Published online Sep 19, 2023. doi: 10.5498/wjp.v13.i9.654
Peer-review started: June 30, 2023
First decision: July 18, 2023
Revised: July 25, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: September 19, 2023
Processing time: 77 Days and 1.4 Hours
It is positive to integrate and evaluate the risk factors for postpartum depression in patients with pregnancy-induced hypertension syndrome and to detect high-risk patients as early as possible, which has application value for the clinical development of personalized prevention programs and prognosis of patients.
To analyze factors related to postpartum depression in patients with pregnancy-induced hypertension and construct and evaluate a nomogram model.
The clinical data of 276 patients with pregnancy-induced hypertension admitted to Huzhou Maternity and Child Health Care Hospital between January 2017 and April 2022 were retrospectively analyzed. We evaluated the depression incidence at 6 wk postpartum. The depression group included patients with postpartum depression, and the remainder were in the non-depression group. Multivariate logistic regression analysis and the LASSO regression model were applied to analyze the factors related to postpartum depression in patients with pregnancy-induced hypertension. After that, a risk prediction model nomogram was constructed and evaluated.
Multivariate logistic regression analysis showed that vitamin A deficiency (VAD) during pregnancy and puerperium, family history of hypertension, maternal intestinal flora imbalance, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) were independent risk factors for postpartum depression in patients with pregnancy-induced hypertension (P < 0.05). We constructed the nomogram model based on these five risk factors. The area under the curve, specificity, and sensitivity of the model in predicting postpartum depression in patients with pregnancy-induced hypertension was 0.867 (95% confidence interval: 0.828–0.935), 0.676, and 0.889, respectively. The average absolute error was 0.037 (Hosmer-Lemeshow test χ2 = 10.739, P = 0.217).
VAD during pregnancy and puerperium, family history of hypertension, maternal intestinal flora imbalance, EPA, and DHA affect postpartum depression in patients with pregnancy-induced hypertension.
Core Tip: Pregnancy-induced hypertension usually manifests as an elevation of blood pressure, oedema, multiple organ damage, eclampsia, and even coma. Here, we analyzed the factors related to postpartum depression in patients with pregnancy-induced hypertension. We used vitamin A deficiency during pregnancy and puerperium, family history of hypertension, maternal intestinal flora imbalance, eicosapentaenoic acid, and docosahexaenoic acid to construct a nomogram evaluated and confirmed to have good predictive performance. It is a breakthrough in the prediction of postpartum depression in pregnancy-induced hypertension.
- Citation: Pan JW, Zhao G. Analysis of factors related to postpartum depression in pregnancy-induced hypertension syndrome patients and construction and evaluation of nomograms. World J Psychiatry 2023; 13(9): 654-664
- URL: https://www.wjgnet.com/2220-3206/full/v13/i9/654.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i9.654
Pregnancy-induced hypertension is a syndrome caused by elevated blood pressure, with a prevalence of 12%[1]. Elevation of blood pressure, oedema, multiple organ damage, eclampsia, and even coma are common clinical symptoms in patients with pregnancy-induced hypertension, which can increase the risk of maternal and perinatal death. Postpartum depression includes negative emotions such as pessimism, anxiety, loss, and ease of fright after childbirth. In severe cases, there may be suicidal tendencies and dangerous events such as harming infants or others[2]. The incidence of postpartum depression in patients with pregnancy-induced hypertension is about 9.4%, significantly higher than in non-depressed pregnant women[3]. Women can have both hypertension and depression during pregnancy and puerperium, which are a threat to maternal and infant health. The pathogenesis of postpartum depression is still unknown, and it may be related to genetic, mental, biochemical, social, and other factors. Therefore, it is practical to integrate and evaluate the risk factors for postpartum depression in patients with pregnancy-induced hypertension and to detect high-risk patients as early as possible, which is of guiding effects for the clinical development of personalized prevention programs and prognosis of patients. In this study, by analyzing the risk factors for postpartum depression in patients with pregnancy-induced hypertension, we constructed a personalized nomogram model to provide reference data for reducing the occurrence of clinical postpartum depression.
We conducted a retrospective study on 276 patients with pregnancy-induced hypertension in Huzhou Maternity and Child Health Care Hospital between January 2017 and April 2022. The enrolled patients met the following criteria: Diagnosis of pregnancy-induced hypertension[4]; age ≥ 18 years; and complete clinical data, laboratory examination data, and 5-HT1A receptor gene C (-1019)G [5-HTR1AC(-1019)G] detection data. The exclusion criteria were: Abnormal cognitive function; malignant tumours, myocardial infarction, stroke, or other serious diseases; inability to communicate smoothly with language; or prenatal history of mental illness or family history.
We collected the clinical data by consulting the electronic medical records of patients, including gestational weeks of pregnancy-induced hypertension, adverse delivery outcomes, adverse delivery history, family support status, vitamin A deficiency (VAD) during pregnancy and puerperium, complications, family history of hypertension, delivery mode, delivery times, education level, planned pregnancy, occupational status during pregnancy and puerperium, feeding mode, 5-HTR1AC(-1019)G, intestinal flora imbalance during pregnancy and puerperium, weight gain during late pregnancy, age, family monthly income during pregnancy and puerperium, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), arachidonic acid (AA), hypersensitive C-reactive protein (Hs-CRP), and procalcitonin (PCT).
Adverse birth outcomes included premature birth (gestational age < 37 wk), birth defects, low weight, asphyxia, and infection. The family member support score was measured using the social support rating scale developed by Xiao Shuiyuan to evaluate the support of family members (parents, spouses, compatriots, children, and one other). The total score was 4–16 points, divided into four levels: Full strength, general, very little, and no support. The higher the score, the higher the level of family support[5]. In smears of faecal samples taken from patients during pregnancy and puerperium, a large number of suppressed proto-flora, a decrease in the total number of bacteria, an increase in Gram-negative bacteria, or a decrease/increase in Gram-positive bacteria indicated intestinal flora imbalance.
Weight gain in the third trimester was weight in the third trimester minus the initial weight. The boundaries for the classification of pregnant women with normal and overweight initial weight were an insufficient increase, < 11.5 and < 7 kg; sufficient increase, 11.5-16 and 7-11.5 kg; and excess increase, > 16 and > 11.5 kg[6]. At 16 wk of gestation and 6 wk after delivery, the venous peripheral blood serum VA content was determined by HPLC. Serum VA concentration < 0.7 μmol/L was determined as VAD[7]. Complications included thyroid disease, kidney disease, heart disease, and diabetes. The concentration of EPA, DHA, and AA in venous blood serum was determined by the double antibody sandwich method on day 3 after delivery. Hs-CRP and PCT were measured by Roche Cobas 8000C701 biochemical analyzer and Roche E411 Luminescence analyzer, respectively.
At 6 wk postpartum, the Edinburgh Postpartum Depression Scale (EPDS)[8] was used to assess depression status. The total score was 0-30 points. The higher the score, the more serious the depression. Patients with EPDS scores ≥ 13 were diagnosed with postpartum depression. Women who met the criteria for postpartum depression fell into the depression group, and the remainder into the non-depression group.
We analyzed and processed the data using R version 4.0.3 and performed LASSO regression analysis with the glmnet package. We used the rms package to draw the nomogram and calibration curve and the predictive receiver operating characteristic curve (ROC) package to draw the ROC. We verified the resulting nomogram model by bootstrap self-sampling 500 times and evaluated its efficacy by the area under the ROC curve (AUC). The test level was α = 0.05.
Among the 276 patients with pregnancy-induced hypertension, 54 were in the depression group and 222 in the non-depression group. The incidence of postpartum depression in patients with pregnancy-induced hypertension was 19.57% (54/276). We compared the number of gestational weeks, adverse delivery outcomes, family support status, VAD during pregnancy and puerperium, complications, family history of hypertension, maternal intestinal flora imbalance, weight gain in the third trimester, EPA and DHA between the two groups (Table 1).
| Variables | Depression group (n = 54) | Non-depression group (n = 222) | t/χ2/Z | P value |
| Gestational weeks of pregnancy-induced hypertension | -2.902 | 0.004 | ||
| < 28 | 20 (37.04) | 58 (26.13) | ||
| 28–34 | 30 (55.56) | 100 (45.05) | ||
| > 34 | 4 (7.41) | 64 (28.83) | ||
| Adverse birth outcomes | 16.283 | < 0.001 | ||
| Yes | 16 (29.63) | 20 (9.01) | ||
| No | 38 (70.37) | 202 (90.99) | ||
| History of adverse childbirth | 0.310 | 0.578 | ||
| Yes | 8 (14.81) | 40 (18.02) | ||
| No | 46 (85.19) | 182 (81.98) | ||
| Family support status | -3.349 | 0.001 | ||
| Very little or no support | 6 (11.11) | 4 (1.80) | ||
| General support | 26 (48.15) | 78 (35.14) | ||
| Full support | 22 (40.74) | 140 (63.06) | ||
| VAD during pregnancy and puerperium | 29.504 | < 0.001 | ||
| Yes | 14 (25.93) | 8 (3.60) | ||
| No | 40 (74.07) | 214 (96.40) | ||
| Complications | 12.325 | < 0.001 | ||
| Yes | 28 (51.85) | 60 (27.03) | ||
| No | 26 (48.15) | 162 (72.97) | ||
| Asthma during pregnancy | 3.140 | 0.076 | ||
| Yes | 10 (18.52) | 22 (9.91) | ||
| No | 44 (81.48) | 200 (90.09) | ||
| Family history of hypertension | 15.471 | < 0.001 | ||
| Yes | 12 (22.22) | 12 (5.41) | ||
| No | 42 (77.78) | 210 (94.59) | ||
| Mode of delivery | -0.285 | 0.775 | ||
| Cesarean section | 16 (29.63) | 74 (33.33) | ||
| Natural birth | 32 (59.26) | 120 (54.05) | ||
| Vaginal midwifery | 6 (11.11) | 28 (12.61) | ||
| Number of deliveries (times) | 0.591 | 0.442 | ||
| 1 (primiparous) | 32 (59.26) | 144 (64.86) | ||
| ≥ 2 (multiparous) | 22 (40.74) | 78 (35.14) | ||
| Education level | -1.490 | 0.136 | ||
| High school and below | 18 (33.33) | 52 (23.42) | ||
| Junior college | 24 (44.44) | 106 (47.75) | ||
| Bachelor's degree and above | 12 (22.22) | 64 (28.83) | ||
| Plan a pregnancy | 0.409 | 0.522 | ||
| No | 10 (18.52) | 50 (22.52) | ||
| Yes | 44 (81.48) | 172 (77.48) | ||
| Occupational status during pregnancy | -0.459 | 0.646 | ||
| Not in office | 10 (18.52) | 50 (22.52) | ||
| Intermittent rest | 32 (59.26) | 124 (55.86) | ||
| On-the-job | 12 (22.22) | 48 (21.62) | ||
| Feeding methods | -0.180 | 0.857 | ||
| Artificial feeding | 10 (18.52) | 46 (20.72) | ||
| Mixed feeding | 24 (44.44) | 94 (42.34) | ||
| Pure breastfeeding | 20 (37.04) | 82 (36.94) | ||
| 5-HTR1AC (-1019) G | ||||
| Genotype | -1.269 | 0.205 | ||
| GG | 30 (55.56) | 117 (52.70) | ||
| CG | 12 (22.22) | 17 (7.66) | ||
| CC | 12 (22.22) | 88 (39.64) | ||
| Allelic | 3.676 | 0.055 | ||
| G | 72 (66.67) | 251 (56.63) | ||
| C | 36 (33.33) | 193 (43.47) | ||
| Intestinal flora imbalance during pregnancy and childbirth | 16.283 | < 0.001 | ||
| Yes | 16 (29.63) | 20 (9.01) | ||
| No | 38 (70.37) | 202 (90.99) | ||
| Weight gain in late pregnancy | -2.122 | 0.034 | ||
| Increase excess | 22 (40.74) | 44 (19.82) | ||
| Insufficient increase | 12 (22.22) | 80 (36.04) | ||
| Adequate increase | 20 (37.04) | 98 (44.14) | ||
| Age (years) | 33.25 ± 9.46 | 32.87 ± 10.64 | 0.240 | 0.810 |
| Maternal family monthly income (yuan) | 3766.15 ± 1348.26 | 4200.85 ± 1622.26 | 1.821 | 0.070 |
| EPA (pg/mL) | 1.75 ± 0.31 | 2.02 ± 0.33 | 7.475 | < 0.001 |
| DHA (pg/mL) | 1.83 ± 0.27 | 2.08 ± 0.36 | 4.784 | < 0.001 |
| AA (lgx, pg/mL) | 1.08 ± 0.25 | 0.98 ± 0.36 | 1.930 | 0.055 |
| Hs-CRP (mg/L) | 21.57 ± 5.39 | 22.04 ± 5.12 | 0.599 | 0.550 |
| PCT (μg/L) | 2.02 ± 0.59 | 1.95 ± 0.46 | 0.946 | 0.345 |
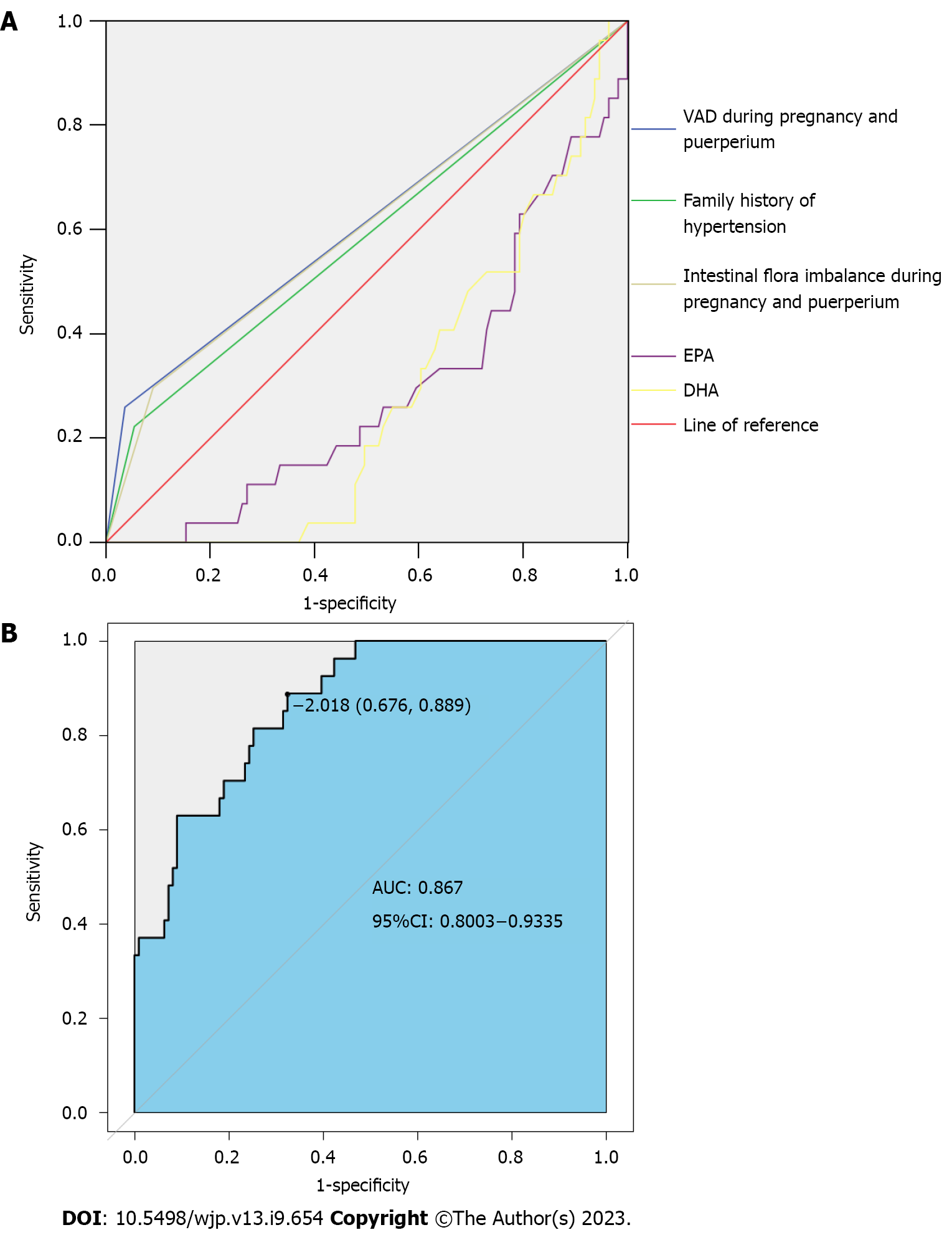
We put independent variables (P < 0.05) and dependent variables (grouping, 0 = non-depression group, 1 = depression group) into a multivariate logistic regression analysis model (Table 2). VAD during pregnancy and puerperium, family history of hypertension, intestinal flora imbalance during pregnancy and puerperium, EPA, and DHA were independent risk factors for postpartum depression (P < 0.05) (Table 3). The AUC of VAD during pregnancy and puerperium, family history of hypertension, intestinal flora imbalance during pregnancy and puerperium, EPA, and DHA in predicting postpartum depression in patients with pregnancy-induced hypertension were 0.612, 0.584, 0.603, 0.293, and 0.281, respectively. The best cut-off values of EPA and DHA were 1.82 pg/mL and 2.18 pg/mL, respectively. Figure 1A shows the ROC curve of each index to predict postpartum depression in patients with pregnancy-induced hypertension.
| Variable | Assignment |
| Gestational weeks of pregnancy-induced hypertension | 0 > 34; 1, 28–34; 2 < 28. |
| Adverse birth outcomes | 0 = No, 1 = Yes |
| Family support status | 0 = full support, 1 = general support, 2 = little or no support |
| VAD during pregnancy and puerperium | 0 = No, 1 = Yes |
| Complications | 0 = none, 1 = have |
| Family history of hypertension | 0 = none, 1 = have |
| Intestinal flora imbalance during pregnancy and childbirth | 0 = none, 1 = have |
| Weight gain in late pregnancy | 0 = increase sufficient, 1 = increase insufficient, 2 = increase excessive |
| EPA | Actual value |
| DHA | Actual value |
| Independent variable | B | Wald | SE | P value | OR (95%CI) |
| Gestational weeks of pregnancy-induced hypertension | - | - | 4.062 | 0.131 | - |
| No. of weeks of pregnancy (1) | -1.564 | 0.816 | 3.678 | 0.055 | 0.209 (0.042–1.035) |
| Gestational weeks of pregnancy-induced hypertension (2) | -0.592 | 0.457 | 1.677 | 0.195 | 0.553 (0.226–1.355) |
| Adverse birth outcomes | 0.261 | 0.684 | 0.145 | 0.703 | 1.298 (0.340–4.959) |
| Family support status | - | - | 1.545 | 0.462 | - |
| Family support status (1) | -1.365 | 1.155 | 1.397 | 0.237 | 0.255 (0.027–2.456) |
| Family support status (2) | -0.630 | 0.898 | 0.492 | 0.483 | 0.533 (0.092–3.095) |
| VAD during pregnancy and puerperium | 2.159 | 0.642 | 11.296 | 0.001 | 8.662 (2.459–30.506) |
| Complications | -0.526 | 0.73 | 0.520 | 0.471 | 0.591 (0.141–2.470) |
| Family history of hypertension | 1.868 | 0.675 | 7.667 | 0.006 | 6.474 (1.726–24.289) |
| Intestinal flora imbalance during pregnancy and childbirth | 1.877 | 0.559 | 11.273 | 0.001 | 6.535 (2.185–19.551) |
| Weight gain in late pregnancy | - | - | 4.060 | 0.131 | - |
| Weight gain in late pregnancy (1) | -0.977 | 0.490 | 3.980 | 0.046 | 0.377 (0.144–0.983) |
| Weight gain in late pregnancy (2) | -0.359 | 0.538 | 0.446 | 0.504 | 0.698 (0.243–2.003) |
| EPA | -3.125 | 0.780 | 16.04 | < 0.001 | 0.044 (0.010–0.203) |
| DHA | -2.568 | 0.644 | 15.88 | < 0.001 | 0.077 (0.022–0.271) |
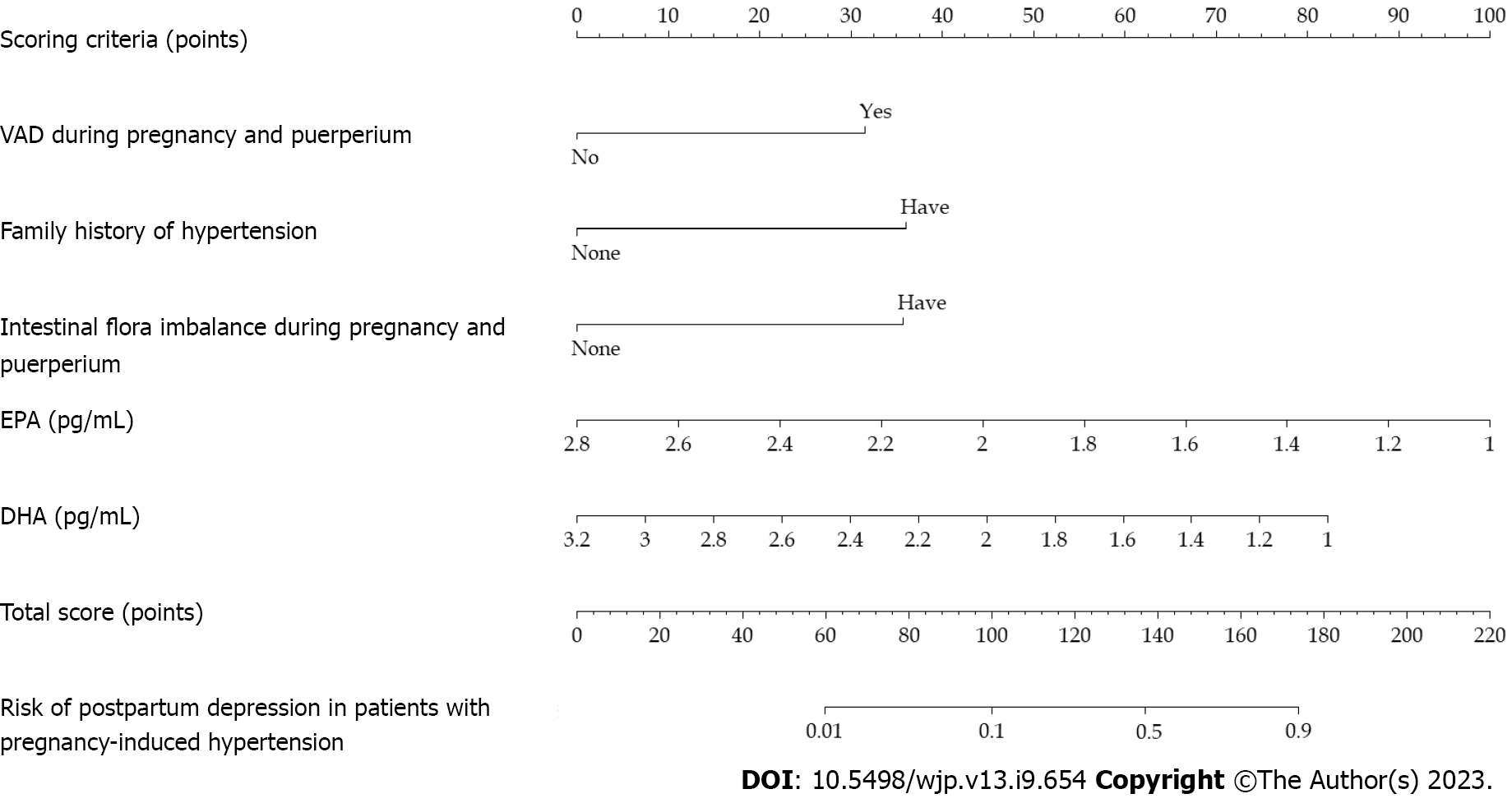
The independent risk factors for postpartum depression in patients with pregnancy-induced hypertension were used as predictors to construct a nomogram model (Figure 2). The internal verification was carried out by bootstrap self-sampling 500 times. The slope of the calibration curve was similar to that of the diagonal line, suggesting that the degree of fitting between the calibration curve and the ideal curve was high, indicating that there was no significant difference between the predicted and measured values. The average absolute error (0.037) was small, which suggested that the nomogram model had a predictive effect (Figure 3).
The AUC predicted by the nomogram for each index was 0.867 greater than the AUC of each index in Figure 1A. The specificity and sensitivity were 0.676 and 0.889, respectively. The Hosmer–Lemeshow test statistic was 10.739, and there was no significant difference between the predicted value and actual (P = 0.217) (Figure 1B).
Postpartum depression in patients with pregnancy-induced hypertension can cause neurological and endocrine system disorders, often making them lose their ability to care for themselves and their infants, which is not conducive to them and their infant’s health[9]. Other studies have shown that for infants aged 1.5-2 mo, maternal postpartum depression may lead to developmental delays in gross motor, fine motor, and communication areas. Research has suggested that postpartum depression harms mothers and adversely affects children’s cognition, behaviour, temperament, and physical development[10]. The risk of postpartum depression in patients with pregnancy-induced hypertension is higher than that in normal pregnant, which may be related to many factors such as VAD during pregnancy and puerperium, family history of hypertension, and intestinal flora imbalance during pregnancy and puerperium. Therefore, integrating the risk factors for postpartum depression in patients with pregnancy-induced hypertension is of clinical significance for identifying high-risk patients.
The postpartum depression incidence in 138 patients with pregnancy-induced hypertension was 19.57%, similar to previous studies[11]. Multivariate logistic regression analysis showed that patients with pregnancy-induced hypertension and VAD during pregnancy and puerperium, family history of hypertension, maternal intestinal flora imbalance, low EPA level, or low DHA level had an increased risk of postpartum depression. We speculated that progesterone level in pregnant women in early pregnancy decreases. Because progesterone can promote the release of VA stored in the liver and adipose tissue into blood, this function of progesterone is weakened after progesterone deficiency, resulting in a decrease in serum VA concentration and the development of VAD. VAD during puerperium may also be related to insufficient progesterone secretion. Long-term continuous VAD during pregnancy and puerperium can promote postpartum depression[12]. In the present study, the proportion of VAD during pregnancy and puerperium in depressed patients was higher than in non-depressed patients, indicating that perinatal VAD also increased the risk of postpartum depression in patients with pregnancy-induced hypertension. There is no consensus on the mechanism of VAD in postpartum depression development. Some scholars believe that VA can affect the proliferation and differentiation of nerve cells, synapse formation, and axon growth. Studies have also found that VAD may be the basis of the pathogenesis of Alzheimer’s disease, which can increase hippocampal Aβ deposition, resulting in hippocampal plasticity and memory function[13]. Therefore, VA is involved in related activities of cranial nerves. These activities may be factors that maintain a good mood. Once the body has continuous VAD, it may increase the incidence of depression. For pregnancy-induced hypertension patients with a family history of hypertension, the psychological burden may be aggravated by similar diseases in their relatives, resulting in anxiety, such as poor pregnancy and poor fetal birth due to unsatisfactory blood pressure control, so it is more likely to induce postpartum depression. Research has also shown a significant difference in the family history of hypertension between the postpartum depression group and the non-depression group of pregnant women with gestational hypertension[14], which is consistent with the results of our study. In recent years, more studies on the mechanism of postpartum depression have shifted from social environmental factors to biological factors (such as gene polymorphism). Abnormal intestinal flora structure can cause abnormal fluctuations in the central nervous system, thus promoting the occurrence of mental diseases such as depression. Intestinal flora imbalance can induce an immune response, and then the hypothalamus-pituitary-adrenal (HPA) axis is abnormally active, and depression occurs. Relevant studies have also shown that after bifidobacteria supplementation to people with intestinal flora imbalance, the HPA axis activity weakens. Also, the concentration of neurotrophic factors in the brain that regulate neuronal development increases, and the HPA axis improves[15]. Therefore, intestinal flora imbalance is a risk factor for postpartum depression in patients with pregnancy-induced hypertension. Clinically, we can reduce the occurrence of postpartum depression by improving the composition of intestinal flora in patients with pregnancy-induced hypertension. EPA and DHA are fatty acids with multiple double bonds and are chief components of phospholipids in the human brain. Phospholipids ensure the integrity and fluidity of nerve cell membranes and participate in the signal transduction of various substances in nerve cells. Therefore, EPA and DHA are closely related to the brain's nervous system. The lack of both may cause neurological and psychological disorders and damage the cerebellum, vision, and cognition. The lower the level of EPA or DHA, the more prone patients are to depression. Serum EPA and DHA levels affect the formation of postpartum depression and negative correlation[16,17]. These show that ensuring adequate EPA or DHA intake during pregnancy may be a protective factor for postpartum depression in patients with pregnancy-induced hypertension. This study also analyzed the 5-HTR1AC (-1019) G genotype and allele in patients with pregnancy-induced hypertension. The results showed that there was no difference in the gene polymorphism of this locus between patients with postpartum depression and non-depressed patients, which was inconsistent with relevant research[18]. It may be related to the different sample sizes and inclusion/exclusion criteria of the two studies. Therefore, we speculated that the occurrence of postpartum depression in patients with pregnancy-induced hypertension may be slightly influenced by biological factors.
This study summarized the risk factors related to postpartum depression in patients with pregnancy-induced hypertension and applied these five risk factors as predictive variables to construct a predictive nomogram model. After the model verification, we found that the degree of fitting between the calibration and the ideal curve was high. The AUC predicted by the nomogram model was higher, indicating that the model had better discriminant ability. The Hosmer–Lemeshow test showed that the predictive data of the model were not much different from the actual data, suggesting that the model had high calibration or predictive accuracy. The nomogram model showed the relationship between the five variables of VAD during pregnancy and puerperium, family history of hypertension, intestinal flora imbalance during pregnancy and puerperium, EPA, and DHA with the calibrated lines, which was readable and easy to evaluate. This study explored the nomogram model of postpartum depression in post-inflammatory hyperpigmentation (PIH) patients, which can help clinicians quickly and accurately identify patients with PIH who are at risk of depression after high production, help guide them to take targeted intervention measures, and thus reduce the possibility of postpartum depression in patients. It has high clinical application value.
There were some limitations to this study. All the women were from a single centre, and there may have been potential selection bias. The risk prediction nomogram model for postpartum depression in patients with pregnancy-induced hypertension was only internally validated, and there was a lack of external validation from other central data. Therefore, we need sufficient external evidence before the practical application of the model.
Postpartum depression in patients with pregnancy-induced hypertension is affected by VAD during pregnancy and puerperium, family history of hypertension, intestinal flora imbalance during pregnancy, and puerperium, EPA, and DHA. The established nomogram model can effectively assess its risk.
The clinical prediction of postpartum depression in patients with pregnancy-induced hypertension is still insufficient, and the application of the nomogram model in predicting postpartum depression in patients with pregnancy-induced hypertension is rarely reported.
Compared with normal pregnant, pregnancy-induced hypertension patients have a higher risk of postpartum depression, which is related to several factors. By integrating risk factors of postpartum depression in pregnancy-induced hypertension and constructing predictive models, this study guides identifying high-risk patients and early clinical intervention.
The study's purpose was to integrate the risk factors of postpartum depression in patients with pregnancy-induced hypertension, construct a graph prediction model, and evaluate the predictive effect of the model.
Multivariate logistic regression analysis and LASSO regression were used to analyze the factors related to postpartum depression in pregnancy-induced hypertension. R version 4.0.3 was used to construct a line graph risk predictive model. The area under the receiver operating curve was used to evaluate effectiveness.
Vitamin A deficiency (VAD) during pregnancy and puerperium, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are independent risk factors for postpartum depression in pregnancy-induced hypertension. The histogram model established by this method had good predictive efficacy and could guide clinical prevention and intervention.
Postpartum depression in pregnancy-induced hypertension is related to VAD during pregnancy and puerperium, family history of hypertension, intestinal flora disorders during pregnancy and perinatal period, EPA, and DHA. The predictive efficacy of the risk model established by this method has clinical application value.
Future research directions should increase the sample size or multicenter study to verify the results, enhance the reliability of the conclusions, and better carry out clinical prevention interventions.
| 1. | Zhou Z, Deng C, Xiang X. Blood glucose related to pregnancy induced hypertension syndrome. Am J Transl Res. 2021;13:5301-5307. [PubMed] |
| 2. | Wallace K, Bean C, Bowles T, Spencer SK, Randle W, Kyle PB, Shaffery J. Hypertension, Anxiety, and Blood-Brain Barrier Permeability Are Increased in Postpartum Severe Preeclampsia/Hemolysis, Elevated Liver Enzymes, and Low Platelet Count Syndrome Rats. Hypertension. 2018;72:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | March WA, Whitrow MJ, Davies MJ, Fernandez RC, Moore VM. Postnatal depression in a community-based study of women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2018;97:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Stepan H, Hund M, Andraczek T. Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia: The Angiogenic-Placental Syndrome. Hypertension. 2020;75:918-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 5. | Ribé JM, Salamero M, Pérez-Testor C, Mercadal J, Aguilera C, Cleris M. Quality of life in family caregivers of schizophrenia patients in Spain: caregiver characteristics, caregiving burden, family functioning, and social and professional support. Int J Psychiatry Clin Pract. 2018;22:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Lin LH, Weng YL, Lin YY, Huang XX, Lin Y, Xiu XY, Yan JY, Lin J. Examining the effects of second-and third-trimester gestational weight gain rates on the perinatal outcomes among Chinese twin pregnancies: a retrospective cohort study. BMC Pregnancy Childbirth. 2022;22:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Alves JG, Souza ASR, Figueiroa JN, de Araújo CAL, Guimarães A, Ray JG. Visceral Adipose Tissue Depth in Early Pregnancy and Gestational Diabetes Mellitus - a Cohort Study. Sci Rep. 2020;10:2032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Matsuoka H, Iwami S, Maeda M, Suizu A, Fujii T. Edinburgh Postnatal Depression Scale scores at 2-week post-partum may reflect those at 4-week post-partum: A single-center retrospective observational study. J Obstet Gynaecol Res. 2021;47:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Schaadt G, Zsido RG, Villringer A, Obrig H, Männel C, Sacher J. Association of Postpartum Maternal Mood With Infant Speech Perception at 2 and 6.5 Months of Age. JAMA Netw Open. 2022;5:e2232672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Gou M, Li L, Fu W, Gong X, Wei Y, Zhou G, Schwarzer R. Prenatal maternal depressive symptoms of Chinese pregnant women and twin newborns' physical health: the moderating role of infant sex. Psychol Health Med. 2022;27:1682-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Ye Y, Chen L, Xu J, Dai Q, Luo X, Shan N, Qi H. Preeclampsia and Its Complications Exacerbate Development of Postpartum Depression: A Retrospective Cohort Study. Biomed Res Int. 2021;2021:6641510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Ribamar A, Almeida B, Soares A, Peniche B, Jesus P, Cruz SPD, Ramalho A. Relationship between vitamin D deficiency and both gestational and postpartum depression. Nutr Hosp. 2020;37:1238-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Chen BW, Zhang KW, Chen SJ, Yang C, Li PG. Vitamin A Deficiency Exacerbates Gut Microbiota Dysbiosis and Cognitive Deficits in Amyloid Precursor Protein/Presenilin 1 Transgenic Mice. Front Aging Neurosci. 2021;13:753351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Roberts L, Davis GK, Homer CSE. Depression, Anxiety, and Post-traumatic Stress Disorder Following a Hypertensive Disorder of Pregnancy: A Narrative Literature Review. Front Cardiovasc Med. 2019;6:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Jahnke JR, Roach J, Azcarate-Peril MA, Thompson AL. Maternal precarity and HPA axis functioning shape infant gut microbiota and HPA axis development in humans. PLoS One. 2021;16:e0251782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Urech C, Eussen SRBM, Alder J, Stahl B, Boehm G, Bitzer J, Bartke N, Hoesli I. Levels of n-3 and n-6 Fatty Acids in Maternal Erythrocytes during Pregnancy and in Human Milk and Its Association with Perinatal Mental Health. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Osuna E, Symington EA, Malan L, Ricci C, Zandberg L, Smuts CM, Baumgartner J. Higher n-3 polyunsaturated fatty acid status during early pregnancy is associated with lower risk for depression at 12 months postpartum: The NuPED study. Prostaglandins Leukot Essent Fatty Acids. 2023;190:102528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Zhang XL, Li JF, Wang Xia, Zhang YZ. [Effects of postpartum depression on serotonin 1A receptor gene C(-1019)G polymorphism and environmental factors]. Zhongguo Fuyoubaojian. 2014;29:448-450. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kathirvel N, Singapore; Tulloch HE, Canada S-Editor: Fan JR L-Editor: A P-Editor: Fan JR