coordinate | ALE value | Volume in mm3 | ||
| X | Y | Z | ||||
| FA decrease | Right caudate head | 24 | 28 | -4 | 0.015717618 | 1096 |
| Right insula | 32 | 26 | -8 | 0.012060626 | 656 | |
| Right lentiform nucleus putamen | 24 | 24 | -12 | 0.010999768 | 696 | |
The outcomes of the sensitivity analysis demonstrated reproducibility in the findings, with the right caudate head, right insula, and right lentiform nucleus putamen showing consistent alterations in 11 of 14 analyses, 10 of 14 analyses, and 11 of 14 analyses, respectively (Table 3).
| Discarded article | Right caudate head | Right insula | Right lentiform nucleus putamen |
| Cullen et al[14], 2010 | N | N | N |
| Liu et al[15], 2010 | Y | Y | Y |
| Henderson et al[16], 2013 | Y | Y | Y |
| Bessette et al[17], 2014 | N | N | N |
| Jiang et al[18], 2015 | Y | Y | Y |
| Xiao et al[19], 2015 | Y | Y | Y |
| Geng et al[20], 2016 | Y | N | Y |
| Tatham et al[21], 2016 | Y | Y | Y |
| Chang et al[22], 2018 | Y | Y | Y |
| Wu et al[23], 2018 | N | N | N |
| Wei et al[24], 2020 | Y | Y | Y |
| Wang et al[25], 2020 | Y | Y | Y |
| Lee et al[26], 2021 | Y | Y | Y |
| Roelofs et al[27], 2022 | Y | Y | Y |
To the best of our knowledge, this meta-analysis represents the inaugural exploration of multimodal brain structural abnormalities in adolescents and young adults with depression by employing the ALE methodology, with the utilization of both DTI and VBM. The findings of this meta-analysis underscore the nuanced neurobiological underpinnings of MDD among adolescents and young adults, thus revealing significant alterations in brain structure and function. The observed reductions in FA within specific brain regions, such as the right caudate head, right insula, and right lentiform nucleus putamen, align with literature suggesting the pivotal role that these areas play in emotional regulation, cognitive processing, and the reward system[43,44]. Notably, no regions exhibited augmented FA values, thus suggesting a pervasive trend toward decreased white matter integrity across the affected neural domains. Alterations in white matter microstructure, particularly in the uncinate fasciculus, are associated with emotional dysregulation[45]. Additionally, a study on sex incongruence revealed lower FA in the inferior fronto-occipital fasciculus, thus emphasizing the role of white matter organization in sex identity[46]. Furthermore, findings in adolescents at high risk for bipolar disorder (BD) suggest decreased FA in various brain areas, thus emphasizing the potential role of FA as an endophenotype for BD[47]. The insula, which is a component of the limbic system, plays a pivotal role in memory and emotion processing[48]. The lack of areas showing increased FA values further emphasizes the potential of these structural changes as being neural markers of MDD, thus being potentially indicative of disrupted white matter integrity and altered neural connectivity.
However, contrary to the alterations observed in white matter through DTI analysis, this study did not identify any regions exhibiting significant changes in gray matter volume among adolescents and young adults with depression. This indicates a more consistent pattern of brain structural changes characterized by reduced integrity, particularly in regions such as the right caudate head, right insula, and right lentiform nucleus putamen, compared to HCs. The absence of significant gray matter volume changes further underscores the potential of these structural alterations as being neurobiomarkers for MDD, thus being potentially indicative of impaired white matter integrity and altered neural connectivity. These findings provide objective neuroimaging evidence, which enriches our understanding of the neurobiological mechanisms underlying depression in adolescents and young adults.
The putamen and globus pallidus together form the lentiform nucleus, which, along with the caudate nucleus, constitutes the striatum. The striatum, which is a subcortical structure, is divided into the ventral and dorsal striatum. The dorsal striatum includes the caudate nucleus and putamen, whereas the ventral striatum comprises the nucleus accumbens[49]. The caudate nucleus is believed to control cognitive aspects within the striatum, thus playing a role in emotional processing, motor control, and particularly in regulating cognitive functions[50,51]. The putamen, which is a critical component of the basal ganglia, is involved in learning, controlling bodily movements, and processing visual information and is associated with emotional processing, cognitive processes, motivation, and motor regulation, thus playing a key role in action selection[52,53].
Recent research has elucidated the structural abnormalities within the striatum in MDD patients, particularly in adolescents and young adults. For instance, studies have reported of lower FA values in the right caudate nucleus among MDD patients than among HCs, thus highlighting white matter integrity impairment[54]. Furthermore, Ding et al[55] reported that individuals with MDD exhibit reduced FA values across the striatum bilaterally, thus suggesting widespread structural changes within this brain region. Moreover, volumetric studies have noted a reduction in the size of the striatum among MDD patients, with one study demonstrating volume reductions in the ventral striatum and putamen even before the onset of the disorder in adolescents at risk due to parental MDD history[56,57]. This familial predisposition is further evidenced by findings of increased right striatum gray matter volume among first-degree relatives of MDD patients, thus suggesting that striatal structural abnormalities may be a risk factor for developing MDD[58].
These findings, although predominantly cross-sectional, provide a foundational basis for future longitudinal studies aimed at elucidating the causal relationships between striatal volume abnormalities and the progression of MDD. The consistent observation of striatal volume reductions in MDD patients compared to HCs across various studies underlines the potential of the striatum as being a key neural substrate in the pathophysiology of depression.
This meta-analysis demonstrated a decrease in FA values within the right insula among adolescents and young adults with MDD. The insula, which is a cortical structure with extensive connections to various cortical and limbic regions, plays a critical role in diverse cognitive, emotional, and regulatory functions, including interoceptive awareness, emotional responses, and empathic processes[59]. Several meta-analyses based on structural MRI studies have reported of bilateral reductions in insular gray matter volume in individuals with MDD[60,61]. Lu et al[62] observed a decrease in the gray matter volume of the right insula with the progression of MDD. Furthermore, functional MRI studies have identified alterations in functional connectivity within significant networks centered on the insula, such as the salience network, default mode network (DMN), and central executive network (CEN), in MDD patients[63,64]. The insula’s mediation of dynamic switches between the DMN and CEN facilitates the allocation of cognitive resources, such as attention and working memory. Hence, alterations in the connectivity strength within these networks in affective disorders can lead to cognitive impairments[65].
Task-based functional MRI studies have demonstrated atypical insula activity during executive function and emotional processing tasks in individuals with MDD, thus potentially revealing the difficulties in cognitive-emotional integration observed in individuals with adolescent depression[66]. Positron emission tomography studies, such as those conducted by Delaveau et al[67], have also noted reduced insula activation in MDD patients. Moreover, changes in insula activity have been observed following various MDD treatments, including pharmacotherapy, deep brain stimulation, and cognitive behavioral therapy, which suggests that the region has a broader role in mediating antidepressant responses and remission[68].
Collectively, these findings, which are derived from diverse neuroimaging methodologies, consistently underscore the critical role of the insula in the neuropathology of depression[69]. These findings further highlight functional and structural abnormalities in the insula as being key neurobiological features of affective disorders.
This meta-analysis did not identify changes in gray matter volume in the brains of adolescents or young adults with depression, which may be attributed to the relatively small number of reported brain regions with altered gray matter volume in the included studies. Among the eight studies that were analyzed, all of them reported regions of reduced gray matter volume, yet five of these studies had fewer than ten central coordinates for the reported areas of abnormal activity. The ALE meta-analysis, which functions as a probability distribution, is more influenced by studies reporting of a greater number of activation points. The scarcity of coordinates may fail to meet the threshold criteria, or the spatial information of these activity regions may be too dispersed to allow for the consolidation of results in areas proximal to the activation points. This dispersion could also explain the absence of increased activity findings in the DTI-based meta-analysis[70]. Therefore, whether there are specific brain regions in adolescents and young adults with depression that exhibit reduced gray matter volume relative to HCs and whether isolated DTI meta-analyses can demonstrate regions of increased activity warrant further exploration.
First, the adoption of stringent exclusion criteria, particularly the exclusion of studies involving pharmacological or acupuncture treatments, has resulted in a smaller amount of literature for analysis. Second, although the ALE metho
In conclusion, the structural alterations observed in the striatum and insula among adolescents and young adults with MDD may be indicative of the recurrent nature of the disorder. These findings underscore the potential of these structural changes as being neural markers for MDD, thus offering insights into the neuropathology of this disorder. Future research should aim to elucidate the longitudinal implications of these structural alterations.
We thank Hui Ding for his crucial data analysis and insights that greatly enhanced this study.
| 1. | Cui L, Li S, Wang S, Wu X, Liu Y, Yu W, Wang Y, Tang Y, Xia M, Li B. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther. 2024;9:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 481] [Article Influence: 240.5] [Reference Citation Analysis (0)] |
| 2. | Shorey S, Ng ED, Wong CHJ. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br J Clin Psychol. 2022;61:287-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 801] [Article Influence: 160.2] [Reference Citation Analysis (0)] |
| 3. | The Lancet. Ensuring care for people with depression. Lancet. 2022;399:885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Sampogna G, Caraci F, Carmassi C, Dell'Osso B, Ferrari S, Martinotti G, Sani G, Serafini G, Signorelli MS, Fiorillo A. Efficacy and tolerability of desvenlafaxine in the real-world treatment of patients with major depression: a narrative review and an expert opinion paper. Expert Opin Pharmacother. 2023;24:1511- 1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Wu Y, Chiu MYL, Wu W, Han S, Wang J. What makes Chinese adolescents "trapped" in severe mental illness? An interactionist perspective on self and identity. Int J Qual Stud Health Well-being. 2023;18:2250093. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Karow A, Lambert M, Finter C, Hohmann S. [Mental Diseases in Adolescence - Approaches of Treatment and Clinical Insights]. Prax Kinderpsychol Kinderpsychiatr. 2022;71:658-676. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Videtta G, Squarcina L, Prunas C, Brambilla P, Delvecchio G. White matter integrity and medication response to antidepressants in major depressive disorder: a review of the literature. Front Psychiatry. 2023;14:1335706. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Kalin NH. Using Neuroimaging to Characterize Brain Alterations Associated With Psychopathology. Am J Psychiatry. 2019;176:495-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Xiao J, Wu J. Effectiveness of the Neuroimaging Techniques in the Recognition of Psychiatric Disorders: A Systematic Review and Meta-analysis of RCTs. Curr Med Imaging. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Cattarinussi G, Gugliotta AA, Hirjak D, Wolf RC, Sambataro F. Brain mechanisms underlying catatonia: A systematic review. Schizophr Res. 2024;263:194-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Kloiber S, Rosenblat JD, Husain MI, Ortiz A, Berk M, Quevedo J, Vieta E, Maes M, Birmaher B, Soares JC, Carvalho AF. Neurodevelopmental pathways in bipolar disorder. Neurosci Biobehav Rev. 2020;112:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 12. | Ma H, Zhang D, Wang Y, Ding Y, Yang J, Li K. Prediction of early improvement of major depressive disorder to antidepressant medication in adolescents with radiomics analysis after ComBat harmonization based on multiscale structural MRI. BMC Psychiatry. 2023;23:466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 13. | Pareek V, Nath B, Roy PK. Role of Neuroimaging Modality in the Assessment of Oxidative Stress in Brain: A Comprehensive Review. CNS Neurol Disord Drug Targets. 2019;18:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, Kurma S, Lim KO. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49:173-83.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Liu X, Wang Y, Liu H, Liu Z, Zhou W. [Diffusion tensor imaging and resting state functional magnetic resonance imaging on young patients with major depressive disorder]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 16. | Henderson SE, Johnson AR, Vallejo AI, Katz L, Wong E, Gabbay V. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front Psychiatry. 2013;4:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Bessette KL, Nave AM, Caprihan A, Stevens MC. White matter abnormalities in adolescents with major depressive disorder. Brain Imaging Behav. 2014;8:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Jiang W, Gong G, Wu F, Kong L, Chen K, Cui W, Ren L, Fan G, Sun W, Ma H, Xu K, Tang Y, Wang F. The papez circuit in first-episode, treatment-naive adults with major depressive disorder: combined atlas-based tract-specific quantification analysis and voxel-based analysis. PLoS One. 2015;10:e0126673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Xiao J, He Y, McWhinnie CM, Yao S. Altered white matter integrity in individuals with cognitive vulnerability to depression: a tract-based spatial statistics study. Sci Rep. 2015;5:9738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Geng H, Wu F, Kong L, Tang Y, Zhou Q, Chang M, Zhou Y, Jiang X, Li S, Wang F. Disrupted Structural and Functional Connectivity in Prefrontal-Hippocampus Circuitry in First-Episode Medication-Naïve Adolescent Depression. PLoS One. 2016;11:e0148345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Tatham EL, Ramasubbu R, Gaxiola-Valdez I, Cortese F, Clark D, Goodyear B, Foster J, Hall GB. White matter integrity in major depressive disorder: Implications of childhood trauma, 5-HTTLPR and BDNF polymorphisms. Psychiatry Res Neuroimaging. 2016;253:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Chang M, Womer FY, Edmiston EK, Bai C, Zhou Q, Jiang X, Wei S, Wei Y, Ye Y, Huang H, He Y, Xu K, Tang Y, Wang F. Neurobiological Commonalities and Distinctions Among Three Major Psychiatric Diagnostic Categories: A Structural MRI Study. Schizophr Bull. 2018;44:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Wu F, Kong L, Zhu Y, Zhou Q, Jiang X, Chang M, Zhou Y, Cao Y, Xu K, Wang F, Tang Y. The Influence of Myelin Oligodendrocyte Glycoprotein on White Matter Abnormalities in Different Onset Age of Drug-Naïve Depression. Front Psychiatry. 2018;9:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Wei S, Womer FY, Edmiston EK, Zhang R, Jiang X, Wu F, Kong L, Zhou Y, Tang Y, Wang F. Structural alterations associated with suicide attempts in major depressive disorder and bipolar disorder: A diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;98:109827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Wang XJ, Ding J, Jiao Q, Guo YX, Cao WF, Cui D, SU LY, Lu GM. Four-dimensional (spatio-temporal) consistency of local neural activities in adolescent patients with major depression disorder: a functional magnetic resonance imaging study. Taishan Yixueyuan Xuebao. 2020;41:481-485. |
| 26. | Lee JH, Chi S, Ko M, Song M, Ham BJ, Ko YH, Suh SI, Lee MS. Prospective study on microstructure in medication-naïve adolescents with first-episode major depressive disorder. J Affect Disord. 2021;293:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Roelofs EF, Bas-Hoogendam JM, van der Werff SJA, Valstar SD, van der Wee NJA, Vermeiren RRJM. Exploring the course of adolescent anxiety and depression: associations with white matter tract microstructure. Eur Arch Psychiatry Clin Neurosci. 2022;272:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Ding J, Su LY, Zhang ZQ, Lu GM, Ma J, Zhang Y, Huang W, Liu XY. Magnetic Resonance Imaging Case Control Study on Brain Three Dimension Structural Abnormalities of First-episode Medication-naive Adolescents with Major Depressive Disorder. Zhongguo Linchuang Xinlixue Zazhi. 2010;18:403-406. [DOI] [Full Text] |
| 29. | Shad MU, Muddasani S, Rao U. Gray matter differences between healthy and depressed adolescents: a voxel-based morphometry study. J Child Adolesc Psychopharmacol. 2012;22:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Vulser H, Lemaitre H, Artiges E, Miranda R, Penttilä J, Struve M, Fadai T, Kappel V, Grimmer Y, Goodman R, Stringaris A, Poustka L, Conrod P, Frouin V, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Büchel C, Flor H, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Lawrence C, Loth E, Mann K, Nees F, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Schumann G, Martinot JL, Paillère-Martinot ML; IMAGEN Consortium (www. imagen-europe.com); IMAGEN Consortium www imagen-europe com. Subthreshold depression and regional brain volumes in young community adolescents. J Am Acad Child Adolesc Psychiatry. 2015;54:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Straub J, Brown R, Malejko K, Bonenberger M, Grön G, Plener PL, Abler B. Adolescent depression and brain development: evidence from voxel-based morphometry. J Psychiatry Neurosci. 2019;44:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Chen Y, Chen Y, Zheng R, Jiang Y, Zhou B, Xue K, Li S, Pang J, Li H, Zhang Y, Han S, Cheng J. Convergent molecular and structural neuroimaging signatures of first-episode depression. J Affect Disord. 2023;320:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 33. | Vulser H, Lemaître HS, Guldner S, Bezivin-Frère P, Löffler M, Sarvasmaa AS, Massicotte-Marquez J, Artiges E, Paillère Martinot ML, Filippi I, Miranda R, Stringaris A, van Noort BM, Penttilä J, Grimmer Y, Becker A, Banaschewski T, Bokde ALW, Desrivières S, Fröhner JH, Garavan H, Grigis A, Gowland PA, Heinz A, Papadopoulos Orfanos D, Poustka L, Smolka MN, Spechler PA, Walter H, Whelan R, Schumann G, Flor H, Martinot JL, Nees F; IMAGEN Consortium. Chronotype, Longitudinal Volumetric Brain Variations Throughout Adolescence, and Depressive Symptom Development. J Am Acad Child Adolesc Psychiatry. 2023;62:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Deng J, He JB, Wang YT, Yuan YZ, Guo X, Huang B, Wang XR, Yang HL, Mei L, Qiu LH. Changes of gray matter volume in adolescents with first-episode untreated depression. Zhongguo Yixue Yingxiangjishu Zazhi. 2023;39:17-21. [DOI] [Full Text] |
| 35. | Kang W, Kang Y, Kim A, Kim H, Han KM, Ham BJ. Gray and white matter abnormalities in major depressive disorder patients and its associations with childhood adversity. J Affect Disord. 2023;330:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 36. | Wang W, Jia S, Zhao Q, Yang L. Diagnosis of Neural Activity among Abnormal Brain Regions in Patients with Major Depressive Disorder by Magnetic Resonance Imaging Features. Comput Math Methods Med. 2022;2022:3044010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 37. | Pereira-Sanchez V, Castellanos FX. Neuroimaging in attention-deficit/hyperactivity disorder. Curr Opin Psychiatry. 2021;34:105-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 38. | Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 39. | Yuan J, Yu H, Yu M, Liang X, Huang C, He R, Lei W, Chen J, Chen J, Tan Y, Liu K, Zhang T, Luo H, Xiang B. Altered spontaneous brain activity in major depressive disorder: An activation likelihood estimation meta-analysis. J Affect Disord. 2022;314:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48652] [Article Influence: 2861.9] [Reference Citation Analysis (3)] |
| 41. | Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907-2926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1655] [Cited by in RCA: 1553] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 42. | Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 1165] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 43. | Rappaport BI, Kandala S, Luby JL, Barch DM. Brain Reward System Dysfunction in Adolescence: Current, Cumulative, and Developmental Periods of Depression. Am J Psychiatry. 2020;177:754-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Burgetova R, Dusek P, Burgetova A, Pudlac A, Vaneckova M, Horakova D, Krasensky J, Varga Z, Lambert L. Age-related magnetic susceptibility changes in deep grey matter and cerebral cortex of normal young and middle-aged adults depicted by whole brain analysis. Quant Imaging Med Surg. 2021;11:3906-3919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 45. | Xu EP, Nguyen L, Leibenluft E, Stange JP, Linke JO. A meta-analysis on the uncinate fasciculus in depression. Psychol Med. 2023;53:2721-2731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | van Heesewijk J, Steenwijk MD, Kreukels BPC, Veltman DJ, Bakker J, Burke SM. Alterations in the inferior fronto-occipital fasciculus - a specific neural correlate of gender incongruence? Psychol Med. 2023;53:3461-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 47. | Camkurt MA, Melicher T, Mwangi B, Wu MJ, Cao B, Zeni CP, Tannous J, Zunta-Soares G, Hasan K, Sanches M, Soares JC. Investigation of endophenotype potential of decreased fractional anisotropy in pediatric bipolar disorder patients and unrelated offspring of bipolar disorder patients. CNS Spectr. 2022;27:709-715. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Labrakakis C. The Role of the Insular Cortex in Pain. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 49. | Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci. 2003;3:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 298] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 50. | An J, Li L, Wang L, Su YA, Wang Y, Li K, Zeng Y, Kong Q, Yan C, Si T. Striatal Functional Connectivity Alterations After Two-Week Antidepressant Treatment Associated to Enduring Clinical Improvement in Major Depressive Disorder. Front Psychiatry. 2019;10:884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Choi KW, Han KM, Kim H, Kim A, Kang W, Kang Y, Tae WS, Ham BJ. Comparison of shape alterations of the thalamus and caudate nucleus between drug-naïve major depressive disorder patients and healthy controls. J Affect Disord. 2020;264:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Kunimatsu J, Maeda K, Hikosaka O. The Caudal Part of Putamen Represents the Historical Object Value Information. J Neurosci. 2019;39:1709-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Jenni NL, Rutledge G, Floresco SB. Distinct Medial Orbitofrontal-Striatal Circuits Support Dissociable Component Processes of Risk/Reward Decision-Making. J Neurosci. 2022;42:2743-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | He M, Cheng Y, Chu Z, Wang X, Xu J, Lu Y, Shen Z, Xu X. White Matter Network Disruption Is Associated With Melancholic Features in Major Depressive Disorder. Front Psychiatry. 2022;13:816191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 55. | Ding H, Tian B, Shu YP, Li SQ. A diffusion tensor imaging study on the microstructure of different brain regions in adolescent depression patients with attempted suicide. Shiyong Fangshexue Zazhi. 2022;38:10-13. [DOI] [Full Text] |
| 56. | Ho TC. Editorial: Toward Neurobiological-Based Treatments of Depression and Anxiety: A Potential Case for the Nucleus Accumbens. J Am Acad Child Adolesc Psychiatry. 2022;61:136-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Pagliaccio D, Alqueza KL, Marsh R, Auerbach RP. Brain Volume Abnormalities in Youth at High Risk for Depression: Adolescent Brain and Cognitive Development Study. J Am Acad Child Adolesc Psychiatry. 2020;59:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 58. | Zhang W, Sweeney JA, Yao L, Li S, Zeng J, Xu M, Tallman MJ, Gong Q, DelBello MP, Lui S, Nery FG. Brain structural correlates of familial risk for mental illness: a meta-analysis of voxel-based morphometry studies in relatives of patients with psychotic or mood disorders. Neuropsychopharmacology. 2020;45:1369-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 59. | Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4387] [Cited by in RCA: 4197] [Article Influence: 262.3] [Reference Citation Analysis (0)] |
| 60. | Sha Z, Wager TD, Mechelli A, He Y. Common Dysfunction of Large-Scale Neurocognitive Networks Across Psychiatric Disorders. Biol Psychiatry. 2019;85:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 255] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 61. | Zhang H, Li L, Wu M, Chen Z, Hu X, Chen Y, Zhu H, Jia Z, Gong Q. Brain gray matter alterations in first episodes of depression: A meta-analysis of whole-brain studies. Neurosci Biobehav Rev. 2016;60:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 62. | Lu F, Cui Q, Chen Y, He Z, Sheng W, Tang Q, Yang Y, Luo W, Yu Y, Chen J, Li D, Deng J, Zeng Y, Chen H. Insular-associated causal network of structural covariance evaluating progressive gray matter changes in major depressive disorder. Cereb Cortex. 2023;33:831-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Gong J, Wang J, Qiu S, Chen P, Luo Z, Wang J, Huang L, Wang Y. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl Psychiatry. 2020;10:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 64. | Wang J, Wang Y, Wu X, Huang H, Jia Y, Zhong S, Wu X, Zhao L, He Y, Huang L, Huang R. Shared and specific functional connectivity alterations in unmedicated bipolar and major depressive disorders based on the triple-network model. Brain Imaging Behav. 2020;14:186-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 65. | Namkung H, Kim SH, Sawa A. The Insula: An Underestimated Brain Area in Clinical Neuroscience, Psychiatry, and Neurology. Trends Neurosci. 2017;40:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 319] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 66. | Miller CH, Hamilton JP, Sacchet MD, Gotlib IH. Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth. JAMA Psychiatry. 2015;72:1045-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 67. | Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord. 2011;130:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 68. | McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 353] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 69. | Savitz J, Nugent AC, Cannon DM, Carlson PJ, Davis R, Neumeister A, Rallis-Frutos D, Fromm S, Herscovitch P, Drevets WC. Effects of arterial cannulation stress on regional cerebral blood flow in major depressive disorder. Sci Rep. 2012;2:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Zhang DS, Gao J, Zhe X, Yan XJ, Tang M, Yang J, Zhang XL. Altered spontaneous brain activity in type 2 diabetes mellitus: an activation likelihood estimation Meta-analysis. Zhonghua Fangshexue Zazhi. 2018;52:241-246. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/