Published online Jun 19, 2024. doi: 10.5498/wjp.v14.i6.884
Revised: April 28, 2024
Accepted: May 20, 2024
Published online: June 19, 2024
Processing time: 107 Days and 4.9 Hours
Patients with schizophrenia may have various disease manifestations, most of which gradually tend toward incurable chronic decline, leading to mental disa
To investigate the effects of Computerized Cognitive Remediation Therapy (CCRT) on cognitive and social functioning in patients with chronic schizophrenia.
A retrospective analysis of 120 patients with chronic schizophrenia in Shanghai Pudong New Area Mental Health Center was performed. They were divided into an intervention group (60 cases treated with CCRT combined with conventional medication) and a control group (60 cases treated with conventional medication). After treatment, effects on cognitive function and social roles were observed in both groups. The Positive and Negative Syndrome Scale (PANSS) was used to assess the patients' psychiatric symptoms. The Wisconsin Card Sorting Test (WCST) was used to assess the patients' cognitive functioning, and the Social Functioning Scale for Psychiatric Inpatients (SSPI) was used to assess the social functioning of the inpatient psychiatric patients.
No significant differences were observed in the PANSS, WCST, and SSPI intergroup scores before treatment (P > 0.05). After 2, 4, and 6 wk of therapy, general psychopathological factors, positive symptoms, negative symptoms, and total PANSS scores of PANSS in the intervention group were lower than in the control group (P < 0.05). After 2, 4, and 6 wk of treatment, the number of false responses, number of persistent bugs, and total responses in the WCST were significantly lower in the intervention group than in the control group (P < 0.05), and the amount of completed classification was significantly higher than in the control group (P < 0.05). After 2, 4, and 6 wk of therapy, the SSPI scores were significantly greater than those of the controls (P < 0.05). After 6 wk of treatment, the efficacy rates of the control and intervention groups were 81.67% and 91.67%, respectively. The curative effect in the intervention group was significantly higher than that in the control group (P < 0.05).
CCRT can significantly improve cognitive function and social abilities in patients with chronic schizophrenia.
Core Tip: Chronic schizophrenia is generally an unconscious disorder with obvious intellectual disabilities. Insidious onset, prolonged course, repeated aggravation or deterioration, and negative symptoms of mental illness are common manifestations of the disease. Cognitive function and social life ability are severely impaired. In this study, we investigated the effect of Computerized Cognitive Remediation Therapy on cognitive impairment and society using the Patient and Negative Syndrome Scale, Wisconsin Card Sorting Test, and Scale of Social Function in Psychosis Inpatients scores in 120 patients with chronic schizophrenia.
- Citation: Hu JJ, Sun XR, Ni SM, Kong Y. Computerized cognitive remediation therapy on cognitive impairment and social function in patients with chronic schizophrenia. World J Psychiatry 2024; 14(6): 884-893
- URL: https://www.wjgnet.com/2220-3206/full/v14/i6/884.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i6.884
Schizophrenia is a common major mental disorder, the cause of which remains unclear[1]. According to relevant epidemiological investigations, schizophrenia affects a broad spectrum of patients and can occur in individuals of various age groups. It is complicated by mental activity abnormalities in many aspects, such as perception, thinking, behavior, and emotional responses. The disease course is characterized by prolonged and repeated episodes that seriously affect social and cognitive function[2]. More severe patients may also have suicidal and violent tendencies, endangering the lives of patients and others[3].
Currently, in addition to the two core symptoms of schizophrenia-positive and negative-a third symptom group has gradually entered the field of vision and gained attention among scholars in the field of psychiatry: Cognitive dysfunc
Clinically, schizophrenia is mainly treated with antipsychotic drugs; however, a single drug treatment can only control the mental symptoms of patients and does not have a significant impact on their cognitive and social features; the treatment requires a long duration and is prone to adverse reactions, resulting in poor treatment compliance[6]. Computerized Cognitive Remediation Therapy (CCRT) is a training method based on behavioral training that improves thinking abilities[7]. Studies have confirmed[8] that applying CCRT for cognitive improvement in patients can effectively improve language, memory, executive power, and other functions. This is because computer cognitive correction therapy is a type of rehabilitation software that is highly targeted for patients with schizophrenia. In the software system, training and intervention are carried out on the patients' working memory, attention, reasoning and problem-solving, processing speed, social cognitive, and computing abilities. The ability of each part of the patient to interfere in a modular manner enhances cognitive performance and social function. In conclusion, as a new therapy, CCRT can effectively enhance the cognitive dysfunction of patients, and the effect is significant; however, few reports are available on CCRT treatment in patients with chronic schizophrenia. Therefore, this study aimed to examine how computerized therapy corrects cognitive deficits and affects the social functioning of patients with chronic schizophrenia.
One hundred and twenty patients with chronic deficit schizophrenia diagnosed at Shanghai Pudong New Area Mental Health Center between April 2021 and July 2022 were selected as study participants, including 69 males and 51 females, who were divided into an intervention (60 patients) and control groups (60 patients). Inclusion criteria: (1) All participants met the diagnostic criteria for schizophrenia[9]; (2) stable symptoms, current maintenance treatment with comparable antipsychotic medication, and Positive and Negative Symptom Scale (PANSS) scores of ≤ 70; (3) hospitalization > 2 times, with a total duration of illness of 2-10 years; age of 20-58 years old, and age of education > 8 years old; and (4) complete clinical data on patients. The exclusion criteria were as follows: (1) Comorbid chronic organic diseases; (2) mental and learning disabilities; (3) psychoactive substance use disorders or alcohol addiction; and (4) pregnant or lactating women.
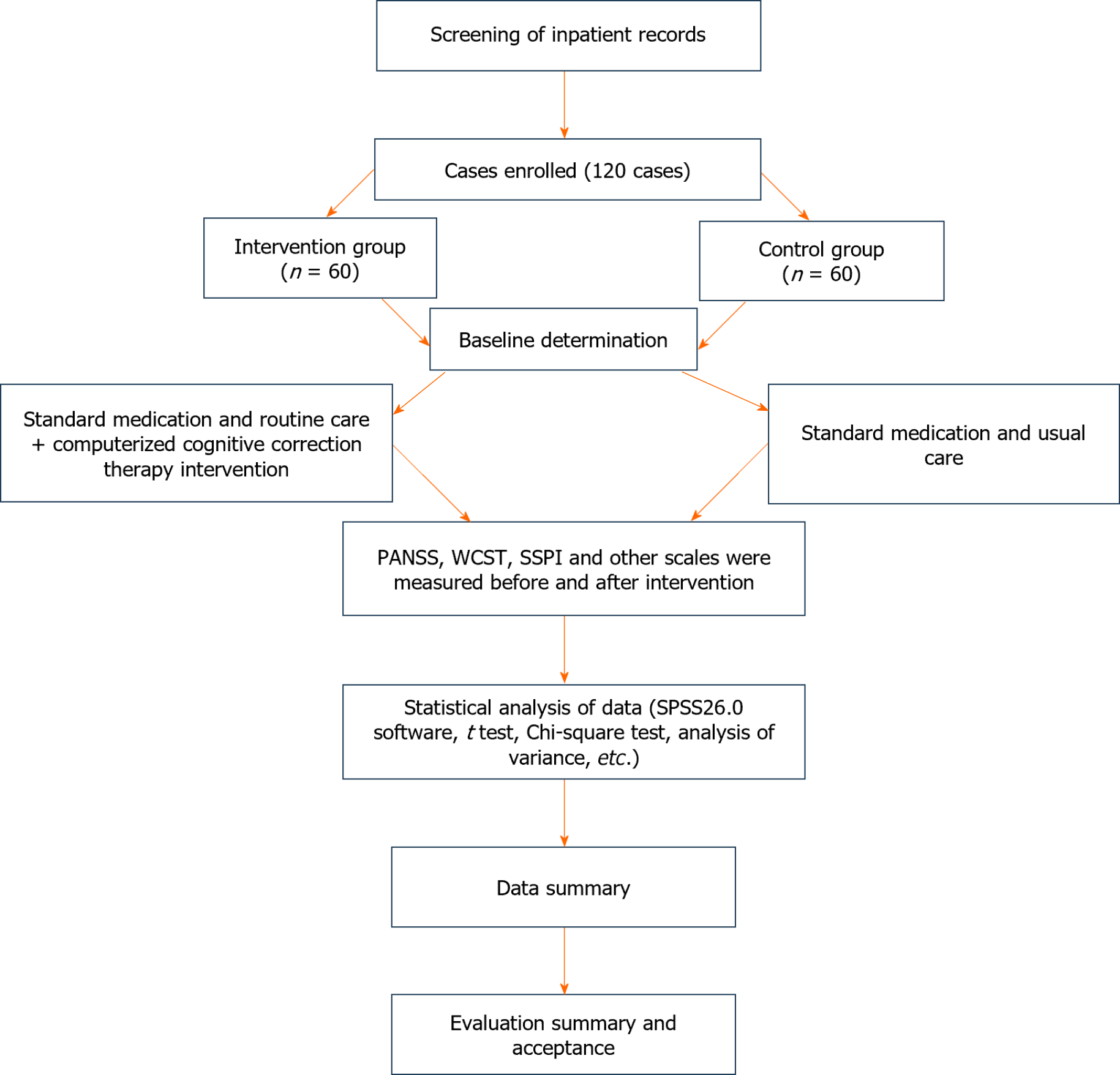
The control group was administered a small dose of the atypical antipsychotic drug lorazepam (Jiangsu Enhua Pharmaceutical Co., Ltd., State Pharmaceutical License H20223573, 10 mg) at a dosage of 10 mg/d once daily. Handicrafts, recreational activities, health exercises, playing poker, and watching television were also used for routine treatment. The intervention group used CCRT to provide therapeutic interventions to the patients based on the control group, which comprised four parts: Planning training, social cognition, working memory, and cognitive flexibility, each of which comprised 8-16 exercises, each of which had 8-16 cognitive correction tasks with different levels of difficulty. Patients were first trained by nurses on CCRT-related knowledge and computer operation. Under the therapist's guidance, the CCRT treatment underwent a structured sequence, starting with cognitive flexibility exercises designed to improve the ability to adapt to changing rules and situations. These tasks varied in difficulty and enhanced the patient’s responsiveness to diverse cognitive demands. Next, the working memory was targeted with exercises like n-back tasks, where the complexity increased as patients demonstrated improved recall capabilities. This was followed by planning training, using strategic tasks such as the Tower of London, gradually intensifying the challenge to bolster problem-solving skills. The final component, social cognition, involved identifying emotions and interpreting social cues through progressively complex scenarios to enhance interpersonal understanding and interactions. Each session was tailored to the individual patient’s level, ensuring consistent cognitive engagement. The intervention was administered over 12 wk, with sessions held five times per week, each lasting 45 min. This structured approach, detailed in Figure 1, evaluated the sustained impact of CCRT on improving cognitive and social outcomes, offering insights into its long-term efficacy and broader applicability in non-pharmacological interventions for chronic schizophrenia.
Efficacy, PANSS, Scale of Social Function in Psychosis Inpatients (SSPI), and Wisconsin Card Sorting Test (WCST) scores.
Psychiatric symptom: Patients’ mental symptoms were evaluated using the three-factor model of the PANSS[10], which has 30 items and a total score between 30 and 210 points. There were 7 positive signs, 7 negative signs, and 16 general psychopathology signs. The higher the number, the more severe the condition.
Cognitive function: All patients were assessed for function using the WCST[11]. The WCST is a neuropsychological test comprising 128 responses and four stimulus cards. This test measures error responses (RE), perseverative response errors (RPE), response answers (RA), and categories completed (CC). Among them, the scores of the three indexes (RE, RPE, and RA) were inversely proportional to the cognitive operation score, and the higher the score, the worse the cognitive function. The index of CC is proportional to cognitive function rating, and the greater the score, the better cognitive function.
Social function: SSPI[12] assesses the social functioning of the two groups of patients. This scale comprises three factors: Daily living ability, activity and communication status, and social activity skills. The higher the total score on the SSPI and each factor score, the lower the social functional deficits.
Data were analyzed using SPSS version 26.0 statistical software. For measurement data, mean ± SD was used (± SD), and for inter-group comparisons, t-test was applied. Counting data are shown as percentages, and comparisons between groups were assessed using a chi-square test. Correlations were analyzed using Pearson's coefficients, and P < 0.05 displays a significant difference.
The general data of the two patient groups were compared, and the differences were not statistically significant (P > 0.05; Table 1).
| Item | Intervention group (n = 60) | Control group (n = 60) | t | P value |
| Sex ratio | 1.67 | 1.96 | ||
| Male | 38 (63.3) | 31 (51.67) | ||
| Female | 22 (36.7) | 29 (48.33) | ||
| Age (yr) | 45.11 ± 10.37 | 46.14 ± 9.97 | -0.58 | 0.57 |
| Nation | 0 | 1 | ||
| Han nationality | 55 (91.67) | 55 (91.67) | ||
| Other | 5 (8.33) | 5 (8.33) | ||
| Duration of disease (yr) | 9.26 ± 3.02 | 9.36 ± 3.74 | -0.16 | 0.87 |
| Number of hospitalizations (times) | 3.97 ± 1.63 | 4.13 ± 1.42 | -0.58 | 0.56 |
| Years of schooling (yr) | 9.97 ± 1.71 | 10.03 ± 1.36 | -0.23 | 0.82 |
| Smoking history | 0.13 | 0.72 | ||
| Exist | 32 (53.33) | 30 (50.00) | ||
| None | 28 (46.67) | 30 (50.00) | ||
| Drinking history | 0.33 | 0.56 | ||
| Exist | 22 (36.67) | 19 (31.67) | ||
| None | 38 (66.3) | 41 (68.33) | 0.56 | |
| Marital status | 0.33 | 0.67 | ||
| Married | 41 (68.33) | 38 (63.33) | ||
| Unmarried | 19 (31.67) | 22 (36.67) | ||
| Fertility | 0.19 | 0.66 | ||
| Infertile | 13 (21.67) | 16 (26.67) | ||
| Rear | 47 (78.33) | 44 (73.33) | ||
| SBP (mmHg) | 128.06 ± 15.03 | 129.81 ± 18.28 | -0.57 | 0.56 |
| DBP (mmHg) | 80.53 ± 12.41 | 83.15 ± 11.40 | -1.2 | 0.23 |
| HR | 75.09 ± 15.98 | 77.51 ± 14.78 | -0.87 | 0.39 |
| Educational level | 0.39 | 0.53 | ||
| Less than high school | 27 (45.00) | 24 (40.00) | ||
| High school and above | 33 (55.00) | 36 (60.00) | ||
| BMI (kg/m2) | 21.16 ± 1.23 | 21.31 ± 1.41 | -0.64 | 0.53 |
| Family history of mental illness | 0.87 | 0.35 | ||
| Exist | 26 (43.33) | 21 (35.00) | ||
| None | 34 (56.67) | 39 (65.00) | ||
| Professional status | 0.3 | 0.58 | ||
| Incumbency | 28 (46.67) | 25 (41.67) | ||
| Non-working | 32 (53.33) | 35 (58.33) | ||
| Residence | 0.03 | 0.85 | ||
| Towns | 33 (55.00) | 34 (56.67) | ||
| Countryside | 27 (45.00) | 26 (43.33) | ||
| Forms of onset | 0.55 | 0.46 | ||
| Acute onset | 23 (38.33) | 27 (45.00) | ||
| Chronic onset | 37 (61.67) | 33 (55.00) |
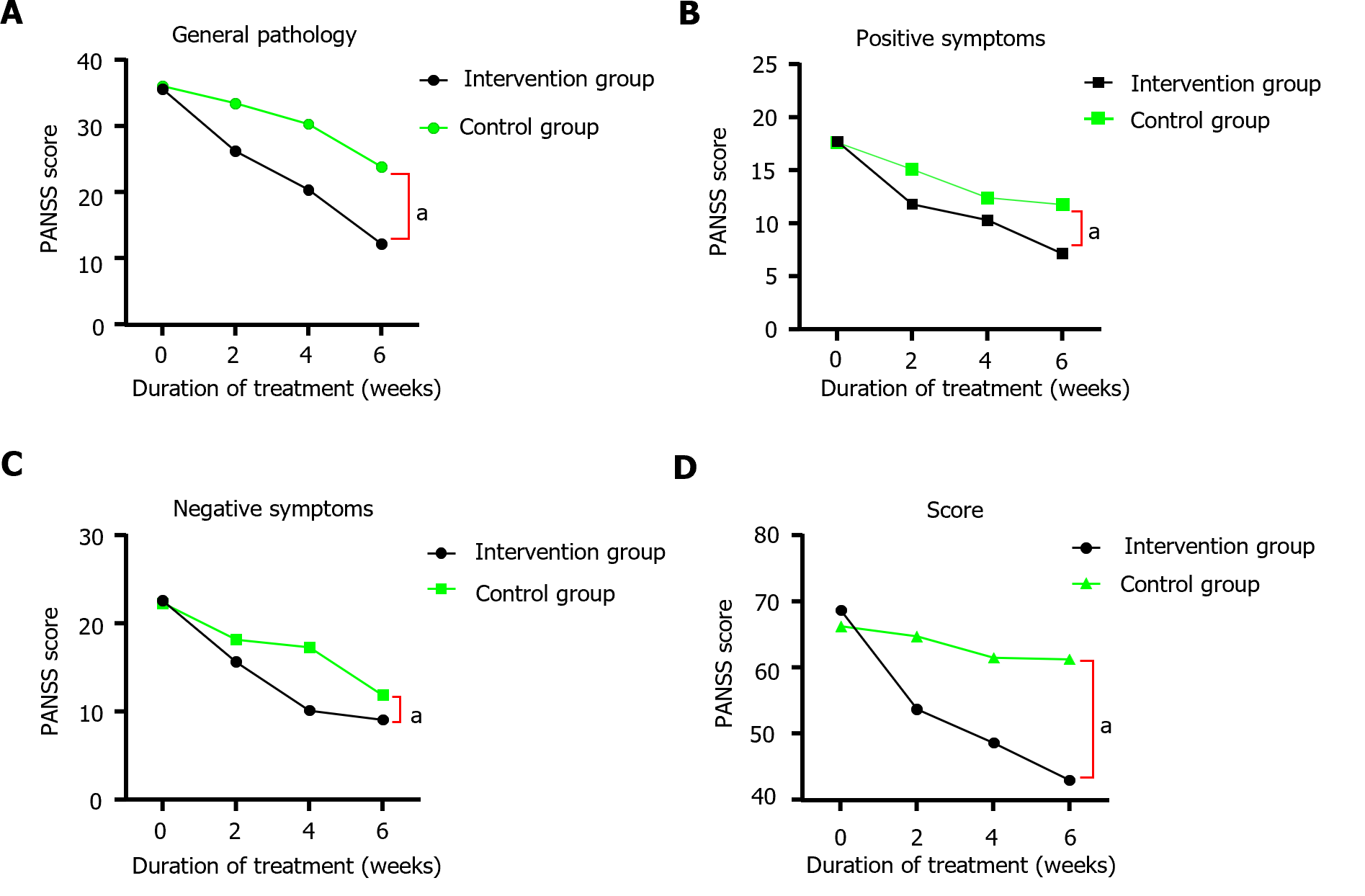
The differences in PANSS scores between the control and intervention groups before treatment were not statistically significant (P > 0.05). After 2, 4, and 6 wk of treatment, significant decreases were observed in positive symptoms, negative symptoms, common psychopathological symptoms, and total PANSS scores in both groups compared to those before treatment (P < 0.05), and the decreases in the intervention group were greater than those in the control group. Differences were considered statistically significant (Table 2 and Figure 2).
| Times | Groups | General pathology | Positive symptom | Negative symptom | Totals |
| Pre-treatment | Intervention group (n = 60) | 35.63 ± 2.03 | 17.77 ± 1.50 | 22.58 ± 1.95 | 68.65 ± 7.63 |
| Control groups (n = 60) | 36.02 ± 1.31 | 17.63 ± 1.58 | 22.28 ± 1.33 | 66.25 ± 6.94 | |
| 2 wk of treatment | Intervention group (n = 60) | 26.22 ± 2.49a | 11.85 ± 2.29 a | 15.63 ± 1.59a | 53.70 ± 3.46a |
| Control groups (n = 60) | 33.42 ± 3.34 | 15.12 ± 1.84 | 18.18 ± 3.65 | 64.72 ± 5.53 | |
| 4 wk of treatment | Intervention group (n = 60) | 20.39 ± 2.08a | 10.35 ± 2.91a | 10.13 ± 1.55a | 48.65 ± 2.67a |
| Control groups (n = 60) | 30.28 ± 1.44 | 12.45 ± 3.08 | 17.31 ± 3.26 | 61.50 ± 2.17 | |
| 6 wk of treatment | Intervention group (n = 60) | 12.21 ± 5.72a | 7.18 ± 6.48a | 9.12 ± 1.79a | 43.02 ± 7.62a |
| Control groups (n = 60) | 23.85 ± 5.67 | 10.90 ± 2.36 | 11.91 ± 2.09 | 56.95 ± 6.94 |
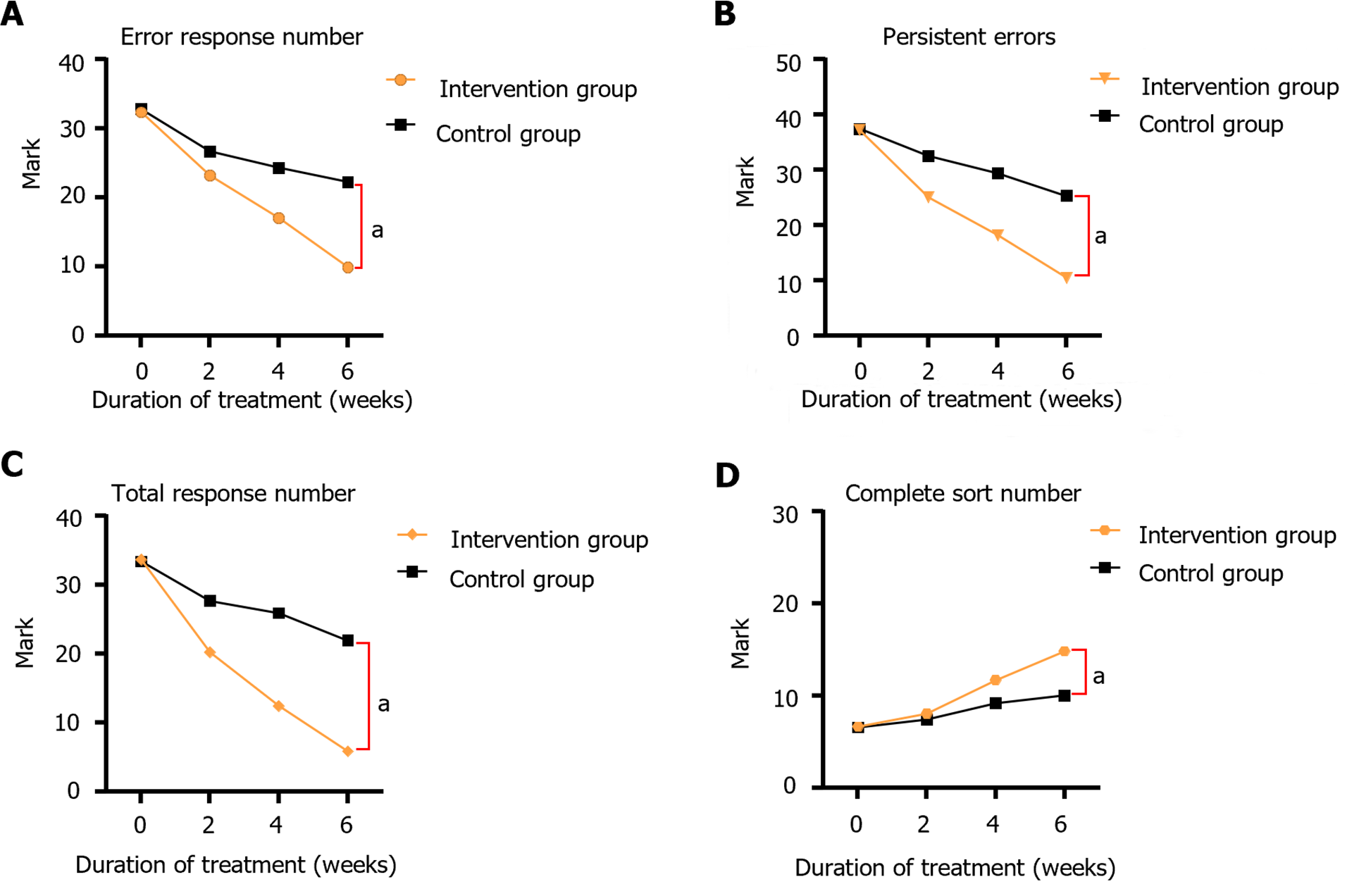
Before treatment, no significant difference was observed between the control and intervention groups in the RE, RPE, RA, and CC levels (P > 0.05); however, after 2-6 wk of continuous treatment, the amount of CC gradually increased. The RE, RPE, and RA scores of the intervention group gradually decreased, and the intervention group was significantly lower than the control group (P < 0.05), and the difference was statistically significant. Further details are provided in Table 3 and Figure 3.
| Times | Groups | RE | RPE | RA | CC |
| Pre-treatment | Intervention group | 62.38 ± 1.76 | 37.28 ± 1.26 | 103.60 ± 1.76 | 3.33 ± 0.89 |
| Control groups | 62.85 ± 3.63 | 37.48 ± 1.20 | 103.35 ± 1.88 | 3.28 ± 0.80 | |
| 2 wk of treatment | Intervention group | 53.23 ± 2.06a | 25.07 ± 2.39a | 90.20 ± 4.28a | 4.03 ± 0.84a |
| Control groups | 56.73 ± 3.68 | 32.62 ± 4.39 | 97.65 ± 5.43 | 3.97 ± 0.89 | |
| 4 wk of treatment | Intervention group | 47.04 ± 1.66a | 18.21 ± 3.19a | 82.46 ± 3.53a | 5.86 ± 2.07a |
| Control groups | 54.35 ± 1.20 | 29.47 ± 2.82 | 95.88 ± 2.34 | 4.78 ± 2.76 | |
| 6 wk of treatment | Intervention group | 39.92 ± 2.29a | 10.50 ± 3.68a | 75.89 ± 1.58a | 7.42 ± 2.24a |
| Control groups | 52.24 ± 3.71 | 25.41 ± 1.36 | 91.92 ± 4.39 | 6.23 ± 1.72 |
In the control group, after 6 wk of treatment, 10 cases were cured, 22 cases were improved, 17 cases were better, and 11 cases were invalid, with an effective rate of 81.67%. In the intervention group, after 6 wk of treatment, 24 cases were cured, 18 were significantly improved, 13 were improved, and 5 were invalid, with an effective rate of 91.67%. The treatment efficiency in the intervention group was significantly better than that in the control group (P < 0.05), and the difference was statistically significant (P < 0.05; Table 4).
| Groups | Precedent | Heal | Significant progress | Progress | Null |
| Intervention group | 60 | 24 (40.00) | 18 (30.00) | 13 (21.67) | 5 (8.33) |
| Control groups | 60 | 10 (16.67) | 22 (36.67) | 17 (28.33) | 11 (18.33) |
| t | 8.95 | ||||
| P value | 0.03 |
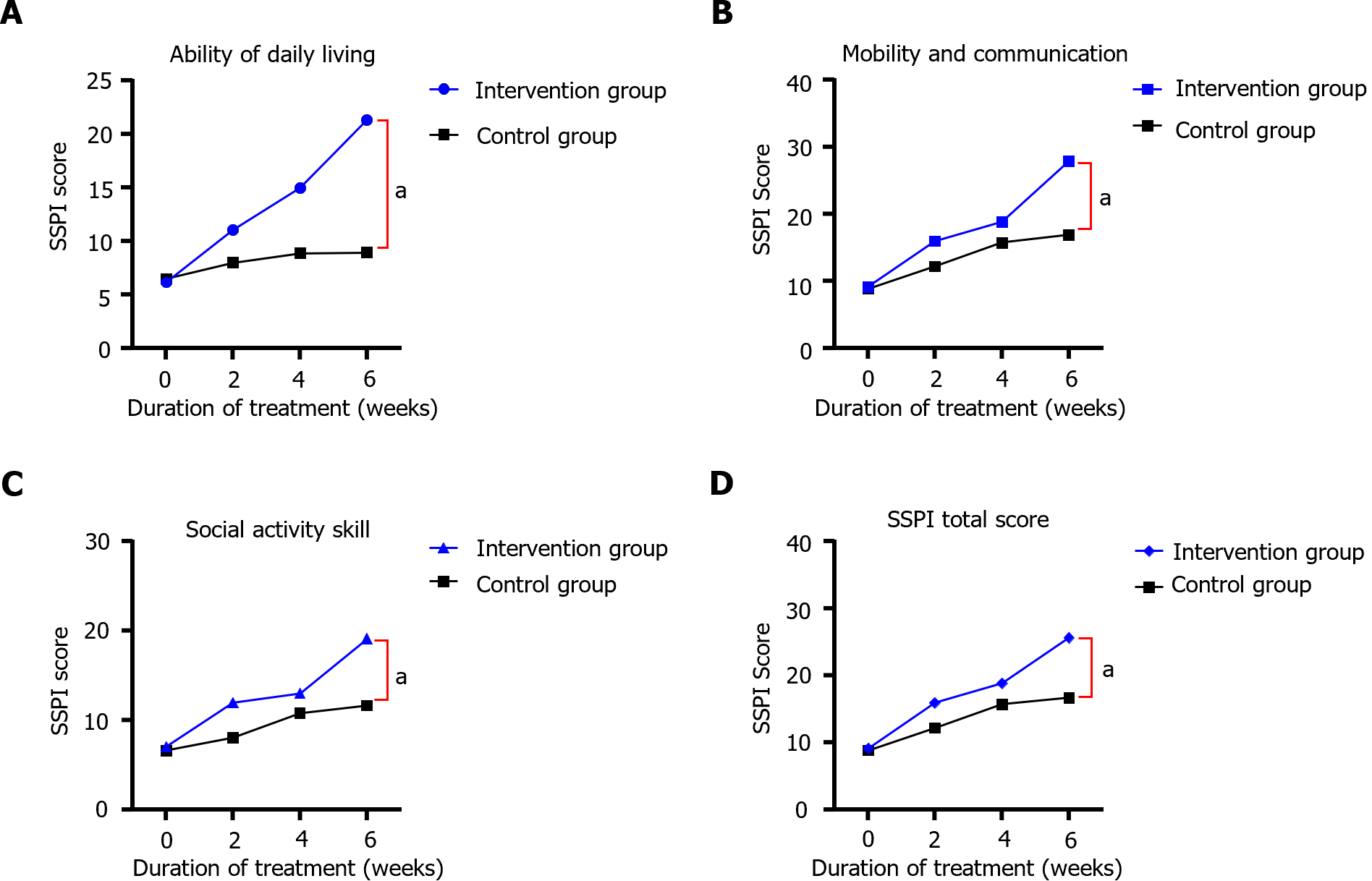
Before treatment, no statistically significant difference was observed between the SSPI scores of the two groups (P > 0.05); after treatment, the daily living skills, motivation and socialization, social mobility skills, and SSPI total scores of the two groups were higher than those before treatment, and the intervention group had significantly higher scores than the control group (P < 0.05). After treatment, the daily living skills, motivation and socialization, social mobility skills, and SSPI total scores of both groups were higher than before treatment, and the intervention group had higher scores than the control group (Table 5 and Figure 4).
| Times | Groups | Daily living skills | Motivation and socialization | Social mobility skills | SSPI total score |
| Pre-treatment | Intervention group (n = 60) | 6.15 ± 2.22 | 9.15 ± 2.31 | 7.04 ± 1.14 | 9.15 ± 2.31 |
| Control groups (n = 60) | 6.52 ± 1.99 | 8.81 ± 2.44 | 6.62 ± 3.59 | 8.81 ± 2.44 | |
| 2 wk of treatment | Intervention group (n = 60) | 11.06 ± 4.56a | 15.96 ± 3.80a | 11.99 ± 4.47a | 15.96 ± 3.80a |
| Control groups (n = 60) | 8.00 ± 3.29 | 12.19 ± 3.78 | 8.03 ± 2.21 | 12.19 ± 3.78 | |
| 4 wk of treatment | Intervention group (n = 60) | 14.97 ± 3.29a | 18.85 ± 2.68a | 13.01 ± 2.95a | 18.85 ± 2.68a |
| Control groups (n = 60) | 11.32 ± 2.79 | 15.72 ± 2.63 | 10.79 ± 2.78 | 15.72 ± 2.63 | |
| 6 wk of treatment | Intervention group (n = 60) | 21.32 ± 1.29a | 27.92 ± 5.80a | 19.13 ± 4.68a | 25.66 ± 5.45a |
| Control groups (n = 60) | 14.78 ± 3.77 | 17.69 ± 3.51 | 14.13 ± 4.29 | 18.84 ± 3.98 |
Deficit schizophrenia is a distinct subtype of schizophrenia that is characterized by primary and persistent negative symptoms[13]. Currently, cognitive dysfunction in patients with schizophrenia and impairment mainly includes four types: Attention, abstract thinking, memory, and information integration disorders[14]. Cognitive impairment not only affects the rehabilitation of patients but also causes various aggressive behaviors in severe cases, which has important implications for patients' social skills and future well-being[15].
The study found that following 6 wk of CCRT treatment, significant improvement was observed in the general psychopathological factors and positive symptoms. The surgery group had lower PANSS scores and fewer negative symptoms than the control group, and the negative symptom score of the intervention group was more significantly decreased than that in the control group (P < 0.05). Currently, the primary treatment methods are modified electroconvulsive therapy and antipsychotic medication; however, the clinical efficacy of these two methods is limited. Compared with conventional treatment, computer cognitive correction therapy combined with drug therapy can not only significantly improve the cognitive and social functions of patients with schizophrenia but also improve their negative symptoms of patients[16,17]. After 6 wk of treatment, the number of false responses, sustained mistakes, and the total number of responses were substantially reduced in the treated group compared to the uncontrolled group (P < 0.01). Computer cognitive correction therapy has been suggested to effectively improve the cognitive function of patients with chronic schizophrenia and is superior to conventional treatment. This indicates that patients with schizophrenia have greatly improved in many aspects of sensory perception after CCRT, which can significantly improve their working memory, task execution ability, and episodic memory ability and positively impact their attention and information processing efficiency[18]. After 6 wk of CCRT, the intervention group's SSPI score was significantly higher than that before treatment (P < 0.05); however, no significant change was observed in the control group (P > 0.05), and the curative effect in the intervention group was significantly higher than that in the control group (P < 0.05). Studies have shown that this treatment can improve brain activation and gray matter density, fundamentally altering neurobiological abnormalities that may be the root cause of cognitive dysfunction, suggesting that after significant improvement in cognitive correction therapy, patients significantly improve their social and cognitive abilities. It can also alleviate clinical symptoms, improve daily living abilities, and improve social activities[19,20]. After 6 wk of treatment, the actual rate of the control group was 81.67%. The effective rate of the intervention group was 91.67%. The curative effect of the intervention group was significantly higher than that of the control group (P < 0.05). These results indicate that CCRT can significantly improve clinical efficacy in patients with schizophrenia.
Summarily, CCRT treatment for patients with chronic deficit schizophrenia can not only improve their mental symptoms and cognitive function but also help improve their social function, which is worthy of clinical promotion and application. However, additional research is needed to understand its long-term effectiveness and applicability across different settings fully. Future studies should investigate the use of CCRT in varied demographic and geographic populations to determine its generalizability and effectiveness in diverse clinical environments. Moreover, the durability of the cognitive improvements post-treatment needs to be assessed. Long-term follow-up studies can help in determining whether the cognitive and social gains are sustained over extended periods, providing additional insights into the potential of CCRT as a routine intervention in the management of schizophrenia. This study had some limitations. First, because of the limitations of manpower, material resources, and other factors, this study only selected patients with schizophrenia from one hospital for research investigation; therefore, the sampling was not comprehensive enough. Second, the duration of computer cognitive correction therapy for patients was limited, with a maximum of six weeks. In subsequent studies, the duration of treatment should be extended, and patients should be followed up continuously after treatment to clarify the impact of long-term computer-assisted cognitive correction therapy on cognitive improvement in patients.
Additionally, in a follow-up study, the number of enrolled patients should continue to increase, and the sample size should be expanded to further increase the certainty of the treatment effect.
CCRT can effectively improve cognitive and social functions in patients with chronic schizophrenia and has a significant therapeutic effect.
| 1. | Yolland COB, Phillipou A, Castle DJ, Neill E, Hughes ME, Galletly C, Smith ZM, Francis PS, Dean OM, Sarris J, Siskind D, Harris AWF, Rossell SL. Improvement of cognitive function in schizophrenia with N-acetylcysteine: A theoretical review. Nutr Neurosci. 2020;23:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Opoka SM, Lincoln TM. The Effect of Cognitive Behavioral Interventions on Depression and Anxiety Symptoms in Patients with Schizophrenia Spectrum Disorders: A Systematic Review. Psychiatr Clin North Am. 2017;40:641-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Driver DI, Thomas S, Gogtay N, Rapoport JL. Childhood-Onset Schizophrenia and Early-onset Schizophrenia Spectrum Disorders: An Update. Child Adolesc Psychiatr Clin N Am. 2020;29:71-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Danenberg R, Ruimi L, Shelef A, Paleacu Kertesz D. A Pilot Study of Cognitive Impairment in Longstanding Electroconvulsive Therapy-treated Schizophrenia Patients Versus Controls. J ECT. 2021;37:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Altman RAE, Tan EJ, Rossell SL. Factors Impacting Access and Engagement of Cognitive Remediation Therapy for People with Schizophrenia: A Systematic Review. Can J Psychiatry. 2023;68:139-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Modabbernia A, Heidari P, Soleimani R, Sobhani A, Roshan ZA, Taslimi S, Ashrafi M, Modabbernia MJ. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | García-Casal JA, Loizeau A, Csipke E, Franco-Martín M, Perea-Bartolomé MV, Orrell M. Computer-based cognitive interventions for people living with dementia: a systematic literature review and meta-analysis. Aging Ment Health. 2017;21:454-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 394] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 9. | Parnas J, Parnas AU. Refining the Diagnostic Criteria for Schizophrenia: An Infinite Task. Schizophr Bull. 2024;50:12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Guaiana G, Abbatecola M, Aali G, Tarantino F, Ebuenyi ID, Lucarini V, Li W, Zhang C, Pinto A. Cognitive behavioural therapy (group) for schizophrenia. Cochrane Database Syst Rev. 2022;7:CD009608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Chang SH, Shie JJ, Yu NY. Enhancing Executive Functions and Handwriting with a Concentrative Coordination Exercise in Children with ADHD: A Randomized Clinical Trial. Percept Mot Skills. 2022;129:1014-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Hasselbring TS, Glaser CH. Use of computer technology to help students with special needs. Future Child. 2000;10:102-122. [PubMed] |
| 13. | Liu J, Wang D, Zhou H, Zhao NO, Wu HE, Zhang X. Deficit syndrome in Chinese patients with first-episode drug naïve schizophrenia: Prevalence, demographic and clinical characteristics. Asian J Psychiatr. 2021;65:102861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Morice R, Delahunty A. Frontal/executive impairments in schizophrenia. Schizophr Bull. 1996;22:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 98] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Hyde TM, Nawroz S, Goldberg TE, Bigelow LB, Strong D, Ostrem JL, Weinberger DR, Kleinman JE. Is there cognitive decline in schizophrenia? A cross-sectional study. Br J Psychiatry. 1994;164:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Isaac C, Januel D. Neural correlates of cognitive improvements following cognitive remediation in schizophrenia: a systematic review of randomized trials. Socioaffect Neurosci Psychol. 2016;6:30054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Abi-Dargham A. Schizophrenia: overview and dopamine dysfunction. J Clin Psychiatry. 2014;75:e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Trapp W, Landgrebe M, Hoesl K, Lautenbacher S, Gallhofer B, Günther W, Hajak G. Cognitive remediation improves cognition and good cognitive performance increases time to relapse--results of a 5 year catamnestic study in schizophrenia patients. BMC Psychiatry. 2013;13:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Zheutlin AB, Viehman RW, Fortgang R, Borg J, Smith DJ, Suvisaari J, Therman S, Hultman CM, Cannon TD. Cognitive endophenotypes inform genome-wide expression profiling in schizophrenia. Neuropsychology. 2016;30:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Flores AT, Hogarty SS, Mesholam-Gately RI, Barrio C, Keshavan MS, Eack SM. Cognitive gains as a mechanism of functional capacity improvement in schizophrenia: Results from a multi-site randomized controlled trial. J Psychiatr Res. 2022;151:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: Https://creativecommons.org/Licenses/by-nc/4.0/