Published online May 19, 2024. doi: 10.5498/wjp.v14.i5.644
Revised: March 26, 2024
Accepted: April 15, 2024
Published online: May 19, 2024
Processing time: 125 Days and 4.3 Hours
Cerebral infarction (CI) is characterized by a high prevalence, disability, and mortality. Timely or improper treatment greatly affects patient prognosis.
To explore the drug efficacy of aspirin plus edaravone and to explore their effect on quality of life (QOL), anxiety and depression in CI patients.
We retrospectively analyzed the records of 124 CI patients treated between June 2019 and February 2021 who were assigned to an observation group (OG) (com
Compared with the CG, the OG had markedly better therapeutic effects, greater improvements in activities of daily living, and better alleviation in cognitive dysfunction after treatment, as well as lower posttreatment NIHSS scores and serum NSE, GFAP, S-100B, hs-CRP, IL-6, and TNF-α levels; the OG was similar to the CG in terms of adverse reactions but was better than the CG in terms of posttreatment QOL; and the OG also had lower SDS and SAS scores than the CG after treatment.
Aspirin plus edaravone had a good curative effect on CI. It can reverse cranial nerve damage in patients, improve neurological function and prognosis, and alleviate inflammation, anxiety, and depression; thus, it is considered safe and worthy of clinical application.
Core Tip: Edaravone, a commonly used free radical scavenger in clinical practice, can reduce the concentration of hydroxyl radicals and inhibit lipid peroxidation activity, ultimately achieving effects such as reducing endothelial cell damage and delaying neuronal cell death. It is currently the main drug used for the treatment of acute cerebral infarction. In addition, it can theoretically play a synergistic role with aspirin to improve therapeutic effectiveness, but there are few relevant studies to confirm our view. This study confirmed that aspirin combined with edaravone has more prominent efficacy and other clinical advantages in the treatment of cerebral infarction patients.
- Citation: Wang TS, Jing LJ. Therapeutic effect and psychological impact of aspirin plus edaravone on patients with cerebral infarction. World J Psychiatry 2024; 14(5): 644-652
- URL: https://www.wjgnet.com/2220-3206/full/v14/i5/644.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i5.644
Cerebral infarction (CI), or ischemic stroke, is a localized area of brain tissue necrosis or softening caused by cerebral atherosclerosis, vascular intimal injury or foreign body obstruction resulting in cerebrovascular lumen stenosis and regional cerebral blood oxygen deficiency[1]. The onset of the disease is often acute, with focal neurological signs peaking within minutes to hours, and there is associated functional loss in the associated sites[2]. CI has a high prevalence and high disability and fatality rates and is a serious threat to the health and life safety of elderly people, the main group at which it causes morbidity[3,4]. If not treated in time or if it is handled improperly, various neurological sequelae can occur, which can greatly affect patient prognosis[5].
At present, the clinical treatment principle for CI is to improve blood oxygen circulation in the brain as soon as possible and promote the recovery of neurological function[6]. Apart from thrombolysis, aspirin is the most effective treatment and exerts antiplatelet effects by inhibiting the release of thromboxane A2 and prostaglandins[7]. However, its long-term use was found to cause many adverse reactions, and some patients experienced no improvement or even worsening of CI symptoms after taking it. These findings may be related to the single mechanism of action of aspirin and the complex mechanism of platelet aggregation, leading to the occurrence of drug resistance[8]. Edaravone, an extensively applied free radical scavenger, can reduce the hydroxyl free radical concentration and inhibit lipid peroxidation activity to reduce vascular endothelial cell damage and delay nerve cell death and is currently the main drug used to treat acute CI[9]. In theory, its combined use with aspirin can have synergistic effects and improve therapeutic effects, but there are few related studies.
This study aimed to provide additional clinical evidence for CI treatment by analyzing the efficacy of aspirin plus edaravone therapy in 124 CI patients admitted between June 2019 and February 2021.
This study retrospectively analyzed the case records of 124 CI patients (64 men and 60 women) treated between June 2019 and February 2021 who were assigned to an observation group (OG) (combination therapy of aspirin and edaravone, 65 patients) or a control group (CG) (aspirin monotherapy, 59 patients). The inclusion criteria were as follows: (1) Met the acute CI diagnostic criteria; (2) Had an initial onset of CI; (3) Had a neurological deficit score (National Institutes of Health Stroke Scale, NIHSS) of 6 or above; (4) Had a blood pressure < 180/100 mmHg; (5) Had not undergone major surgery recently or had a hemorrhagic tendency; and (6) Had undergone neurological factor testing. The exclusion criteria were as follows: (1) Intracranial hemorrhage and no early large-area CI imaging changes; (2) Severe heart, liver, or liver insufficiency; (3) Intracranial hemorrhage within the past 3 months; (4) History of head trauma or myocardial infarction; (5) Severe trauma or active hemorrhage; (6) Platelet count < 100×109/L; and (7) Incomplete clinical data.
Both groups of patients were given routine supportive treatment, such as treatments to control blood pressure, maintain blood sugar, prevent infection, and maintain the electrolyte balance, and streptokinase thrombolysis was also performed. During this period, the keeping of real-time clinical records and nursing were performed. On this basis, CG patients were given 100 mg oral aspirin (Sichuan Pacific Pharmaceutical Co., Ltd., H51021475) once daily. Based on the treatments of the CG, OG patients received 30 mg of edaravone (Sinopharm Group Guorui Pharmaceutical Co., Ltd.; H20080056) plus 250 mL of normal saline by intravenous drip twice a day. The drug efficacy obtained in the two groups was compared after two 7-d courses of treatment.
(1) Therapeutic efficacy was evaluated and divided into recovery (the patient’s symptoms and signs basically disappeared after treatment, the NIHSS score decreased by ≥ 90% compared with that before treatment, and the degree of disability was 0); marked effectiveness (the patient’s symptoms after treatment were significantly relieved, the NIHSS score was reduced by 46% to 90% compared with that before treatment, and the degree of disability was 1% to 2%); effectiveness (the patient’s symptoms and signs were relieved to a certain extent after treatment, the NIHSS score was reduced by 18% to 46% compared with that before treatment, and the disability level was 2-3) and ineffective (the symptoms and signs and NIHSS scores of the patients after treatment did not change significantly or even worsened compared with those before treatment). The overall effective rate was the percentage of patients who were categorized as cured, markedly effective, and effective among the total number of patients; (2) Patients were scored by the NIHSS (score range: 0-42)[10] before and after treatment to assess their neurological function in the visual domain, facial paralysis domain, limb movement domain, etc. Higher scores indicate more severe neurological deficits; (3) Before and after treatment, patients’ activities of daily living (ADL) were assessed with the Barthel index[11] from the dressing, grooming, bathing, bladder function, bowel function, toilet use, transfer (bed or wheelchair), mobility, and stairs domains, with 10 items out of 100. The score is proportional to the number of ADLs; (4) Before and after treatment, the Mini Mental State Evaluation Scale (MMSE, score range: 0-30) was used for cognitive dysfunction assessment, with lower scores suggesting more serious cognitive dysfunction; (5) Fasting venous blood (3 mL) was also drawn before and after treatment, and the samples were centrifuged for biochemical analysis with a Toshiba TBA-2000FR for enzyme-linked immunosorbent assay (ELISA) quantification of neuron-specific enolase (NSE), glial fibrillary acidic protein (GFAP), and S-100B protein levels; (6) The levels of serum high-sensitivity C-reactive protein (hs-CRP), interleukin (IL)-6 and tumor necrosis factor (TNF)-α, which are inflammatory factors, were compared before and after treatment via an ELISA; (7) Adverse reactions, including skin ecchymosis, melena, nausea, dizziness, and gastric bleeding, were recorded; (8) The Medical Outcomes Study 36- Item Short Form Health Survey Scale[12], which assesses four dimensions of physical function, social function, physiological state, and psychological state, was used to assess quality of life (QOL) after treatment. A higher score suggests a better QOL; And (9) Patients’ anxiety and depression before and after treatment were assessed using the Self-rating Anxiety Scale (SAS) and Depression Scale (SDS), respectively. The scores ranged from 0-80 points; higher scores are associated with more severe anxiety and depression.
SPSS 19.0 and GraphPad 7 were used for the statistical analysis and visualization of the collected data, respectively. Enumeration data, expressed as percentages, were statistically analyzed with chi-square tests. Intergroup comparisons were conducted with Student’s t test, and post hoc comparisons were performed with paired t tests. This study used P < 0.05 as the significance level.
There were no significant differences in sex, age, body mass index, or other clinical information between OG and CG patients (P > 0.05; Table 1).
| Factors | Observation group (n = 65) | Control group (n = 59) | χ2 | P value |
| Sex | 0.002 | 0.065 | ||
| Male | 35 (53.85) | 32 (54.24) | ||
| Female | 30 (46.15) | 27 (45.76) | ||
| Age (yr) | 0.026 | 0.871 | ||
| ≤ 65 | 31 (47.69) | 29 (49.15) | ||
| > 65 | 34 (52.31) | 30 (50.85) | ||
| BMI (kg/m2) | 0.039 | 0.844 | ||
| ≤ 23 | 32 (49.23) | 28 (47.46) | ||
| > 23 | 33 (50.77) | 31 (52.54) | ||
| History of smoking | 0.009 | 0.923 | ||
| Yes | 38 (58.46) | 35 (59.32) | ||
| No | 27 (41.54) | 24 (40.68) | ||
| Diabetes | 0.009 | 0.993 | ||
| Yes | 32 (49.23) | 29 (49.15) | ||
| No | 33 (50.77) | 30 (50.85) | ||
| Hypertension | 0.084 | 0.772 | ||
| Yes | 38 (58.46) | 36 (61.02) | ||
| No | 27 (41.54) | 23 (38.98) | ||
| Drinking habit | 0.001 | 0.981 | ||
| Yes | 42 (64.62) | 38 (64.41) | ||
| No | 23 (35.38) | 21 (35.59) |
After treatment, 38, 24, and 3 patients were categorized as obtaining markedly effective, effective and ineffective treat
| Curative effect | Observation group (n = 65) | Control group (n = 59) | χ2 | P value |
| Markedly effective | 38 (58.46) | 25 (42.37) | - | - |
| Effective | 24 (36.92) | 21 (35.59) | - | - |
| Ineffective | 3 (4.62) | 13 (22.03) | - | - |
| Effectiveness of treatment | 62 (95.38) | 46 (77.97) | 8.350 | 0.004 |
The OG and CG had similar pretreatment NIHSS scores (P > 0.05); the posttreatment scores decreased in both groups, and the OG exhibited a more prominent decrease than did the CG (P < 0.05; Table 3).
| Time | Observation group (n = 65) | Control group (n = 59) | t | P value |
| Before treatment | 22.18 ± 1.01 | 22.24 ± 1.13 | 0.312 | 0.755 |
| After treatment | 11.25 ± 1.05 | 17.00 ± 1.10 | 29.772 | < 0.001 |
The pretreatment Barthel index scores were also not significantly different between the OG and CG (P > 0.05); both groups had improved Barthel index scores after treatment; and the OG had markedly greater scores than the CG (P < 0.05; Table 4).
| Time | Observation group (n = 65) | Control group (n = 59) | t | P value |
| Before treatment | 52.34 ± 1.53 | 52.42 ± 1.80 | 0.267 | 0.790 |
| After treatment | 67.20 ± 1.54 | 59.37 ± 1.68 | 27.079 | < 0.001 |
Before treatment, there was little difference in MMSE scores between the OG and CG (P > 0.05); the posttreatment MMSE scores increased in both groups, especially in the OG (P < 0.05; Table 5).
| Time | Observation group (n = 65) | Control group (n = 59) | t | P value |
| Before treatment | 21.06 ± 1.07 | 21.15 ± 1.22 | 0.438 | 0.663 |
| After treatment | 24.94 ± 1.09 | 23.20 ± 1.00 | 9.232 | < 0.001 |
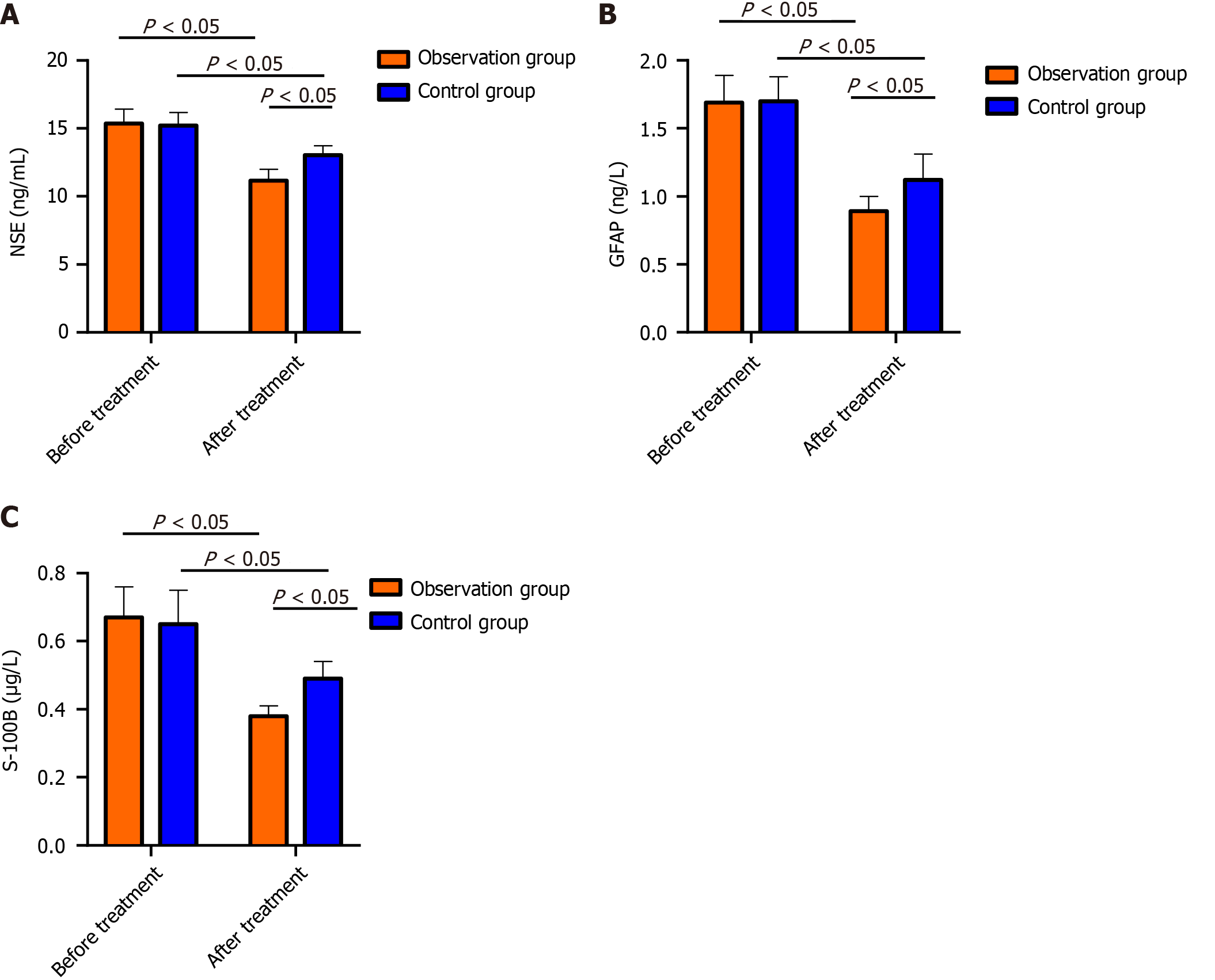
The pretreatment protein levels of NSE, GFAP and S-100B were similar in both groups (P > 0.05); after treatment, the above indicators decreased in both the OG and CG, and the levels were markedly lower in the OG than in the CG (P < 0.05; Figure 1).
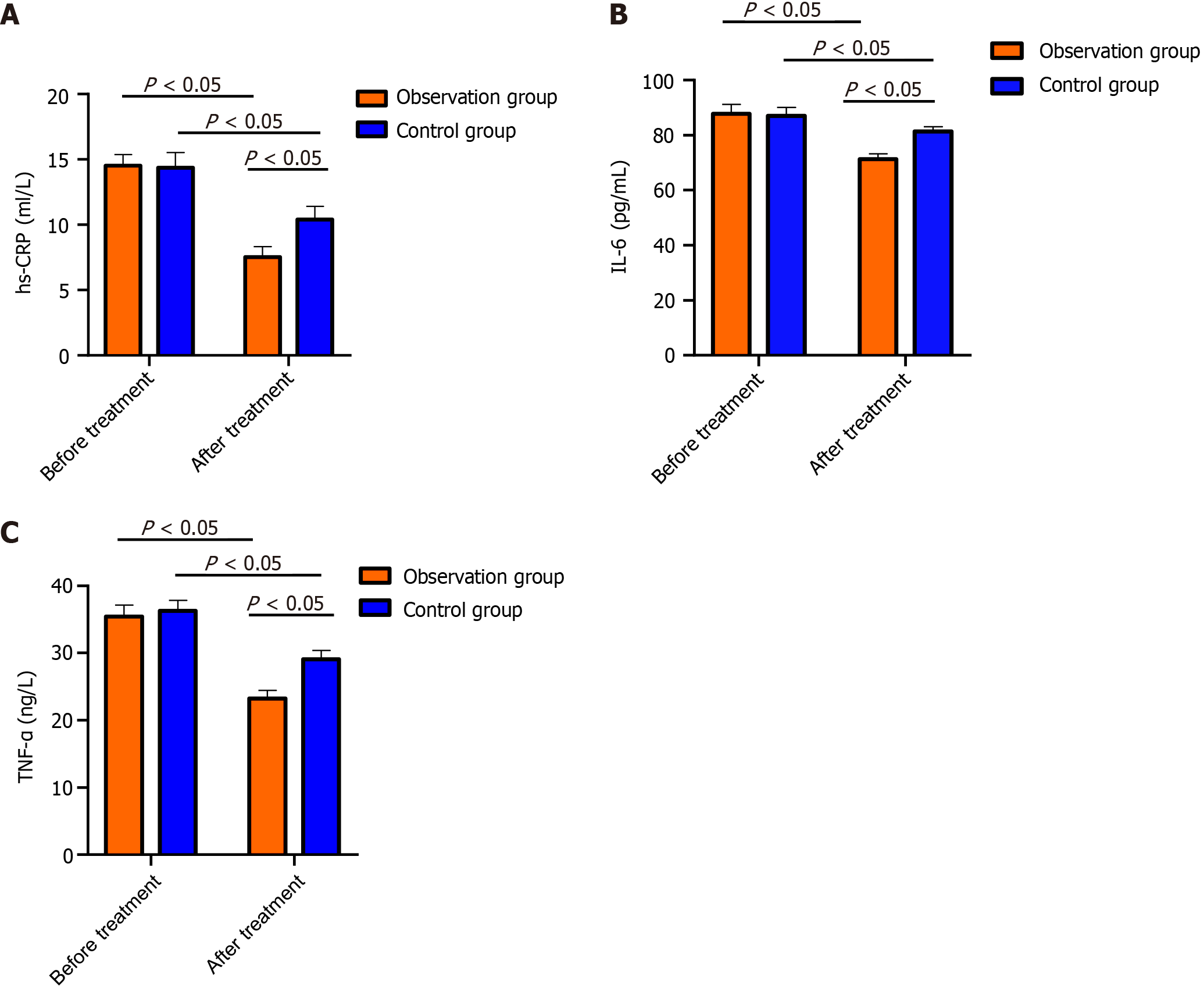
Before treatment, no marked intergroup differences were detected in TNF-α, IL-6, or hs-CRP (P > 0.05); after treatment, the above indices were lower in the OG than in the CG (P < 0.05; Figure 2).
During treatment, a slightly greater total adverse reaction rate was detected in the OG compared with the CG, but without statistical inter-group difference (P > 0.05). Patients in both groups had mild adverse reactions that subsided naturally without special treatment, which did not affect the follow-up treatment, as shown in Table 6.
| Adverse reactions | Observation group (n = 65) | Control group (n = 59) | χ2 | P value |
| Skin ecchymosis | 2 (3.08) | 1 (1.69) | - | - |
| Black stool | 2 (3.08) | 2 (3.39) | - | - |
| Dizziness | 3 (4.62) | 1 (1.69) | - | - |
| Stomach bleeding | 2 (3.08) | 1 (1.69) | - | - |
| Total incidence | 9 (13.85) | 5 (8.47) | 0.891 | 0.345 |
After treatment, the OG exhibited markedly better physical function, social function, physiological state and psychological state than did the CG (P < 0.05; Table 7).
| Quality of life | Observation group (n = 65) | Control group (n = 59) | t | P value |
| Body function | 62.28 ± 1.39 | 49.49 ± 1.60 | 47.625 | < 0.001 |
| Social function | 65.09 ± 1.44 | 51.07 ± 1.57 | 51.868 | < 0.001 |
| Physiological state | 63.25 ± 1.35 | 52.00 ± 1.13 | 50.041 | < 0.001 |
| Mental state | 65.09 ± 1.38 | 53.19 ± 1.33 | 48.788 | < 0.001 |
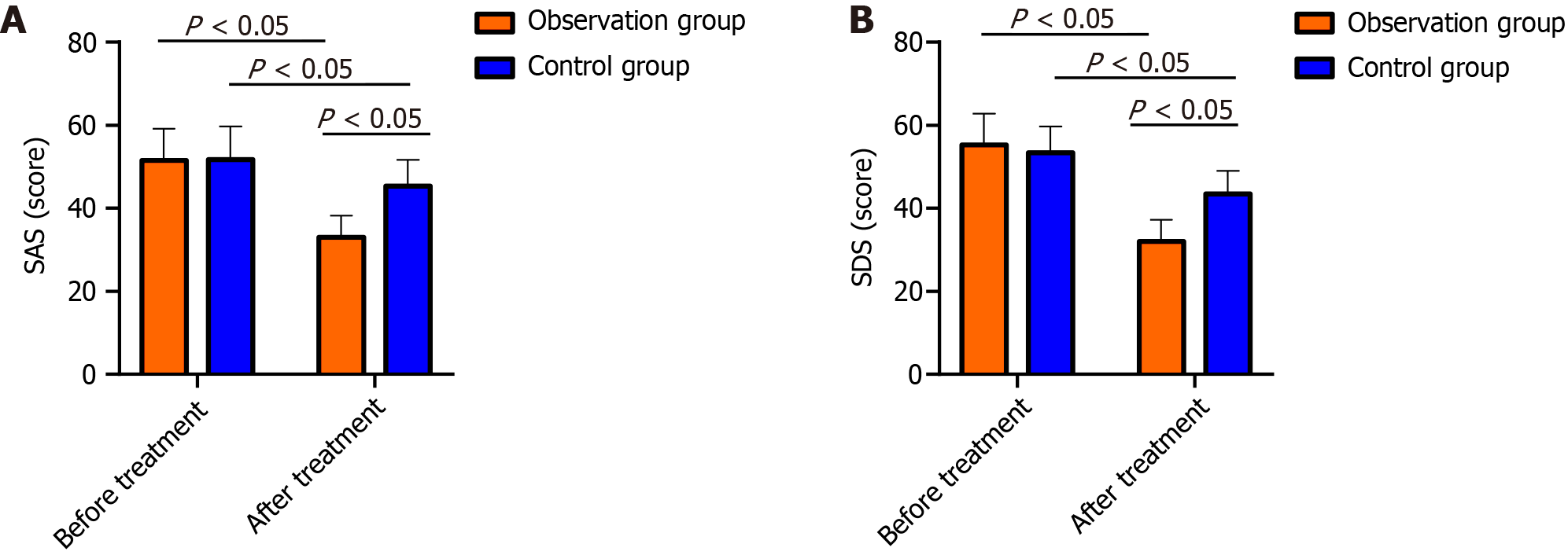
Compared with the CG, the OG had significantly lower SDS and SAS scores after treatment (P < 0.05; Figure 3).
As people’s living and eating habits continue to change and the aging population in China increases, the incidence of geriatric diseases has skyrocketed[13]. CI is a frequently occurring clinical disease commonly known as stroke that is affected by different factors and often has a high incidence, morbidity and mortality[14]. CI, characterized by acute onset and rapid disease progression, is believed to be directly associated with hypertension, arrhythmia, diabetes, obesity, etc., with a predilection for middle-aged and elderly individuals (45-70 years old). If the treatment is not timely, intracranial hypertension or severe cerebral edema are very likely to be induced, which will have a great impact on patients’ safety[15]. The pathogenesis of CI is complex, and CI exerts a serious impact on patients’ vigor and lifespans. Therefore, it is essential to adopt effective treatment measures, such as restoring nerve function and improving blood circulation to the lesion[16].
In clinical practice, antiplatelet agglutination drugs and drugs that lower blood lipid levels in patients are often used to restore the smooth flow of brain microcirculation as soon as possible and actively repair brain cell damage caused by hypoxia[17]. The repair and protection of nerve cells and their systems in the brain can effectively reduce microcirculation disturbance, reduce infarct size, and greatly relieve the symptoms of CI[18]. Aspirin is an anti-platelet aggregation drug and a key drug for CI treatment that can inhibit cyclooxygenase and reduce the conversion of arachidonic acid to thromboxane A2 in platelets, thereby inhibiting platelet aggregation and promoting blood microcirculation[19]. However, aspirin alone can lead to upper gastrointestinal bleeding or liver and kidney damage to a certain extent, which affects its efficacy[20]. Edaravone is a drug with neurotrophic, protective and reparative functions. After edaravone enters brain tissue, it can be embedded in damaged cell membranes and antagonize neurotoxic effects through the inhibition of lipid peroxidation and oxygen free radicals, and excitatory amino acids. Edaravone has unique free radical scavenging effects and can alleviate ischemia-reperfusion, ameliorate lipid peroxidation, and delay neuronal apoptosis, thus alleviating cerebral ischemia symptoms[21]. This study investigated the efficacy of aspirin plus edaravone in treating CI patients. First, we observed that, compared with those of CG patients, the therapeutic efficacy, neurological deficit scores, living ability scores and cognitive function scores of OG patients were markedly greater. This suggested that aspirin plus edaravone could improve drug synergy, improve antiplatelet aggregation while controlling the progression of the patient’s disease, and promote the recovery of damaged nerve function. Although aspirin and edaravone both have protective effects on brain tissue, their mechanisms of action are different, and combined application could reduce brain tissue damage through different mechanisms, thereby promoting the recovery of neurological function in patients[22].
NSE is a marker enzyme for nerve cell damage. When the structure of the neuronal cell membrane is damaged after brain tissue ischemia and hypoxia, NSE is released into the blood through the blood-cerebrospinal fluid barrier, causing an increase in NSE[23]. GFAP can induce an inflammatory response in acute anterior circulation CI and aggravate the development of the disease. Damaged brain tissue also leads to an increase in the level of GFAP[24]. S-100B is a marker reflecting brain injury[25]. Deduced posttreatment NSE, GFAP and S-100B protein levels were detected in both groups, with more marked reductions in the OG. Previous studies have shown that edaravone can inhibit the production of arachidonic acid and leukotrienes, relieve cerebral edema and cerebral vasospasm, protect vascular endothelial cell function, enhance cerebral microvssel circulation, and inhibit ischemic cascade reactions, thereby alleviating the symptoms of neurological deficits. In addition, edaravone can also reduce intracellular calcium overload and inhibit the toxic effects of excitatory amino acids, thereby improving nerve loss and exerting neuroprotective effects[26], which explains our observations. Inflammation, which is very important in CI pathogenesis and progression, is positively correlated with disease severity and is an independent etiological factor for assessing patient prognosis and disease outcomes[27]. Our study revealed that both groups had reduced levels of the inflammatory factors hs-CRP, TNF-α, and IL-6 after treatment, and the inflammatory factors decreased more in the OG than in the control group, suggesting that aspirin plus edaravone could improve the inflammatory response in CI patients and reduce inflammatory factor expression. Subsequently, we comparatively analyzed adverse reactions and QOL. No notable changes in the occurrence of adverse reactions were detected, while the quality of life of OG patients markedly improved after treatment compared with that of CG patients, suggesting that aspirin plus edaravone was safe and did not cause additional adverse reactions. Finally, the evaluation of patients’ negative emotions revealed that patients in the OG had significantly lower SAS and SDS than the patients in the CG after treatment, indicating that aspirin plus edaravone is more effective in relieving patients’ negative emotions.
In conclusion, aspirin plus edaravone is of good curative effect in the treatment of CI patients, which can reverse the cra
| 1. | Feske SK. Ischemic Stroke. Am J Med. 2021;134:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 527] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 2. | Herpich F, Rincon F. Management of Acute Ischemic Stroke. Crit Care Med. 2020;48:1654-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 584] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 3. | Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 406] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 4. | Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp Neurol. 2021;335:113518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 526] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 5. | Pluta R, Januszewski S, Czuczwar SJ. The Role of Gut Microbiota in an Ischemic Stroke. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 6. | Rabinstein AA. Update on Treatment of Acute Ischemic Stroke. Continuum (Minneap Minn). 2020;26:268-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | Conway J, Friedman BW. Aspirin after Acute Ischemic Stroke. Am Fam Physician. 2020;102:Online. [PubMed] |
| 8. | Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Knutsson M, Ladenvall P, Molina CA, Wang Y, Johnston SC; THALES Steering Committee and Investigators*. Ticagrelor Added to Aspirin in Acute Nonsevere Ischemic Stroke or Transient Ischemic Attack of Atherosclerotic Origin. Stroke. 2020;51:3504-3513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Ren Y, Wei B, Song X, An N, Zhou Y, Jin X, Zhang Y. Edaravone's free radical scavenging mechanisms of neuroprotection against cerebral ischemia: review of the literature. Int J Neurosci. 2015;125:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Pan Y, Li H, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Birve F, Ladenvall P, Molina CA, Johnston SC; THALES Steering Committee and Investigators. Efficacy and Safety of Ticagrelor and Aspirin in Patients With Moderate Ischemic Stroke: An Exploratory Analysis of the THALES Randomized Clinical Trial. JAMA Neurol. 2021;78:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Yang H, Chen Y, Wang J, Wei H, Jin J. Activities of daily living measurement after ischemic stroke: Rasch analysis of the modified Barthel Index. Medicine (Baltimore). 2021;100:e24926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Boudokhane S, Migaou H, Kalai A, Jellad A, Borgi O, Bouden A, Sriha Belguith A, Ben Salah Frih Z. Predictors of Quality of Life in Stroke Survivors: A 1-year Follow-Up Study of a Tunisian Sample. J Stroke Cerebrovasc Dis. 2021;30:105600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Ekkert A, Šliachtenko A, Grigaitė J, Burnytė B, Utkus A, Jatužis D. Ischemic Stroke Genetics: What Is New and How to Apply It in Clinical Practice? Genes (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Tietjen GE, Maly EF. Migraine and Ischemic Stroke in Women. A Narrative Review. Headache. 2020;60:843-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Marto JP, Strambo D, Livio F, Michel P. Drugs Associated With Ischemic Stroke: A Review for Clinicians. Stroke. 2021;52:e646-e659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | An H, Zhou B, Ji X. Mitochondrial quality control in acute ischemic stroke. J Cereb Blood Flow Metab. 2021;41:3157-3170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 17. | Lun R, Dhaliwal S, Zitikyte G, Roy DC, Hutton B, Dowlatshahi D. Comparison of Ticagrelor vs Clopidogrel in Addition to Aspirin in Patients With Minor Ischemic Stroke and Transient Ischemic Attack: A Network Meta-analysis. JAMA Neurol. 2022;79:141-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Yang Y, Zhou M, Zhong X, Wang Y, Zhao X, Liu L. Dual versus mono antiplatelet therapy for acute non-cardioembolic ischaemic stroke or transient ischaemic attack: a systematic review and meta-analysis. Stroke Vasc Neurol. 2018;3:107-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Lusk JB, Xu H, Peterson ED, Bhatt DL, Fonarow GC, Smith EE, Matsouaka R, Schwamm LH, Xian Y. Antithrombotic Therapy for Stroke Prevention in Patients With Ischemic Stroke With Aspirin Treatment Failure. Stroke. 2021;52:e777-e781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Diener HC, Chutinet A, Easton JD, Granger CB, Kleine E, Marquardt L, Meyerhoff J, Zini A, Sacco RL. Dabigatran or Aspirin After Embolic Stroke of Undetermined Source in Patients With Patent Foramen Ovale: Results From RE-SPECT ESUS. Stroke. 2021;52:1065-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Ahmad A, Khan MM, Javed H, Raza SS, Ishrat T, Khan MB, Safhi MM, Islam F. Edaravone ameliorates oxidative stress associated cholinergic dysfunction and limits apoptotic response following focal cerebral ischemia in rat. Mol Cell Biochem. 2012;367:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Yang HQ, Yin WJ, Liu K, Liu MC, Zuo XC. Renal safety evaluation of aspirin plus edaravone in patients with ischaemic stroke: a retrospective cohort study. BMJ Open. 2022;12:e055469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Isgrò MA, Bottoni P, Scatena R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:125-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 383] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 24. | Liu M, Xu Z, Wang L, Zhang L, Liu Y, Cao J, Fu Q, Li H, Lou J, Hou W, Mi W, Ma Y. Cottonseed oil alleviates ischemic stroke injury by inhibiting the inflammatory activation of microglia and astrocyte. J Neuroinflammation. 2020;17:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 25. | Kokocinska D, Wieczorek P, Partyka R, Jarzab J, Jałowiecki P, Sikora J. The diagnostic utility of S-100B protein and TPA in patients with ischemic stroke. Neuro Endocrinol Lett. 2007;28:693-698. [PubMed] |
| 26. | Hua K, Sheng X, Li TT, Wang LN, Zhang YH, Huang ZJ, Ji H. The edaravone and 3-n-butylphthalide ring-opening derivative 10b effectively attenuates cerebral ischemia injury in rats. Acta Pharmacol Sin. 2015;36:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Armstead WM, Hekierski H, Pastor P, Yarovoi S, Higazi AA, Cines DB. Release of IL-6 After Stroke Contributes to Impaired Cerebral Autoregulation and Hippocampal Neuronal Necrosis Through NMDA Receptor Activation and Upregulation of ET-1 and JNK. Transl Stroke Res. 2019;10:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Cialowicz M, Poland S-Editor: Zhang L L-Editor: A P-Editor: Zhao S