Published online Jun 19, 2024. doi: 10.5498/wjp.v14.i6.794
Revised: April 28, 2024
Accepted: May 17, 2024
Published online: June 19, 2024
Processing time: 99 Days and 5.7 Hours
Accumulating evidence suggests that the inflammatory cytokine interleukin-6 (IL-6) contributes to the pathophysiology of psychiatric disorders. However, there was no study concerning the relationship between IL-6 concentrations and clinical features in the chronic phase of early-onset schizophrenia (EOS).
To investigate the relationship between serum IL-6 concentration and the clinical features of EOS.
We measured serum IL-6 Levels from 74 patients with chronic schizophrenia, including 33 with age at onset < 21 years (EOS group) and 41 with onset ≥ 21 years in [adult-onset schizophrenia (AOS) group], and from 41 healthy controls. Symptom severities were evaluated using the Positive and Negative Syndrome Scale (PANSS).
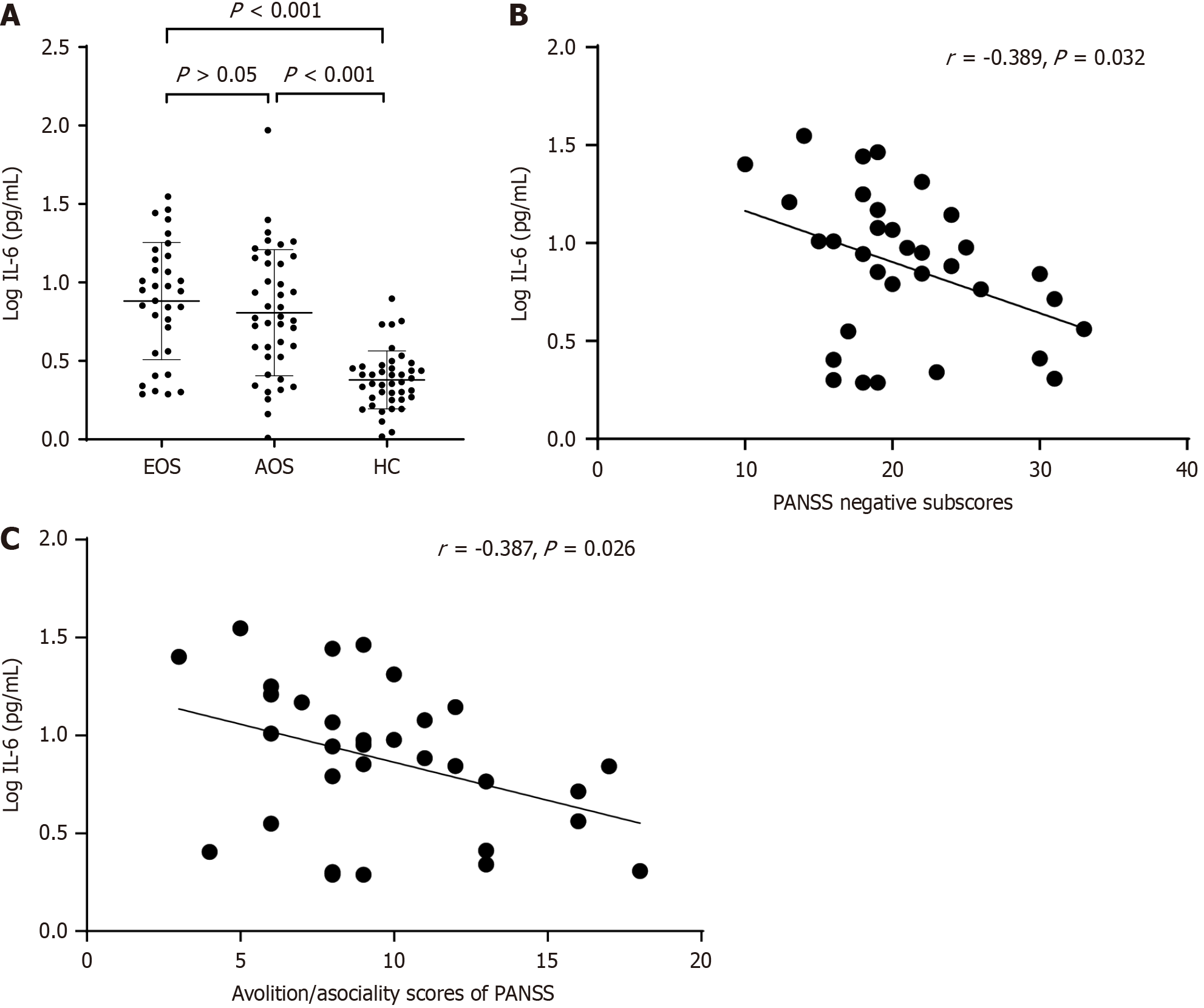
Serum IL-6 concentrations were higher in both EOS and AOS groups than healthy controls (F = 22.32, P < 0.01), but did not differ significantly between EOS and AOS groups (P > 0.05) after controlling for age, body mass index, and other covariates. Negative symptom scores were higher in the EOS group than the AOS group (F = 6.199, P = 0.015). Serum IL-6 concentrations in the EOS group were negatively correlated with both total PANSS-negative symptom score (r = -0.389, P = 0.032) and avolition/asociality subscore (r = -0.387, P = 0.026).
Patients with EOS may have more severe negative symptoms than those with adult-onset schizophrenia during the chronic phase of the illness. IL-6 signaling may regulate negative symptoms and its avolition/asociality subsym
Core Tip: In this study, we focus on the negative symptoms and inflammatory levels in the chronic stage of early-onset schizophrenia. Patients' clinical symptoms were assessed by the Positive and Negative Syndrome Scale, and the level of inflammation was assessed by serum interleukin-6 (IL-6) Levels. Our study found that patients with early-onset schizophrenia may have more severe negative symptoms than those with adult-onset schizophrenia during the chronic phase of the illness. IL-6 signaling may regulate negative symptoms and its avolition/asociality subsymptoms among the early-onset chronic schizophrenic patients.
- Citation: Chen P, Yang HD, Wang JJ, Zhu ZH, Zhao HM, Yin XY, Cai Y, Zhu HL, Fu JL, Zhang XZ, Sun WX, Hui L, Zhang XB. Association of serum interleukin-6 with negative symptoms in stable early-onset schizophrenia. World J Psychiatry 2024; 14(6): 794-803
- URL: https://www.wjgnet.com/2220-3206/full/v14/i6/794.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i6.794
Schizophrenia is a neuropsychiatric disorder of complex etiology with lifetime incidence of about 0.4% and total global prevalence of approximately 1%[1]. Symptoms of the disease are stratified into three domains, positive, negative, and cognitive, with substantial variation in severity among individual patients. Furthermore, these distinct symptom domains can have unique effects on clinical course and outcome. For instance, negative symptoms such as flat affect and asociality impair social functioning[2]. Several schizophrenia subtypes have been defined based on predominant symptom profiles and clinical course, including early-onset schizophrenia (EOS) with age of onset before 21 years[3,4]. According to an der Heiden and Häfner[5], about 41% of patients develop their first symptoms before the age of 20, and numerous studies have found that EOS was associated with more severe negative symptoms, more frequent relapses, poorer social functioning, and worse overall prognosis[6-8]. While the pathological mechanisms contributing to poor outcome among EOS patients are still largely unknown, Fraguas et al[9] reported more severe inflammation and oxidative stress in this patient group.
Exploratory factor analyses of Position and Negative Syndrome Scale (PANSS) scores have revealed two negative sub-symptom clusters[10-13], expression deficits and avoidance/asociality, with possibly distinct underlying pathological mechanisms. Therefore, uniform treatment strategies for all EOS patients regardless of individual negative symptom profile may produce suboptimal clinical outcomes[11]. The expression cluster includes flat affect, poor rapport, lack of spontaneity, mannerisms and posturing, motor retardation, and avolition, while the asociality factor consists of emotional withdrawal, passive/apathetic social withdrawal, and active social avoidance. The first factor reflects a loss of initiative, and the second factor social amotivation related to community interaction[14]. Based on these findings, we speculated that indices of neuroinflammation will be larger in chronic EOS patients compared to adult-onset schizophrenia (AOS) patients and associated with negative symptom severity. The current study focused primarily on expression deficits and avolition/asociality symptoms.
The inflammatory hypothesis of schizophrenia posits that low grade inflammation contributes to disease course and symptom expression[15-18], and is supported by studies showing higher levels of pro-inflammatory factors such as cytokines in the blood and cerebrospinal fluid of schizophrenia patients. A large epidemiological study also found that severe infections and autoimmune disorders were risk factors for schizophrenia[15,19], while the largest genome-wide association study to date identified 108 Low effect-size risk loci, most of which were associated with inflammatory responses[20].
Interleukin-6 (IL-6) is a highly pleiotropic cytokine involved in multiple aspects of the inflammatory response, in
Schizophrenia inpatients were recruited from Guangji Hospital, Suzhou, China, from January 2016 to October 2019. Inclusion criteria were as follows: (1) Age 18–75 years; (2) Meeting he Diagnostic and Statistical Manual of Mental Disorders-IV diagnostic criteria for schizophrenia as determined by two psychiatrists using the Structured Clinical Interview for he Diagnostic and Statistical Manual of Mental Disorders (SCID); (3) Disease duration of at least 5 years; (4) Han Chinese ethnicity; (5) Taking stable doses of antipsychotic drugs for at least one year (primarily clozapine, phenazine, risperidone, sulpiride, haloperidol, and chlorpromazine (CPZ); and (6) Agreeing to voluntarily participation with informed written consent. Exclusion criteria were: (1) Comorbid somatic disorders or substance dependence; and (2) Not completing primary education. A demographic assessment questionnaire was used to gather enrollment data, including sex, age, years of education, smoking status/history, body mass index (BMI), age of onset, and duration of illness.
Forty-one healthy controls (HCs) were recruited during the same period from Suzhou local communities through media and pamphlet advertisements. A psychiatrist confirmed health status and family psychiatric history using an unstructured clinical interview. Candidates with relevant physical and mental health problems were excluded. Enrolled candidates provided written informed consent for participation. The study protocol and informed consent form were approved by the Institutional Review Board of a Suzhou City-owned Mental Hospital (No. 2022005).
Participants with schizophrenia were asked to identify the age at first acute psychotic symptoms during the SCID, and this information was confirmed by medical records. We divided these patients into an EOS group if age at first psychotic episode occurred before age 21 and an AOS group if first psychotic episode occurred at 21 years or older[3,4,33]. The EOS group included 33 patients (23 males and 10 females) and the AOS group included 41 patients (24 males and 24 females).
Exploratory factor analysis yielded a two-factor structure of negative symptoms. The first factor, expressive deficits, consisted of PANSS items N1, N3, N6, G5, G7, and G13, while the second factor, avolition/asociality, consisted of PANSS items N2, N4, and G16[10,34].
To ensure the reliability and consistency of assessments across the study period, two psychiatrists with at least 5 years of clinical experience attended a training course on the use of the PANSS. After training, the interobserver correlation coefficient for the PANSS score was maintained above 0.8.
Peripheral venous blood samples were drawn from patients and HCs between 7 and 9 AM after an overnight fast, and centrifuged at 3000 rpm for 15 min in procoagulant and anticoagulant tubes to isolate serum and plasma fractions, respectively. The samples were then stored at -80 °C until analysis. Serum IL-6 concentrations were measured using a BDTM FACSCanto Flow Cytometer and BDTM Cytometric Bead Array (CBA) Human Inflammatory Cytokines Kit (BD Biosciences, San Jose, CA, United States) according to the manufacturers’ instructions. A standard curve was also constructed for each sample batch from triplicate measurements of known IL-6 concentrations using the supplied BDTM CBA Human Inflammatory Cytokine Standards. All measurements were performed by the same technician who was blinded to donor identity and clinical information.
Statistical analyses were conducted using Statistical Product and Service Solutions 25.00. All datasets were first examined for normality using the Kolmogorov-Smirnov test. Based on results indicating non-normally distributed serum IL-6 concentrations for both EOS and AOS patients and HCs (all P < 0.05 by Kolmogorov-Smirnov test), values were converted to natural logarithm values for analysis. Serum log IL-6 concentrations were compared among EOS patients, AOS patients, and HCs by multivariate analysis of covariance (MANCOVA) with diagnosis as a fixed factor, and age, sex, and BMI as covariates. Other continuous variables were compared by Student’s t-test or one-way analysis of variance, while categorical variables were compared by chi-square test. Results were corrected for multiple comparisons using the Bonferroni method. Associations between variables were evaluated by calculating Pearson’s correlation coefficients. Exploratory multiple regression analysis was also performed to examine the relationship between serum log IL-6 and PANSS scores after controlling for age, gender, and BMI as covariates. A corrected P < 0.05 (two-tailed) was considered statistically significant for all tests.
Chronic schizophrenia inpatients were divided into an early-onset group (EOS group, n = 33) with first psychotic episode before 21 years of age and an adult-onset group (AOS group, n = 41) with first psychotic episode after 21 years of age. A HC group (HC group, n = 41) was also recruited as a control. The demographic and clinical characteristics of all three study groups are summarized in Table 1. There was no significant difference in sex ratio and number of smokers among groups (P > 0.05). As expected, there were significant group differences in age, years of education, and BMI (P < 0.05), but neither years of education, duration of disease, nor CPZ equivalent dose differed between EOS and AOS groups (P > 0.05). According to the criterion for group stratification, age at schizophrenia onset was significantly older in the AOS group. Mean BMI was higher in the EOS group than the AOS group (P < 0.05), and so was included as a fixed effect covariate in subsequent analyses.
| | Early-onset schizophrenia | Adult-onset schizophrenia | Healthy control | Statistic (F/χ2) | P value |
| Sex | |||||
| Male | 23 | 24 | 23 | 1.57 | 0.461 |
| Female | 10 | 17 | 18 | ||
| Smoking | |||||
| Smoker | 7 | 13 | 15 | 2.09 | 0.351 |
| Nonsmoker | 26 | 28 | 26 | ||
| Age (yr)a | 38.79 ± 9.21 | 46.14 ± 7.26 | 42.61 ± 10.30 | 5.00 | 0.0082 |
| Educations (yr)b | 9.64 ± 2.50 | 9.10 ± 2.96 | 12.24 ± 2.61 | 20.78 | < 0.0012 |
| BMI (kg/m2)b | 28.57 ± 4.87 | 25.56 ± 3.74 | 23.26 ± 2.72 | 17.88 | < 0.0012 |
| Age of onset (yr)b | 17.00 ± 2.75 | 28.22 ± 5.69 | 107.67 | < 0.0012 | |
| Duration of illness (yr) | 21.61 ± 9.22 | 17.88 ± 7.29 | 3.78 | 0.0562 | |
| Dose of CPZ equivalent (mg/d) | 653.37 ± 300.04 | 704.85 ± 311.41 | 0.52 | 0.4752 | |
| PANSS | |||||
| Positive subscores | 14.54 ± 5.95 | 14.73 ± 6.71 | 1.60 | 0.213 | |
| Negative subscoresa | 20.85 ± 5.56 | 18.15 ± 3.86 | 6.20 | 0.0153 | |
| General subscores | 33.21 ± 9.32 | 31.05 ± 8.24 | 0.16 | 0.6883 | |
| Total scores | 68.61 ± 17.90 | 63.93 ± 14.23 | 0.004 | 0.9533 | |
| Expressive deficits | 16.33 ± 4.77 | 14.17 ± 3.26 | 3.26 | 0.0753 | |
| Avolition/asociality | 9.52 ± 3.72 | 8.44 ± 2.44 | 2.05 | 0.1573 | |
| Log IL-6 levelb | 0.88 ± 0.37 | 0.81 ± 0.40 | 0.18 ± 0.03 | 22.32 | < 0.0013 |
As shown in Figure 1A, serum log IL-6 concentrations were higher in both EOS and AOS groups compared to the HC group (P < 0.001) but did not differ between EOS and AOS groups (P > 0.05).
In the EOS group, serum log IL-6 concentration was negatively correlated with total PANSS-negative symptom score (r = -0.389, P = 0.032; Figure 1B) and with avolition/asociality subscore (r = -0.387, P = 0.026; Figure 1C). Furthermore, the correlation between total PANSS-negative score and serum log IL-6 concentration remained significant after controlling for age, years of education, BMI, smoking status, age of onset, duration of illness, and CPZ equivalence dose (R2 = 0.151, P = 0.025). This association was also significant in stepwise multiple regression analysis (R2 = 0.150, P = 0.026). In contrast, no such association was found between clinical factors and serum log IL-6 concentration in the AOS group.
The main findings of the present study were as follows: (1) Serum IL-6 concentrations were elevated in both EOS and AOS patient groups compared to a control group but did not differ between patient groups; (2) total PANSS-negative score (sum of subscores) was significantly higher in the EOS group than the AOS group, indicating more severe negative symptoms; and (3) total PANSS-negative symptom score and Avolition/Asociality subscore were correlated with serum log IL-6 concentration in the EOS group but not the AOS group. These results suggested that IL-6 inflammatory signaling regulated negative symptom severity in EOS patients. To our knowledge, this is the first study to find an association between serum IL-6 concentration and PANSS-negative symptom severity in EOS patients during chronic stabilization.
Many patients receiving antipsychotic treatments experience unhealthy weight gain, and we found a significant BMI elevation among EOS patients during the first unmedicated phase compared to AOS patients. Lang et al[35] found no significant difference in BMI between EOS patients during the first unmedicated phase and AOS patients, but did identify BMI as a risk factor for metabolic syndrome in EOS. A prospective study by Ratzoni et al[36] also found that olanzapine and risperidone induced greater weight gain in Israeli adolescent patients than adult patients. Thus, weight gain should be closely monitored in EOS patients. We speculate that elevated BMI in the chronic phase of EOS may contribute to poorer outcome. Obesity is also associated with elevated serum IL-6, although the correlation between IL-6 and total PANSS-negative symptoms among EOS patients remained significant after controlling for BMI as well as other covariates. Nonetheless, further studies are needed to comprehensively assess the influences of confounding factors on BMI in schizophrenia, such as different types of medications, age, ethnicity, duration of illness, and sex.
Negative symptom scores were higher in the EOS group that the AOS group, consistent with several previous relevant studies[8,37-40] but at odds with several others reporting no difference[41-43]. These disparities may be related to sample heterogeneity (e.g., ethnic background, first-episode or relapse, anti-psychotic drug dose, hospitalized vs outpatient, and illness severity). Nonetheless, the current research suggests that intervention for the chronic phase of EOS should place greater emphasis on negative symptoms.
Serum IL-6 concentrations were higher in both EOS and AOS patient groups compared to healthy matched controls, suggesting that schizophrenia was associated with a chronic inflammatory response independent of onset age and consistent with the inflammation hypothesis of schizophrenia. Of the few previous studies on serum IL-6 in EOS patients, one found no difference between first-episode EOS and healthy individuals[44], while a clinical two-sample Mendelian randomized study found increased soluble IL-6 receptor levels in patients, which can be explained as a compensatory response to IL-6 elevation[45]. Elevated serum IL-6 suggests microglial cell hyperactivity[26] in the chronic phases of EOS and AOS. While additional studies with larger samples are needed for verification, it appears that elevated serum IL-6 concentration is a ubiquitous feature of stable chronic schizophrenia.
Numerous studies have found strong associations between elevated IL-6 and both the development and progression of first-episode psychosis in acute and chronic stages of schizophrenia, suggesting that inflammatory cascade responses contribute to the underlying pathogenesis and symptom expression[18,46]. Indeed, some investigations have found correlations with positive symptoms, negative symptoms, depressive symptoms, and cognitive deficits[47]. A meta-analysis concluded that the IL-6 elevation in patients with first-episode psychosis or acute relapse normalized after antipsychotic treatment[48]. Thus, variations in IL-6 Levels may reflect complex immunoregulatory functions at different stages of the disease. However, we found that higher serum IL-6 concentrations in stably medicated EOS patients were associated with both lower overall PANSS-negative symptom severity and avolition/asociality severity, while most previous investigations have found either a positive correlation or no relationship[48-50]. Stojanovic et al[51] did report a negative correlation with PANSS positive subscale scores (but positive correlations with PANSS-negative subscale scores) among outpatients with psychotic disorders, while Gibson et al[52] found a negative correlation between serum IL-6 and PANSS total score as well as positive and negative subscales in patients with cannabinoid-positive acute psychiatric disorders. As mentioned, Golimbet et al[32] found a link between the IL-6 -174 G/C polymorphism and both dementia and apathy scores that approached significance. To our best knowledge, the current case-control study is the first to find negative correlations between serum IL-6 and both total PANSS-negative symptom score and avolition/asociality subscore among patients with chronic stage EOS.
These discrepancies across studies may reflect the pleiotropic activity of IL-6 signaling in immune regulation[21-23,53,54]. IL-6 can modulate cellular responses in two ways. In the classical signaling pathway, IL-6 binds to its cognate cell membrane receptor (IL-6R) and triggers a heterodimeric association with two membrane-bound gp130 molecules, which in turn initiates downstream pro- and anti-inflammatory responses through activation of three signaling cascades, most prominently the JAK-STAT pathway. Alternatively, in the trans-signaling pathway, IL-6 binds to the soluble form of its receptor (slL-6R) before forming a complex with membrane-bound gp130. This complex then activates downstream pathways leading to pro-inflammatory responses. However, soluble gp130 (sgp130) inhibits trans-signaling by blocking the association of the IL-6/slL-6R complex with membrane-bound gp130 molecules[54]. Szabo et al[53] reported sig
We examined serum IL-6 concentrations in patients with EOS and AOS in the chronic phase. Our study demonstrated that serum IL-6 concentrations were elevated in both EOS and AOS patient groups compared to a control group but did not differ between patient groups. Moreover, total PANSS-negative score was significantly higher in the EOS group than the AOS group, indicating more severe negative symptoms. In addition, we found that the mean BMI was higher in the EOS group than the AOS group. And total PANSS-negative symptom score and Avolition/Asociality subscore were correlated with serum log IL-6 concentration in the EOS group but not the AOS group. These results suggested that IL-6 inflammatory signaling regulated negative symptom severity in EOS patients. The present study had several limitations. First, the sample size was relatively small, limiting statistical power. Therefore, more subtle associations and differences may have been missed. Second, although we did not find a relationship between drug treatment (in clozapine equi
We would like to thank all participants and all co-authors in this study.
| 1. | McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1519] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 2. | Hunter R, Barry S. Negative symptoms and psychosocial functioning in schizophrenia: neglected but important targets for treatment. Eur Psychiatry. 2012;27:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Häfner H, Nowotny B. Epidemiology of early-onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1995;245:80-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 102] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Basso MR, Nasrallah HA, Olson SC, Bornstein RA. Cognitive deficits distinguish patients with adolescent- and adult-onset schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:107-112. [PubMed] |
| 5. | an der Heiden W, Häfner H. The epidemiology of onset and course of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2000;250:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Immonen J, Jääskeläinen E, Korpela H, Miettunen J. Age at onset and the outcomes of schizophrenia: A systematic review and meta-analysis. Early Interv Psychiatry. 2017;11:453-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 7. | Naguy A, AlShalabi SR, AlKhadhari S. Betahistine-Associated Weight Loss and Improved Cognitive and Negative Symptoms: Domain in Early-Onset Schizophrenia. Am J Ther. 2019;26:e790-e792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Harvey RC, James AC, Shields GE. A Systematic Review and Network Meta-Analysis to Assess the Relative Efficacy of Antipsychotics for the Treatment of Positive and Negative Symptoms in Early-Onset Schizophrenia. CNS Drugs. 2016;30:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, Arango C. Oxidative Stress and Inflammation in Early Onset First Episode Psychosis: A Systematic Review and Meta-Analysis. Int J Neuropsychopharmacol. 2017;20:435-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Liemburg E, Castelein S, Stewart R, van der Gaag M, Aleman A, Knegtering H; Genetic Risk and Outcome of Psychosis (GROUP) Investigators. Two subdomains of negative symptoms in psychotic disorders: established and confirmed in two large cohorts. J Psychiatr Res. 2013;47:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 340] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 12. | Lincoln TM, Dollfus S, Lyne J. Current developments and challenges in the assessment of negative symptoms. Schizophr Res. 2017;186:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5:664-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 384] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 14. | Stentebjerg-Olesen M, Pagsberg AK, Fink-Jensen A, Correll CU, Jeppesen P. Clinical Characteristics and Predictors of Outcome of Schizophrenia-Spectrum Psychosis in Children and Adolescents: A Systematic Review. J Child Adolesc Psychopharmacol. 2016;26:410-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci. 2015;9:372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (1)] |
| 16. | van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, Kahn RS, Sommer IE. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7:e1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 283] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 17. | Misiak B, Bartoli F, Carrà G, Stańczykiewicz B, Gładka A, Frydecka D, Samochowiec J, Jarosz K, Hadryś T, Miller BJ. Immune-inflammatory markers and psychosis risk: A systematic review and meta-analysis. Psychoneuroendocrinology. 2021;127:105200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | Halstead S, Siskind D, Amft M, Wagner E, Yakimov V, Shih-Jung Liu Z, Walder K, Warren N. Alteration patterns of peripheral concentrations of cytokines and associated inflammatory proteins in acute and chronic stages of schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry. 2023;10:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 19. | Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 435] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 20. | Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6882] [Cited by in RCA: 5874] [Article Influence: 489.5] [Reference Citation Analysis (0)] |
| 21. | Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 326] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 22. | Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 561] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 23. | García-Juárez M, Camacho-Morales A. Defining the Role of Anti- and Pro-inflammatory Outcomes of Interleukin-6 in Mental Health. Neuroscience. 2022;492:32-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Kong X, Gong Z, Zhang L, Sun X, Ou Z, Xu B, Huang J, Long D, He X, Lin X, Li Q, Xu L, Xuan A. JAK2/STAT3 signaling mediates IL-6-inhibited neurogenesis of neural stem cells through DNA demethylation/methylation. Brain Behav Immun. 2019;79:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1869] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 26. | Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1837] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 27. | Perry BI, Upthegrove R, Kappelmann N, Jones PB, Burgess S, Khandaker GM. Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: A bi-directional two-sample mendelian randomization study. Brain Behav Immun. 2021;97:176-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 28. | Williams JA, Burgess S, Suckling J, Lalousis PA, Batool F, Griffiths SL, Palmer E, Karwath A, Barsky A, Gkoutos GV, Wood S, Barnes NM, David AS, Donohoe G, Neill JC, Deakin B, Khandaker GM, Upthegrove R; PIMS Collaboration. Inflammation and Brain Structure in Schizophrenia and Other Neuropsychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry. 2022;79:498-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 209] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 29. | Gallego JA, Blanco EA, Husain-Krautter S, Madeline Fagen E, Moreno-Merino P, Del Ojo-Jiménez JA, Ahmed A, Rothstein TL, Lencz T, Malhotra AK. Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: New data and an updated meta-analysis. Schizophr Res. 2018;202:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Kim YK, Kim L, Lee MS. Relationships between interleukins, neurotransmitters and psychopathology in drug-free male schizophrenics. Schizophr Res. 2000;44:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Luo Y, He H, Zhang J, Ou Y, Fan N. Changes in serum TNF-α, IL-18, and IL-6 concentrations in patients with chronic schizophrenia at admission and at discharge. Compr Psychiatry. 2019;90:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Golimbet V, Lezheiko T, Mikhailova V, Korovaitseva G, Kolesina N, Plakunova V, Kostyuk G. A study of the association between polymorphisms in the genes for interleukins IL-6 and IL-10 and negative symptoms subdomains in schizophrenia. Indian J Psychiatry. 2022;64:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Xu H, Wang J, Zhou Y, Chen D, Xiu M, Wang L, Zhang X. BDNF affects the mediating effect of negative symptoms on the relationship between age of onset and cognition in patients with chronic schizophrenia. Psychoneuroendocrinology. 2021;125:105121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Fernandez-Egea E, Mucci A, Lee J, Kirkpatrick B. A new era for the negative symptoms of schizophrenia. Br J Psychiatry. 2023;223:269-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 35. | Lang X, Zhou Y, Zhao L, Gu Y, Wu X, Zhao Y, Li Z, Zhang X. Differences in patterns of metabolic abnormality and metabolic syndrome between early-onset and adult-onset first-episode drug-naive schizophrenia patients. Psychoneuroendocrinology. 2021;132:105344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Ratzoni G, Gothelf D, Brand-Gothelf A, Reidman J, Kikinzon L, Gal G, Phillip M, Apter A, Weizman R. Weight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective study. J Am Acad Child Adolesc Psychiatry. 2002;41:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 166] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 37. | Coulon N, Godin O, Bulzacka E, Dubertret C, Mallet J, Fond G, Brunel L, Andrianarisoa M, Anderson G, Chereau I, Denizot H, Rey R, Dorey JM, Lançon C, Faget C, Roux P, Passerieux C, Dubreucq J, Leignier S, Capdevielle D, André M, Aouizerate B, Misdrahi D, Berna F, Vidailhet P, Leboyer M, Schürhoff F. Early and very early-onset schizophrenia compared with adult-onset schizophrenia: French FACE-SZ database. Brain Behav. 2020;10:e01495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Li ZT, Li SB, Wen JF, Zhang XY, Hummel T, Zou LQ. Early-Onset Schizophrenia Showed Similar but More Severe Olfactory Identification Impairment Than Adult-Onset Schizophrenia. Front Psychiatry. 2020;11:626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Ballageer T, Malla A, Manchanda R, Takhar J, Haricharan R. Is adolescent-onset first-episode psychosis different from adult onset? J Am Acad Child Adolesc Psychiatry. 2005;44:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Biswas P, Malhotra S, Malhotra A, Gupta N. Comparative study of neurological soft signs in schizophrenia with onset in childhood, adolescence and adulthood. Acta Psychiatr Scand. 2007;115:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Schimmelmann BG, Conus P, Cotton S, McGorry PD, Lambert M. Pre-treatment, baseline, and outcome differences between early-onset and adult-onset psychosis in an epidemiological cohort of 636 first-episode patients. Schizophr Res. 2007;95:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Hintze B, Rowicka M, Barczak A. Are Executive Functions Deficits in Early-Onset Chronic Schizophrenia More Severe than in Adult-Onset Chronic Schizophrenia? Clin Neuropsychiatry. 2022;19:54-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 43. | Cobia D, Rich C, Smith MJ, Engel Gonzalez P, Cronenwett W, Csernansky JG, Wang L. Thalamic Shape Abnormalities Differentially Relate to Cognitive Performance in Early-Onset and Adult-Onset Schizophrenia. Front Psychiatry. 2022;13:803234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 44. | Şimşek Ş, Yıldırım V, Çim A, Kaya S. Serum IL-4 and IL-10 Levels Correlate with the Symptoms of the Drug-Naive Adolescents with First Episode, Early Onset Schizophrenia. J Child Adolesc Psychopharmacol. 2016;26:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. Inflammatory Biomarkers and Risk of Schizophrenia: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry. 2017;74:1226-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 46. | Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R, Frei R, Garbani M, Globinska A, Hess L, Huitema C, Kubo T, Komlosi Z, Konieczna P, Kovacs N, Kucuksezer UC, Meyer N, Morita H, Olzhausen J, O'Mahony L, Pezer M, Prati M, Rebane A, Rhyner C, Rinaldi A, Sokolowska M, Stanic B, Sugita K, Treis A, van de Veen W, Wanke K, Wawrzyniak M, Wawrzyniak P, Wirz OF, Zakzuk JS, Akdis CA. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138:984-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 659] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 47. | Borovcanin MM, Jovanovic I, Radosavljevic G, Pantic J, Minic Janicijevic S, Arsenijevic N, Lukic ML. Interleukin-6 in Schizophrenia-Is There a Therapeutic Relevance? Front Psychiatry. 2017;8:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1395] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 49. | Kim DJ, Kim W, Yoon SJ, Go HJ, Choi BM, Jun TY, Kim YK. Effect of risperidone on serum cytokines. Int J Neurosci. 2001;111:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Chase KA, Cone JJ, Rosen C, Sharma RP. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 51. | Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E, Labad J. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology. 2014;41:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 52. | Gibson CL, Bassir Nia A, Spriggs SA, DeFrancisco D, Swift A, Perkel C, Zhong X, Mazumdar M, Fernandez N, Patel M, Kim-Schulze S, Hurd YL. Cannabinoid use in psychotic patients impacts inflammatory levels and their association with psychosis severity. Psychiatry Res. 2020;293:113380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Szabo A, Akkouh IA, Ueland T, Lagerberg TV, Dieset I, Bjella T, Aukrust P, Le Hellard S, Stavrum AK, Melle I, Andreassen OA, Djurovic S. Cannabis Use Is Associated With Increased Levels of Soluble gp130 in Schizophrenia but Not in Bipolar Disorder. Front Psychiatry. 2020;11:642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1902] [Cited by in RCA: 2403] [Article Influence: 160.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/